CDOM Spatiotemporal Variability in the Mediterranean Sea: A Modelling Study
Abstract
1. Introduction
2. Materials and Methods
2.1. The Transport Reaction Model
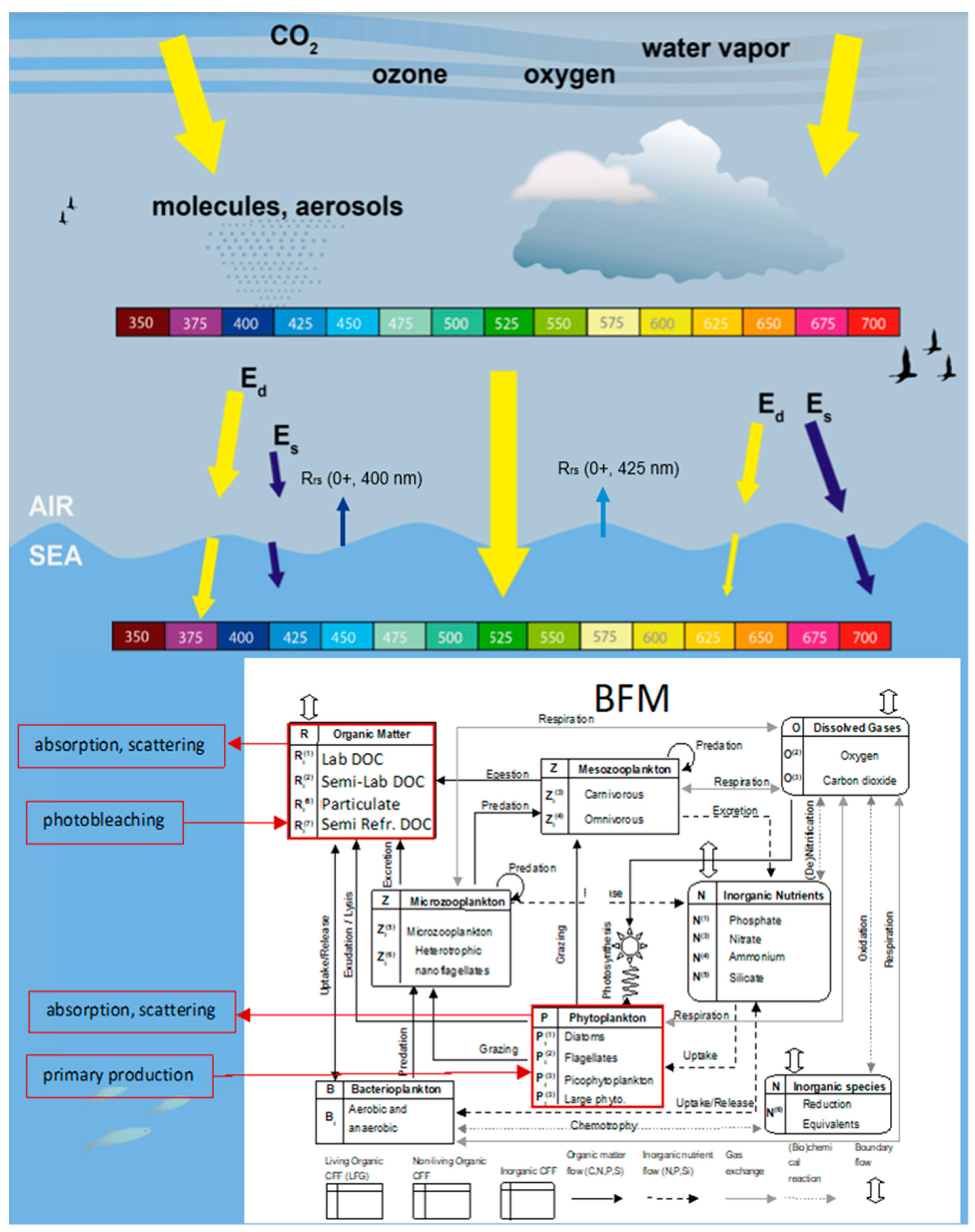
2.2. The Biogeochemical Model
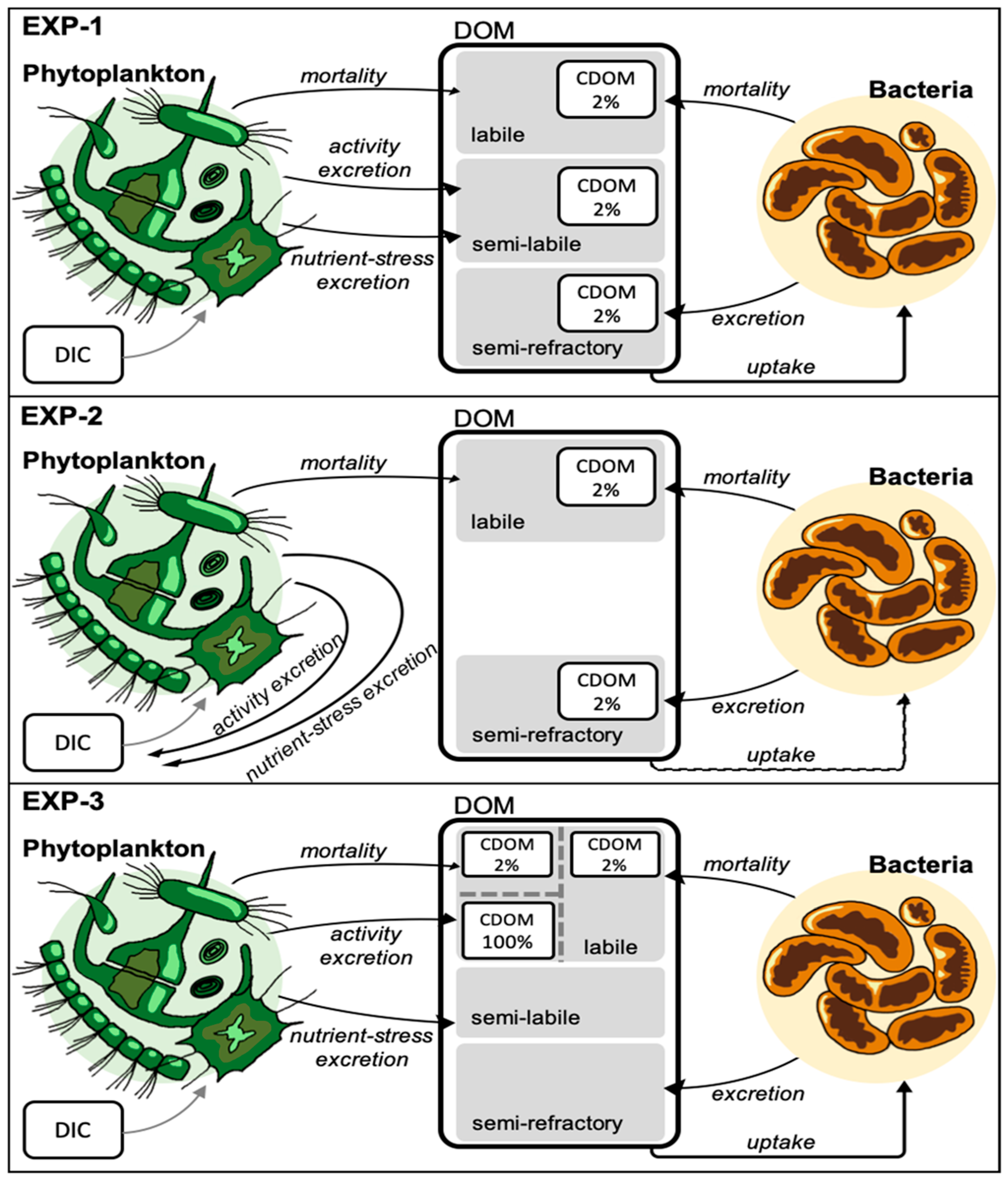
- EXP-2: the CDOM is partitioned as 2% of DOC in all the fluxes but the nutrient stress limitation is in part accounted as non-realized production [31] and in part channeled to the production of semi-labile DOC. In this experiment we chose to remove the flux to semi-labile DOC to explore an extreme condition;
- EXP-3: the CDOM production is related to activity exudation and is considered colored, the semi-refractory component of CDOM in not considered.
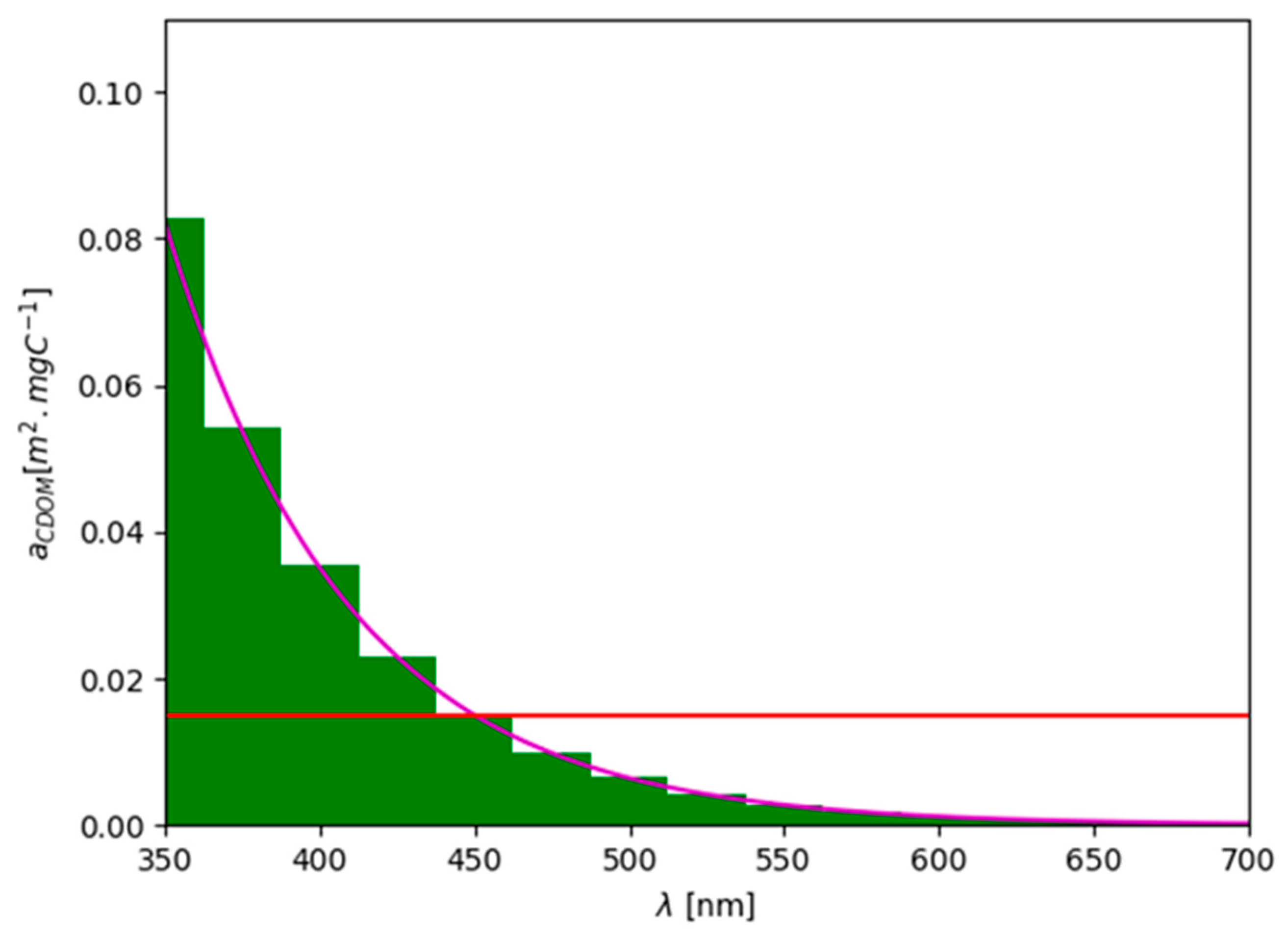
2.3. The Radiative Transfer Model
2.4. Dataset for Model Validation
2.5. Simulation Protocol
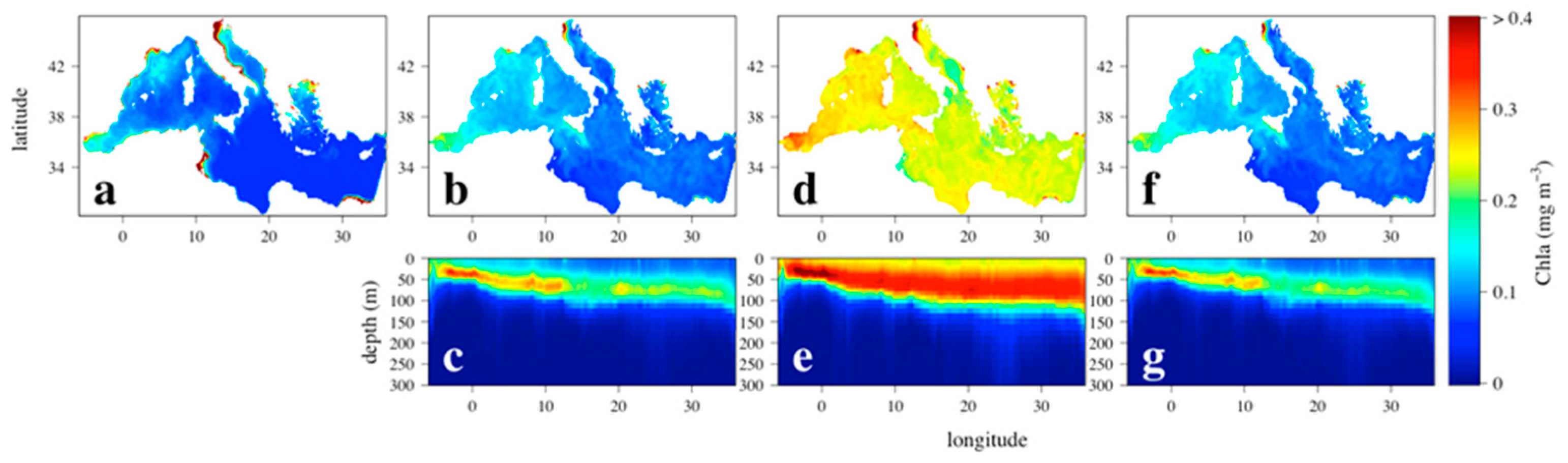
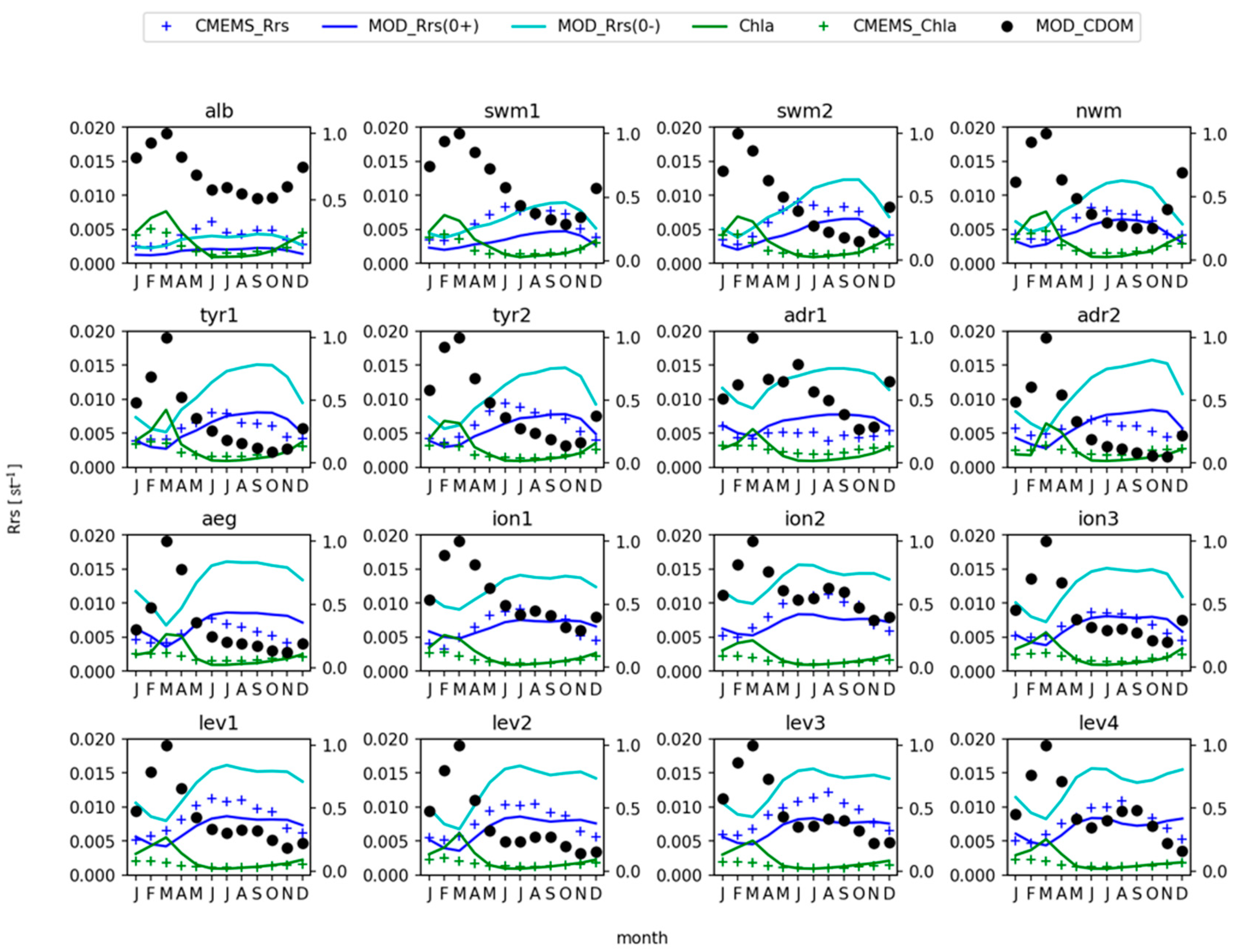
3. Results
3.1. Chlorophyll Spatial Distribution
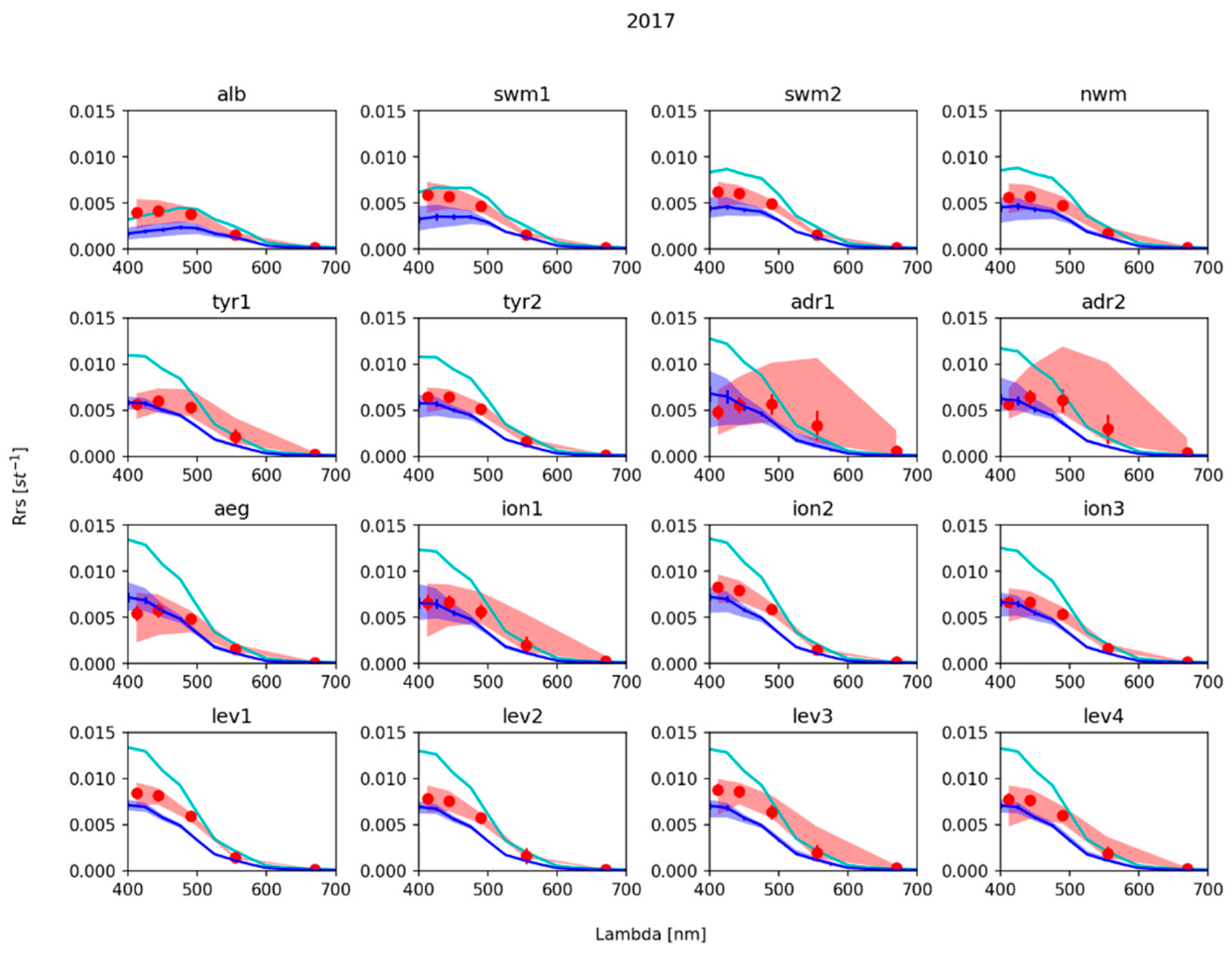
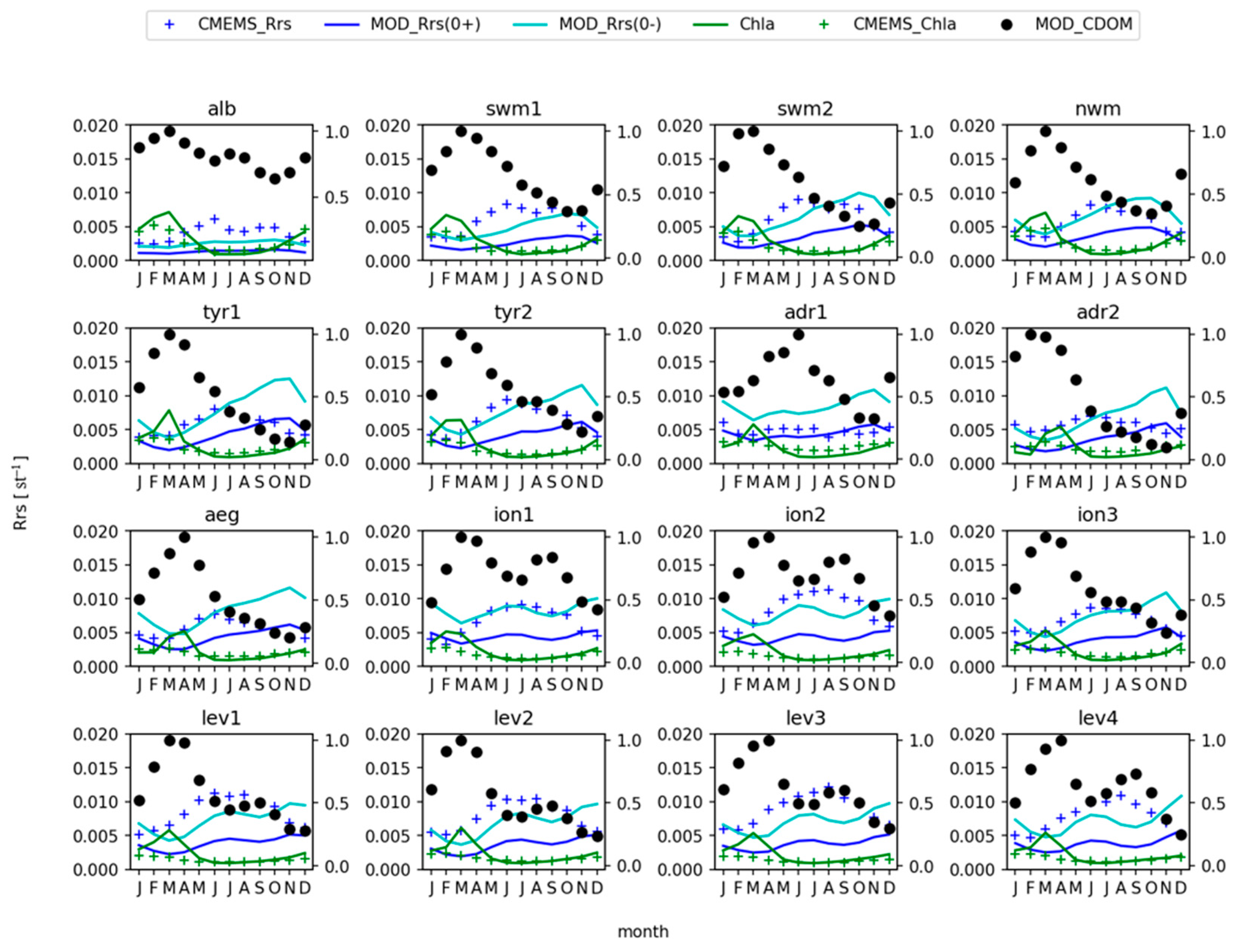
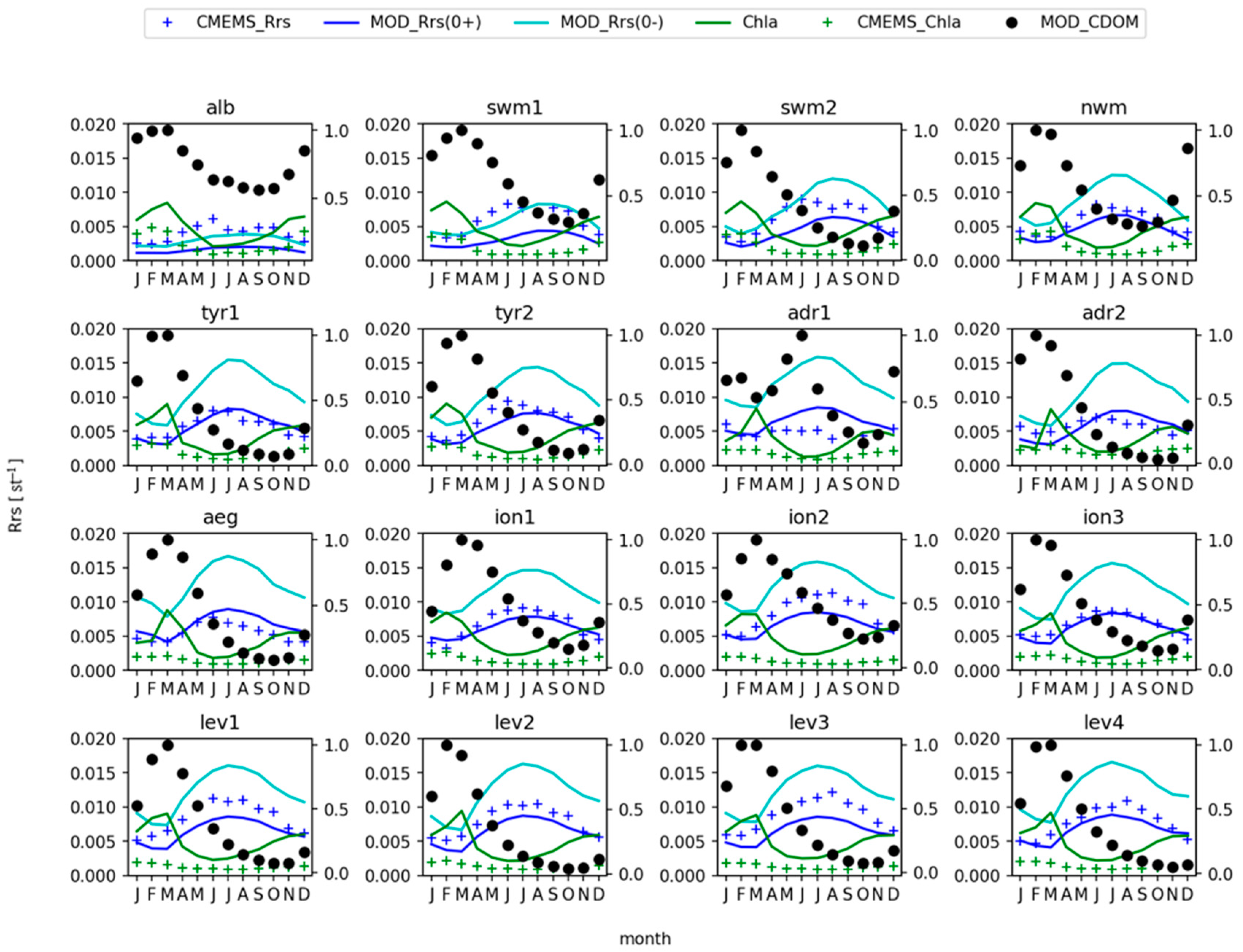
3.2. Remote Sensing Reflectance
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hansell, D.A.; Carlson, C.A. (Eds.) Biogeochemistry of Marine Dissolved Organic Matter, 2nd ed.; Academic Press: Amsterdam, The Netherlands; Boston, MA, USA, 2015; ISBN 978-0-12-405940-5. [Google Scholar]
- Galletti, Y.; Gonnelli, M.; Retelletti Brogi, S.; Vestri, S.; Santinelli, C. DOM Dynamics in Open Waters of the Mediterranean Sea: New Insights from Optical Properties. Deep Sea Res. Part Oceanogr. Res. Pap. 2019, 144, 95–114. [Google Scholar] [CrossRef]
- Santinelli, C. DOC in the Mediterranean Sea. In Biogeochemistry of Marine Dissolved Organic Matter; Elsevier: Amsterdam, The Netherlands, 2015; pp. 579–608. [Google Scholar]
- Morel, A.; Gentili, B. The Dissolved Yellow Substance and the Shades of Blue in the Mediterranean Sea. Biogeosciences 2009, 6, 2625–2636. [Google Scholar] [CrossRef]
- Claustre, H.; Morel, A.; Hooker, S.B.; Babin, M.; Antoine, D.; Oubelkheir, K.; Bricaud, A.; Leblanc, K.; Quéguiner, B.; Maritorena, S. Is Desert Dust Making Oligotrophic Waters Greener: Saharan Dust and Ocean Color. Geophys. Res. Lett. 2002, 29, 107-1–107-4. [Google Scholar] [CrossRef]
- Gitelson, A.; Karnieli, A.; Goldman, N.; Yacobi, Y.Z.; Mayo, M. Chlorophyll Estimation in the Southeastern Mediterranean Using CZCS Images: Adaptation of an Algorithm and Its Validation. J. Mar. Syst. 1996, 9, 283–290. [Google Scholar] [CrossRef]
- D’Ortenzio, F.; Marullo, S.; Ragni, M.; Ribera d’Alcalà, M.; Santoleri, R. Validation of Empirical SeaWiFS Algorithms for Chlorophyll-a Retrieval in the Mediterranean Sea. Remote Sens. Environ. 2002, 82, 79–94. [Google Scholar] [CrossRef]
- Morel, A.; Huot, Y.; Gentili, B.; Werdell, P.J.; Hooker, S.B.; Franz, B.A. Examining the Consistency of Products Derived from Various Ocean Color Sensors in Open Ocean (Case 1) Waters in the Perspective of a Multi-Sensor Approach. Remote Sens. Environ. 2007, 111, 69–88. [Google Scholar] [CrossRef]
- Organelli, E.; Claustre, H.; Bricaud, A.; Barbieux, M.; Uitz, J.; D’Ortenzio, F.; Dall’Olmo, G. Bio-Optical Anomalies in the World’s Oceans: An Investigation on the Diffuse Attenuation Coefficients for Downward Irradiance Derived from Biogeochemical Argo Float Measurements: World’s Ocean Bio-Optical Anomalies. J. Geophys. Res. Oceans 2017, 122, 3543–3564. [Google Scholar] [CrossRef]
- Organelli, E.; Bricaud, A.; Antoine, D.; Matsuoka, A. Seasonal Dynamics of Light Absorption by Chromophoric Dissolved Organic Matter (CDOM) in the NW Mediterranean Sea (BOUSSOLE Site). Deep Sea Res. Part Oceanogr. Res. Pap. 2014, 91, 72–85. [Google Scholar] [CrossRef]
- Pérez, G.L.; Galí, M.; Royer, S.-J.; Sarmento, H.; Gasol, J.M.; Marrasé, C.; Simó, R. Bio-Optical Characterization of Offshore NW Mediterranean Waters: CDOM Contribution to the Absorption Budget and Diffuse Attenuation of Downwelling Irradiance. Deep Sea Res. Part Oceanogr. Res. Pap. 2016, 114, 111–127. [Google Scholar] [CrossRef]
- Xing, X.; Claustre, H.; Wang, H.; Poteau, A.; D’Ortenzio, F. Seasonal Dynamics in Colored Dissolved Organic Matter in the Mediterranean Sea: Patterns and Drivers. Deep Sea Res. Part Oceanogr. Res. Pap. 2014, 83, 93–101. [Google Scholar] [CrossRef]
- Mühlenbruch, M.; Grossart, H.P.; Eigemann, F.; Voss, M. Mini-Review: Phytoplankton-Derived Polysaccharides in the Marine Environment and Their Interactions with Heterotrophic Bacteria. Environ. Microbiol. 2018, 20, 2671–2685. [Google Scholar] [CrossRef] [PubMed]
- Stedmon, C.A.; Nelson, N.B. Chapter 10—The Optical Properties of DOM in the Ocean. In Biogeochemistry of Marine Dissolved Organic Matter; Hansell, D.A., Carlson, C.A., Eds.; Academic Press: Amsterdam, The Netherlands, 2015; pp. 481–508. [Google Scholar]
- Azam, F.; Fenchel, T.; Field, J.G.; Gray, J.C.; Meyer-Reil, L.A.; Thingstad, F. The Ecological Role of Water-Column Microbes in the Sea. Mar. Ecol. Prog. Ser. 1983, 10, 257–264. [Google Scholar] [CrossRef]
- Romera-Castillo, C.; Sarmento, H.; Álvarez-Salgado, X.A.; Gasol, J.M.; Marrasé, C. Production of Chromophoric Dissolved Organic Matter by Marine Phytoplankton. Limnol. Oceanogr. 2010, 55, 446–454. [Google Scholar] [CrossRef]
- Lazzari, P.; Solidoro, C.; Ibello, V.; Salon, S.; Teruzzi, A.; Béranger, K.; Colella, S.; Crise, A. Seasonal and Inter-Annual Variability of Plankton Chlorophyll and Primary Production in the Mediterranean Sea: A Modelling Approach. Biogeosciences 2012, 9, 217–233. [Google Scholar] [CrossRef]
- Krom, M.D.; Woodward, E.M.S.; Herut, B.; Kress, N.; Carbo, P.; Mantoura, R.F.C.; Spyres, G.; Thingstad, T.F.; Wassmann, P.; Wexels-Riser, C.; et al. Nutrient Cycling in the South East Levantine Basin of the Eastern Mediterranean: Results from a Phosphorus Starved System. Deep Sea Res. Part II Top. Stud. Oceanogr. 2005, 52, 2879–2896. [Google Scholar] [CrossRef]
- Lazzari, P.; Solidoro, C.; Salon, S.; Bolzon, G. Spatial Variability of Phosphate and Nitrate in the Mediterranean Sea: A Modeling Approach. Deep Sea Res. Part Oceanogr. Res. Pap. 2016, 108, 39–52. [Google Scholar] [CrossRef]
- Thingstad, T.F.; Krom, M.D.; Mantoura, R.F.C.; Flaten, G.F.; Groom, S.; Herut, B.; Kress, N.; Law, C.S.; Pasternak, A.; Pitta, P. Nature of Phosphorus Limitation in the Ultraoligotrophic Eastern Mediterranean. Science 2005, 309, 1068–1071. [Google Scholar] [CrossRef]
- Kirk, J.T.O. Light and Photosynthesis in Aquatic Ecosystems, 2nd ed.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 1994; ISBN 978-0-521-45353-0. [Google Scholar]
- Teruzzi, A.; Dobricic, S.; Solidoro, C.; Cossarini, G. A 3-D Variational Assimilation Scheme in Coupled Transport-biogeochemical Models: Forecast of Mediterranean Biogeochemical Properties. J. Geophys. Res. Oceans 2014, 119, 200–217. [Google Scholar] [CrossRef]
- Salon, S.; Cossarini, G.; Bolzon, G.; Feudale, L.; Lazzari, P.; Teruzzi, A.; Solidoro, C.; Crise, A. Novel Metrics Based on Biogeochemical Argo Data to Improve the Model Uncertainty Evaluation of the CMEMS Mediterranean Marine Ecosystem Forecasts. Ocean Sci. 2019, 15, 997–1022. [Google Scholar] [CrossRef]
- Escudier, R.; Clementi, E. Quality Information Document of the Mediterranean Reanalysis Product Medsea_Multiyear_Phy_006_004. 2020. Available online: https://resources.marine.copernicus.eu/documents/QUID/CMEMS-MED-QUID-006-004.pdf (accessed on 10 December 2020).
- Vichi, M.; Lovato, T.; Lazzari, P.; Cossarini, G.; Gutierrez Mlot, E.; Mattia, G.; Masina, S.; McKiver, W.J.; Pinardi, N.; Solidoro, C.; et al. The Biogeochemical Flux Model (BFM): Equation Description and User Manual, BFM Version 5.2; BFM Report Series 1; BFM System Team: Bologna, Italy, 2020; p. 104. Available online: http://bfm-community.eu (accessed on 1 December 2020).
- Cossarini, G.; Lazzari, P.; Solidoro, C. Spatiotemporal Variability of Alkalinity in the Mediterranean Sea. Biogeosciences 2015, 12, 1647–1658. [Google Scholar] [CrossRef]
- Melaku Canu, D.; Ghermandi, A.; Nunes, P.A.L.D.; Lazzari, P.; Cossarini, G.; Solidoro, C. Estimating the Value of Carbon Sequestration Ecosystem Services in the Mediterranean Sea: An Ecological Economics Approach. Glob. Environ. Chang. 2015, 32, 87–95. [Google Scholar] [CrossRef]
- Dutkiewicz, S.; Hickman, A.E.; Jahn, O.; Gregg, W.W.; Mouw, C.B.; Follows, M.J. Capturing Optically Important Constituents and Properties in a Marine Biogeochemical and Ecosystem Model. Biogeosciences 2015, 12, 4447–4481. [Google Scholar] [CrossRef]
- Teira, E.; José Pazó, M.; Serret, P.; Fernández, E. Dissolved Organic Carbon Production by Microbial Populations in the Atlantic Ocean. Limnol. Oceanogr. 2001, 46, 1370–1377. [Google Scholar] [CrossRef]
- Organelli, E.; Claustre, H. Small Phytoplankton Shapes Colored Dissolved Organic Matter Dynamics in the North Atlantic Subtropical Gyre. Geophys. Res. Lett. 2019, 46, 12183–12191. [Google Scholar] [CrossRef] [PubMed]
- Fogg, G.E. The Ecological Significance of Extracellular Products of Phytoplankton Photosynthesis. Bot. Mar. 1983, 26. [Google Scholar] [CrossRef]
- Lazzari, P.; Salon, S.; Terzić, E.; Gregg, W.W.; D’Ortenzio, F.; Vellucci, V.; Organelli, E.; Antoine, D. Assessment of the Spectral Downward Irradiance at the Surface of The Mediterranean Sea Using the OASIM Ocean-Atmosphere Radiative Model. Ocean Sci. Discuss. 2020. In Review. [Google Scholar] [CrossRef]
- Terzić, E.; Lazzari, P.; Miró, A.; Organelli, E.; D’Ortenzio, F. Radiative Transfer Modeling with BGC-Argo Float Data in The Mediterranean Sea. Biogeosci. Discuss. 2021. In Review. [Google Scholar] [CrossRef]
- Babin, M.; Stramski, D.; Ferrari, G.M.; Claustre, H.; Bricaud, A.; Obolensky, G.; Hoepffner, N. Variations in the Light Absorption Coefficients of Phytoplankton, Nonalgal Particles, and Dissolved Organic Matter in Coastal Waters around Europe. J. Geophys. Res. Oceans 2003, 108. [Google Scholar] [CrossRef]
- Aas, E.; Højerslev, N.K. Analysis of Underwater Radiance Observations: Apparent Optical Properties and Analytic Functions Describing the Angular Radiance Distribution. J. Geophys. Res. Oceans 1999, 104, 8015–8024. [Google Scholar] [CrossRef]
- Lee, Z.; Carder, K.L.; Arnone, R.A. Deriving Inherent Optical Properties from Water Color: A Multiband Quasi-Analytical Algorithm for Optically Deep Waters. Appl. Opt. 2002, 41, 5755. [Google Scholar] [CrossRef] [PubMed]
- Dutkiewicz, S.; Hickman, A.E.; Jahn, O. Modelling Ocean-Colour-Derived Chlorophyll a. Biogeosciences 2018, 15, 613–630. [Google Scholar] [CrossRef]
- Storm, T.; Boettcher, M.; Grant, M.; Zühlke, M.; Fomferra, N.; Jackson, T.; Sathyendranath, S. Product User Guide, Ocean Colour Climate Change Initiative; Plymouth Marine Laboratory: Plymouth, UK, 2013. [Google Scholar]
- Volpe, G.; Pitarch, J.; Colella, S.; Brando, V.E.; Forneris, V.; Bracaglia, M.; Benincasa, M. Quality Information Document for the OCTAC Products—Ocean Colour Mediterranean and Black Sea Observation Product, Copernicus Monitoring Environment Marine Service. 2017. Available online: http://Resources.Marine.Copernicus.Eu/Documents/QUID/CMEMS-OC-QUID-009-038to045-071-073-078-079-095-096.Pdf (accessed on 1 December 2020).
- Belo Couto, A.; Brotas, V.; Mélin, F.; Groom, S.; Sathyendranath, S. Inter-Comparison of OC-CCI Chlorophyll—A Estimates with Precursor Data Sets. Int. J. Remote Sens. 2016, 37, 4337–4355. [Google Scholar] [CrossRef]
- Cmems Global Ocean Nrrs, Bbp, Cdm, Kd, Zsd, Spm (Copernicus-Globcolour) from Satellite Observations: Monthly and Daily Interpolated (Reprocessed from 1997). Available online: https://resources.marine.copernicus.eu/documents/QUID/CMEMS-OC-QUID-009-030-032-033-037-081-082-083-085-086-098.pdf (accessed on 3 January 2021).
- D’Ortenzio, F.; D’Alcalà, M.R. On the trophic regimes of the Mediterranean Sea: A satellite analysis. Biogeosciences 2009, 543, 139–148. [Google Scholar] [CrossRef]
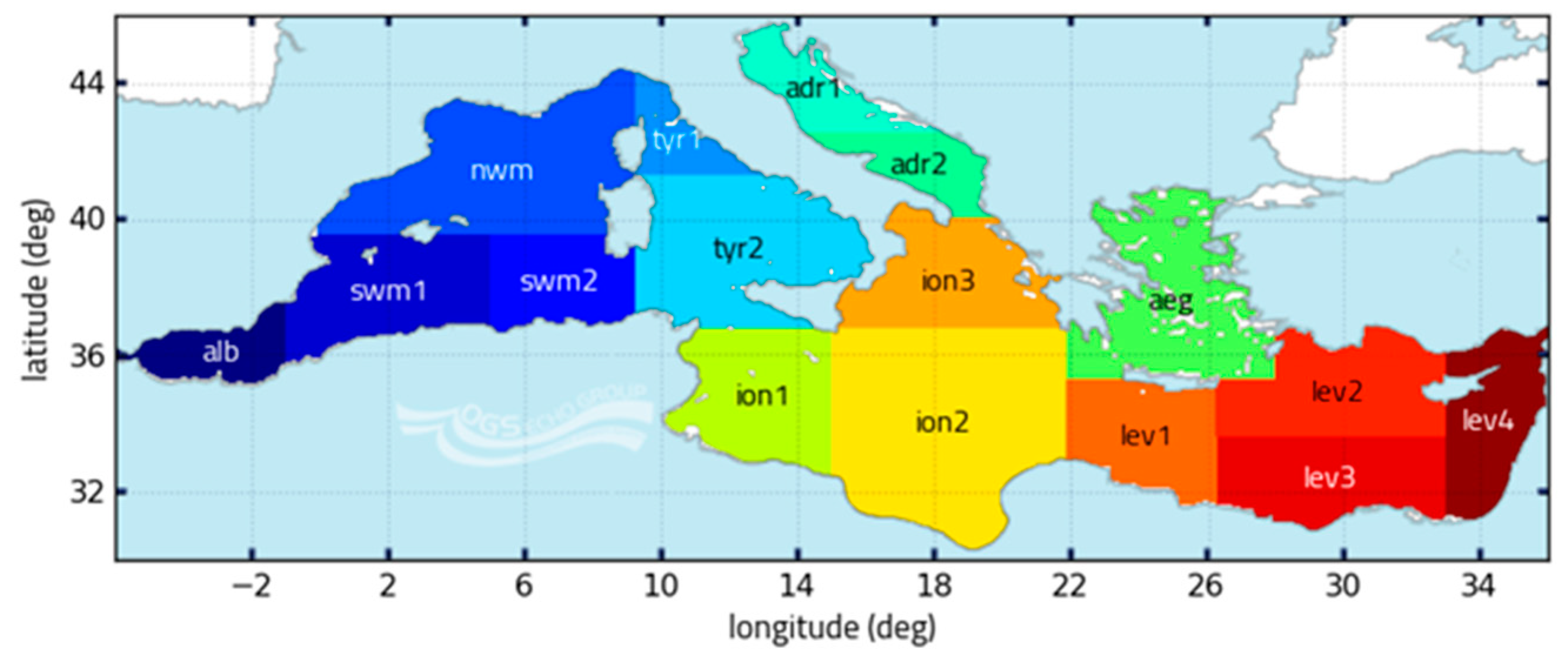
- Ayata, S.D.; Irisson, J.O.; Aubert, A.; Berline, L.; Dutay, J.C.; Mayot, N.; Nieblas, A.; D’Ortenzio, F.; Palmiéri, J.; Reygondeau, G.; et al. Regionalisation of the Mediterranean basin, a MERMEX synthesis. Prog. Oceanogr. 2018, 504, 7–20. [Google Scholar] [CrossRef]
- Volpe, G.; Colella, S.; Forneris, V.; Tronconi, C.; Santoleri, R. The Mediterranean Ocean Colour Observing System—System Development and Product Validation. Ocean Sci. 2012, 8, 869–883. [Google Scholar] [CrossRef]
- Lavigne, H.; D’Ortenzio, F.; Ribera D’Alcalà, M.; Claustre, H.; Sauzède, R.; Gacic, M. On the Vertical Distribution of the Chlorophyll a; Concentration in the Mediterranean Sea: A Basin-Scale and Seasonal Approach. Biogeosciences 2015, 12, 5021–5039. [Google Scholar] [CrossRef]
- Morel, A.; Prieur, L. Analysis of Variations in Ocean Color1: Ocean Color Analysis. Limnol. Oceanogr. 1977, 22, 709–722. [Google Scholar] [CrossRef]
- Retelletti Brogi, S.; Balestra, C.; Casotti, R.; Cossarini, G.; Galletti, Y.; Gonnelli, M.; Vestri, S.; Santinelli, C. Time Resolved Data Unveils the Complex DOM Dynamics in a Mediterranean River. Sci. Total Environ. 2020, 733, 139212. [Google Scholar] [CrossRef] [PubMed]
- Polimene, L.; Allen, J.; Zavatarelli, M. Model of Interactions between Dissolved Organic Carbon and Bacteria in Marine Systems. Aquat. Microb. Ecol. 2006, 43, 127–138. [Google Scholar] [CrossRef]
- Polimene, L.; Pinardi, N.; Zavatarelli, M.; Colella, S. The Adriatic Sea Ecosystem Seasonal Cycle: Validation of a Three-Dimensional Numerical Model. J. Geophys. Res. 2007, 112, C03S19. [Google Scholar] [CrossRef]
- Catalá, T.S.; Martínez-Pérez, A.M.; Nieto-Cid, M.; Álvarez, M.; Otero, J.; Emelianov, M.; Reche, I.; Arístegui, J.; Álvarez-Salgado, X.A. Dissolved Organic Matter (DOM) in the Open Mediterranean Sea. I. Basin—Wide Distribution and Drivers of Chromophoric DOM. Prog. Oceanogr. 2018, 165, 35–51. [Google Scholar] [CrossRef]
- Mobley, C.; Sundman, L.K. Hydrolight 5 Ecolight 5; Sequoia Scientific Inc.: Bellevue, WA, USA, 2008. [Google Scholar]
- Thornton, D.C.O. Dissolved Organic Matter (DOM) Release by Phytoplankton in the Contemporary and Future Ocean. Eur. J. Phycol. 2014, 49, 20–46. [Google Scholar] [CrossRef]
- Nelson, N.B.; Siegel, D.A. The Global Distribution and Dynamics of Chromophoric Dissolved Organic Matter. Annu. Rev. Mar. Sci. 2013, 5, 447–476. [Google Scholar] [CrossRef] [PubMed]
- Claustre, H.; Johnson, K.S.; Takeshita, Y. Observing the Global Ocean with Biogeochemical-Argo. Annu. Rev. Mar. Sci. 2020, 12, 23–48. [Google Scholar] [CrossRef] [PubMed]
- Terzić, E.; Lazzari, P.; Organelli, E.; Solidoro, C.; Salon, S.; D’Ortenzio, F.; Conan, P. Merging Bio-Optical Data from Biogeochemical-Argo Floats and Models in Marine Biogeochemistry. Biogeosciences 2019, 16, 2527–2542. [Google Scholar] [CrossRef]
| EXP-1 | EXP-2 | EXP-3 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 412 | 443 | 490 | CHL | 412 | 443 | 490 | CHL | 412 | 443 | 490 | CHL | |
| alb | 0.75 | 0.82 | 0.84 | 0.05 | 0.83 | 0.89 | 0.88 | 0.05 | 0.89 | 0.94 | 0.91 | 0.06 |
| swm1 | 0.47 | 0.52 | 0.55 | 0.05 | 0.77 | 0.83 | 0.84 | 0.06 | 0.77 | 0.83 | 0.94 | 0.06 |
| swm2 | 0.52 | 0.59 | 0.61 | 0.05 | 0.84 | 0.90 | 0.90 | 0.06 | 0.8 | 0.86 | 0.93 | 0.06 |
| nwm | 0.66 | 0.78 | 0.80 | 0.05 | 0.96 | 0.97 | 0.95 | 0.06 | 0.91 | 0.94 | 0.81 | 0.06 |
| tyr1 | 0.24 | 0.44 | 0.43 | 0.07 | 0.87 | 0.95 | 0.89 | 0.08 | 0.68 | 0.83 | 0.84 | 0.08 |
| tyr2 | 0.33 | 0.48 | 0.48 | 0.07 | 0.88 | 0.94 | 0.91 | 0.08 | 0.73 | 0.82 | 0.91 | 0.07 |
| adr1 | 0.07 | 0.14 | −0.19 | 0.06 | −0.09 | −0.37 | –0.68 | 0.08 | −0.04 | −0.3 | −0.68 | 0.06 |
| adr2 | −0.08 | 0.21 | −0.05 | 0.06 | 0.59 | 0.71 | −0.08 | 0.08 | 0.32 | 0.52 | −0.18 | 0.07 |
| aeg | −0.04 | 0.18 | 0.19 | 0.06 | 0.86 | 0.93 | 0.92 | 0.08 | 0.52 | 0.71 | 0.86 | 0.06 |
| ion1 | −0.12 | 0.23 | 0.45 | 0.05 | 0.95 | 0.96 | 0.88 | 0.08 | 0.7 | 0.83 | 0.95 | 0.05 |
| ion2 | 0.03 | 0.27 | 0.28 | 0.05 | 0.96 | 0.96 | 0.91 | 0.09 | 0.79 | 0.86 | 0.83 | 0.05 |
| ion3 | 0.23 | 0.39 | 0.34 | 0.05 | 0.94 | 0.97 | 0.94 | 0.08 | 0.76 | 0.85 | 0.82 | 0.06 |
| lev1 | 0.28 | 0.38 | 0.30 | 0.07 | 0.93 | 0.94 | 0.88 | 0.10 | 0.73 | 0.8 | 0.76 | 0.06 |
| lev2 | 0.34 | 0.49 | 0.49 | 0.07 | 0.93 | 0.96 | 0.95 | 0.10 | 0.72 | 0.83 | 0.8 | 0.07 |
| lev3 | 0.19 | 0.32 | 0.27 | 0.06 | 0.94 | 0.95 | 0.92 | 0.09 | 0.72 | 0.8 | 0.77 | 0.05 |
| lev4 | −0.06 | 0.13 | 0.16 | 0.06 | 0.92 | 0.94 | 0.92 | 0.09 | 0.57 | 0.73 | 0.77 | 0.05 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lazzari, P.; Álvarez, E.; Terzić, E.; Cossarini, G.; Chernov, I.; D’Ortenzio, F.; Organelli, E. CDOM Spatiotemporal Variability in the Mediterranean Sea: A Modelling Study. J. Mar. Sci. Eng. 2021, 9, 176. https://doi.org/10.3390/jmse9020176
Lazzari P, Álvarez E, Terzić E, Cossarini G, Chernov I, D’Ortenzio F, Organelli E. CDOM Spatiotemporal Variability in the Mediterranean Sea: A Modelling Study. Journal of Marine Science and Engineering. 2021; 9(2):176. https://doi.org/10.3390/jmse9020176
Chicago/Turabian StyleLazzari, Paolo, Eva Álvarez, Elena Terzić, Gianpiero Cossarini, Ilya Chernov, Fabrizio D’Ortenzio, and Emanuele Organelli. 2021. "CDOM Spatiotemporal Variability in the Mediterranean Sea: A Modelling Study" Journal of Marine Science and Engineering 9, no. 2: 176. https://doi.org/10.3390/jmse9020176
APA StyleLazzari, P., Álvarez, E., Terzić, E., Cossarini, G., Chernov, I., D’Ortenzio, F., & Organelli, E. (2021). CDOM Spatiotemporal Variability in the Mediterranean Sea: A Modelling Study. Journal of Marine Science and Engineering, 9(2), 176. https://doi.org/10.3390/jmse9020176







