Performance of a Potentially Invasive Species of Ornamental Seaweed Caulerpa sertularioides in Acidifying and Warming Oceans
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation and Species Identification
2.2. Experimental Conditions and Indoor Mesocosm System
2.3. Determination of Carbonate Chemistry
2.4. Oxygenic Photosynthesis and In Vivo Fluorescence
2.5. Growth Rate
2.6. Statistical Analysis
3. Results
3.1. Carbonate Chemistry
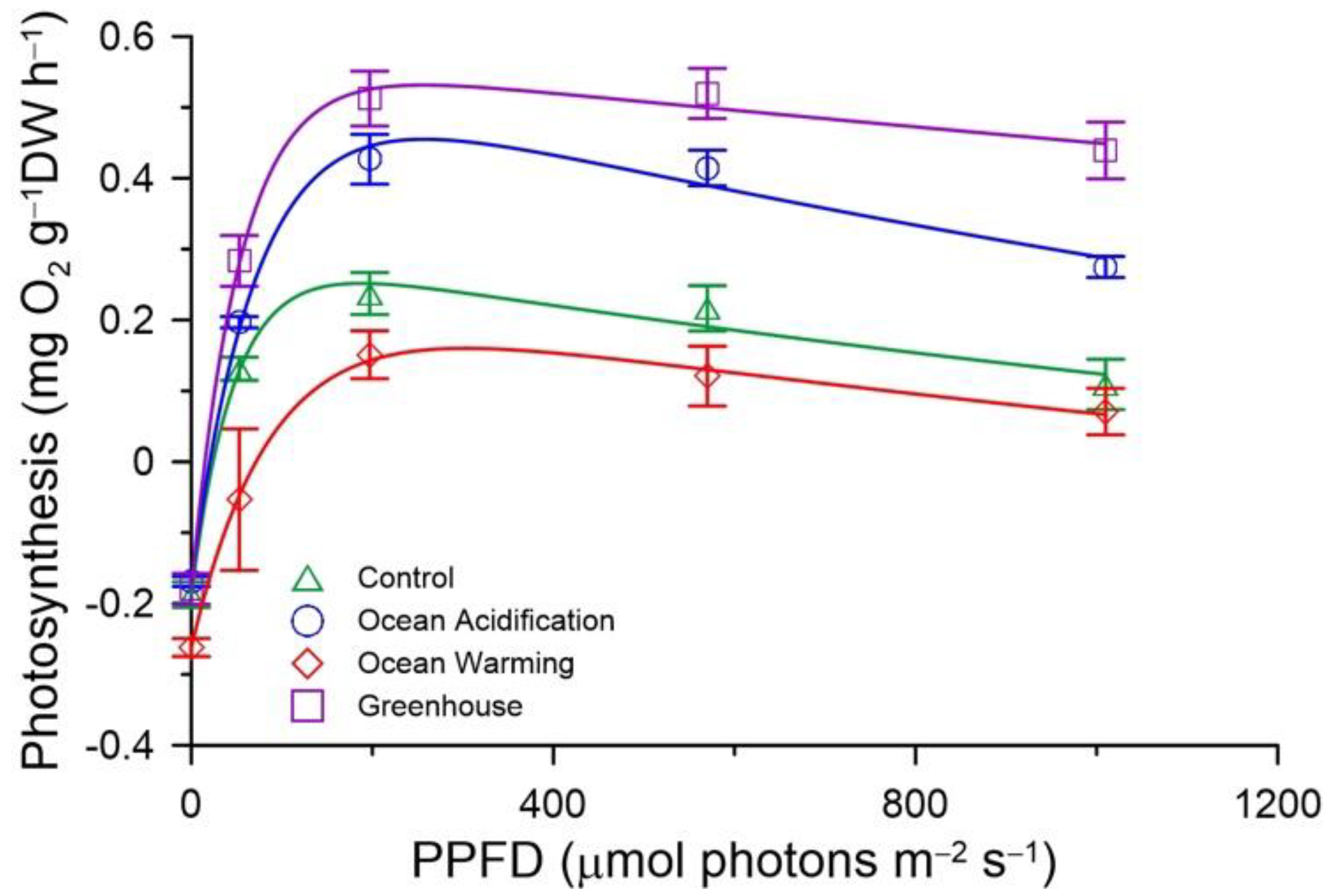
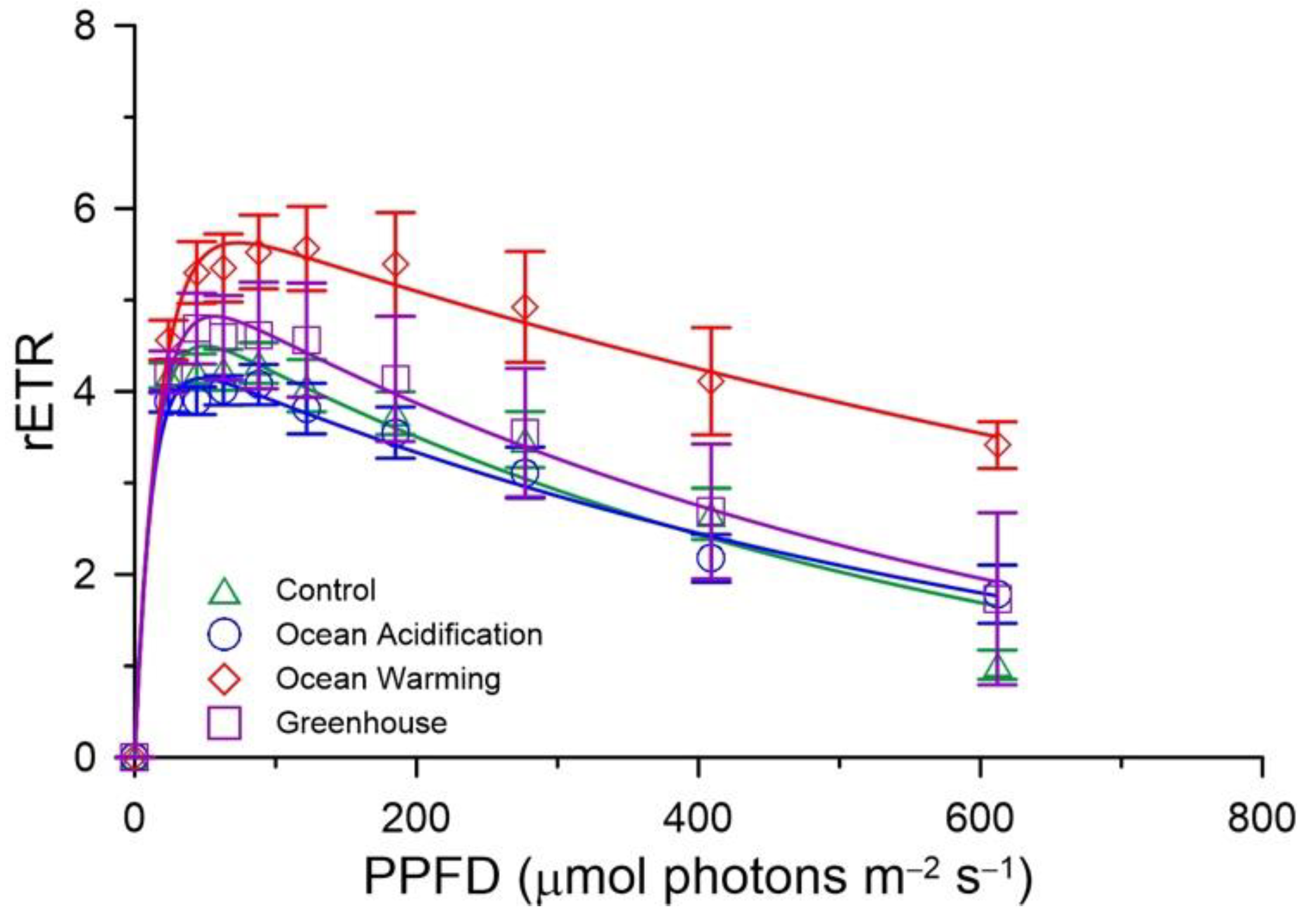
3.2. Photosynthesis
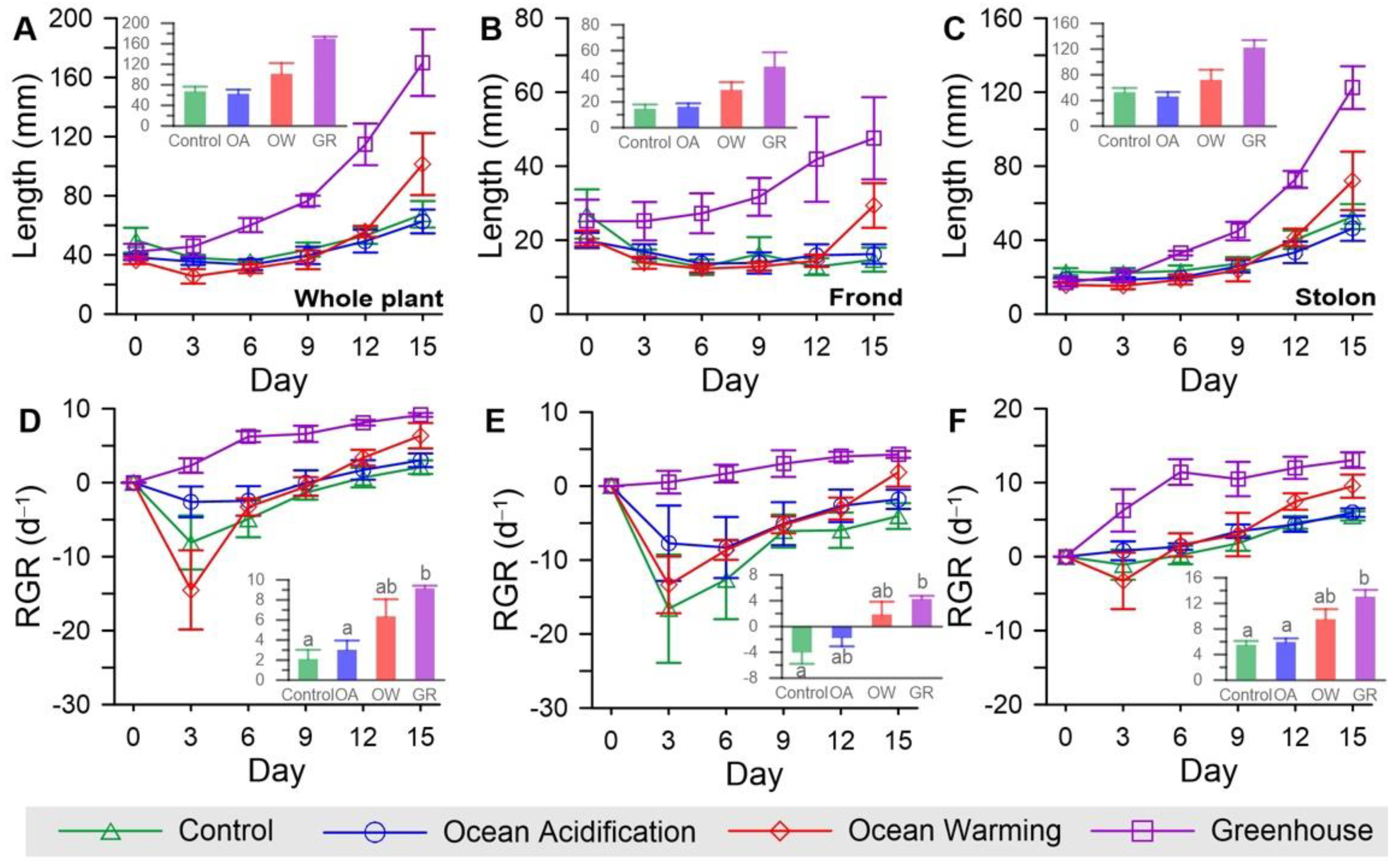
3.3. Growth Rate
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guy-Haim, T.; Lyons, D.A.; Kotta, J.; Ojaveer, H.; Queirós, A.M.; Chatzinikolaou, E.; Arvanitidis, C.; Como, S.; Magni, P.; Blight, A.J.; et al. Diverse Effects of Invasive Ecosystem Engineers on Marine Biodiversity and Ecosystem Functions: A Global Review and Meta-Analysis. Glob. Chang. Biol. 2018, 24, 906–924. [Google Scholar] [CrossRef] [PubMed]
- Doney, S.C.; Ruckelshaus, M.; Duffy, J.E.; Barry, J.P.; Chan, F.; English, C.A.; Galindo, H.M.; Grebmeier, J.M.; Hollowed, A.B.; Knowlton, N.; et al. Climate Change Impacts on Marine Ecosystems. Annu. Rev. Mar. Sci. 2012, 4, 11–37. [Google Scholar] [CrossRef] [PubMed]
- Pinsky, M.L.; Selden, R.L.; Kitchel, Z.J. Climate-Driven Shifts in Marine Species Ranges: Scaling from Organisms to Communities. Annu. Rev. Mar. Sci. 2020, 12, 153–179. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, N.; Moon, H.; Lee, S.; Jeong, S.Y.; Diaz-Pulido, G.; Edwards, M.S.; Kang, J.H.; Kang, E.J.; Oh, H.J.; et al. Global Warming Offsets the Ecophysiological Stress of Ocean Acidification on Temperate Crustose Coralline Algae. Mar. Pollut. Bull. 2020, 157, 111324. [Google Scholar] [CrossRef] [PubMed]
- Kang, E.J.; Han, A.R.; Kim, J.H.; Kim, I.N.; Lee, S.; Min, J.O.; Nam, B.R.; Choi, Y.J.; Edwards, M.S.; Diaz-Pulido, G.; et al. Evaluating Bloom Potential of the Green-Tide Forming Alga Ulva ohnoi Under Ocean Acidification and Warming. Sci. Total Environ. 2021, 769, 144443. [Google Scholar] [CrossRef]
- IPCC. Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. In Climate Change; Stocker, T.F., Qin, D., Plattner, G.-K., Tignor, M., Allen, S.K., Boschung, J., Nauels, A., Xia, Y., Bex, V., Midgley, P.M., Eds.; Cambridge University Press: Cambridge; UK; New York, NY, USA, 2013. [Google Scholar]
- Harley, C.D.G.; Anderson, K.M.; Demes, K.W.; Jorve, J.P.; Kordas, R.L.; Coyle, T.A.; Graham, M.H. Effects of Climate Change on Global Seaweed Communities. J. Phycol. 2012, 48, 1064–1078. [Google Scholar] [CrossRef] [PubMed]
- Kroeker, K.J.; Kordas, R.L.; Crim, R.; Hendriks, I.E.; Ramajo, L.; Singh, G.S.; Duarte, C.M.; Gattuso, J.P. Impacts of Ocean Acidification on Marine Organisms: Quantifying Sensitivities and Interaction with Warming. Glob. Chang. Biol. 2013, 19, 1884–1896. [Google Scholar] [CrossRef]
- Blackburn, T.M.; Pyšek, P.; Bacher, S.; Carlton, J.T.; Duncan, R.P.; Jarošík, V.; Wilson, J.R.U.; Richardson, D.M. A Proposed Unified Framework for Biological Invasions. Trends Ecol. Evol. 2011, 26, 333–339. [Google Scholar] [CrossRef]
- Bax, N.; Williamson, A.; Aguero, M.; Gonzalez, E.; Geeves, W. Marine Invasive Alien Species: A Threat to Global Biodiversity. Mar. Policy 2003, 27, 313–323. [Google Scholar] [CrossRef]
- Epstein, G.; Smale, D.A. Undaria pinnatifida: A Case Study to Highlight Challenges in Marine Invasion Ecology and Management. Ecol. Evol. 2017, 7, 8624–8642. [Google Scholar] [CrossRef]
- Sullaway, G.H.; Edwards, M.S. Impacts of the Non-Native Alga Sargassum horneri on Benthic Community Production in a California Kelp Forest. Mar. Ecol. Prog. Ser. 2020, 637, 45–57. [Google Scholar] [CrossRef]
- Fidai, Y.A.; Dash, J.; Tompkins, E.L.; Tonon, T. A Systematic Review of Floating and Beach Landing Records of Sargassum Beyond the Sargasso Sea. Environ. Res. Commun. 2020, 2, 122001. [Google Scholar] [CrossRef]
- Kwon, K.; Choi, B.-J.; Kim, K.Y.; Kim, K. Tracing the Trajectory of Pelagic Sargassum Using Satellite Monitoring and Lagrangian Transport Simulations in the East China Sea and Yellow Sea. Algae 2019, 34, 315–326. [Google Scholar] [CrossRef]
- Kim, H.M.; Jo, J.; Park, C.; Choi, B.-J.; Lee, H.-G.; Kim, K.Y. Epibionts Associated with Floating Sargassum horneri in the Korea Strait. Algae 2019, 34, 303–313. [Google Scholar] [CrossRef]
- Hewitt, C.L.; Campbell, M.L.; Schaffelke, B. Introductions of Seaweeds: Accidental Transfer Pathways and Mechanisms. Bot. Mar. 2007, 50, 326–337. [Google Scholar] [CrossRef]
- Williams, S.L.; Grosholz, E.D. Preliminary Reports from the Caulerpa taxifolia Invasion in Southern California. Mar. Ecol. Prog. Ser. 2002, 233, 307–310. [Google Scholar] [CrossRef]
- Meinesz, A.; de Vaugelas, J.; Hesse, B.; Mari, X. Spread of the Introduced Tropical Green Alga Caulerpa taxifolia in Northern Mediterranean Waters. J. Appl. Phycol. 1993, 5, 141–147. [Google Scholar] [CrossRef]
- Kim, G.H.; Klochkova, T.A. Brypsidales. Algal Flora Korean. In Chlrophyta. Ulvophyceae: Ulotrichales, Ulvales, Cladophorales, Bryopsidales, Marine Green Algae; Bae, E.H., Kim, H.-S., Kwon, C.-J., Hwang, I.-K., Kim, G.H., Klochkova, T.A., Eds.; National Institute of Biological Resources: Incheon, Korean, 2010; Volume 1, pp. 157–209. [Google Scholar]
- Gao, X.; Choi, H.G.; Park, S.K.; Sun, Z.M.; Nam, K.W. Assessment of Optimal Growth Conditions for Cultivation of the Edible Caulerpa okamurae (Caulerpales, Chlorophyta) From Korea. J. Appl. Phycol. 2019, 31, 1855–1862. [Google Scholar] [CrossRef]
- Fernandez, C.; Cortes, J. Caulerpa sertularioides, a Green Alga Spreading Aggressively Over Coral Reef Communities in Culebra Bay, North Pacific of Costa Rica. Coral Reefs 2005, 24, 10. [Google Scholar] [CrossRef]
- Klein, J.; Verlaque, M. The Caulerpa Racemose Invasion: A Critical Review. Mar. Pollut. Bull. 2008, 56, 205–225. [Google Scholar] [CrossRef] [PubMed]
- Famà, P.; Wysor, B.; Kooistra, W.H.C.F.; Zuccarello, G.C. Molecular Phylogeny of the Genus Caulerpa (Caulerpales, Chlorophyta) Inferred from Chloroplast Tufa Gene. J. Phycol. 2002, 38, 1040–1050. [Google Scholar] [CrossRef]
- Dickson, A.G.; Sabine, C.L.; Christian, J.R. Guide to Best Practices for Ocean CO2 Measurement [PICES Special Publication]; North Pacific Marine Science Organization: Sydney, Canada, 2007; Volume 3, p. 191. [Google Scholar]
- Dickson, A.G. The Measurement of Sea Water pH. Mar. Chem. 1993, 44, 131–142. [Google Scholar] [CrossRef]
- Millero, F.J.; Zhang, J.Z.; Lee, K.; Campbell, D.M. Titration Alkalinity of Seawater. Mar. Chem. 1993, 44, 153–165. [Google Scholar] [CrossRef]
- Kim, H.-C.; Lee, K.; Choi, W. Contribution of Phytoplankton and Bacterial Cells to the Measured Alkalinity of Seawater. Limnol. Oceanogr. 2006, 51, 331–338. [Google Scholar] [CrossRef]
- Platt, T.; Gallegos, C.L.; Harrison, W.G. Photoinhibition of Photosynthesis in Natural Assemblage of Marine Phytoplankton. J. Mar. Res. 1980, 38, 687–701. [Google Scholar]
- Kim, J.B.; Park, J.-I.; Jung, C.-S.; Lee, P.-Y.; Lee, K.-S. Distributional Range Extension of the Seagrass Halophila nipponica into Coastal Waters off the Korean Peninsula. Aquat. Bot. 2009, 90, 269–272. [Google Scholar] [CrossRef]
- Walters, L.J.; Brown, K.R.; Stam, W.T.; Olsen, J.L. Commerce and Caulerpa: Unregulated Dispersal of Invasive Species. Front. Ecol. Environ. 2006, 4, 75–79. [Google Scholar] [CrossRef]
- Frisch Zaleski, S.F.; Murray, S.N. Taxonomic Diversity and Geographic Distributions of Aquarium-Traded Species of Caulerpa (Chlorophyta: Caulerpaceae) in Southern California, USA. Mar. Ecol. Prog. Ser. 2006, 314, 97–108. [Google Scholar] [CrossRef][Green Version]
- Masojídek, J.; Grobbelaar, J.U.; Pechar, L.; Koblížek, M. Photosystem II Electron Transport Rates and Oxygen Production in Natural Waterblooms of Freshwater Cyanobacteria During a Diel Cycle. J. Plankton Res. 2001, 23, 57–66. [Google Scholar] [CrossRef]
- Giordano, M.; Beardall, J.; Raven, J.A. CO2 Concentrating Mechanisms in Algae: Mechanisms, Environmental Modulation and Evolution. Annu. Rev. Plant. Biol. 2005, 56, 99–131. [Google Scholar] [CrossRef]
- Kim, J.-H.; Kim, K.Y.; Kang, E.J.; Lee, K.; Kim, J.-M.; Park, K.-T.; Shin, K.; Hyun, B.; Jeong, H.J. Enhancement of Photosynthetic Carbon Assimilation Efficiency by Phytoplankton in the Future Coastal Ocean. Biogeosciences 2013, 10, 7525–7535. [Google Scholar] [CrossRef]
- Kim, J.-H.; Kang, E.J.; Edwards, M.S.; Lee, K.; Jeong, H.J.; Kim, K.Y. Species-Specific Responses of Temperate Macroalgae with Different Photosynthetic Strategies to Ocean Acidification: A Mesocosm Study. Algae 2016, 31, 243–256. [Google Scholar] [CrossRef]
- Ceccherelli, G.; Piazzi, L. Dispersal of Caulerpa racemose Fragments in the Mediterranean: Lack of Detachment Time Effect on Establishment. Bot. Marina 2001, 44, 209–213. [Google Scholar] [CrossRef]
- Roth-Schulze, A.J.; Thomas, T.; Steinberg, P.; Deveney, M.R.; Tanner, J.E.; Wiltshire, K.H.; Papantoniou, S.; Runcie, J.W.; Gurgel, C.F.D. The Effects of Warming and Ocean Acidification on Growth, Photosynthesis, and Bacterial Communities for the Marine Invasive Macroalga Caulerpa taxifolia. Limnol. Oceanogr. 2018, 63, 459–471. [Google Scholar] [CrossRef]
- Ceccherelli, G.; Cinelli, F. The Role of Vegetative Fragmentation in Dispersal of the Invasive Alga Caulerpa taxifolia in the Mediterranean. Mar. Ecol. Prog. Ser. 1999, 182, 299–303. [Google Scholar] [CrossRef]
- Wright, J.T. Differences Between Native and Invasive Caulerpa taxifolia: A Link Between Asexual Fragmentation and Abundance in Invasive Populations. Mar. Biol. 2005, 147, 559–569. [Google Scholar] [CrossRef]
- Piazzi, L.; Ceccherelli, G.; Cinelli, F. Expansion de Caulerpa taxifolia et de Caulerpa racemosa le long des côtes toscanes (Italie), situation en 1998. In Fourth International Workshop on Caulerpa taxifolia; Gravez, V., Ruitton, S., Boudouresque, C.F., Le Direach, L., Meinesz, A., Scabbia, G., Verlaque, M., Eds.; GIS Posidonie Publisher: Marseille, France, 2001; pp. 71–77. [Google Scholar]
| Treatments | Temp. | pH | AT (µmol kg−1SW) | DIC (µmol kg−1SW) | pCO2 (µatm CO2) | HCO3− (µmol kg−1SW) | CO32− (µmol kg−1SW) |
|---|---|---|---|---|---|---|---|
| Control | 20 °C | 7.919 ± 0.002 | 2308.2 ± 2.9 | 2074.5 ± 3.3 | 474.2 ± 3.2 | 1911.0 ± 3.6 | 162.6 ± 0.3 |
| Acidification (OA) | 20 °C | 7.647 ± 0.003 | 2390.3 ± 7.2 | 2249.0 ± 7.4 | 963.2 ± 10.6 | 2150.0 ± 7.6 | 98.7 ± 0.9 |
| Warming (OW) | 25 °C | 8.020 ± 0.002 | 2632.0 ± 5.0 | 2291.8 ± 3.3 | 476.6 ± 2.7 | 2049.4 ± 2.0 | 242.3 ± 1.3 |
| Greenhouse (GR) | 25 °C | 7.771 ± 0.004 | 2430.7 ± 3.5 | 2239.1 ± 3.1 | 879.5 ± 5.5 | 2104.0 ± 3.3 | 134.9 ± 1.1 |
| Treatments | Control | Acidification | Warming | Greenhouse | |
|---|---|---|---|---|---|
| P-E curves | GPmax | 0.440 ± 0.033 ab | 0.624 ± 0.044 bc | 0.416 ± 0.045 a | 0.711 ± 0.053 c |
| NPmax | 0.252 ± 0.031 a | 0.455 ± 0.037 b | 0.154 ± 0.037 a | 0.532 ± 0.036 b | |
| 𝛼 | 0.0101 ± 0.0008 | 0.0096 ± 0.0002 | 0.0072 ± 0.0027 | 0.0137 ± 0.0017 | |
| Ek | 43.9 ± 2.7 | 65.0 ± 3.6 | 81.5 ± 32.8 | 52.9 ± 3.9 | |
| Rd | 0.188 ± 0.018 | 0.169 ± 0.007 | 0.262 ± 0.013 * | 0.179 ± 0.022 | |
| RLCs | rETRmax | 4.523 ± 0.226 ab | 4.238 ± 0.191 a | 5.719 ± 0.413 b | 4.854 ± 0.485 ab |
| 𝛼RLC | 0.3925 ± 0.0298 | 0.3737 ± 0.0335 | 0.3465 ± 0.0306 | 0.3910 ± 0.0274 | |
| Ek,RLC | 12.0 ± 01.2 | 12.0 ± 1.3 | 17.0 ± 2.3 | 12.9 ± 1.9 | |
| Parameter | Source | Type III SS | Df | MS | F | p | |
|---|---|---|---|---|---|---|---|
| P-E curves | GPmax | CO2 | 0.173 | 1 | 0.173 | 29.226 | 0.001 |
| Temp. | 0.003 | 1 | 0.003 | 0.510 | 0.495 | ||
| CO2 × Temp. | 0.009 | 1 | 0.009 | 1.555 | 0.248 | ||
| Error | 0.047 | 8 | 0.006 | ||||
| NPmax | CO2 | 0.253 | 1 | 0.253 | 66.747 | <0.001 | |
| Temp. | 0.000 | 1 | 0.000 | 0.089 | 0.773 | ||
| CO2 × Temp. | 0.023 | 1 | 0.023 | 6.044 | 0.039 | ||
| Error | 0.030 | 8 | 0.004 | ||||
| 𝛼 | CO2 | 0.0000267 | 1 | 0.0000267 | 3.279 | 0.108 | |
| Temp. | 0.000001107 | 1 | 0.000001107 | 0.136 | 0.722 | ||
| CO2 × Temp. | 0.00003604 | 1 | 0.00003604 | 4.426 | 0.069 | ||
| Error | 0.00006515 | 8 | 0.00000814 | ||||
| Ek | CO2 | 41.642 | 1 | 41.642 | 0.050 | 0.829 | |
| Temp. | 489.028 | 1 | 489.028 | 0.587 | 0.466 | ||
| CO2 × Temp. | 1853.107 | 1 | 1853.107 | 2.224 | 0.174 | ||
| Error | 6665.503 | 8 | 833.188 | ||||
| Rd | CO2 | 0.008 | 1 | 0.008 | 10.157 | 0.013 | |
| Temp. | 0.005 | 1 | 0.005 | 7.079 | 0.029 | ||
| CO2 × Temp. | 0.003 | 1 | 0.003 | 4.074 | 0.078 | ||
| Error | 0.006 | 8 | 0.001 | ||||
| RLCs | rETRmax | CO2 | 1.324 | 1 | 1.324 | 2.681 | 0.127 |
| Temp. | 3.286 | 1 | 3.286 | 6.655 | 0.024 | ||
| CO2 × Temp. | 0.335 | 1 | 0.335 | 0.680 | 0.426 | ||
| Error | 5.924 | 12 | 0.494 | ||||
| 𝛼RLC | CO2 | 0.001 | 1 | 0.001 | 0.178 | 0.681 | |
| Temp. | 0.001 | 1 | 0.001 | 0.222 | 0.646 | ||
| CO2 × Temp. | 0.004 | 1 | 0.004 | 1.081 | 0.319 | ||
| Error | 0.044 | 12 | 0.004 | ||||
| Ek,RLC | CO2 | 17.270 | 1 | 17.270 | 1.415 | 0.257 | |
| Temp. | 35.575 | 1 | 35.575 | 2.916 | 0.113 | ||
| CO2 × Temp. | 17.663 | 1 | 17.663 | 1.448 | 0.252 | ||
| Error | 146.421 | 12 | 12.202 | ||||
| Parameter | Source | Type III SS | Df | MS | F | p | |
|---|---|---|---|---|---|---|---|
| Growth | RGRfrond | CO2 | 21.798 | 1 | 21.798 | 2.478 | 0.141 |
| Temp. | 143.972 | 1 | 143.972 | 16.368 | 0.002 | ||
| CO2 × Temp. | 0.018 | 1 | 0.018 | 0.002 | 0.965 | ||
| Error | 105.551 | 12 | 8.796 | ||||
| RGRstolon | CO2 | 15.654 | 1 | 15.654 | 3.655 | 0.080 | |
| Temp. | 123.672 | 1 | 123.672 | 28.875 | <0.001 | ||
| CO2 × Temp. | 9.493 | 1 | 9.493 | 2.216 | 0.162 | ||
| Error | 51.397 | 12 | 4.283 | ||||
| RGRwhole | CO2 | 13.942 | 1 | 13.942 | 2.947 | 0.112 | |
| Temp. | 107.949 | 1 | 107.949 | 22.814 | <0.001 | ||
| CO2 × Temp. | 3.550 | 1 | 3.550 | 0.750 | 0.403 | ||
| Error | 56.779 | 12 | 4.732 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, E.J.; Lee, S.; Kang, J.; Moon, H.; Kim, I.-N.; Kim, J.-H. Performance of a Potentially Invasive Species of Ornamental Seaweed Caulerpa sertularioides in Acidifying and Warming Oceans. J. Mar. Sci. Eng. 2021, 9, 1368. https://doi.org/10.3390/jmse9121368
Kang EJ, Lee S, Kang J, Moon H, Kim I-N, Kim J-H. Performance of a Potentially Invasive Species of Ornamental Seaweed Caulerpa sertularioides in Acidifying and Warming Oceans. Journal of Marine Science and Engineering. 2021; 9(12):1368. https://doi.org/10.3390/jmse9121368
Chicago/Turabian StyleKang, Eun Ju, Sukyeon Lee, Juhyun Kang, Hanbi Moon, Il-Nam Kim, and Ju-Hyoung Kim. 2021. "Performance of a Potentially Invasive Species of Ornamental Seaweed Caulerpa sertularioides in Acidifying and Warming Oceans" Journal of Marine Science and Engineering 9, no. 12: 1368. https://doi.org/10.3390/jmse9121368
APA StyleKang, E. J., Lee, S., Kang, J., Moon, H., Kim, I.-N., & Kim, J.-H. (2021). Performance of a Potentially Invasive Species of Ornamental Seaweed Caulerpa sertularioides in Acidifying and Warming Oceans. Journal of Marine Science and Engineering, 9(12), 1368. https://doi.org/10.3390/jmse9121368






