Substrate Selection of Ascidian Larva: Wettability and Nano-Structures
Abstract
1. Introduction
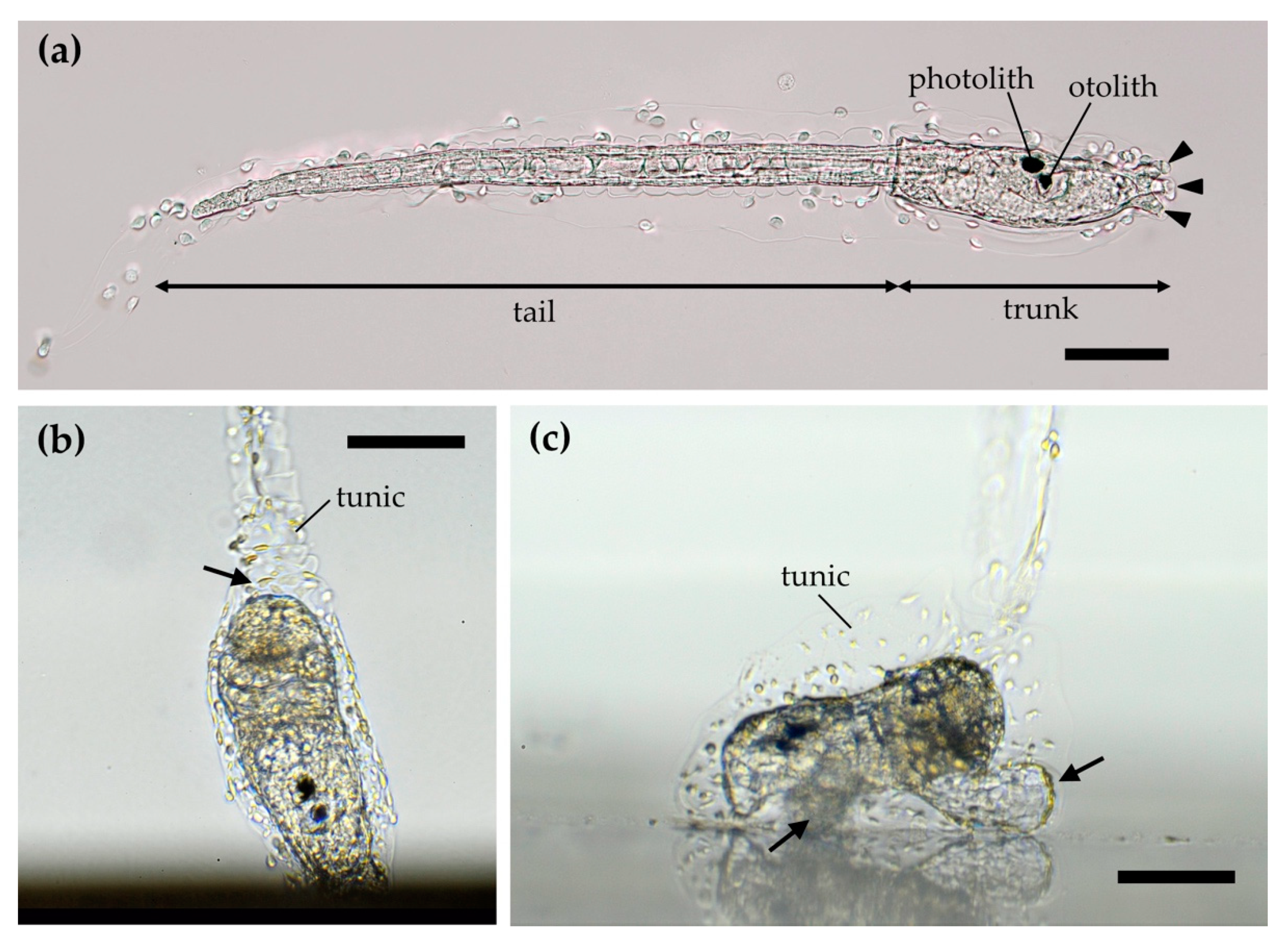
2. Ascidians and Their Larvae
3. Water Wettability
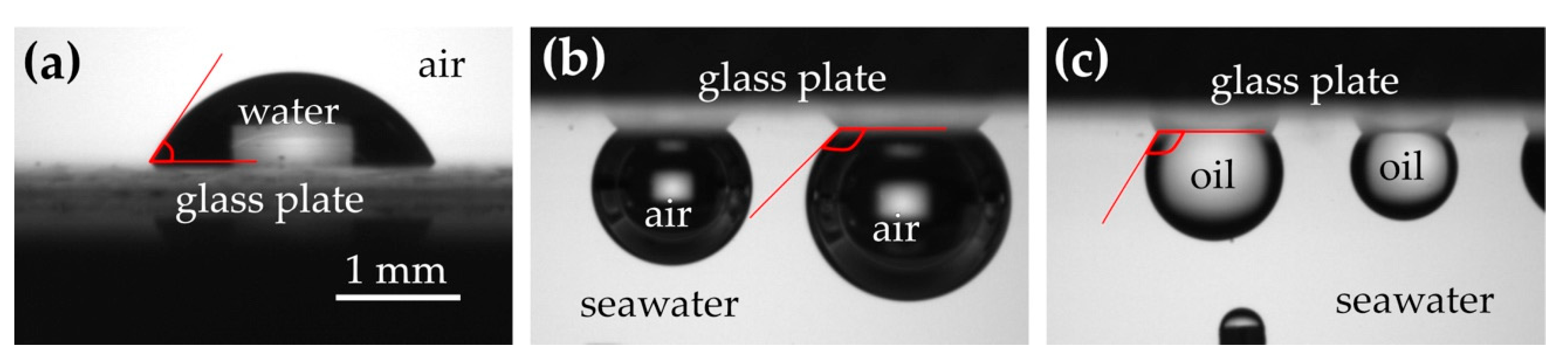
3.1. Measurement of Wettability
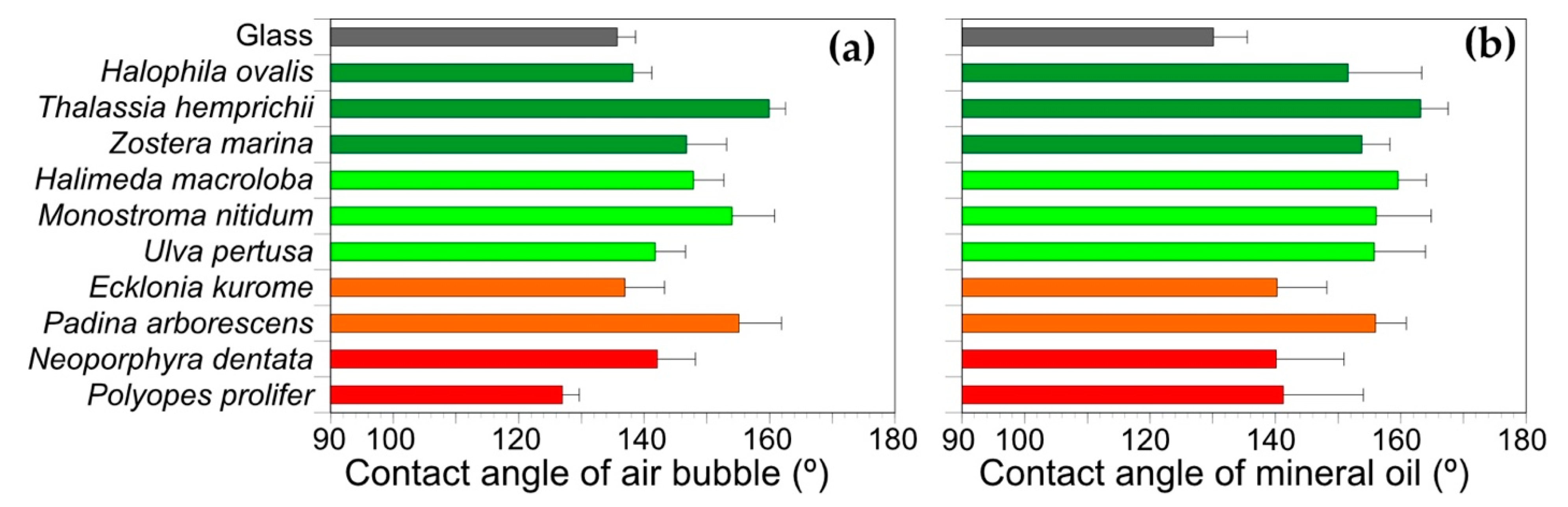
3.2. Substrate Preference in Wettability
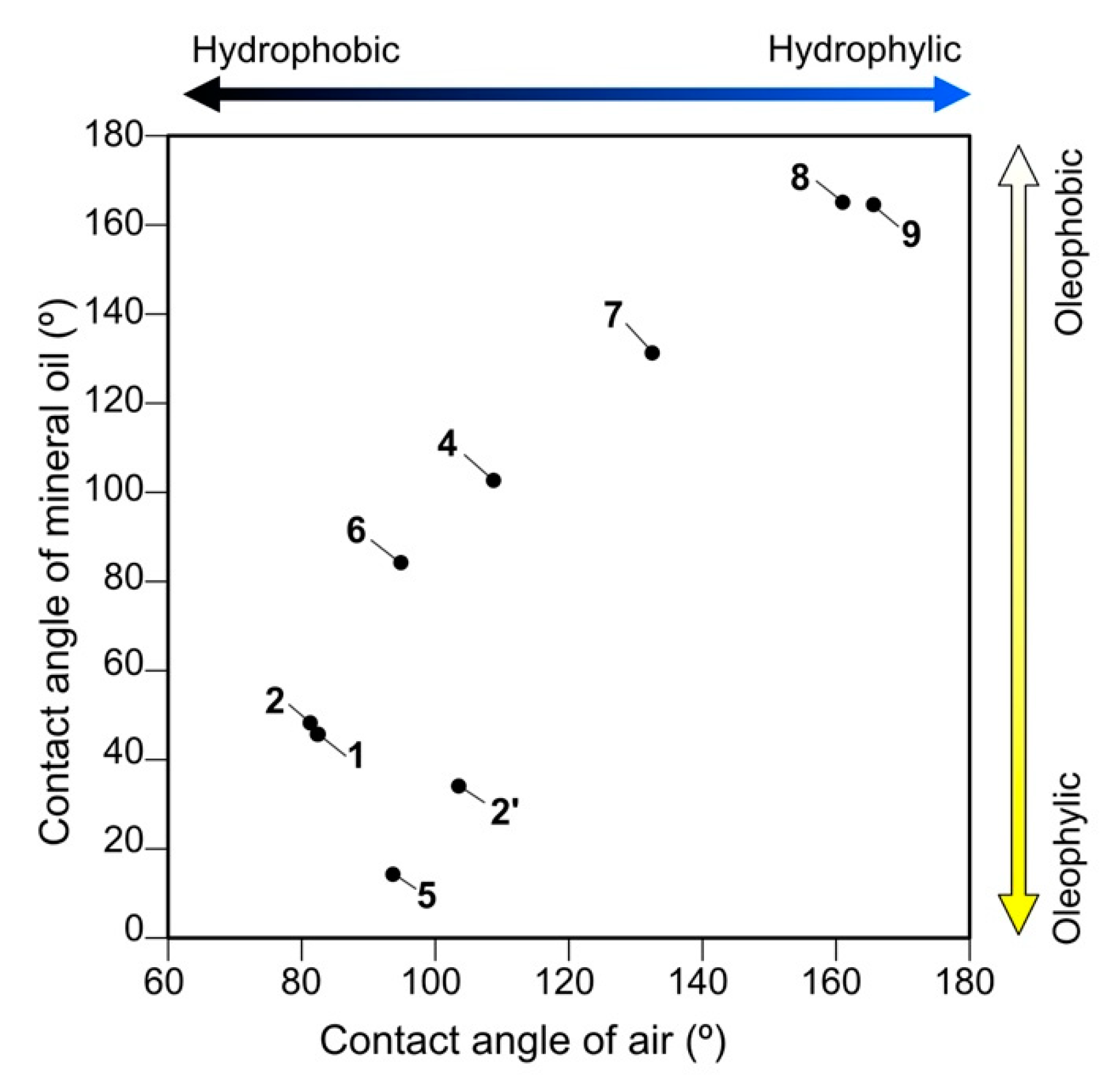
3.3. “Silicone Paradox”
4. Surface Structures
4.1. Preference in Surface Structure
4.2. Nano-Scale Nipple Array
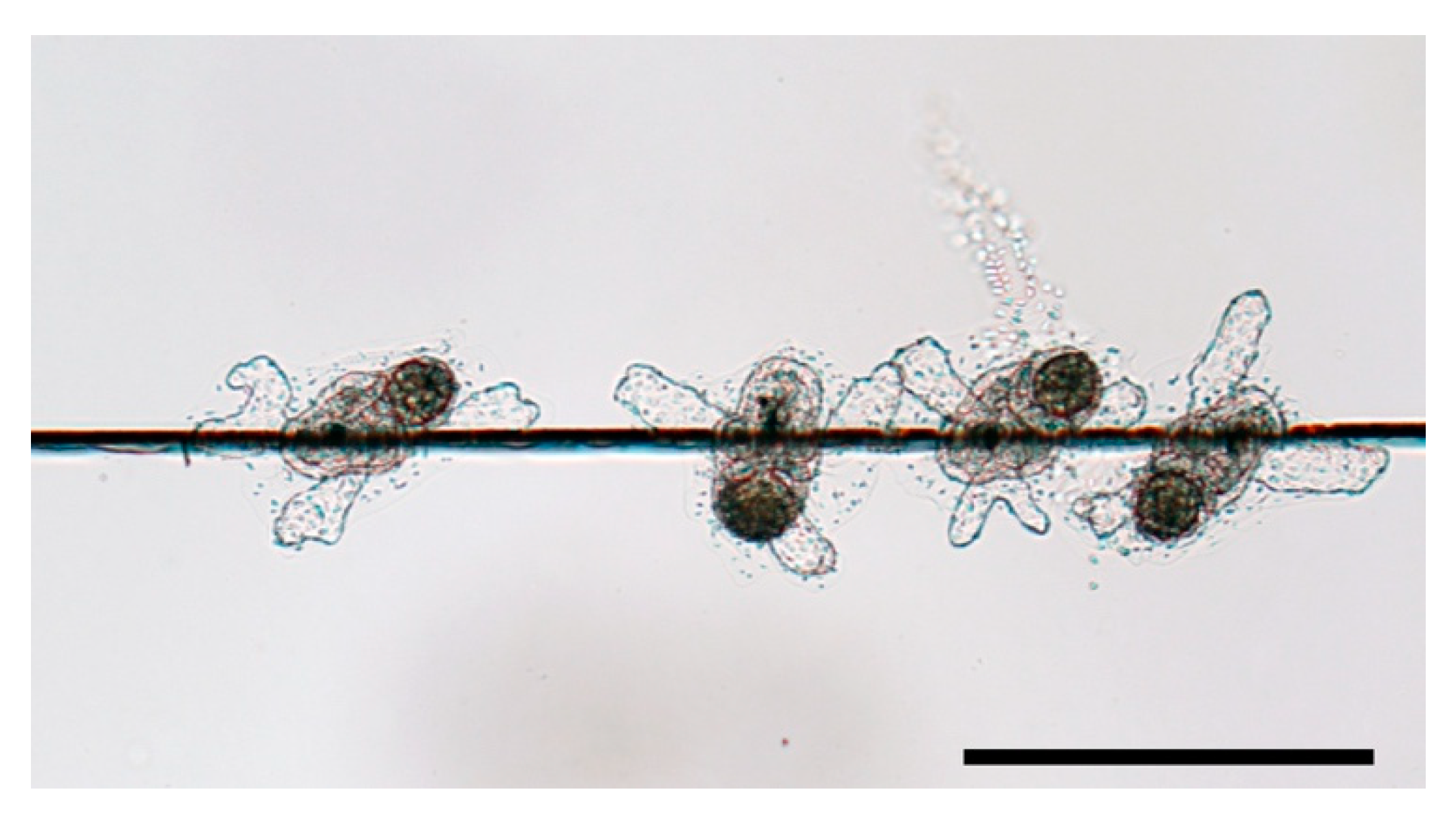
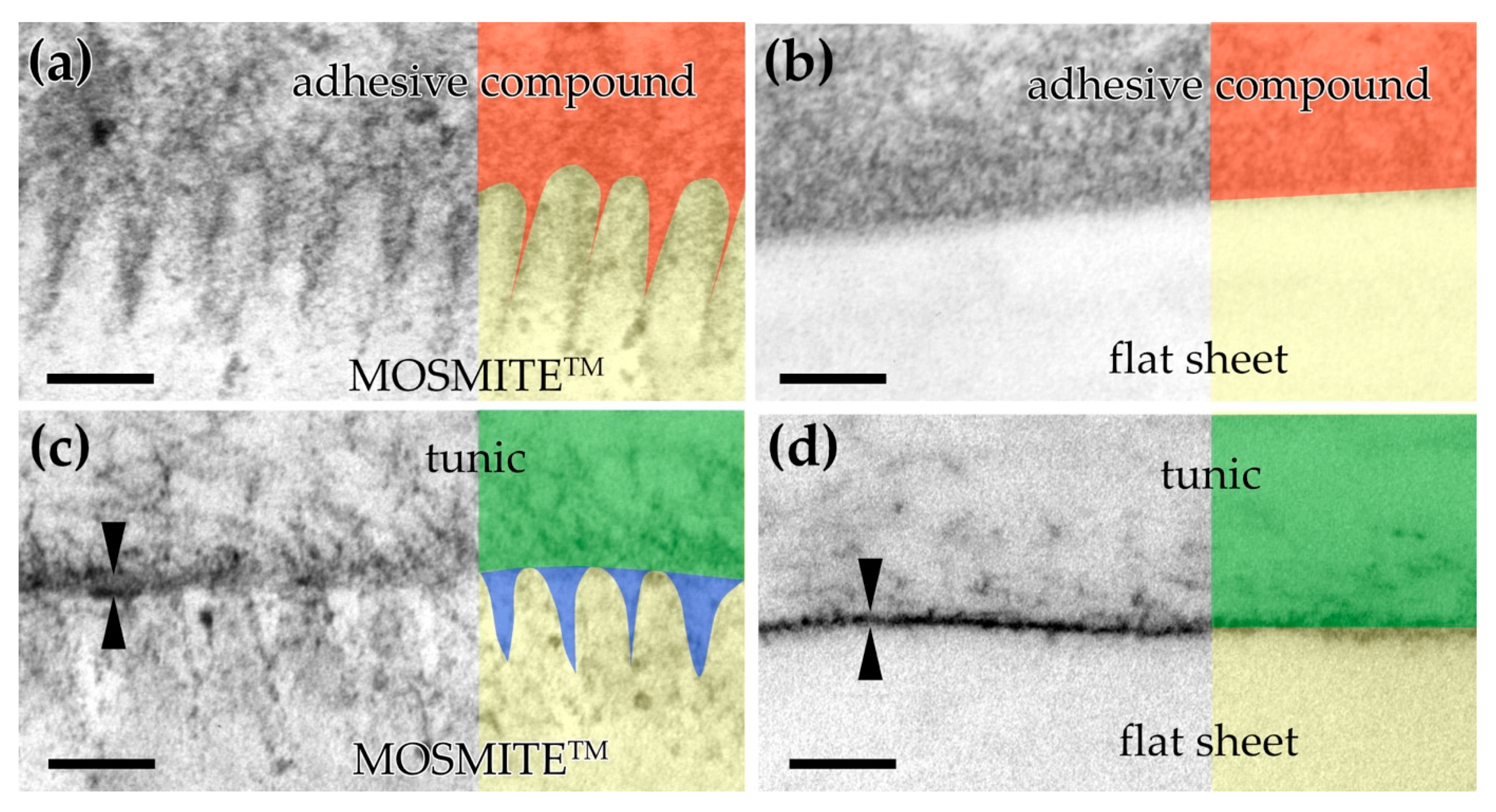
4.3. Settlement Test on Synthetic Nipple Array
5. Flypapers May Mitigate Invasive Fouling
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, H.; Lamb, R.; Lewis, J. Engineering nanoscale roughness on hydrophobic surface—Preliminary assessment of fouling behaviour. Sci. Technol. Adv. Mater. 2005, 6, 236–239. [Google Scholar] [CrossRef]
- Siddik, A.A.; Al-Sofyani, A.A.; Ba-Akdah, M.A.; Satheesh, S. Invertebrate recruitment on artificial substrates in the Red Sea: Role of substrate type and orientation. J. Mar. Biol. Assoc. U. K. 2019, 99, 741–750. [Google Scholar] [CrossRef]
- Rittschof, D.; Costlow, J.D. Bryozoan and barnacle settlement in relation to initial surface wettability: A comparison of laboratory and field studies. Sci. Mar. 1989, 53, 411–416. [Google Scholar]
- Gerhart, D.J.; Rittschof, D.; Hooper, I.R.; Eisenman, K.; Meyer, A.E.; Baier, R.E.; Young, C. Rapid and inexpensive quantifications of the combined polar components of surface wettability application to biofouling. Biofouling 1992, 5, 251–259. [Google Scholar] [CrossRef]
- Dahlström, M.; Jonsson, H.; Jonsson, P.R.; Elwing, H. Surface wettability as a determinant in the settlement of the barnacle Balanus Improvisus (DARWIN). J. Exp. Mar. Bio. Ecol. 2004, 305, 223–232. [Google Scholar] [CrossRef]
- Carl, C.; Poole, A.J.; Sexton, B.A.; Glenn, F.L.; Vucko, M.J.; Williams, M.R.; Whalan, S.; de Nys, R. Enhancing the settlement and attachment strength of pediveligers of Mytilus galloprovincialis by changing surface wettability and microtopography. Biofouling 2012, 28, 175–186. [Google Scholar] [CrossRef]
- Scardino, A.J.; Zhang, H.; Cookson, D.J.; Lamb, R.N.; de Nys, R. The role of nano-roughness in antifouling. Biofouling 2009, 25, 757–767. [Google Scholar] [CrossRef]
- Hirose, E.; Sensui, N. Does a nano-scale nipple array (moth-eye structure) suppress the settlement of ascidian larvae? J. Mar. Biol. Assoc. U. K. 2019, 99, 1393–1397. [Google Scholar] [CrossRef]
- Chase, A.L.; Dijkstra, J.A.; Harris, L.G. The influence of substrate material on ascidian larval settlement. Mar. Pollut. Bull. 2016, 106, 35–42. [Google Scholar] [CrossRef]
- Zeng, F.; Wunderer, J.; Salvenmoser, W.; Ederth, T.; Rothbächer, U. Identifying adhesive components in a model tunicate. Philos. Trans. R. Soc. B Biol. Sci. 2019, 374, 20190197. [Google Scholar] [CrossRef]
- Sensui, N.; Hirose, E. Wettability and substrate selection in the larval settlement of the solitary ascidian Phallusia philippinensis (Phlebobranchia: Ascidiidae). Zool. Sci. 2020, 37, 366–370. [Google Scholar] [CrossRef]
- Matsunobu, S.; Sasakura, Y. Time course for tail regression during metamorphosis of the ascidian Ciona intestinalis. Dev. Biol. 2015, 405, 71–81. [Google Scholar] [CrossRef]
- Bannister, J.; Sievers, M.; Bush, F.; Bloecher, N. Biofouling in marine aquaculture: A review of recent research and developments. Biofouling 2019, 35, 631–648. [Google Scholar] [CrossRef] [PubMed]
- Chambers, L.D.; Stokes, K.R.; Walsh, F.C.; Wood, R.J.K. Modern approaches to marine antifouling coatings. Surf. Coat. Technol. 2006, 201, 3642–3652. [Google Scholar] [CrossRef]
- Turner, A. Marine pollution from antifouling paint particles. Mar. Pollut. Bull. 2010, 60, 159–171. [Google Scholar] [CrossRef] [PubMed]
- Lawes, J.C.; Clark, G.F.; Johnston, E.L. Disentangling settlement responses to nutrient-rich contaminants: Elevated nutrients impact marine invertebrate recruitment via water-borne and substrate-bound cues. Sci. Total Environ. 2018, 645, 984–992. [Google Scholar] [CrossRef] [PubMed]
- Lambert, G. Ecology and natural history of the protochordates. Can. J. Zool. 2005, 83, 34–50. [Google Scholar] [CrossRef]
- Hirose, E. Ascidian tunic cells: Morphology and functional diversity of free cells outside the epidermis. Invertebr. Biol. 2009, 128, 83–96. [Google Scholar] [CrossRef]
- Burighel, P.; Cloney, R.A. Urochordata: Ascidiacea. In Microscopic Anatomy of Invertebrates Hemichordata, Chaetognatha, and the Invertebrate Chordates; Harrison, F.W., Ruppert, E., Eds.; Wiley-Liss, Inc.: New York, NY, USA, 1997; Volume 15, pp. 221–347. ISBN 0471561223. [Google Scholar]
- Huber, J.L.; da Silva, K.B.; Bates, W.R.; Swalla, B.J. The evolution of anural larvae in molgulid ascidians. Semin. Cell Dev. Biol. 2000, 11, 419–426. [Google Scholar] [CrossRef]
- Tsuda, M.; Sakurai, D.; Goda, M. Direct evidence for the role of pigment cells in the brain of ascidian larvae by laser ablation. J. Exp. Biol. 2003, 206, 1409–1417. [Google Scholar] [CrossRef] [PubMed]
- Bingham, B.L.; Reyns, N.B. Ultraviolet radiation and distribution of the solitary ascidian Corella inflata (Huntsman). Biol. Bull. 1999, 196, 94–104. [Google Scholar] [CrossRef]
- Zeng, F.; Wunderer, J.; Salvenmoser, W.; Hess, M.W.; Ladurner, P.; Rothbächer, U. Papillae revisited and the nature of the adhesive secreting collocytes. Dev. Biol. 2019, 448, 183–198. [Google Scholar] [CrossRef] [PubMed]
- Hadfield, M.; Paul, V. Natural chemical cues for settlement and metamorphosis of marine-invertebrate larvae. In Marine Chemical Ecology; McClintock, J.B., Baker, B.J., Eds.; CRC Press: Cambridge, UK, 2001; pp. 431–461. ISBN 9781420036602. [Google Scholar]
- Tsukamoto, S.; Kato, H.; Hirota, H.; Fusetani, N. Lumichrome. A larval metamorphosis-inducing substance in the ascidian Halocynthia roretzi. Eur. J. Biochem. 1999, 264, 785–789. [Google Scholar] [CrossRef] [PubMed]
- Grosberg, R.K.; Quinn, J.F. The genetic control and consequences of kin recognition by the larvae of a colonial marine invertebrate. Nature 1986, 322, 456–459. [Google Scholar] [CrossRef]
- Mihm, J.W.; Banta, W.C.; Loeb, G.I. Effects of adsorbed organic and primary fouling films on bryozoan settlement. J. Exp. Mar. Biol. Ecol. 1981, 54, 167–179. [Google Scholar] [CrossRef]
- Callow, M.E.; Callow, J.A.; Ista, L.K.; Coleman, S.E.; Nolasco, A.C.; Lopez, G.P. Use of self-assembled monolayers of different wettabilities to study surface selection and primary adhesion processes of green algal (Enteromorpha) zoospores. Appl. Environ. Microbiol. 2000, 66, 3249–3254. [Google Scholar] [CrossRef]
- Hodson, S.L.; Burke, C.M.; Bissett, A.P. Biofouling of fish-cage netting: The efficacy of a silicone coating and the effect of netting colour. Aquaculture 2000, 184, 277–290. [Google Scholar] [CrossRef]
- Terlizzi, A.; Conte, E.; Zupo, V.; Mazzella, L. Biological succession on silicone fouling-release surfaces: Long-term exposure tests in the Harbour of Ischia, Italy. Biofouling 2000, 15, 327–342. [Google Scholar] [CrossRef]
- Tettelbach, S.T.; Tetrault, K.; Carroll, J. Efficacy of Netminder® silicone release coating for biofouling reduction in bay scallop grow-out and comparative effects on scallop survival, growth and reproduction. Aquac. Res. 2014, 45, 234–242. [Google Scholar] [CrossRef]
- Flemming, H.C. Biofouling in water systems—Cases, causes and countermeasures. Appl. Microbiol. Biotechnol. 2002, 59, 629–640. [Google Scholar] [CrossRef]
- Swain, G.W.; Schultz, M.P. The testing and evaluation of non-toxic antifouling coatings. Biofouling 1996, 10, 187–197. [Google Scholar] [CrossRef]
- Krishnan, S.; Wang, N.; Ober, C.K.; Finlay, J.A.; Callow, M.E.; Callow, J.A.; Hexemer, A.; Sohn, K.E.; Kramer, E.J.; Fischer, D.A. Comparison of the fouling release properties of hydrophobic fluorinated and hydrophilic PEGylated block copolymer surfaces: Attachment strength of the diatom Navicula and the green alga Ulva. Biomacromolecules 2006, 7, 1449–1462. [Google Scholar] [CrossRef] [PubMed]
- Scardino, A.J.; de Nys, R. Mini review: Biomimetic models and bioinspired surfaces for fouling control. Biofouling 2011, 27, 73–86. [Google Scholar] [CrossRef]
- Bernhard, C.G. Structural and functional adaptation in a visual system. Endeavour 1967, 26, 79–84. [Google Scholar]
- Wilson, S.J.; Hutley, M.C. The optical properties of “moth eye” antireflection surfaces. Opt. Acta 1982, 29, 993–1009. [Google Scholar] [CrossRef]
- Spalding, A.; Shanks, K.; Bennie, J.; Potter, U.; Ffrench-Constant, R. Optical modelling and phylogenetic analysis provide in butterflies and moths. Insects 2019, 10, 262. [Google Scholar] [CrossRef] [PubMed]
- Watson, G.S.; Myhra, S.; Cribb, B.W.; Watson, J.A. Putative functions and functional efficiency of ordered cuticular nanoarrays on insect wings. Biophys. J. 2008, 94, 3352–3360. [Google Scholar] [CrossRef]
- Peisker, H.; Gorb, S.N. Always on the bright side of life: Anti-adhesive properties of insect ommatidia grating. J. Exp. Biol. 2010, 213, 3457–3462. [Google Scholar] [CrossRef]
- Ivanova, E.P.; Hasan, J.; Webb, H.K.; Truong, V.K.; Watson, G.S.; Watson, J.A.; Baulin, V.A.; Pogodin, S.; Wang, J.Y.; Tobin, M.J.; et al. Natural bactericidal surfaces: Mechanical rupture of Pseudomonas aeruginosa cells by cicada wings. Small 2012, 8, 2489–2494. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, E.P.; Hasan, J.; Webb, H.K.; Gervinskas, G.; Juodkazis, S.; Truong, V.K.; Wu, A.H.F.; Lamb, R.N.; Baulin, V.A.; Watson, G.S.; et al. Bactericidal activity of black silicon. Nat. Commun. 2013, 4, 2838. [Google Scholar] [CrossRef] [PubMed]
- Hirose, E.; Saito, Y.; Hashimoto, K.; Watanabe, H. Minute protrusions of the cuticle: Fine surface structures of the tunic in ascidians. J. Morphol. 1990, 204, 67–73. [Google Scholar] [CrossRef]
- Hirose, E.; Nishikawa, T.; Saito, Y.; Watanabe, H. Minute protrusions of ascidian tunic cuticle: Some implications for ascidian phylogeny. Zool. Sci. 1992, 9, 405–412. [Google Scholar]
- Hirose, E.; Lambert, G.; Kusakabe, T.; Nishikawa, T. Tunic cuticular protrusions in ascidians (Chordata, Tunicata): A perspective of their character-state distribution. Zool. Sci. 1997, 14, 683–689. [Google Scholar] [CrossRef]
- Hirose, E.; Kimura, S.; Itoh, T.; Nishikawa, J. Tunic morphology and cellulosic components of pyrosomas, doliolids, and salps (Thaliacea, Urochordata). Biol. Bull. 1999, 196, 113–120. [Google Scholar] [CrossRef]
- Hirose, E.; Sakai, D.; Shibata, T.; Nishii, J.; Mayama, H.; Miyauchi, A.; Nishikawa, J. Does the tunic nipple array serve to camouflage diurnal salps? J. Mar. Biol. Assoc. U. K. 2015, 95, 1025–1031. [Google Scholar] [CrossRef]
- Sakai, D.; Kakiuchida, H.; Nishikawa, J.; Hirose, E. Physical properties of the tunic in the pinkish-brown salp Pegea confoederata (Tunicata: Thaliacea). Zool. Lett. 2018, 4, 7. [Google Scholar] [CrossRef] [PubMed]
- Hirose, E.; Uyeno, D. Histopathology of a mesoparasitic hatschekiid copepod in hospite: Does Mihbaicola sakamakii (Copepoda: Siphonostomatoida: Hatschekiidae) fast within the host fish tissue? Zool. Sci. 2014, 31, 546–552. [Google Scholar] [CrossRef] [PubMed]
- Holland, N.D.; Nealson, K.H. The fine structure of the echinoderm cuticle and the subcuticular bacteria of echinoderms. Acta Zool. 1978, 59, 169–185. [Google Scholar] [CrossRef]
- Flammang, P.; Jangoux, M. Functional morphology of coronal and peristomeal podia in Sphaerechinus granularis (Echinodermata, Echinoida) Patrick. Zoomorphology 1993, 113, 47–60. [Google Scholar] [CrossRef]
- Hausen, H. Comparative structure of the epidermis in polychaetes (Annelida). Hydrobiologia 2005, 535–536, 25–35. [Google Scholar] [CrossRef]
- Iseto, T.; Hirose, E. Comparative morphology of the foot structure of four genera of Loxosomatidae (Entoprocta): Implications for foot functions and taxonomy. J. Morphol. 2010, 271, 1185–1196. [Google Scholar] [CrossRef] [PubMed]
- Kakiuchida, H.; Sakai, D.; Nishikawa, J.; Hirose, E. Measurement of refractive indices of tunicates’ tunics: Light reflection of the transparent integuments in an ascidian Rhopalaea sp. and a salp Thetys vagina. Zool. Lett. 2017, 3, 7. [Google Scholar] [CrossRef] [PubMed]
- Sakai, D.; Kakiuchida, H.; Harada, K.; Nishikawa, J.; Hirose, E. Parallel plications may enhance surface function: Physical properties of transparent tunics in colonial ascidians Clavelina cyclus and C. obesa. J. Mar. Biol. Assoc. U. K. 2019, 99, 1831–1839. [Google Scholar] [CrossRef]
- Hirose, E.; Mayama, H.; Miyauchi, A. Does the aquatic invertebrate nipple array prevent bubble adhesion? An experiment using nanopillar sheets. Biol. Lett. 2013, 9, 20130552. [Google Scholar] [CrossRef] [PubMed]
- Nomura, S.; Kojima, H.; Ohyabu, Y.; Kuwabara, K.; Miyauchi, A.; Uemura, T. Cell culture on nanopillar sheet: Study of HeLa cells on nanopillar sheet. Jpn. J. Appl. Phys. 2005, 44, 1184–1186. [Google Scholar] [CrossRef]
- Ballarin, L.; Franchi, N.; Gasparini, F.; Caicci, F.; Miyauchi, A.; Hirose, E. Suppression of cell-spreading and phagocytic activity on nano-pillared surface: In vitro experiment using hemocytes of the colonial ascidian Botryllus schlosseri. Invertebr. Surviv. J. 2015, 12, 82–88. [Google Scholar]
- Moth Eyes High-Performance Film MOSMITETM. Available online: https://www.m-chemical.co.jp/en/products/departments/mcc/hp-films-pl/product/1201267_7568.html (accessed on 11 April 2021).
- Marmur, A. Wetting on hydrophobic rough surfaces: To be heterogeneous or not to be? Langmuir 2003, 19, 8343–8348. [Google Scholar] [CrossRef]
- Cloney, R.A. Ascidian larvae and the events of metamorphosis. Am. Zool. 1982, 22, 817–826. [Google Scholar] [CrossRef]
- Karaiskou, A.; Swalla, B.J.; Sasakura, Y.; Chambon, J. Metamorphosis in solitary ascidians. Genesis 2015, 47, 34–47. [Google Scholar] [CrossRef] [PubMed]
- Simkanin, C.; Davidson, I.C.; Dower, J.F.; Jamieson, G.; Therriault, T.W. Anthropogenic structures and the infiltration of natural benthos by invasive ascidians. Mar. Ecol. Prog. Ser. 2012, 33, 499–511. [Google Scholar] [CrossRef]
- Airoldi, L.; Turon, X.; Perkol-Finkel, S.; Rius, M.; Keller, R. Corridors for aliens but not for natives: Effects of marine urban sprawl at a regional scale. Divers. Distrib. 2015, 21, 755–768. [Google Scholar] [CrossRef]
- Aldred, N.; Clare, A.S. Mini-review: Impact and dynamics of surface fouling by solitary and compound ascidians. Biofouling 2014, 30, 259–270. [Google Scholar] [CrossRef] [PubMed]
- Cima, F.; Bragadin, M.; Ballarin, L. Toxic effects of new antifouling compounds on tunicate haemocytes I. Sea-nine 211 and chlorothalonil. Aquat. Toxicol. 2008, 86, 299–312. [Google Scholar] [CrossRef] [PubMed]
- Menin, A.; Ballarin, L.; Bragadin, M.; Cima, F. Immunotoxicity in ascidians: Antifouling compounds alternative to organotins-II. The case of Diuron and TCMS pyridine. J. Environ. Sci. Health Part B 2008, 43, 644–654. [Google Scholar] [CrossRef] [PubMed]
- Cima, F.; Ballarin, L. Immunotoxicity in ascidians: Antifouling compounds alternative to organotins: III—The case of copper(I) and Irgarol 1051. Chemosphere 2012, 89, 19–29. [Google Scholar] [CrossRef]
- Cima, F.; Ballarin, L. Immunotoxicity in ascidians: Antifouling compounds alternative to organotins—IV. The case of zinc pyrithione. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2015, 169, 16–24. [Google Scholar] [CrossRef]
- Amara, I.; Miled, W.; Ben, R.; Ladhari, N. Antifouling processes and toxicity effects of antifouling paints on marine environment. A review. Environ. Toxicol. Pharmacol. 2018, 57, 115–130. [Google Scholar] [CrossRef]
- Cima, F.; Varello, R. Immunotoxicity in ascidians: Antifouling compounds alternative to organotins—V. the case of dichlofluanid. J. Mar. Sci. Eng. 2020, 8, 396. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hirose, E.; Sensui, N. Substrate Selection of Ascidian Larva: Wettability and Nano-Structures. J. Mar. Sci. Eng. 2021, 9, 634. https://doi.org/10.3390/jmse9060634
Hirose E, Sensui N. Substrate Selection of Ascidian Larva: Wettability and Nano-Structures. Journal of Marine Science and Engineering. 2021; 9(6):634. https://doi.org/10.3390/jmse9060634
Chicago/Turabian StyleHirose, Euichi, and Noburu Sensui. 2021. "Substrate Selection of Ascidian Larva: Wettability and Nano-Structures" Journal of Marine Science and Engineering 9, no. 6: 634. https://doi.org/10.3390/jmse9060634
APA StyleHirose, E., & Sensui, N. (2021). Substrate Selection of Ascidian Larva: Wettability and Nano-Structures. Journal of Marine Science and Engineering, 9(6), 634. https://doi.org/10.3390/jmse9060634





