Evidence for Ecosystem-Level Trophic Cascade Effects Involving Gulf Menhaden (Brevoortia patronus) Triggered by the Deepwater Horizon Blowout
Abstract
1. Introduction
2. Methods
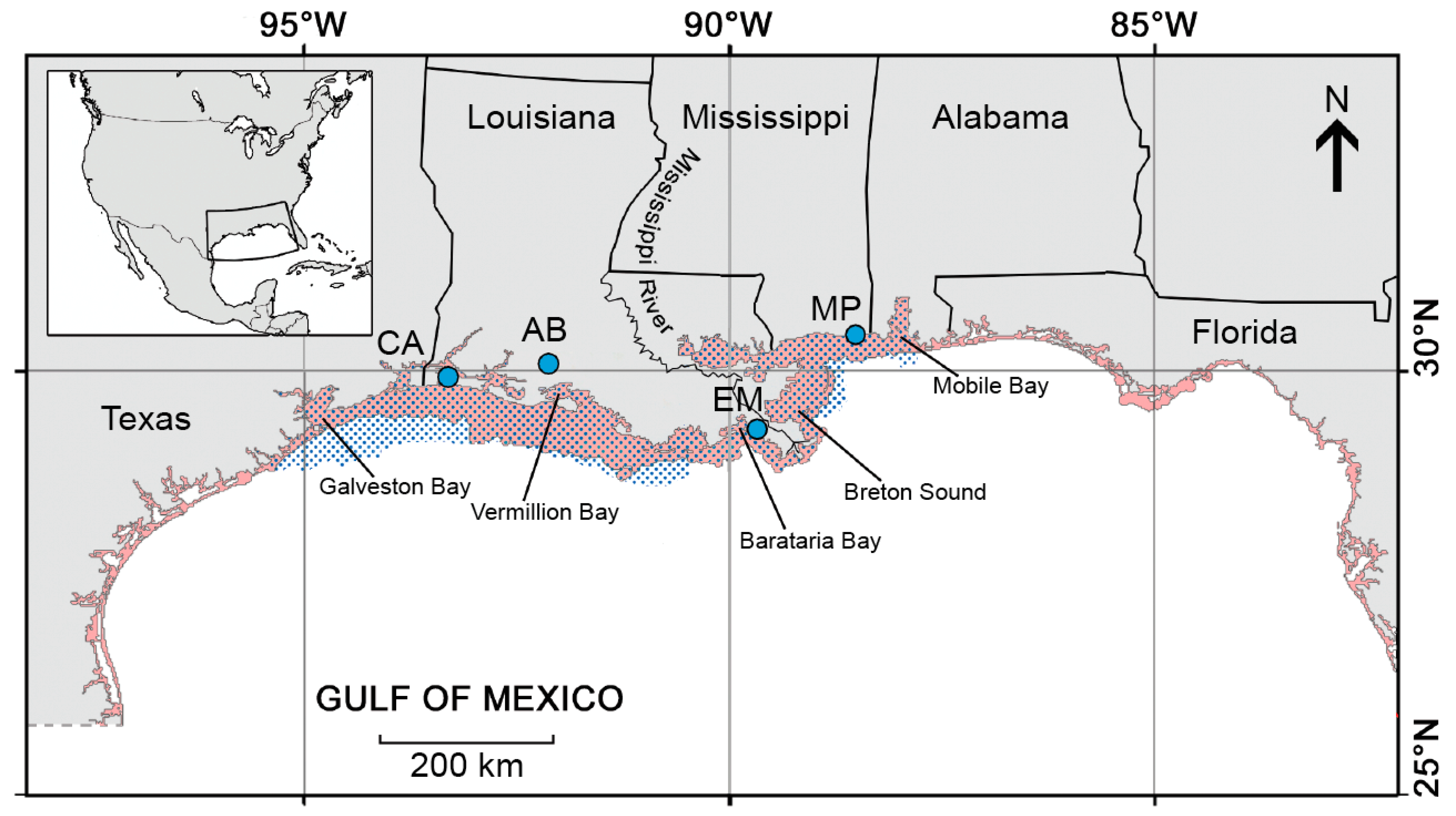
2.1. Study Area, Gulf Menhaden Life History and Fishery
2.2. US National Marine Fisheries Service Port Sampling
2.3. Daybrook Fisheries Sampling
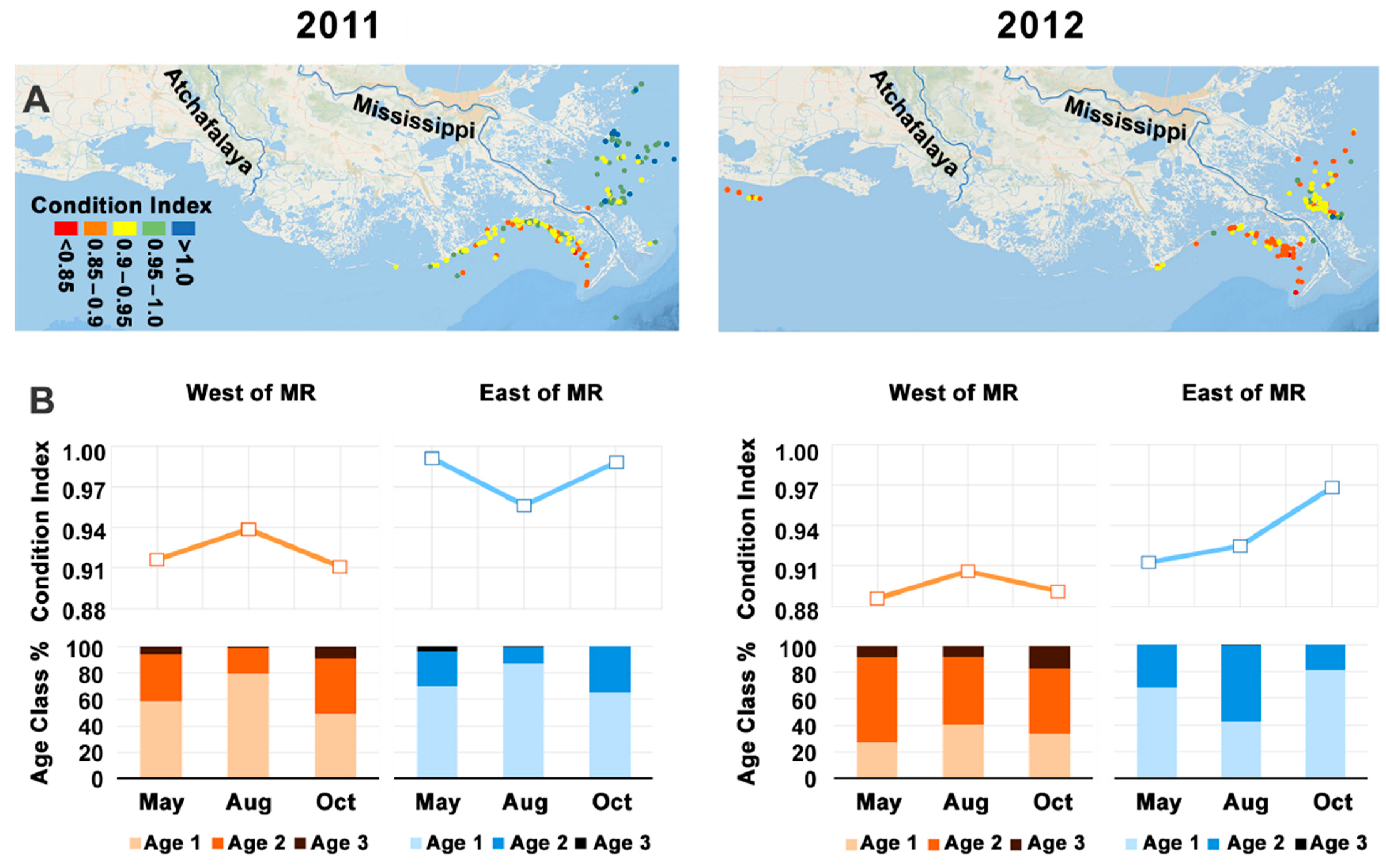
2.4. Gulf Menhaden Condition Index and Oil Content
2.5. Biomass, Productivity and Trophic Transfer Computations
2.6. Data Quality and Parameter Uncertainty
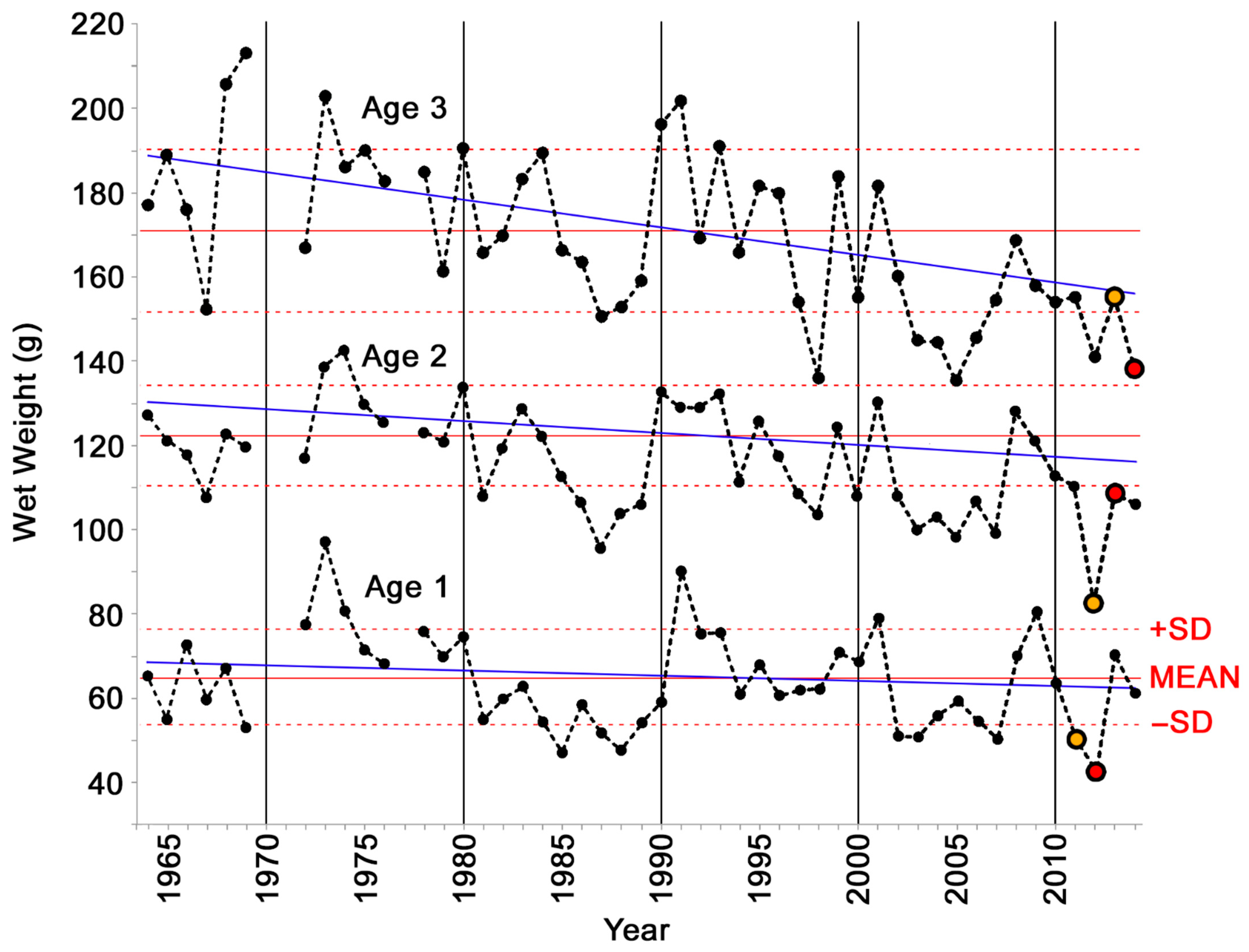
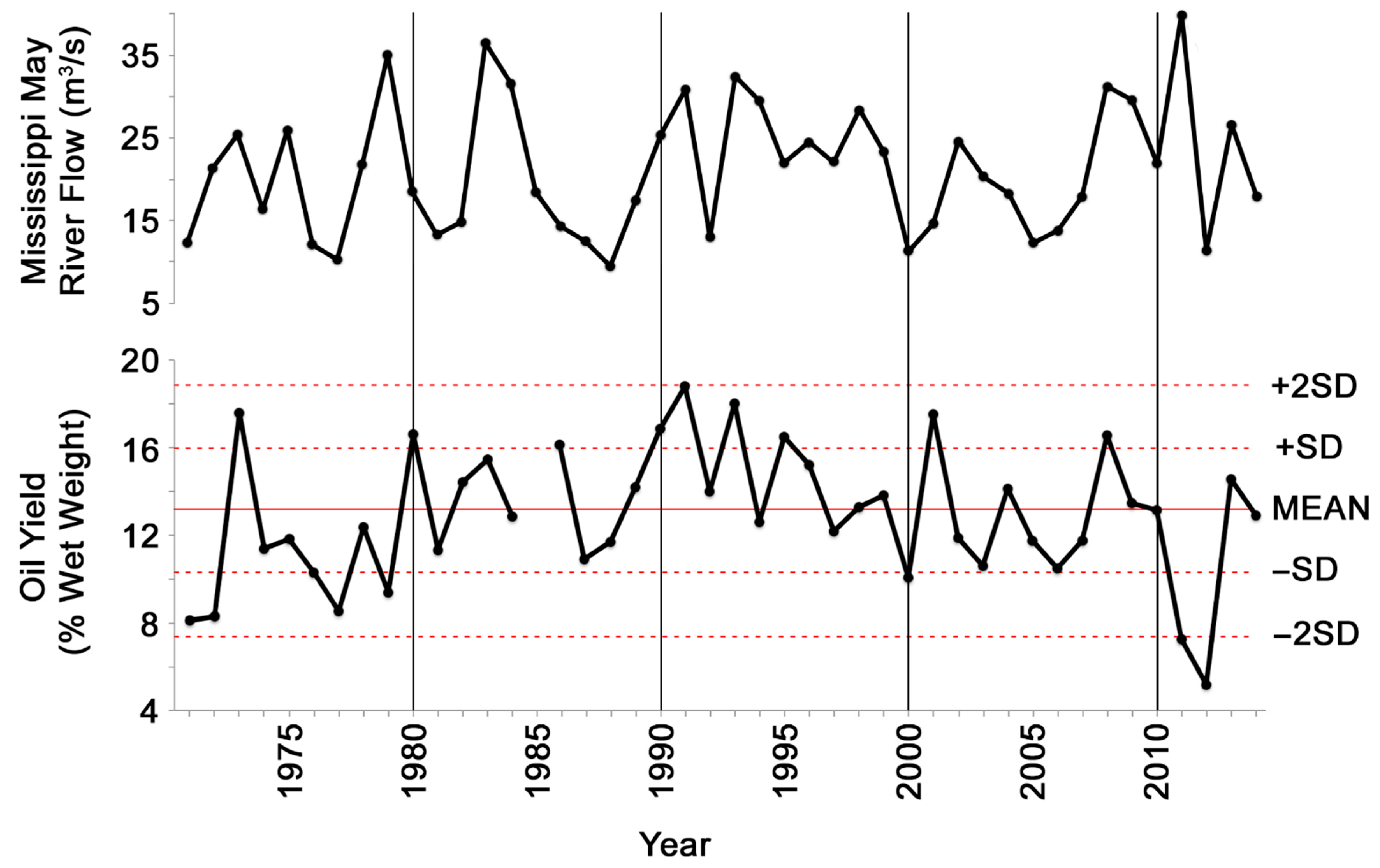
3. Results
4. Discussion
4.1. Decline of Physiological Condition of Gulf Menhaden after the Deepwater Horizon Blowout
4.2. Comparison of Gulf Menhaden Productivity with Total Available Productivity
4.3. Ecological Implications
4.4. Modeling Implications
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Palumbi, S.R.; McLeod, K.L.; Grünbaum, D. Ecosystems in Action: Lessons from Marine Ecology about Recovery, Resistance, and Reversibility. Bioscience 2008, 58, 33–42. [Google Scholar] [CrossRef]
- National Research Council. Committee on Oil in the Sea III: Inputs, Fates, and Effects; The National Academies Press: Washington, DC, USA, 2003. [Google Scholar]
- Peterson, C.H.; Rice, S.D.; Short, J.W.; Esler, D.; Bodkin, J.L.; Ballachey, B.E.; Irons, D.B. Long-Term Ecosystem Response to the Exxon Valdez Oil Spill. Science 2003, 302, 2082–2086. [Google Scholar] [CrossRef]
- Peterson, C.H.; Anderson, S.S.; Cherr, G.N.; Ambrose, R.; Anghera, S.; Bay, S.; Blum, M.; Condon, R.; Dean, T.A.; Graham, M.; et al. A Tale of Two Spills: Novel Science and Policy Implications of an Emerging New Oil Spill Model. Bioscience 2012, 62, 461–469. [Google Scholar] [CrossRef]
- Haney, J.C.; Geiger, H.J.; Short, J.W. Bird mortality from the Deepwater Horizon oil spill. II. Carcass sampling and exposure probability estimates for coastal Gulf of Mexico. Mar. Ecol. Prog. Ser. 2014, 513, 239–252. [Google Scholar] [CrossRef]
- Short, J.W.; Geiger, H.J.; Haney, J.C.; Voss, C.M.; Vozzo, M.L.; Guillory, V.; Peterson, C.H. Anomalously High Recruitment of the 2010 Gulf Menhaden (Brevoortia patronus) Year Class: Evidence of Indirect Effects from the Deepwater Horizon Blowout in the Gulf of Mexico. Arch. Environ. Contam. Toxicol. 2017, 73, 76–92. [Google Scholar] [CrossRef]
- Ahrenholz, D.W. Recruitment and exploitation of Gulf menhaden, Brevoortia patronus. Fish. Bull. 1981, 79, 325–335. [Google Scholar]
- Schueller, A.; Smith, J.; VanderKooy, S. Southeast Data, Assessment, and Review 32A Gulf of Mexico Menhaden Assessment Report; Gulf States Marine Fisheries Commission: Ocean Springs, MS, USA, 2013. [Google Scholar]
- Robinson, K.L.; Ruzicka, J.J.; Hernandez, F.J.; Graham, W.M.; Decker, M.B.; Brodeur, R.D.; Sutor, M. Evaluating energy flows through jellyfish and gulf menhaden (Brevoortia patronus) and the effects of fishing on the northern Gulf of Mexico eco-system. ICES J. Mar. Sci. 2015, 72, 2301–2312. [Google Scholar] [CrossRef]
- Lamb, J.S.; Satgé, Y.G.; Jodice, P.G.R. Diet composition and provisioning rates of nestlings determine reproductive success in a subtropical seabird. Mar. Ecol. Prog. Ser. 2017, 581, 149–164. [Google Scholar] [CrossRef]
- Bakun, A. “Wasp-waist” populations and ecosystem dynamics: Navigating the “predator pit” topographies. Prog. Oceanogr. 2006, 68, 271–288. [Google Scholar] [CrossRef]
- Turner, W.R. Life History of Menhadens in the Eastern Gulf of Mexico. Trans. Am. Fish. Soc. 1969, 98, 216–224. [Google Scholar] [CrossRef]
- Deegan, L.A. Changes in body composition and morphology of young-of-the-year gulf menhaden, Brevoortia patronus Goode, in Fourleague Bay, Louisiana. J. Fish Biol. 1986, 29, 403–415. [Google Scholar] [CrossRef]
- Olsen, Z.; Fulford, R.; Dillon, K.; Graham, W. Trophic role of gulf menhaden Brevoortia patronus examined with carbon and nitrogen stable isotope analysis. Mar. Ecol. Prog. Ser. 2014, 497, 215–227. [Google Scholar] [CrossRef]
- Kroger, R.L.; Pristas, P.J. Movements of tagged juvenile menhaden (Brevoortia patronus) in the Gulf of Mexico. Tex. J. Sci. 1974, 26, 473–477. [Google Scholar]
- Le Cren, E.D. The length-weight relationship and seasonal cycle in gonad weight and condition in the perch (Perca fluviatilis). J. Anim. Ecol. 1951, 20, 201–219. [Google Scholar] [CrossRef]
- Nicholson, W.R.; Schaaf, W.E. Aging of gulf menhaden, Brevoortia patronus. Fish. Bull. 1978, 76, 315–322. [Google Scholar]
- Vaughan, D.S.; Smith, J.W.; Schueller, A.M. Age, Growth, and Reproduction of Gulf Menhaden; SEDAR 27-DW02; South Atlantic Fishery Management Council: North Charleston, SC, USA, 2010. [Google Scholar]
- Durbin, E.G.; Durbin, A.G. Energy and nitrogen budgets for the Atlantic menhaden, Brevoortia tyrannus (Pisces: Clupeidae), a filter-feeding planktivore. Fish. Bull. 1983, 81, 177–199. [Google Scholar]
- Gulland, J.A. Summary. In The Fish Resources of the Ocean; Gulland, J., Ed.; Fishing News: Surrey, UK, 1971; pp. 246–255. [Google Scholar]
- Lohrenz, S.; Fahnenstiel, G.; Redalje, D.; Lang, G.; Chen, X.; Dagg, M. Variations in primary production of northern Gulf of Mexico continental shelf waters linked to nutrient inputs from the Mississippi River. Mar. Ecol. Prog. Ser. 1997, 155, 45–54. [Google Scholar] [CrossRef]
- Cushing, D. Production in the Indian Ocean and the transfer from the primary to the secondary level. In The Biology of the Indian Ocean, Ecological Studies; Zeitchel, B., Gerlach, S.A., Eds.; Springer: Berlin, Germany, 1973; Volume 3, pp. 475–486. [Google Scholar]
- Vaughan, D.S.; Shertzer, K.W.; Smith, J.W. Gulf menhaden (Brevoortia patronus) in the U.S. Gulf of Mexico: Fish characteris-tics and biological reference points for management. Fish. Res. 2007, 83, 263–275. [Google Scholar] [CrossRef]
- Dickinson, W.T. Accuracy of Discharge Determinations; Hydrology Paper #20; Colorado State University: Fort Collins, CO, USA, 1967. [Google Scholar]
- Williams, E.H.; Shertzer, K.W. Technical Documentation of the Beaufort Assessment Model; National Oceanic and Atmospheric Administration Technical Memorandum NMFS-SEFSC-671; National Marine Fisheries Service, Southeast Fisheries Science Center, Beaufort Laboratory: Beaufort, NC, USA, 2015.
- Rabalais, N.N.; Turner, R.E. Gulf of Mexico Hypoxia: Past, Present, and Future. Limnol. Oceanogr. Bull. 2019, 28, 117–124. [Google Scholar] [CrossRef]
- Ramey, T.S.; Miller, M. Omega Protein Corp., 16 August 2011. D.A. Davidson & Co., Two Centerpointe Dr., Suite 400, Lake Oswego, OR 97035; Omega Protein: Reedville, VA, USA, 2011. [Google Scholar]
- Ramey, T.S.; Miller, M. Omega Protein Corp., 14 June 2012. D.A. Davidson & Co., Two Centerpointe Dr., Suite 400, Lake Oswego, OR 97035; Omega Protein: Reedville, VA, USA, 2012. [Google Scholar]
- Broch, O.J.; Nepstad, R.; Ellingsen, I.; Bast, R.; Skeie, G.M.; Carroll, J. Simulating crude oil exposure, uptake and effects in North Atlantic Calanus finmarchicus populations. Mar. Environ. Res. 2020, 162, 105184. [Google Scholar] [CrossRef] [PubMed]
- Guillory, V. Influences of environment and age structure on Gulf menhaden oil yield. In Contributions of the Marine Research Laboratory, 1980–1985; Technical Bulletin 40; Louisiana Department of Wildlife and Fisheries: Grand Terre Island, LA, USA, 1986; pp. 22–31. [Google Scholar]
- Adams, G.; Leaf, R.T.; Wu, W.; Hernandez, F.J. Environmentally driven fluctuations in condition factor of adult Gulf menhaden (Brevoortia patronus) in the northern Gulf of Mexico. ICES J. Mar. Sci. 2018, 75, 1269–1279. [Google Scholar] [CrossRef]
- Carroll, J.; Vikebø, F.B.; Howell, D.; Broch, O.J.; Nepstad, R.; Augustine, S.; Skeie, G.M.; Bast, R.; Juselius, J. Assessing impacts of simulated oil spills on the Northeast Arctic cod fishery. Mar. Pollut. Bull. 2018, 126, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Millemann, D.R.; Portier, R.J.; Olson, G.M.; Bentivegna, C.S.; Cooper, K.R. Particulate accumulations in the vital organs of wild Brevoortia patronus from the northern Gulf of Mexico after the Deepwater Horizon oil spill. Ecotoxicology 2015, 24, 1831–1847. [Google Scholar] [CrossRef] [PubMed]
- Olson, G.M. Three Year Assessment of Nearshore Crude Oil Contamination in the Gulf of Mexico Using Gulf Menhaden (Brevoortia patronus) as an Indicator Species: Menhaden Watch. Ph.D. Thesis, Louisiana State University, Baton Rouge, LA, USA, December 2015. [Google Scholar]
- Turner, R.E. Smaller size-at-age menhaden with coastal warming and fishing intensity. Geo Geogr. Environ. 2017, 4, e00044. [Google Scholar] [CrossRef]
- Cheung, W.W.L.; Sarmiento, J.L.; Dunne, J.P.; Frölicher, T.L.; Lam, V.W.Y.; Palomares, M.L.D.; Watson, R.; Pauly, D. Shrinking of fishes exacerbates impacts of global ocean changes on marine ecosystems. Nat. Clim. Chang. 2013, 3, 254–258. [Google Scholar] [CrossRef]
- Muller-Karger, F.E.; Smith, J.P.; Werner, S.; Chen, R.; Roffer, M.A.; Liu, Y.; Muhling, B.; Lindo-Atichati, D.; Lamkin, J.T.; Cerdeira-Estrada, S.; et al. Natural variability of surface oceanographic conditions in the offshore Gulf of Mexico. Prog. Oceanogr. 2015, 134, 54–76. [Google Scholar] [CrossRef]
- Krausmann, F.; Erb, K.-H.; Gingrich, S.; Haberl, H.; Bondeau, A.; Gaube, V.; Lauk, C.; Plutzar, C.; Searchinger, T.D. Global human appropriation of net primary production doubled in the 20th century. Proc. Natl. Acad. Sci. USA 2013, 110, 10324–10329. [Google Scholar] [CrossRef]
- Fodrie, F.J.; Heck, K.L. Response of Coastal Fishes to the Gulf of Mexico Oil Disaster. PLoS ONE 2011, 6, e21609. [Google Scholar] [CrossRef]
- Schaefer, J.; Frazier, N.; Barr, J. Dynamics of Near-Coastal Fish Assemblages following the Deepwater Horizon Oil Spill in the Northern Gulf of Mexico. Trans. Am. Fish. Soc. 2015, 145, 108–119. [Google Scholar] [CrossRef]
- Cury, P.; Bakun, A.; Crawford, R.J.M.; Jarre, A.; Quiñones, R.A.; Shannon, L.J.; Verheye, H.M. Small pelagics in upwelling systems: Patterns of interaction and structural changes in “wasp-waist” ecosystems. ICES J. Mar. Sci. 2000, 57, 603–618. [Google Scholar] [CrossRef]
- Wanless, S.; Harris, M.P.; Redman, P.; Speakman, J. Low energy values of fish as a probable cause of a major seabird breeding failure in the North Sea. Mar. Ecol. Prog. Ser. 2005, 294, 1–8. [Google Scholar] [CrossRef]
- Brooke, M.d.L.; Balance, L.T.; Pitman, R.L.; Furness, R.W. Fishery impacts on the food supplies of seabirds. Acta Zool. Sin. 2006, 52 (Suppl. 2), 639–640. [Google Scholar]
- Einoder, L.D. A review of the use of seabirds as indicators in fisheries and ecosystem management. Fish. Res. 2009, 95, 6–13. [Google Scholar] [CrossRef]
- Pauly, D.; Christensen, V.; Guénette, S.; Pitcher, T.J.; Sumaila, U.R.; Walters, C.J.; Watson, R.A.; Zeller, D. Towards sustainability in world fisheries. Nature 2002, 418, 689–695. [Google Scholar] [CrossRef]
- Rice, J. Food web theory, marine food webs, and what climate change may do to northern fish populations. Can. Spec. Publ. Fish. Aquat. Sci. 1995, 121, 561–568. [Google Scholar]
- Guillory, V.; Bejarano, R. Evaluation of juvenile menhaden abundance data for prediction of commercial harvest. Southeast. Assoc. Fish Wildl. Agencies 1980, 34, 193–203. [Google Scholar]
- Durbin, A.G.; Durbin, E.G. Effects of Menhaden Predation on Plankton Populations in Narragansett Bay, Rhode Island. Estuaries 1998, 21, 449–465. [Google Scholar] [CrossRef]
- Lynch, P.D.; Brush, M.J.; Latour, R.J. Simulated short-term impacts of the Atlantic menhaden reduction fishery on Chesapeake Bay water quality. N. Am. J. Fish. Manag. 2011, 31, 70–78. [Google Scholar] [CrossRef]
- Schueller, A.; Smith, J.; VanderKooy, S. Southeast Data, Assessment, and Review 63 Gulf of Mexico Menhaden Assessment Report; Gulf States Marine Fisheries Commission: Ocean Springs, MS, USA, 2018. [Google Scholar]
- Casini, M.; Lövgren, J.; Hjelm, J.; Cardinale, M.; Molinero, J.C.; Kornilovs, G. Multi-level trophic cascades in a heavily ex-ploited open marine ecosystem. Proc. R. Soc. B 2008, 275, 1793–1801. [Google Scholar] [CrossRef]
- Österblom, H.; Hansson, S.; Larsson, U.; Hjerne, O.; Wulff, F.; Elmgren, R.; Folke, C. Human-induced Trophic Cascades and Ecological Regime Shifts in the Baltic Sea. Ecosystems 2007, 10, 877–889. [Google Scholar] [CrossRef]
- Österblom, H.; Casini, M.; Olsson, O.; Bignert, A. Fish, seabirds and trophic cascades in the Baltic Sea. Mar. Ecol. Prog. Ser. 2006, 323, 233–238. [Google Scholar] [CrossRef]
- Grémillet, D.; Pichegru, L.; Kuntz, G.; Woakes, A.G.; Wilkinson, S.; Crawford, R.J.; Ryan, P.G. A junk-food hypothesis for gannets feeding on fishery waste. Proc. R. Soc. B Biol. Sci. 2008, 275, 1149–1156. [Google Scholar] [CrossRef] [PubMed]
- Rosen, D.A.S.; Trites, A.W. Pollock and the decline of Steller sea lions: Testing the junk food hypothesis. Can. J. Zool. 2000, 78, 1243–1250. [Google Scholar] [CrossRef]
- Geers, T.M.; Pikitch, E.K.; Frisk, M. An original model of the northern Gulf of Mexico using Ecopath with Ecosim and its implications for the effects of fishing on ecosystem structure and maturity. Deep. Sea Res. Part II Top. Stud. Oceanogr. 2016, 129, 319–331. [Google Scholar] [CrossRef]
- Walters, C.; Martell, S.J.D.; Christensen, V.; Mahmoudi, B. An Ecosim model for exploring Gulf of Mexico ecosystem man-agement options: Implications of including multistanza life-history models for policy predictions. Bull. Mar. Sci. 2008, 83, 251–271. [Google Scholar]

| Weight-at-Age (g) | ||||
|---|---|---|---|---|
| Age: | 0 | 1 | 2 | 3 |
| 0.5 | 45.3 | 95.9 | 145.9 | |
| 45.3 | 95.9 | 149.9 | 187.9 | |
| 10.6 | 69.6 | 121.6 | 168.1 | |
| Abundance-at-Age (109 Fish) | Biomass, B (×109 g Wet wt) | Annual Production, P (×109 g Wet wt/y) | P/B | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Year | 0 | 1 | 2 | 3 | Age 0 | Age 1+ | Sum | ||
| 2000 | 99 | 22 | 5.3 | 0.02 | 1500 | 1700 | 810 | 2500 | 1.7 |
| 2001 | 100 | 20 | 5.3 | 0.09 | 1400 | 1800 | 750 | 2600 | 1.9 |
| 2002 | 80 | 20 | 4.7 | 0.16 | 1400 | 1400 | 730 | 2100 | 1.5 |
| 2003 | 99 | 16 | 4.5 | 0.15 | 1200 | 1700 | 600 | 2300 | 1.9 |
| 2004 | 89 | 20 | 3.6 | 0.15 | 1300 | 1600 | 700 | 2300 | 1.8 |
| 2005 | 83 | 18 | 4.4 | 0.03 | 1200 | 1400 | 670 | 2100 | 1.8 |
| 2006 | 130 | 16 | 4.2 | 0.14 | 1200 | 2300 | 600 | 2900 | 2.4 |
| 2007 | 120 | 27 | 3.9 | 0.08 | 1600 | 2100 | 930 | 3000 | 1.9 |
| 2008 | 47 | 24 | 6.3 | 0.10 | 1700 | 820 | 920 | 1700 | 1.0 |
| 2009 | 100 | 9.3 | 6.1 | 0.64 | 1100 | 1800 | 470 | 2300 | 2.1 |
| 2010 | 170 | 20 | 2.4 | 0.45 | 1200 | 3700 | 680 | 4400 | 1.7 |
| 2011 | 110 | 54 | 4.5 | 0.03 | 2900 | 1900 | 1700 | 3600 | 1.9 |
| 2012 | 120 | 22 | 12 | 0 | 2100 | 2100 | 1000 | 3100 | 1.5 |
| 2013 | 24 | 5.9 | 0 | ||||||
| 2000–2009 Average: | 1400 | 1700 | 720 | 2400 | 1.8 | ||||
| Annual Production | Productivity | % Mean Productivity Available at Trophic Level of: | |||||
|---|---|---|---|---|---|---|---|
| ×109 g C/y | g C/y/m2 | ||||||
| Year | Age 0 | Age 1+ | Age 0 | Age 1+ | Age 0 | Age 1+ | Sum |
| 2000 | 320 | 150 | 5.4 | 2.5 | 8.6 | 13 | 22 |
| 2001 | 340 | 140 | 5.7 | 2.4 | 9.2 | 12 | 21 |
| 2002 | 270 | 140 | 4.5 | 2.4 | 7.3 | 12 | 19 |
| 2003 | 320 | 110 | 5.4 | 1.9 | 8.6 | 9.4 | 18 |
| 2004 | 300 | 130 | 5.1 | 2.2 | 8.1 | 11 | 19 |
| 2005 | 270 | 130 | 4.5 | 2.2 | 7.3 | 11 | 18 |
| 2006 | 440 | 110 | 7.4 | 1.9 | 12 | 9.4 | 21 |
| 2007 | 400 | 180 | 6.7 | 3.0 | 11 | 15 | 26 |
| 2008 | 160 | 170 | 2.7 | 2.9 | 4.3 | 15 | 19 |
| 2009 | 340 | 90 | 5.7 | 1.5 | 9.2 | 7.7 | 17 |
| 2010 | 700 | 130 | 12 | 2.2 | 19 | 11 | 30 |
| 2011 | 360 | 320 | 6.1 | 5.4 | 9.7 | 27 | 37 |
| 2012 | 400 | 190 | 6.7 | 3.2 | 11 | 16 | 27 |
| 2000–2009 Average: | 320 | 140 | 5.3 | 2.3 | 8.6 | 12 | 20 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Short, J.W.; Voss, C.M.; Vozzo, M.L.; Guillory, V.; Geiger, H.J.; Haney, J.C.; Peterson, C.H. Evidence for Ecosystem-Level Trophic Cascade Effects Involving Gulf Menhaden (Brevoortia patronus) Triggered by the Deepwater Horizon Blowout. J. Mar. Sci. Eng. 2021, 9, 190. https://doi.org/10.3390/jmse9020190
Short JW, Voss CM, Vozzo ML, Guillory V, Geiger HJ, Haney JC, Peterson CH. Evidence for Ecosystem-Level Trophic Cascade Effects Involving Gulf Menhaden (Brevoortia patronus) Triggered by the Deepwater Horizon Blowout. Journal of Marine Science and Engineering. 2021; 9(2):190. https://doi.org/10.3390/jmse9020190
Chicago/Turabian StyleShort, Jeffrey W., Christine M. Voss, Maria L. Vozzo, Vincent Guillory, Harold J. Geiger, James C. Haney, and Charles H. Peterson. 2021. "Evidence for Ecosystem-Level Trophic Cascade Effects Involving Gulf Menhaden (Brevoortia patronus) Triggered by the Deepwater Horizon Blowout" Journal of Marine Science and Engineering 9, no. 2: 190. https://doi.org/10.3390/jmse9020190
APA StyleShort, J. W., Voss, C. M., Vozzo, M. L., Guillory, V., Geiger, H. J., Haney, J. C., & Peterson, C. H. (2021). Evidence for Ecosystem-Level Trophic Cascade Effects Involving Gulf Menhaden (Brevoortia patronus) Triggered by the Deepwater Horizon Blowout. Journal of Marine Science and Engineering, 9(2), 190. https://doi.org/10.3390/jmse9020190







