Filtering Activity and Nutrient Release by the Keratose Sponge Sarcotragus spinosulus Schmidt, 1862 (Porifera, Demospongiae) at the Laboratory Scale
Abstract
1. Introduction
2. Materials and Methods
2.1. Studied Species
2.2. Sponge Sampling
2.3. Experimental Procedures
2.4. Filtering Activity Assessment
2.5. Nutrient Analysis
2.6. Statistical Analysis
3. Results
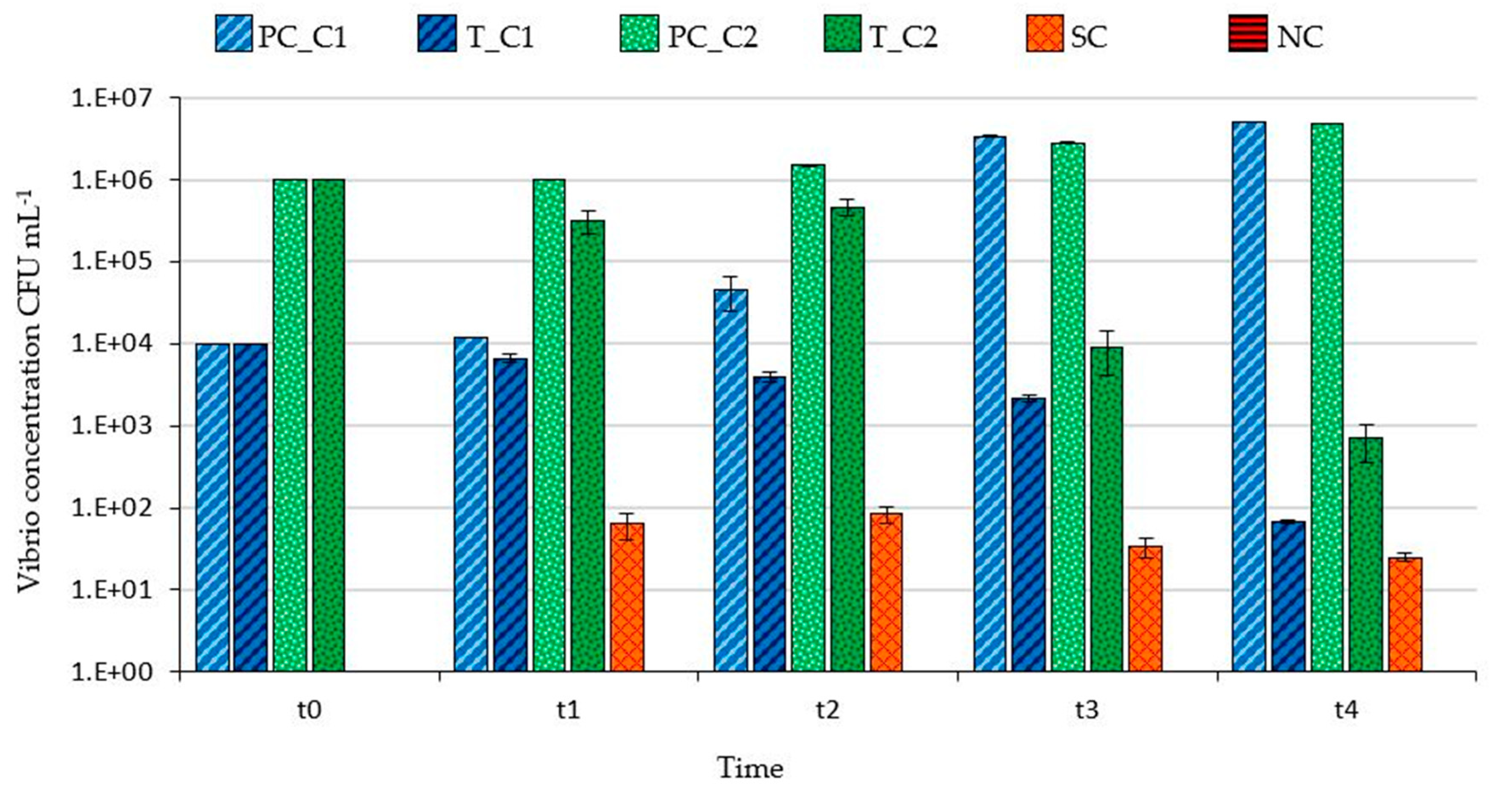
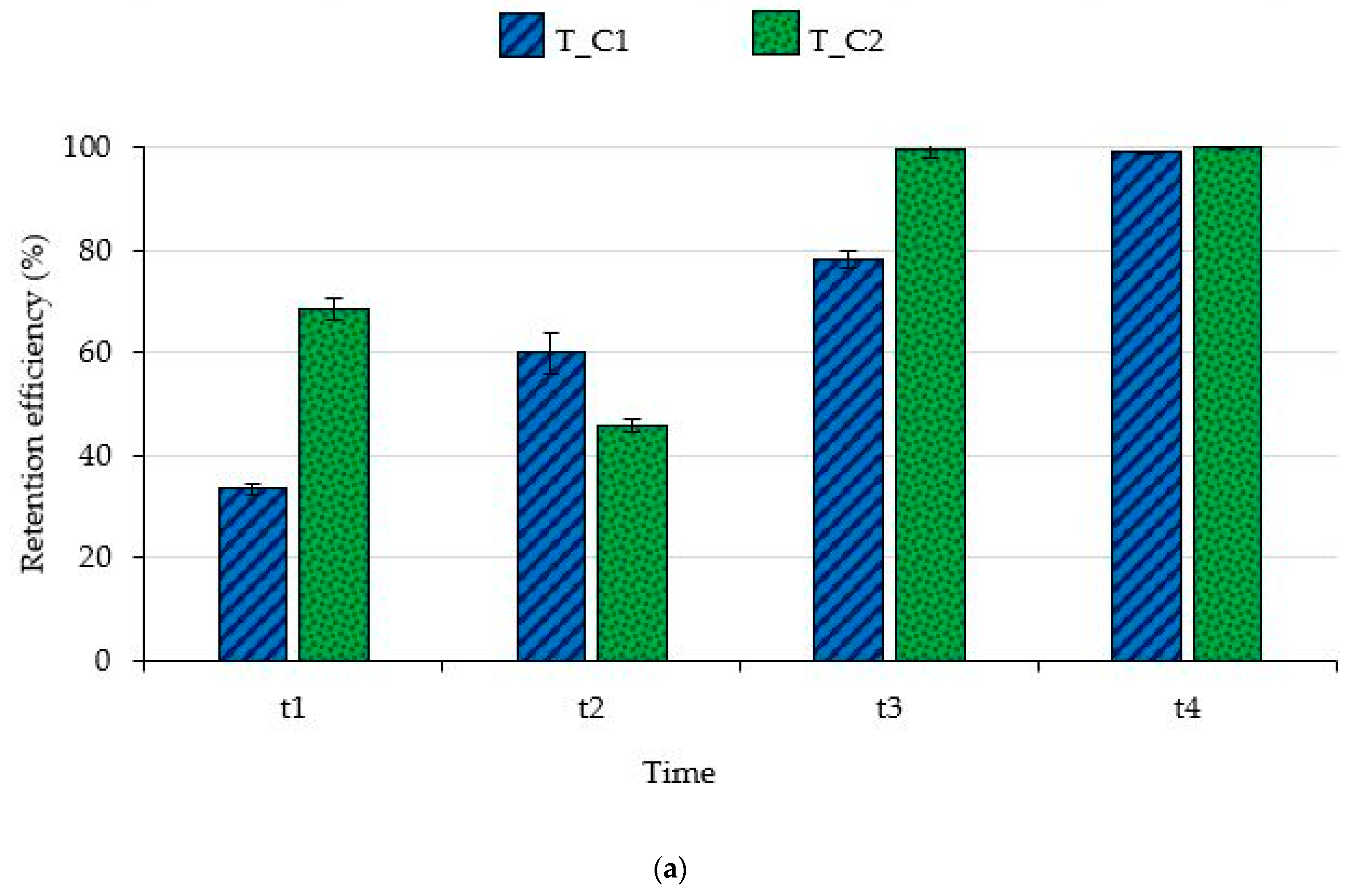
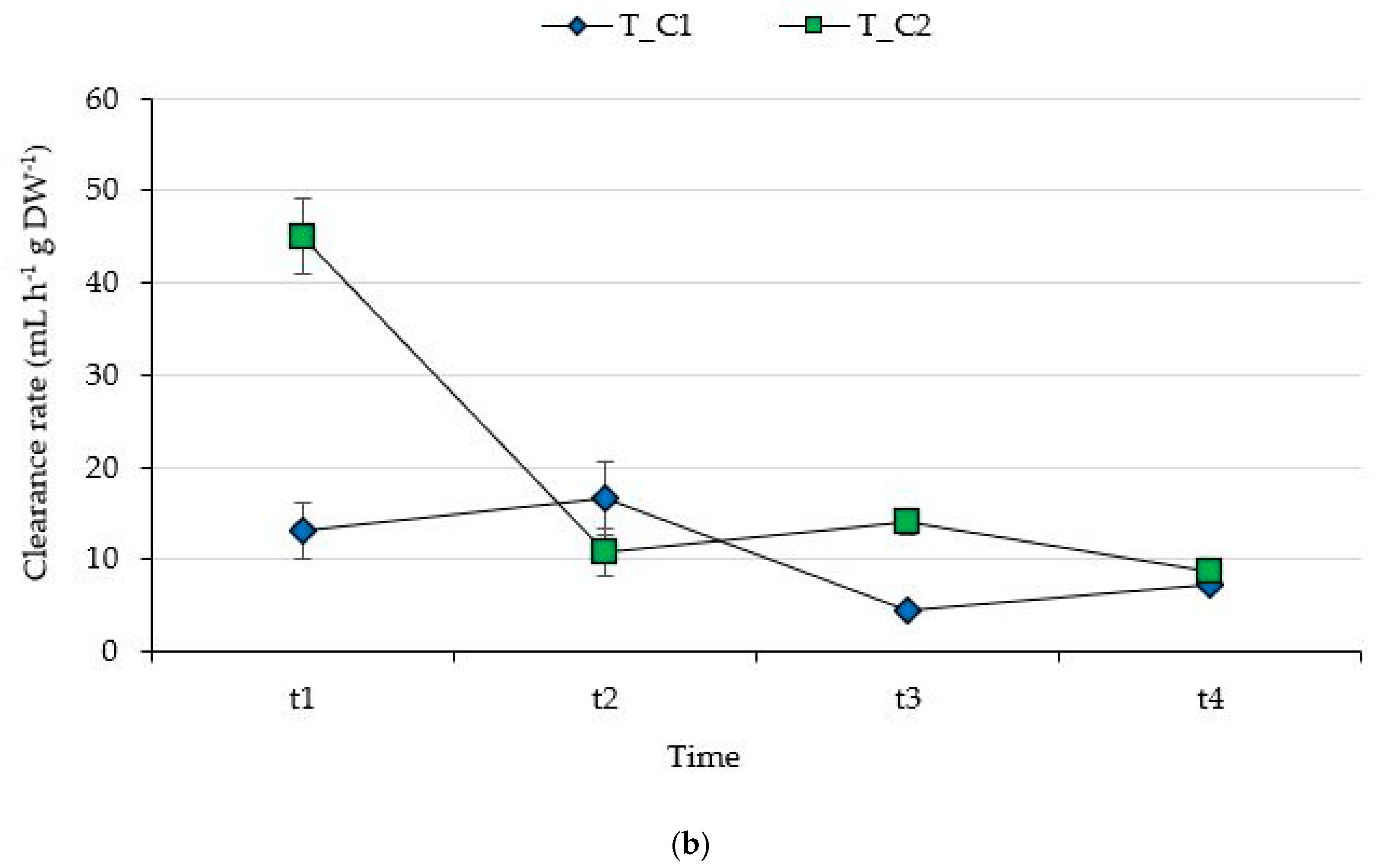
3.1. Filtering Capability
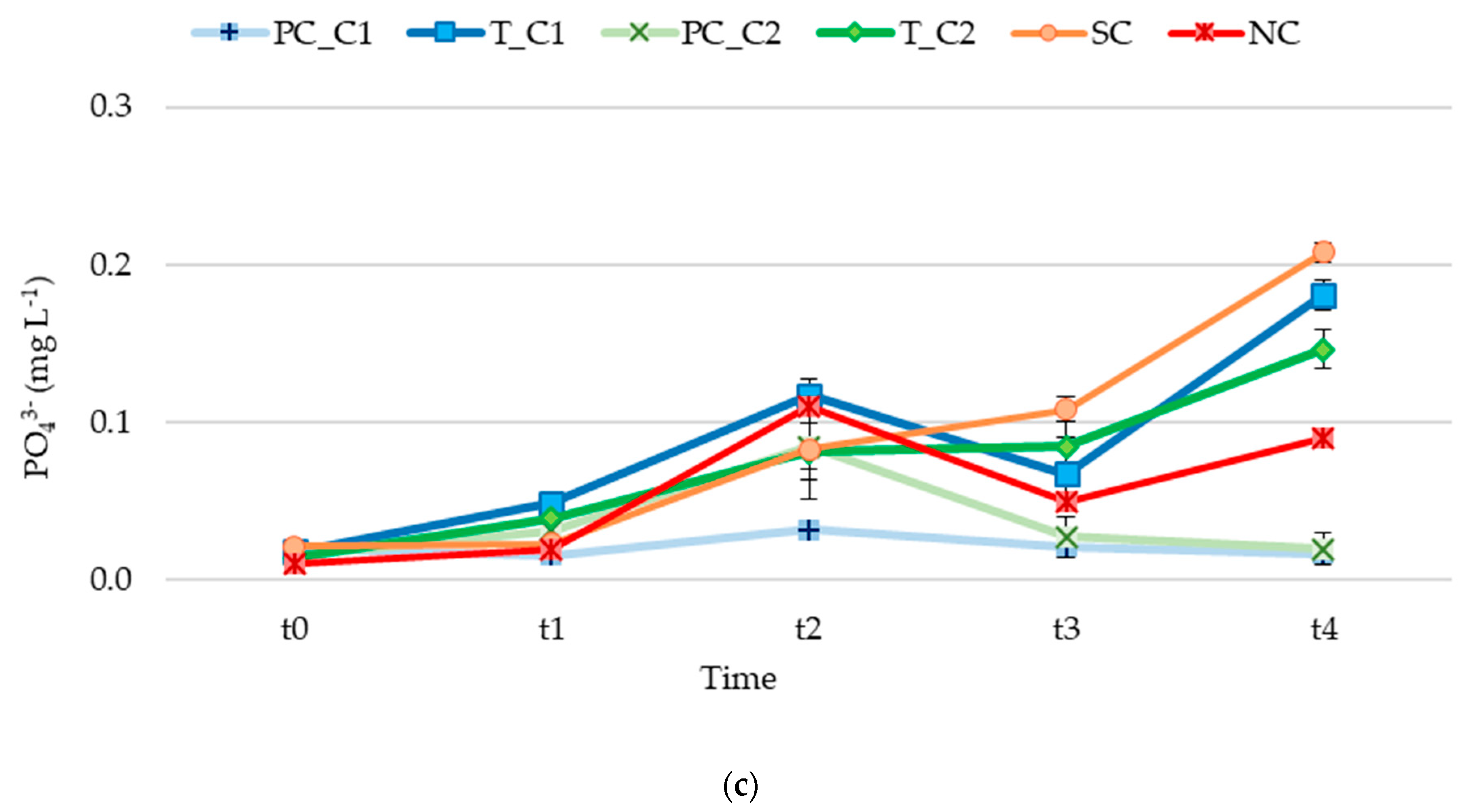
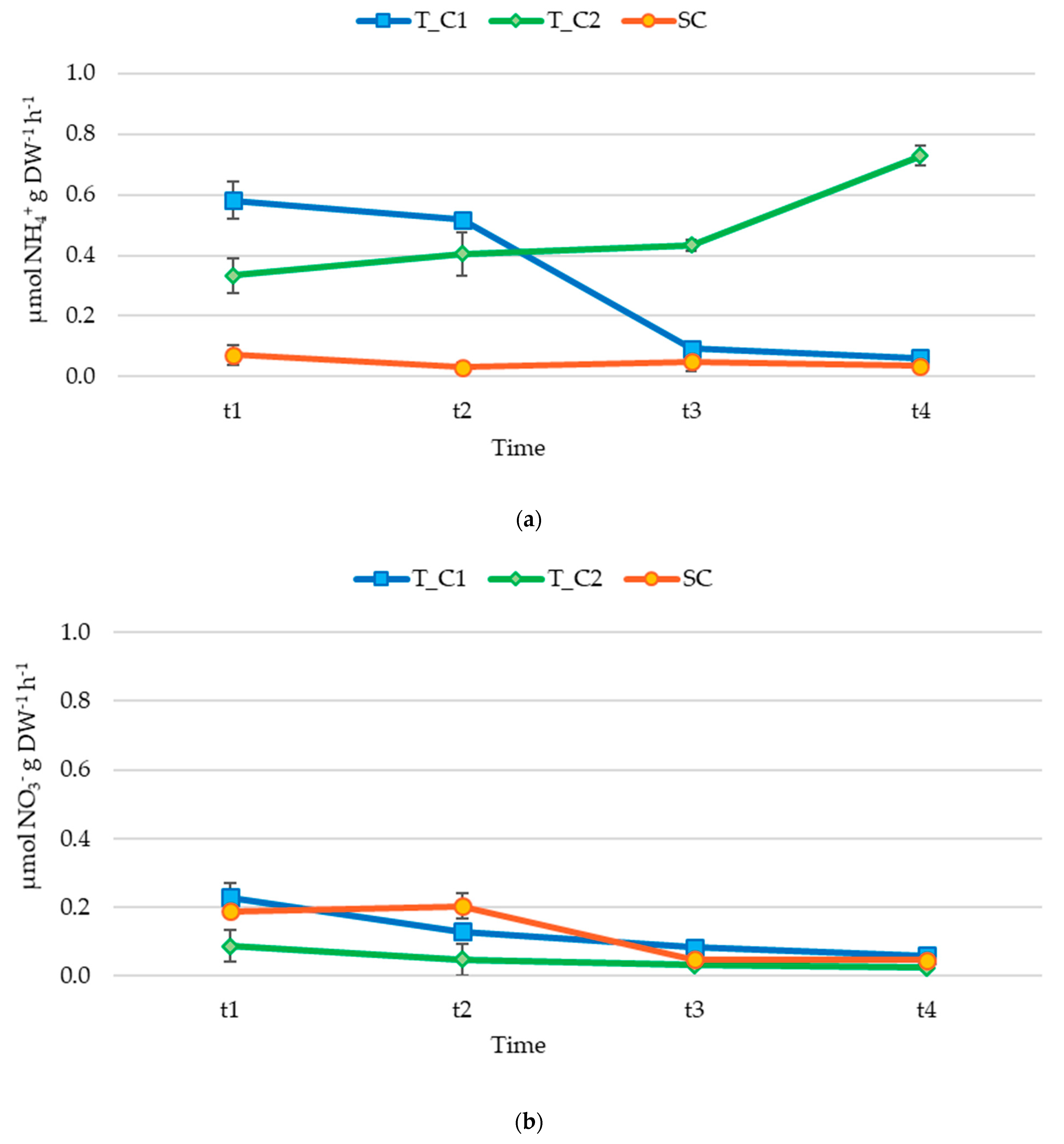
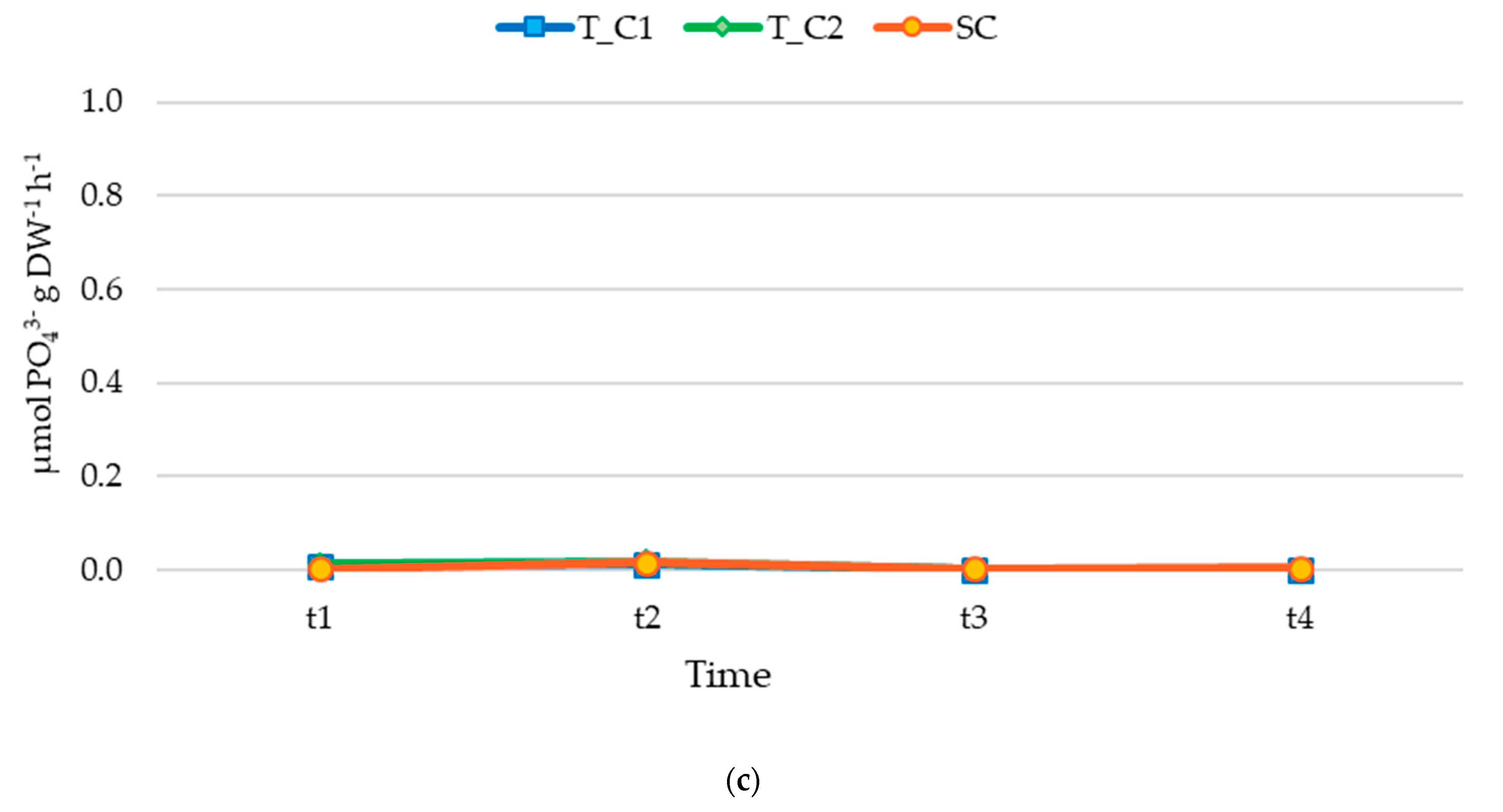
3.2. Nutrient Release
4. Discussion and Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Bell, J.J.; Mcgrath, E.; Biggerstaff, A.; Bates, T.; Cárdenas, C.A.; Bennett, H. Global conservation status of sponges. Conserv. Biol. 2015, 29, 42–53. [Google Scholar] [CrossRef]
- Van Soest, R.W.M.; Boury-Esnault, N.; Vacelet, J.; Dohrmann, M.; Erpenbeck, D.; de Voogd, N.J.; Santodomingo, N.; Vanhoorne, B.; Kelly, M.; Hooper, J.N.A. Global diversity of sponges (Porifera). PLoS ONE 2012, 7, e35105. [Google Scholar] [CrossRef] [PubMed]
- Reiswig, H.M. In Situ Feeding in Two Shallow Water Hexactinellid Sponges; Rützler, K., Ed.; Smithsonian Institution Press: Washington, DC, USA, 1990. [Google Scholar]
- Ribes, M.; Coma, R.; Gili, J.M. Natural diet and grazing rate of the temperate sponge Dysidea avara (Demospongiae, Dendroceratida) throughout an annual cycle. Mar. Ecol. Prog. Ser. 1999, 176, 179–190. [Google Scholar] [CrossRef]
- Weisz, J.B.; Lindquist, N.; Martens, C.S. Do associated microbial abundances impact marine demosponge pumping rates and tissue densities? Oecologia 2008, 155, 367–376. [Google Scholar] [CrossRef] [PubMed]
- Reiswig, H.M. Bacteria as food for temperate-water marine sponges. Can. J. Zool. 1975, 53, 582–589. [Google Scholar] [CrossRef]
- Wilkinson, C.R. Microbial associations in sponges. I. Ecology, physiology and microbial populations of coral reef sponges. Mar. Biol. 1978, 49, 161–167. [Google Scholar] [CrossRef]
- Simpson, T.L. The Cell Biology of Sponges; Springer: New York, NY, USA, 1984. [Google Scholar]
- Larsen, P.S.; Riisgåd, H.U. The sponge pump. J. Theor. Biol. 1994, 168, 53–63. [Google Scholar] [CrossRef]
- Riisgård, H.; Larsen, P. Filter-feeding in marine macro-invertebrates: Pump characteristics, modelling and energy cost. Biol. Rev. 1995, 70, 67–106. [Google Scholar] [CrossRef]
- Pile, A.J.; Patterson, M.R.; Witman, J.D. In situ grazing on plankton <10 μm by the boreal sponge. Mar. Ecol. Prog. Ser. 1996, 141, 95–102. [Google Scholar] [CrossRef]
- Hadas, E.; Marie, D.; Shpigel, M.; Ilan, M. Virus predation by sponges is a new nutrient-flow pathway in coral reef food webs. Limnol. Oceanogr. 2006, 51, 1548–1550. [Google Scholar] [CrossRef]
- Ribes, M.; Coma, R.; Atkinson, M.J.; Kinzie, R.A. Sponges and ascidians control removal of particulate organic nitrogen from coral reef water. Limnol. Oceanogr. 2005, 50, 1480–1489. [Google Scholar] [CrossRef]
- de Goeij, J.M.; van Oevelen, D.; Vermeij, M.J.A.; Osinga, R.; Middelburg, J.J.; de Goeij, A.F.P.M.; Admiraal, W. Surviving in a marine desert: The sponge loop retains resources within coral reefs. Science 2013, 342, 108–110. [Google Scholar] [CrossRef]
- Maldonado, M.; Ribes, M.; van Duyl, F.C. Nutrient Fluxes Through Sponges. Biology, Budgets, and Ecological Implications, 1st ed.; Elsevier Ltd.: Amsterdam, The Netherlands, 2012; Volume 62, ISBN 9780123942838. [Google Scholar]
- Van de Vyver, G.; Vray, B.; Belaouane, S.; Touussaint, D. Efficiency and Selectivity of Microorganism Retention by Ephydatia fluviatilis; New Perspe; Rutzer, K., Ed.; Smithsonian Institution Press: Washington, DC, USA, 1990. [Google Scholar]
- Gifford, S.; Dunstan, R.H.; O’Connor, W.; Koller, C.E.; MacFarlane, G.R. Aquatic zooremediation: Deploying animals to remediate contaminated aquatic environments. Trends Biotechnol. 2007, 25, 60–65. [Google Scholar] [CrossRef]
- Pronzato, R.; Bavestrello, G.; Cerrano, C. Morpho-functional adaptations of three species of Spongia (Porifera, Demospongiae) from a Mediterranean vertical cliff. Bull. Mar. Sci. 1998, 63, 317–328. [Google Scholar]
- Milanese, M.; Chelossi, E.; Manconi, R.; Sarà, A.; Sidri, M.; Pronzato, R. The marine sponge Chondrilla nucula Schmidt, 1862 as an elective candidate for bioremediation in integrated aquaculture. Biomol. Eng. 2003, 20, 363–368. [Google Scholar] [CrossRef]
- Fu, W.; Sun, L.; Zhang, X.; Zhang, W. Potential of the marine sponge Hymeniacidon perleve as a bioremediator of pathogenic bacteria in integrated aquaculture ecosystems. Biotechnol. Bioeng. 2006, 93, 1112–1122. [Google Scholar] [CrossRef]
- Fu, W.; Wu, Y.; Sun, L.; Zhang, W. Efficient bioremediation of total organic carbon (TOC) in integrated aquaculture system by marine sponge Hymeniacidon perleve. Biotechnol. Bioeng. 2007, 97, 1387–1397. [Google Scholar] [CrossRef] [PubMed]
- Osinga, R.; Sidri, M.; Cerig, E.; Gokalp, S.Z.; Gokalp, M. Sponge aquaculture trials in the East-Mediterranean Sea: New approaches to earlier ideas. Open Mar. Biol. J. 2010, 4, 74–81. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, W.; Xue, L.; Zhang, B.; Jin, M.; Fu, W. Bioremediation of bacteria pollution using the marine sponge Hymeniacidon perlevis in the intensive mariculture water system of turbot Scophthalmus maximus. Biotechnol. Bioeng. 2010, 105, 59–68. [Google Scholar] [CrossRef]
- Ledda, F.D.; Pronzato, R.; Manconi, R. Mariculture for bacterial and organic waste removal: A field study of sponge filtering activity in experimental farming. Aquac. Res. 2014, 45, 1389–1401. [Google Scholar] [CrossRef]
- Gökalp, M.; Kooistra, T.; Rocha, M.S.; Silva, T.H.; Osinga, R.; Murk, A.J.; Wijgerde, T. The Effect of Depth on the Morphology, Bacterial Clearance, and Respiration of the Mediterranean Sponge Chondrosia reniformis (Nardo, 1847). Mar. Drugs 2020, 18, 358. [Google Scholar] [CrossRef] [PubMed]
- Stabili, L.; Licciano, M.; Longo, C.; Corriero, G.; Mercurio, M. Evaluation of microbiological accumulation capability of the commercial sponge Spongia officinalis var. adriatica (Schmidt) (Porifera, Demospongiae). Water Res. 2008, 42, 2499–2506. [Google Scholar] [CrossRef] [PubMed]
- Pronzato, R.; Bavestrello, G.; Cerrano, C.; Magnino, G.; Manconi, R.; Pantelis, J.; Sarà, A.; Sidri, M. Sponge farming in the Mediterranean Sea: New perspectives. Mem. Qld. Mus. 1999, 44, 485–491. [Google Scholar]
- Corsi, I.; Iacocca, A.; Mercurio, M.; Longo, C.; Giangrande, A.; Pierri, C.; Lembo, G.; Spedicato, M.; Focardi, S. Promising extractive species for integrated mariculture system: Preliminary results on resistance to organophosphate insecticides. Eur. Aquac. Soc. 2004, 34, 247–248. [Google Scholar]
- Pronzato, R. Sponge-fishing, disease and farming in the Mediterranean Sea. Aquat. Conserv. Mar. Freshw. Ecosyst. 1999, 9, 485–493. [Google Scholar] [CrossRef]
- Nemoy, P.; Ehud, S.D.A. Sustainable cultivation of sponges in the Eastern Mediterranean Sea: Integrated aquaculture with fish farms. In Proceedings of the 10th World Sponge Conference, Book of Abstract. Galway, Ireland, 25–30 June 2017; p. 89. [Google Scholar]
- Benediktsdóttir, E.; Helgason, S.; Sigurjónsdóttir, H. Vibrio spp. isolated from salmonids with shallow skin lesions and reared at low temperature. J. Fish. Dis. 1998, 21, 19–28. [Google Scholar] [CrossRef]
- Hann, P.J.; Altmann, K.; Chen, D.; Smith, A.; Cosic, S.; Moon, P.; Hammond, L.S. Development of monoclonal antibodies for the rapid identification of epizootic Vibrio species. J. Fish. Dis. 1992, 15, 63–69. [Google Scholar] [CrossRef]
- Silva-Aciares, F.; Moraga, D.; Auffret, M.; Tanguy, A.; Riquelme, C. Transcriptomic and cellular response to bacterial challenge (pathogenic Vibrio parahaemolyticus) in farmed juvenile Haliotis rufescens fed with or without probiotic diet. J. Invertebr. Pathol. 2013, 113, 163–176. [Google Scholar] [CrossRef]
- Sung, H.H.; Li, H.C.; Tsai, F.M.; Ting, Y.Y.; Chao, W.L. Changes in the composition of Vibrio communities in pond water during tiger shrimp (Penaeus monodon) cultivation and in the hepatopancreas of healthy and diseased shrimp. J. Exp. Mar. Bio. Ecol. 1999, 236, 261–271. [Google Scholar] [CrossRef]
- Almeida, A.; Cunha, Â.; Gomes, N.C.M.; Alves, E.; Costa, L.; Faustino, M.A.F. Phage therapy and photodynamic therapy: Low environmental impact approaches to inactivate microorganisms in fish farming plants. Mar. Drugs 2009, 7, 268–313. [Google Scholar] [CrossRef] [PubMed]
- Noya, M.; Magariños, B.; Lamas, J. Interactions between peritoneal exudate cells (PECs) of gilthead seabream (Sparus aurata) and Pasteurella piscicida. A morphological study. Aquaculture 1995, 131, 11–21. [Google Scholar] [CrossRef]
- Toranzo, A.E.; Barreiro, S.; Casal, J.F.; Figueras, A.; Magarin˜os, B.; Barja, J.L. Pasteurellosis in cultured gilthead seabream (Sparus aurata): First report in Spain. Aquaculture 1991, 99, 1–15. [Google Scholar] [CrossRef]
- Reilly, A.; Käferstein, F. Food safety hazards and the application of the principles of the hazard analysis and critical control point (HACCP) system for their control in aquaculture production. Aquac. Res. 1997, 28, 735–752. [Google Scholar] [CrossRef]
- Aresta, A.; Marzano, C.N.; Lopane, C.; Corriero, G.; Longo, C.; Zambonin, C.; Stabili, L. Analytical investigations on the lindane bioremediation capability of the demosponge Hymeniacidon perlevis. Mar. Pollut. Bull. 2015, 90, 143–149. [Google Scholar] [CrossRef]
- Longo, C.; Corriero, G.; Licciano, M.; Stabili, L. Bacterial accumulation by the Demospongiae Hymeniacidon perlevis: A tool for the bioremediation of polluted seawater. Mar. Pollut. Bull. 2010, 60, 1182–1187. [Google Scholar] [CrossRef] [PubMed]
- Longo, C.; Cardone, F.; Corriero, G.; Licciano, M.; Pierri, C.; Stabili, L. The co-occurrence of the demosponge Hymeniacidon perlevis and the edible mussel Mytilus galloprovincialis as a new tool for bacterial load mitigation in aquaculture. Environ. Sci. Pollut. Res. 2015, 23, 3736–3746. [Google Scholar] [CrossRef]
- Stabili, L.; Licciano, M.; Giangrande, A.; Longo, C.; Mercurio, M.; Marzano, C.N.; Corriero, G. Filtering activity of Spongia officinalis var. adriatica (Schmidt) (Porifera, Demospongiae) on bacterioplankton: Implications for bioremediation of polluted seawater. Water Res. 2006, 40, 3083–3090. [Google Scholar] [CrossRef] [PubMed]
- Ledda, F.D.; Manconi, R.; Pronzato, R. Retention rates on bacteria and organic matter by Ircinia variabilis (Demospongiae, Dictyoceratida) in experimental sponge farming for bioremediation. Biol. Mar. Mediterr. 2008, 15, 164–165. [Google Scholar]
- Madri, P.P.; Claus, G.; Kunen, S.M.; Moss, E.E. Preliminary studies on the Escherichia coli uptake of the redbeard sponge Microciona prolifera (Verrill). Life Sci. 1967, 6, 889–894. [Google Scholar] [CrossRef]
- Gökalp, M.; Mes, D.; Nederloff, M.; Zhao, H.; de Goeij, J.M.; Osinga, R. The potential roles of sponges in integrated mariculture. Rev. Aquac. 2020. [Google Scholar] [CrossRef]
- Pronzato, R.; Manconi, R.; Corriero, G. Tipologie di Impianto Modulare per la Spongicoltura Subacquea Anche in Policoltura USAMA (Underwater Sponge Aquacolture Modular System). Italian Ministry Patent 0001334230, 2006. [Google Scholar]
- Corriero, G.; Longo, C.; Mercurio, M.; Marzano, C.N.; Lembo, G.; Spedicato, M.T. Rearing performance of Spongia officinalis on suspended ropes off the Southern Italian Coast (Central Mediterranean Sea). Aquaculture 2004, 238, 195–205. [Google Scholar] [CrossRef]
- Gifford, S.; Dunstan, R.H.; O’Connor, W.; Roberts, T.; Toia, R. Pearl aquaculture-profitable environmental remediation? Sci. Total Environ. 2004, 319, 27–37. [Google Scholar] [CrossRef]
- Giangrande, A.; Pierri, C.; Arduini, D.; Borghese, J.; Licciano, M.; Trani, R.; Corriero, G.; Basile, G.; Cecere, E.; Petrocelli, A.; et al. An innovative IMTA system: Polychaetes, sponges and macroalgae co-cultured in a Southern Italian in-shore mariculture plant (Ionian Sea). J. Mar. Sci. Eng. 2020, 8, 733. [Google Scholar] [CrossRef]
- Pérez-López, P.; Ledda, F.D.; Bisio, A.; Feijoo, G.; Perino, E.; Pronzato, R.; Manconi, R.; Moreira, M.T. Life cycle assessment of in situ mariculture in the Mediterranean Sea for the production of bioactive compounds from the sponge Sarcotragus spinosulus. J. Clean. Prod. 2017, 142, 4356–4368. [Google Scholar] [CrossRef]
- Hardoim, C.C.P.; Costa, R. Temporal dynamics of prokaryotic communities in the marine sponge Sarcotragus spinosulus. Mol. Ecol. 2014, 23, 3097–3112. [Google Scholar] [CrossRef] [PubMed]
- Abed, C.; Legrave, N.; Dufies, M.; Robert, G.; Guérineau, V.; Vacelet, J.; Auberger, P.; Amade, P.; Mehiri, M. A new hydroxylated nonaprenylhydroquinone from the mediterranean marine sponge Sarcotragus spinosulus. Mar. Drugs 2011, 9, 1210. [Google Scholar] [CrossRef] [PubMed]
- Cimino, G.; De Stefano, S.; Minale, L.; Fattorusso, E. Ircinin-1 and -2, linear sesterterpenes from the marine sponge Ircinia oros. Tetrahedron 1972, 28, 333–341. [Google Scholar] [CrossRef]
- Mercurio, M.; Scalera Liaci, L.; Corriero, G. La fauna a poriferi del bacino della Strea di Porto Cesareo (LE). Biol. Mar. Mediterr. 2001, 8, 403–412. [Google Scholar]
- Corriero, G. Distribuzione ed Ecologia dei Poriferi in Ambienti ‘Confinati Mediterranei’. Ph.D. Thesis, University of Genova, Genova, Italy, 1990. [Google Scholar]
- Corriero, G.; Gherardi, M.; Giangrande, A.; Longo, C.; Mercurio, M.; Musco, L.; Marzano, C.N. Inventory and distribution of hard bottom fauna from the marine protected area of porto cesareo (ionian sea): Porifera and polychaeta. Ital. J. Zool. 2004, 71, 237–245. [Google Scholar] [CrossRef][Green Version]
- Mercurio, M.; Corriero, G.; Gherardi, M.; Baldacconi, R.; Elda, G. Sexual reproduction in Sarcotragus spinosulus from two different shallow environments. Mar. Ecol. 2013, 34, 394–408. [Google Scholar] [CrossRef]
- Ottaviani, D.; Leoni, F.; Rocchegiani, E.; Santarelli, S.; Masini, L.; D’Annibale, M.L.; Pianetti, A.; Carraturo, A. A severe case of aeromonas veronii biovar sobria travellers’ diarrhoea characterized by vibrio parahaemolyticus co-isolation. J. Med. Microbiol. 2013, 62, 161–164. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Coughlan, J. The estimation of filtering rate from the clearance of suspensions. Mar. Biol. 1969, 2, 356–358. [Google Scholar] [CrossRef]
- Neori, A.; Shpigel, M.; Ben-Ezra, D. A sustainable integrated system for culture of fish, seaweed and abalone. Aquaculture 2000, 186, 279–291. [Google Scholar] [CrossRef]
- Sanz-Lázaro, C.; Marin, A. Benthic recovery during open sea fish farming abatement in Western Mediterranean, Spain. Mar. Environ. Res. 2006, 62, 374–387. [Google Scholar] [CrossRef]
- Carvalho, A.P.; Meireles, L.A.; Malcata, F.X. Rapid spectrophotometric determination of nitrates and nitrites in marine aqueous culture media. Analysis 1998, 26, 347–351. [Google Scholar] [CrossRef]
- Strickland, J.D.H.; Parsons, T.R. A Practical Handbook of Seawater Analysis; the Alger Press L.T.D: Ottawa, ON, Canada, 1972. [Google Scholar]
- De Goeij, J.M.; De Kluijver, A.; Van Duyl, F.C.; Vacelet, J.; Wijffels, R.H.; De Goeij, A.F.R.M.; Cleutjens, J.P.M.; Schutte, B. Cell kinetics of the marine sponge Halisarca caerulea reveal rapid cell turnover and shedding. J. Exp. Biol. 2009, 212, 3892–3900. [Google Scholar] [CrossRef]
- Anderson, M.J. A new method for non-parametric multivariate analysis of variance. Austral Ecol. 2001, 26, 32–46. [Google Scholar] [CrossRef]
- Anderson, M.J.; Gorley, R.N.; Clarke, K.R. PERMANOVA+ for PRIMER: Guide to Software and Statistical Methods; PRIMER-E Ltd.: Plymouth, UK, 2008. [Google Scholar]
- Su, Y.C.; Liu, C. Vibrio parahaemolyticus: A concern of seafood safety. Food Microbiol. 2007, 24, 549–558. [Google Scholar] [CrossRef]
- Colwell, R.R.; Grimes, D.J. Vibrio diseases of marine fish populations. Helgol. Mar. Res. 1984, 37, 265–287. [Google Scholar] [CrossRef]
- Maldonado, M.; Zhang, X.; Cao, X.; Xue, L.; Cao, H.; Zhang, W. Selective feeding by sponges on pathogenic microbes: A reassessment of potential for abatement of microbial pollution. Mar. Ecol. Prog. Ser. 2010, 403, 75–89. [Google Scholar] [CrossRef]
- Reiswig, H.M. Particle Feeding in Natural Populations of Three Marine Demosponges. Biol. Bull. 1971, 141, 568–591. [Google Scholar] [CrossRef]
- Topçu, N.E.; Pérez, T.; Grégori, G.; Harmelin-Vivien, M. In situ investigation of Spongia officinalis (Demospongiae) particle feeding: Coupling flow cytometry and stable isotope analysis. J. Exp. Mar. Bio. Ecol. 2010, 389, 61–69. [Google Scholar] [CrossRef]
- Duckworth, A.R.; Brück, W.M.; Janda, K.E.; Pitts, T.P.; McCarthy, P.J. Retention efficiencies of the coral reef sponges Aplysina lacunosa, Callyspongia vaginalis and Niphates digitalis determined by Coulter counter and plate culture analysis. Mar. Biol. Res. 2006, 2, 243–248. [Google Scholar] [CrossRef]
- Reiswig, H.M. Water transport, respiration and energetics of three tropical marine sponges. J. Exp. Mar. Bio. Ecol. 1974, 14, 231–249. [Google Scholar] [CrossRef]
- Hardoim, C.C.P.; Esteves, A.I.S.; Pires, F.R.; Gonçalves, J.M.S.; Cox, C.J.; Xavier, J.R.; Costa, R. Phylogenetically and Spatially Close Marine Sponges Harbour Divergent Bacterial Communities. PLoS ONE 2012, 7, e53029. [Google Scholar] [CrossRef]
- Jiménez, E.; Ribes, M. Sponges as a source of dissolved inorganic nitrogen: Nitrification mediated by temperate sponges. Limnol. Oceanogr. 2007, 52, 948–958. [Google Scholar] [CrossRef]
- Rix, L.; De Goeij, J.M.; Van Oevelen, D.; Struck, U.; Al-Horani, F.A.; Wild, C.; Naumann, M.S. Reef sponges facilitate the transfer of coral-derived organic matter to their associated fauna via the sponge loop. Mar. Ecol. Prog. Ser. 2018, 589, 85–96. [Google Scholar] [CrossRef]
- Pita, L.; Rix, L.; Slaby, B.M.; Franke, A.; Hentschel, U. The sponge holobiont in a changing ocean: From microbes to ecosystems. Microbiome 2018, 6, 46. [Google Scholar] [CrossRef]
- Hoffmann, F.; Radax, R.; Woebken, D.; Holtappels, M.; Lavik, G.; Rapp, H.T.; Schläppy, M.L.; Schleper, C.; Kuypers, M.M.M. Complex nitrogen cycling in the sponge Geodia barretti. Environ. Microbiol. 2009, 11, 2228–2243. [Google Scholar] [CrossRef]
- Fiore, C.L.; Labrie, M.; Jarett, J.K.; Lesser, M.P. Transcriptional activity of the giant barrel sponge, Xestospongia muta Holobiont: Molecular evidence for metabolic interchange. Front. Microbiol. 2015, 6, 364. [Google Scholar] [CrossRef]
- Schläppy, M.L.; Schöttner, S.I.; Lavik, G.; Kuypers, M.M.M.; de Beer, D.; Hoffmann, F. Evidence of nitrification and denitrification in high and low microbial abundance sponges. Mar. Biol. 2010, 157, 593–602. [Google Scholar] [CrossRef] [PubMed]
- Fiore, C.L.; Baker, D.M.; Lesser, M.P. Nitrogen Biogeochemistry in the Caribbean Sponge, Xestospongia muta: A Source or Sink of Dissolved Inorganic Nitrogen? PLoS ONE 2013, 8, e72961. [Google Scholar] [CrossRef]
- Bayer, K.; Schmitt, S.; Hentschel, U. Physiology, phylogeny and in situ evidence for bacterial and archaeal nitrifiers in the marine sponge Aplysina aerophoba. Environ. Microbiol. 2008, 10, 2942–2955. [Google Scholar] [CrossRef] [PubMed]
- Southwell, M.W.; Weisz, J.B.; Martens, C.S.; Lindquist, N. In situ fluxes of dissolved inorganic nitrogen from the sponge community on Conch Reef, Key Largo, Florida. Limnol. Oceanogr. 2008, 53, 986–996. [Google Scholar] [CrossRef]
- Richter, C.; Wunsch, M.; Rasheed, M.; Kötter, I.; Badran, M.I. Endoscopic exploration of Red Sea coral reefs reveals dense populations of cavity-dwelling sponges. Nature 2001, 413, 726–730. [Google Scholar] [CrossRef]
- Zhang, F.; Blasiak, L.C.; Karolin, J.O.; Powell, R.J.; Geddes, C.D.; Hill, R.T.; Karl, D.M. Phosphorus sequestration in the form of polyphosphate by microbial symbionts in marine sponges. Proc. Natl. Acad. Sci. USA 2015, 112, 4381–4386. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trani, R.; Corriero, G.; de Pinto, M.C.; Mercurio, M.; Pazzani, C.; Pierri, C.; Scrascia, M.; Longo, C. Filtering Activity and Nutrient Release by the Keratose Sponge Sarcotragus spinosulus Schmidt, 1862 (Porifera, Demospongiae) at the Laboratory Scale. J. Mar. Sci. Eng. 2021, 9, 178. https://doi.org/10.3390/jmse9020178
Trani R, Corriero G, de Pinto MC, Mercurio M, Pazzani C, Pierri C, Scrascia M, Longo C. Filtering Activity and Nutrient Release by the Keratose Sponge Sarcotragus spinosulus Schmidt, 1862 (Porifera, Demospongiae) at the Laboratory Scale. Journal of Marine Science and Engineering. 2021; 9(2):178. https://doi.org/10.3390/jmse9020178
Chicago/Turabian StyleTrani, Roberta, Giuseppe Corriero, Maria Concetta de Pinto, Maria Mercurio, Carlo Pazzani, Cataldo Pierri, Maria Scrascia, and Caterina Longo. 2021. "Filtering Activity and Nutrient Release by the Keratose Sponge Sarcotragus spinosulus Schmidt, 1862 (Porifera, Demospongiae) at the Laboratory Scale" Journal of Marine Science and Engineering 9, no. 2: 178. https://doi.org/10.3390/jmse9020178
APA StyleTrani, R., Corriero, G., de Pinto, M. C., Mercurio, M., Pazzani, C., Pierri, C., Scrascia, M., & Longo, C. (2021). Filtering Activity and Nutrient Release by the Keratose Sponge Sarcotragus spinosulus Schmidt, 1862 (Porifera, Demospongiae) at the Laboratory Scale. Journal of Marine Science and Engineering, 9(2), 178. https://doi.org/10.3390/jmse9020178











