1. Introduction
In recent years, the consumption of fish has grown exponentially due to the global population increase and the search for healthier foods with great nutritional quality [
1]. Thus, the groups of the most valuable organisms with significant production, in particular shrimps, showed a maximum level of catches in 2016 [
1].
The capture of shrimps is economically important throughout the Brazilian coast, and also has social and historical implications [
2]. In Brazil, the capture of crustaceans in made up of 67.1% penaeid shrimps, which belong to the order Decapoda and suborder Dendrobranchiata. Among these, the main species caught are the sea bob shrimp
Xiphopenaeus kroyeri (Heller, 1862), the pink shrimp
Farfantepenaeus spp. (Burukovsky, 1997) and the white shrimp
Penaeus schmitti (Burkenroad, 1936), which corresponds to a production of 26.9%, 18% and 7%, respectively [
3].
Among the most captured species, the
P. schmitti, popularly known as the southern white shrimp, is distributed in the Western Atlantic, from the Antilles, in the Caribbean, to the state of Rio Grande do Sul, in Brazil [
4]. They can be found from shallow depths up to depths of 30 m, with some catches recorded at up to 50 m. The species has a high growth rate, high fertility and a short life cycle of approximately 24 months [
4]. Unlike the pink shrimp species and the sea bob shrimp, the white shrimp has an open telycum, and ecdysis is not necessary for reproduction [
5]. It also has a short rostrum, one of the main characteristics of differentiation between these species.
Shrimp fishing is carried out intensively by means of single or double trawl nets, which capture a large amount of juvenile individuals and accompanying fauna, and generate a great amount of damage to biodiversity and the environment [
6]. Due to the economic importance and characteristics of this fishing activity, management measures are essential for the sustainability of the activity and for the protection and maintenance of stocks of
P. schmitti, since, to establish an adequate closed season of a species, it is essential to describe and understand the gonadal development, in order to enable the classification of maturation stages.
In Brazil, studies addressing the aspects of the reproductive dynamics of
P. schmitti have been carried out in the northeast [
5,
7,
8,
9], in the south [
10,
11], and in the southeastern region, though only for the state of São Paulo [
5,
12]. Therefore, in Espírito Santo, there is still no research that incorporates and/or provides information on the reproductive dynamics of the species
P. schmitti.
Research that provides information on the reproductive biology of these natural populations becomes an essential tool for fisheries management and aids in the conservation of the species. In addition, studies on this aspect contribute to the development of technologies for captive breeding and future possibilities of cultivation of the species in the region, since this is one of the most promising species for aquaculture in Brazil. The species has high tolerance to salinity and a large size, among other morphological and reproductive characteristics that make it attractive for cultivation [
13].
Therefore, the present study sought to describe the stages of gonadal development of the white shrimp P. schmitti from the southern coast of Espírito Santo, Brazil, through macroscopic and histological analysis of the ovaries, thus contributing to our knowledge required for appropriate stock management of penaeids and in the reproductive physiology of this species in captivity.
2. Materials and Methods
Samples of the shrimps were obtained during a period of eight months in the fishing areas comprised by the municipalities of Anchieta (20°48′21″ S 40°38′44″ W), Piúma (20°50′7″ S 40°43′42″ W) and Itapemirim (21°0′42″ S 40°50′2″ W), on the southern coast of the state of Espírito Santo, Brazil. The samplings were carried out by trawlers with tandem balloon-type trawl nets. The net used is 12 m long, with mesh of 28 mm in the body of the net and 14 mm in the bagger, the mouth opening varies with the depth, but on average it is 2.5 m wide. In total, 23 trips were carried out, each with two one-hour trawls, in order to capture the specimens.
On the vessel, the shrimps were separated from the accompanying fauna, placed in plastic bags and stored in thermal boxes with ice. In the Mariculture Laboratory (IFES, Campus Piúma), these were first separated according to the species, using identification guides for penaeid shrimps [
14], then separated by sex using external characteristics (presence of telycum in females and petasma in males). The weight and morphometric data of each individual were obtained, by measuring the total length (TL), cephalothorax length (CL) in millimeters, using a caliper and the total weight (TW) in grams, using precision scale (0.001 g).
The ovaries of the females were extracted and classified as to their maturational stage by means of external aspects such as their placement in the body, the degree of turgidity, flaccidity and their coloring (according to the color catalog of the Pantone chart, Pantone Matching System, Pantone, Carlstadt, NJ, USA). After macroscopic identification, the ovaries were segmented, and samples were obtained from their different regions, regarding their anterior, lateral and posterior lobes. They were first fixed in Davidson’s solution, and then cleaved and preserved in a 70% alcohol solution for histological analysis. The sample fragments were dehydrated, cleared in xylene, impregnated and embedded in paraffin. After being embedded in paraffin, the samples were sectioned in rotary microtome (Leica RM2145, Leica Microsystems, Wetzlar, Germany) and the cuts exposed on slides and stained by the method of hematoxylin/eosin-floxin, according to the methodology proposed by Junqueira and Junqueira [
15].
The histological slides were observed under an optical microscope (DM500, Leica), photographed (ICC50 HD camera, Leica) and then classified according to the histological characteristics described for penaeid shrimps [
9]. The photomicrographs of the slides were digitized with Leica LAS EZ Software.
3. Results
A total of 181 females of P. schmitti were captured, which presented a total length amplitude (TL) of 70.0 to 220.0 mm (152.4 ± 27.4 mm), cephalothorax length (CL) between 14.0 and 52.0 mm (31.7 ± 6.9 mm) and total weight ranging from 2.60 to 91.77 g (30.02 ± 15.02 g). A total of 154 females were submitted to histological analysis of the ovary.
Macroscopic and Histological Characteristics of the Ovaries
Significant macroscopic differences were identified in the ovaries of the captured P. schmitti females. It was observed that the ovaries are paired organs that have a tubular structure with bilateral symmetry. In the dorsal region, they are present alongside the intestine and, when they occupy the cephalothorax, the two anterior lobes and the seven short lateral lobes are evident. The posterior lobes begin in the cephalothorax and extend to the antepenultimate abdominal somite when immature and to the last abdominal so-mite when mature. In the course of gonadal development, it is possible to observe differences in the degree of turgidity and coloring of these ovaries. When immature, the ovaries are thin, smooth and translucent and, after development, they acquire a yellowish coloring in the lobes. This coloring is accentuated with development, and thus the ovaries acquire a greater degree of turgidity. It is also possible to visualize these differences along the gonadal region since maturation begins in the anterior lobes, followed by the lateral lobes until reaching the posterior lobes.
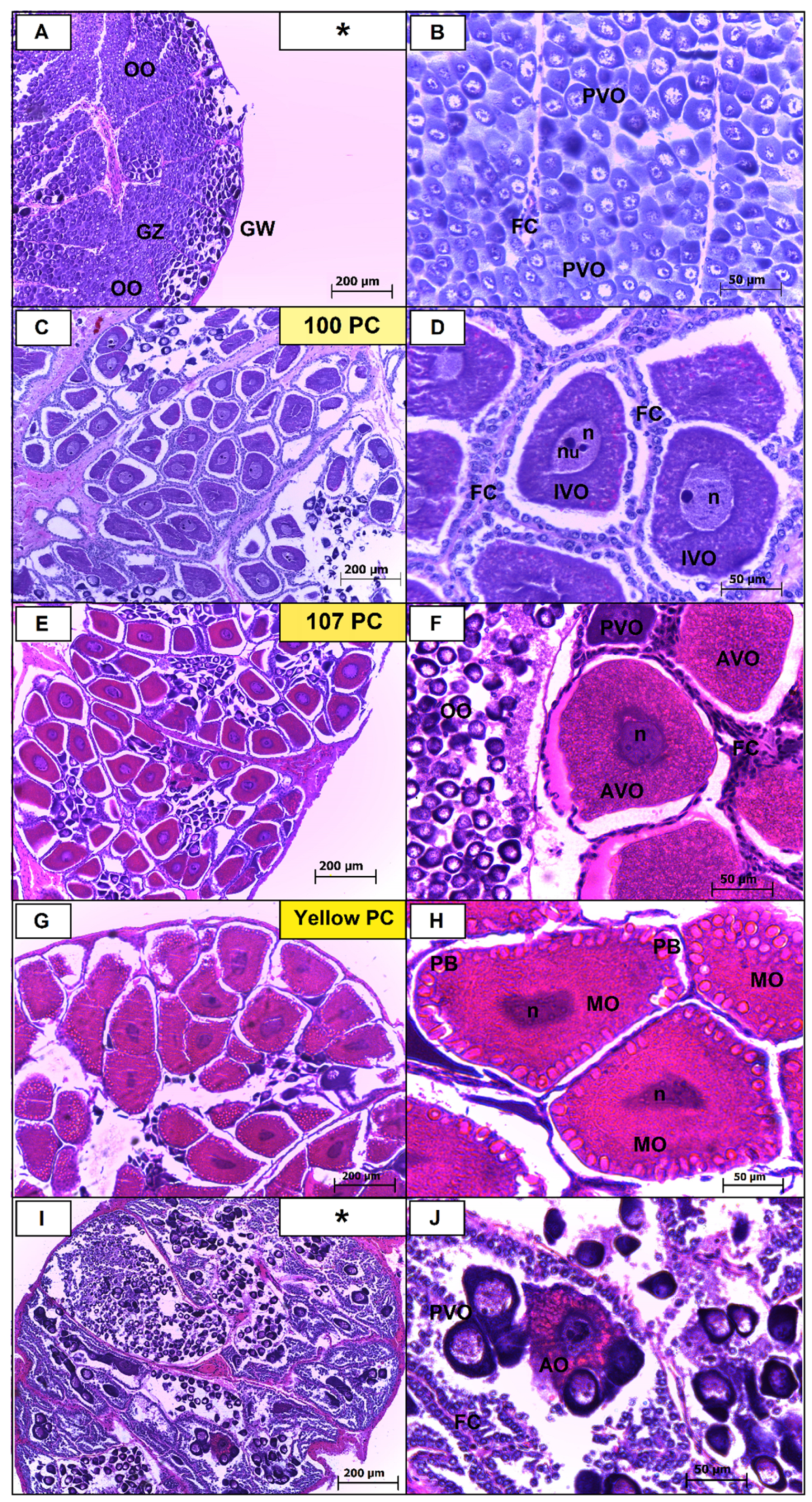
In histological analyses, the germ cells were classified into oogonia (OO), previtellogenic oocytes (PVO), oocytes in initial vitellogenesis (OIV), oocytes in advanced vitellogenesis (OAV), mature oocytes (MO) and atretic oocytes (AO). From this, it was possible to identify a correlation with the differences found in the stages identified through macroscopic observation. Based on macroscopic and histological analysis, five distinct maturation stages were determined for females: immature (I), initial maturation (II), advanced maturation (III), mature (IV), spent (V).
Immature (I): Macroscopically, the gonads are narrow, similar to the intestine, with a smooth surface and are translucent. They cannot be visualized through the cephalothorax, due to their thickness and lack of coloring. Histologically, occur predominance of oogonia grouped in the germinative zone and previtellogenic oocytes. OO are basophilic oocytes found in all stages of gonadal development, with a higher proportion in the immature and spawned stages. They have a spherical shape, with reduced cytoplasm, and a large and well-defined nucleus. During the development process, chromatin is more concentrated around the nucleus. The oogonia are mainly grouped in the center of the ovaries, which is known as the germination zone. PVO is also found at all stages of gonadal development. They have an oval shape, more developed cytoplasm and less dense chromatin compared to the previous stage. In these cells, it is possible to observe nucleoli on the periphery of the nucleus (
Figure 1A,B;
Table 1).
Initial maturation (II): Macroscopically, the ovaries begin to become more turgid in the cephalothorax with light-yellow coloring (Pantone 100 PC) and unobtrusively fill the abdominal region. Histologically, oogonia and previtellogenic oocytes are present occupying the central germinative zone. Presence of cells in initial lipid vitellogenesis (OVI). OIV have lipid vitellogenesis. The cytoplasm is more developed than in the previous stage and has an acidophilic character. The basophilic nucleus has less condensed chromatin compared to the previous stage (
Figure 1C,D;
Table 1).
Advanced maturation (III): the macroscopic analysis was observed as the gonad being slightly more consistent, rougher and thicker compared to the previous stage, presenting development in the portion of the abdominal somites. The medium-yellow coloring (Pantone 107 PC) is more apparent and is present in all lobes of the ovary. Histologically, the presence of oocytes in complete vitellogenesis occurs on the periphery of the lobes of the ovary. OAV have, in addition to lipid vitellogenesis, discrete protein deposition. They are more developed structures than those of the previous stage and acquire a granular appearance due to the lipid droplets (
Figure 1E,F). Lipid droplets are visualized throughout the cell, but in greater concentration in the peripheral region. Oogonia and previtellogenic oocytes are present (
Figure 1E,F;
Table 1).
Mature (IV): Macroscopically, the ovary is easily seen, it fills the entire abdominal and cephalothorax cavity. The gonad is very turgid, with a rough and very thick surface. The coloring at this stage is dark yellow (Pantone yellow PC). Histologically, presence of mature oocytes presenting cortical rods in the extremities. MO differs by presenting complete lipid and protein vitellogenesis and mainly by the presence of peripheral bodies (PB) at the ends of the oocytes (
Figure 1G,H). They are more developed structures than those of the previous stage (
Figure 1G,H;
Table 1).
Spent (V): Macroscopically, the coloring of this stage is similar to the immature stage. However, the ovaries are more flaccid and the surface is slightly rough. Histologically, occur presence of atretic oocytes (AO) in the process of reabsorption. The presence of atretic oocytes is the main component that microscopically distinguishes the immature stage from the spawned stage. It was possible to observe predominance of basophilic oocytes (
Figure 1I,J;
Table 1).
In the macroscopic observation, of the 181 females analyzed, 8 were described as immature, 17 in initial maturation, 9 in advanced maturation, 29 were mature and 40 had spawned. Of the total females analyzed, it was not possible to identify the maturational stage through macroscopic observation of 45 females (30.5%), which was mainly due to the difficulty in distinguishing the immature and spent spawned ovaries, which have similar macroscopic characteristics. In the females in which it was possible to easily differentiate between the spawned stage and the immature, this was possible due to the length of the individual and the size and turgidity of the gonads, for this reason 85% of the samples were for histological analysis (n = 151).
Ovaries were found with shades of dark green to light brown (Pantone: 5763 PC, 384 and 140 PC), which is different from the yellow scale coloration standard observed in most specimens of P. schmitti captured on the coast of Espírito Santo. These were represented by only five females from the total captured (catches of June and early July), in different locations and depths.
No histological differences were found between the segments removed from the different regions of the ovary. Therefore, the same oocyte types were found distributed throughout the gonad, thus characterizing the same stage of development. The two forms of analysis were correlated and the histology of the ovaries validated 53 of the maturational stages (46.90%) previously obtained by macroscopic observation, and demonstrated an error in 17 analyses (15.05%). For the rest of the ovaries analyzed, for about 19 of these (16.81%), it was only possible to define the maturational stage through microscopic analysis, due to the difficulty of visual distinction of some stages of gonadal development. A total of 24 of the ovaries analyzed (21.24%) remained undefined even after microscopic analysis, due to the initial difficulties in standardizing histological processing for the different samples. The ovaries previously mentioned as having greenish tones were classified histologically as being in initial maturation (5763 PC) and advanced maturation (384 and 140 PC).
4. Discussion
The morphology of the ovaries and the location of the anterior, lateral and posterior lobes are in accordance with the description by Dall et al. [
16] for penaeid shrimps. In some immature females, the identification and location of the ovary was only possible when placed whole in the fixing solution for subsequent dissection. This difficulty was corroborated by Santana [
17], who pointed out the similarity of the reproductive organ with the musculature of the cephalothorax and abdominal musculature, as well as the small size of the ovary at this stage.
The macroscopic classification of gonadal development using a coloration scale and by observing the size of the gonad is a widely used methodology. This classification of ovaries from macroscopic characteristics is a practical alternative and is closely related to the development and organization of ovarian constituent cells [
18,
19]. However, these visual criteria can confuse and generate doubts about the real stage of development, since it is possible to observe different shades of coloring in an ovary at the same maturational stage [
20,
21]. Despite the subjectivity in the definition of colors and the limitations in their use, this method is an important tool in laboratory practice and in fieldwork onboard ships that is aimed at reproductive dynamics. In addition, it is very useful in the sampling of reproducers in the wild, since it is easy to recognize the mainly mature females [
20].
The study of Quintero and Gracia [
20] classified the ovaries of
P. brasiliensis as having five colors. However, the spawning stage was characterized by a whitish and nontranslucent coloration. This coloration generated doubts mainly when differentiating the spawned gonads from the initial developing gonads. In the present study, the coloration for
P. schmitti in the immature and spawned stages was translucent and these were the most difficult stages to be differentiated, especially in females of intermediate sizes, due to the similarity in characteristics and for presenting the same coloration. The same difficulty was reported for
P. schmitti in Pernambuco [
8], in São Paulo [
12], and for other species of penaeids shrimps [
12,
18,
19,
20,
22,
23].
In the other stages of gonadal development in which coloration can be observed, specifically stages II, III and IV, the macroscopic yellowish scale classification for
P. schmitti follows what was observed by Peixoto et al. [
8]. However, studies by Gonçalves et al. [
12] and Machado et al. [
11] described a greenish-tinted scale to classify gonadal development for the same species in São Paulo and Santa Catarina, respectively. We can observe that for most stages of development of
P. schmitti of Espírito Santo, the coloring pattern follows that found in the northeast. However, it does present some specimens with coloring patterns related to that found further south of the species distribution in Brazil. This indicates that the Espírito Santo region can be characterized as the beginning of a transition region of environments in which the gonadal coloring pattern is influenced by the feeding of the species.
The study of Machado et al. [
11] classified the shrimps by means of the colors described as follows: white or translucent for the immature and spawned stage, though they cited that these can only be distinguished histologically; gray or green for the developing stage; and dark green to black for the mature stage. The study by Gonçalves et al. [
12] defined a macroscopic scale using transparent for the immature stage, the color light green for the developing stage, dark green for mature and whitish/transparent for empty. The difference in the coloring and size of the ovaries throughout maturation is common for penaeid shrimps [
21], and is observed among different species. There is also a difference in the coloring of the ovaries among individuals of the same species, as was observed for
P. schmitti.
The coloring displayed on the ovaries is due to differences in the carotenoid content. Carotenoids are natural pigments that are not produced by crustaceans and, therefore, are acquired and accumulated through food [
24]. The difference found in the color of the ovaries may be due to the different diets to which individuals of the same species are submitted to in different regions [
25]. Nutritional quality is an important factor in crustacean reproduction and the influence on carotenoid content has been studied, correlating them with gonadal development, reproductive success and other influences for shrimps and other crustaceans [
26].
Standardizing a color scale in ovarian maturation does not exempt the possibility of errors that can be found in the differentiation of the gonadal stage of a species even in the same region. Some nonstandard tones found in the established scale can cause confusion when defining the stage macroscopically. The stages of gonadal development found in the present study with greenish tones were defined only through histology. The differences in coloring found in nearby regions, such as that presented by Gonçalves et al. [
12] in a study conducted in São Paulo, and even in nearby municipalities, demonstrates the limitation in using only macroscopic visual analysis as the main source of information. This emphasizes the importance of using histological analysis whenever possible and, especially, in gonads that do not present the tone of the color scale normally found.
In addition to the influence of feeding on the coloring of the ovaries, another factor that impairs macroscopic analyses is the fact of how these individuals are captured by the trawl nets. When dragging the shrimps and accumulating them at the bottom of the net, the fishing apparatus can cause damage to the structures. Perforation of other organs impairs the classification of the stages when using the macroscopic scale of colors.
Regarding ovarian histology, the histological characteristics observed and the modifications found in the different stages of gonadal development for
P. schmitti are in accordance with the standards observed in the literature. It was observed that there is a germinative zone, which is also cited by other authors as a proliferation zone, in which oogony are concentrated [
8,
9,
12]. Basophilic oocytes (oogonia and previtellogenic oocytes) were found predominantly in the immature and spawned stages. According to Peixoto et al. [
21], these cells are present at these stages at frequencies of almost 100%. The onset of vitellogenin is an important feature that differentiates the immature stage from the initial developing stage. In the initial development stage, one can observe the emergence of initial lipid vitellogenesis in oocytes. In the stage of advanced development, oocytes begin to present lipid and protein vitellogenesis [
27].
The emergence of peripheral bodies (PB) is the main factor that indicates that the ovary is in complete development, and characterizes its mature stage. Gonçalves et al. [
12] and Craveiro et al. [
9] demonstrated clear differentiation of cell structure by the presence of PB in the mature ovary of
P. schmitti, which was corroborated in the study of gonadal development of other penaeid shrimps [
20,
21,
22,
23]. Peripheral bodies were observed in spherical to oval shapes, but can also be found in the form of rods for
P. schmitti [
8,
11]. These structures release a gelatinous product around the oocyte to promote better sperm fixation and form a hatching envelope [
28].
The atretic oocytes found were important in the identification and differentiation of the immature and spawned stages, and were considered the main characteristic in this histological distinction, since the same germinative cells (oogonia and previtellogenic oocytes) are found predominantly in the immature and spawned stages.
The evidence found was sufficient to safely define five stages of gonadal development. There are controversies in the literature regarding the number of ovarian stages even within the same species [
16]. Other authors delimited only four maturational stages for the species [
8,
10,
11,
12]. The classification used in the present study corroborated the studies performed by Craveiro et al. [
9]. It was evident that the classification of ovarian development in five stages is important mainly so that this more detailed information can be incorporated in future studies of reproductive and population dynamics. These studies stand out for their importance in the identification of the maturational stages that will serve to obtain reliable data about the size of maturity and spawning season, which are fundamental when establishing management measures, as well as in order to verify whether the current closed season is appropriate for the species.