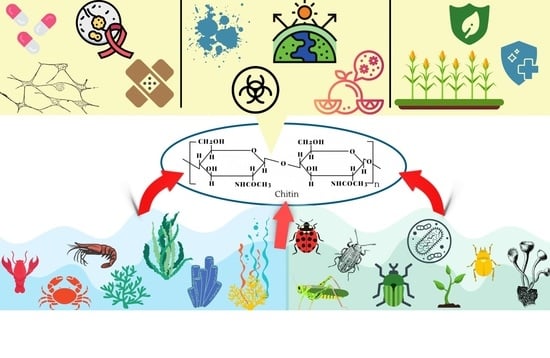
Applications of Chitin in Medical, Environmental, and Agricultural Industries
Abstract
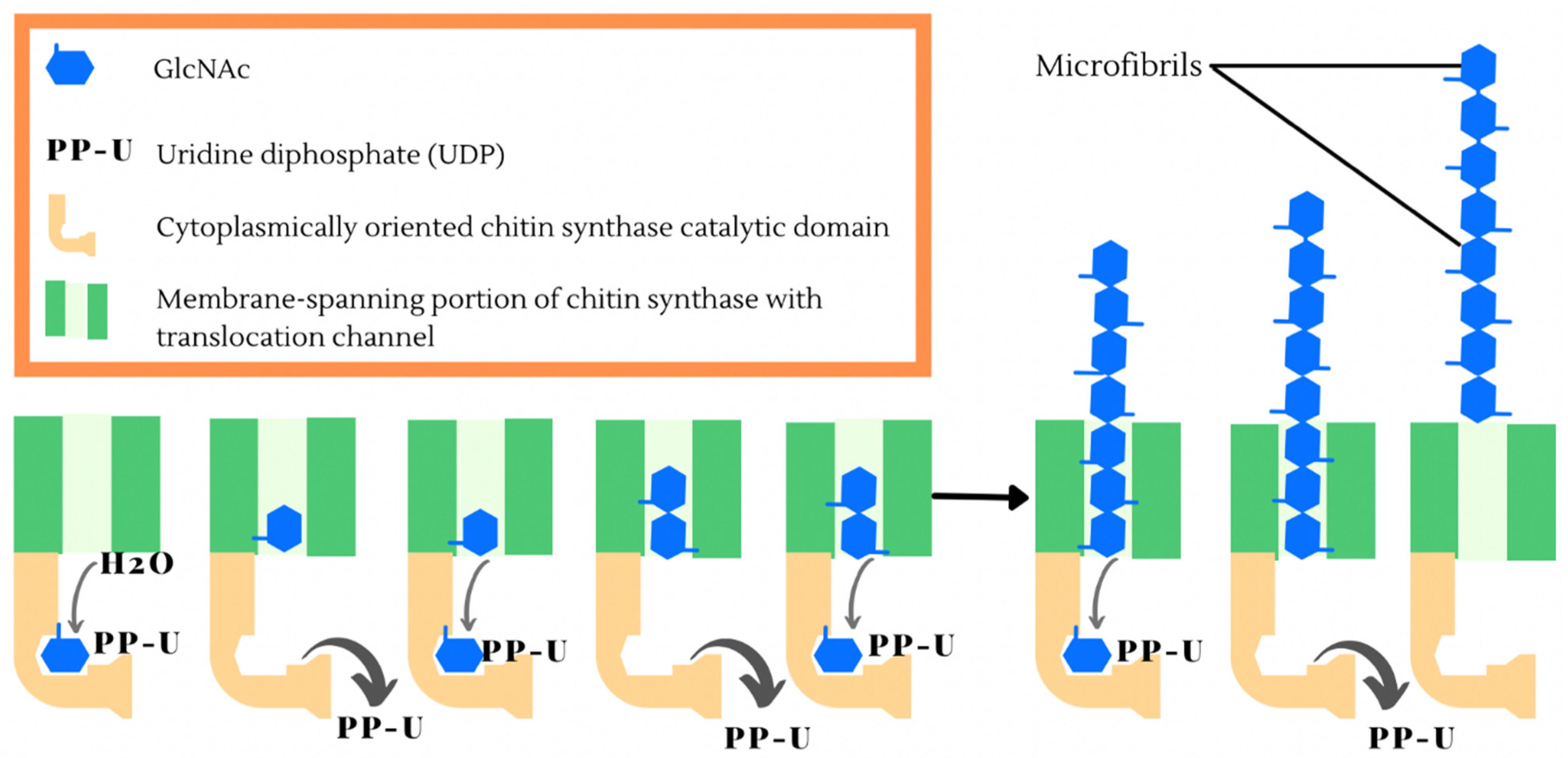
:1. Introduction
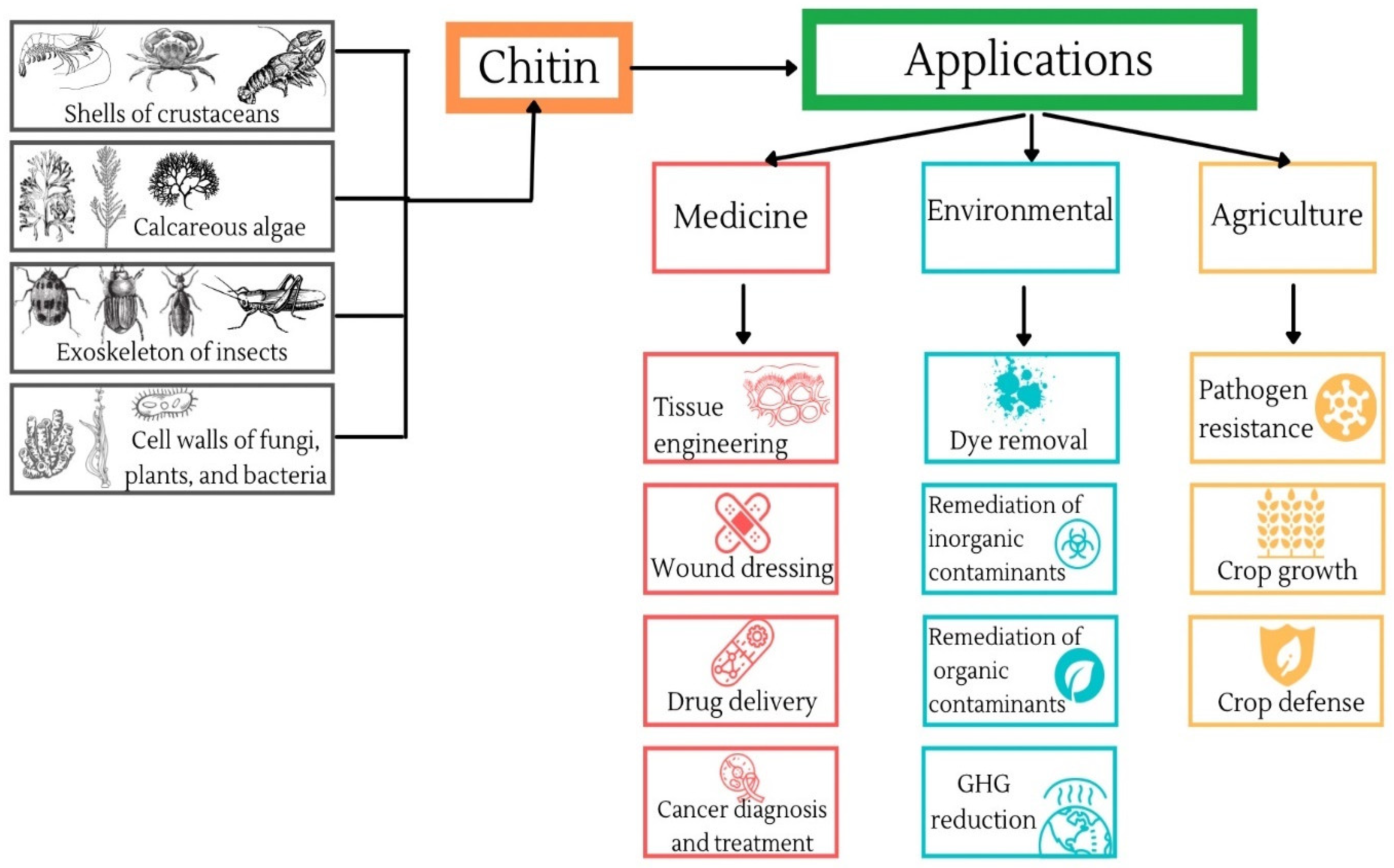
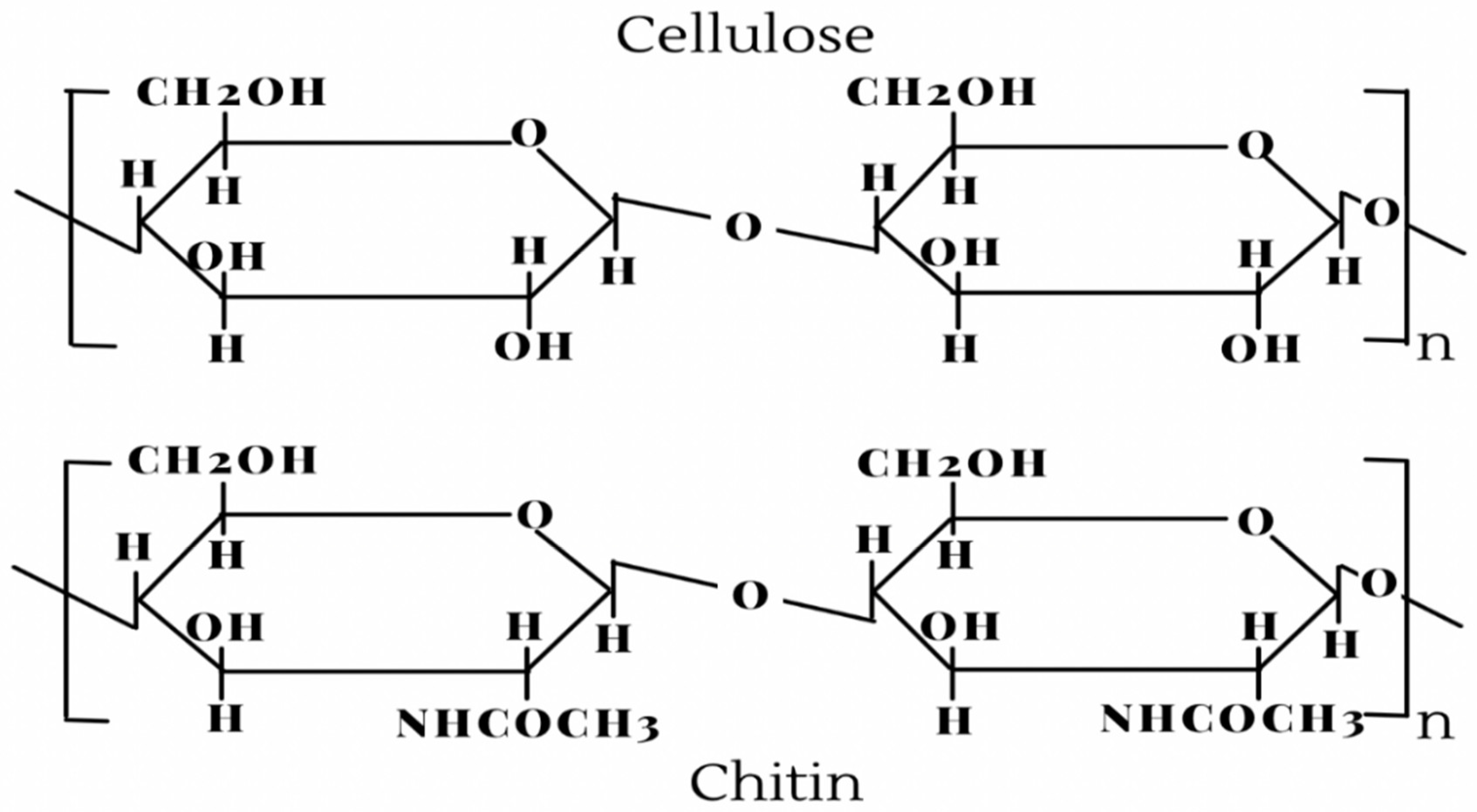
2. Production of Chitin
3. Medical Applications of Chitin
3.1. Tissue Engineering
3.2. Wound Dressing
3.3. Drug Delivery
3.4. Cancer Diagnosis and Treatment
4. Environmental Applications of Chitin
4.1. Dye Removal
4.2. Remediation of Inorganic Contaminants
4.3. Remediation of Organic Contaminants
5. Agricultural Applications of Chitin
5.1. Pathogen Resistance
5.2. Crop Growth
5.3. Crop Defense
6. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rahman, M.A.; Halfar, J. First evidence of chitin in calcified coralline algae: New insights into the calcification process of Clathromorphum compactum. Sci. Rep. 2014, 4, 6162. [Google Scholar] [CrossRef]
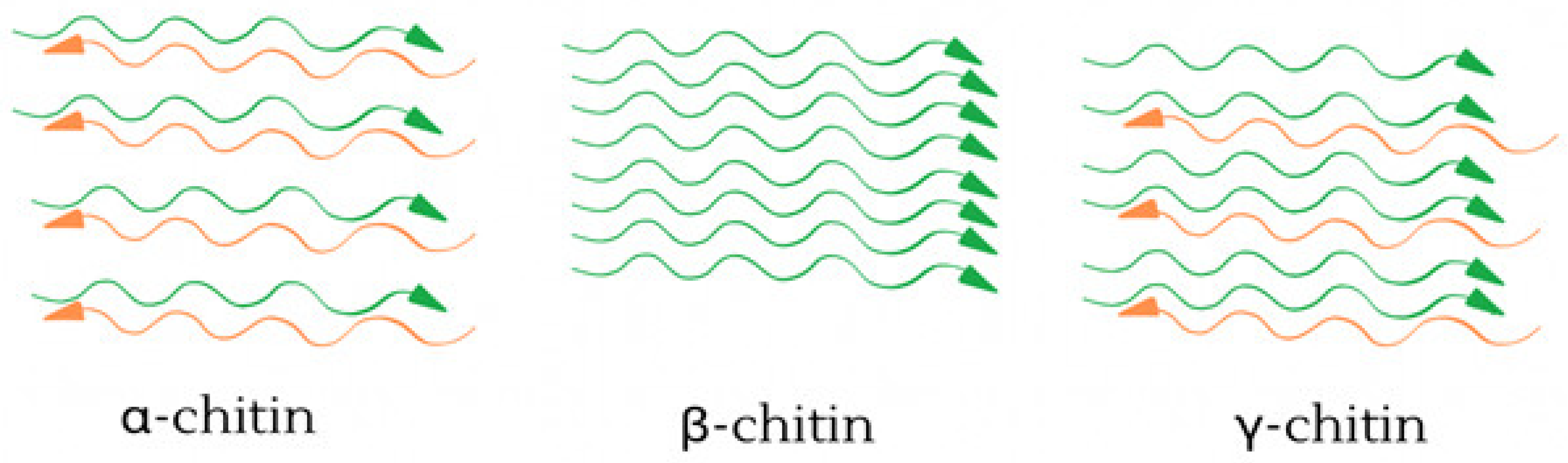
- Jang, M.-K.; Kong, B.-G.; Jeong, Y.-I.; Lee, C.H.; Nah, J.-W. Physicochemical characterization of α-chitin, β-chitin, and γ-chitin separated from natural resources. J. Polym. Sci. Part A Polym. Chem. 2004, 42, 3423–3432. [Google Scholar] [CrossRef]
- Hajji, S.; Younes, I.; Ghorbel-Bellaaj, O.; Hajji, R.; Rinaudo, M.; Nasri, M.; Jellouli, K. Structural differences between chitin and chitosan extracted from three different marine sources. Int. J. Biol. Macromol. 2014, 65, 298–306. [Google Scholar] [CrossRef]
- Moussian, B.; Schwarz, H.; Bartoszewski, S.; Nüsslein-Volhard, C. Involvement of chitin in exoskeleton morphogenesis in Drosophila melanogaster. J. Morphol. 2005, 264, 117–130. [Google Scholar] [CrossRef]
- Rahman, M.A.; Halfar, J.; Adey, W.H.; Nash, M.; Paulo, C.; Dittrich, M. The role of chitin-rich skeletal organic matrix on the crystallization of calcium carbonate in the crustose coralline alga Leptophytum foecundum. Sci. Rep. 2019, 9, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Boßelmann, F.; Romano, P.; Fabritius, H.; Raabe, D.; Epple, M. The composition of the exoskeleton of two crustacea: The American lobster Homarus americanus and the edible crab Cancer pagurus. Thermochim. Acta 2007, 463, 65–68. [Google Scholar] [CrossRef]
- Merzendorfer, H.; Zimoch, L. Chitin metabolism in insects: Structure, function and regulation of chitin synthases and chitinases. J. Exp. Biol. 2003, 206, 4393–4412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, K.Y.; Merzendorfer, H.; Zhang, W.; Zhang, J.; Muthukrishnan, S. Biosynthesis, Turnover, and Functions of Chitin in Insects. Annu. Rev. Èntomol. 2016, 61, 177–196. [Google Scholar] [CrossRef]
- Rocha, J.; García-Carreño, F.; Muhlia-Almazán, A.; Peregrino-Uriarte, A.B.; Yépiz-Plascencia, G.; Cordova-Murueta, J.H. Cuticular chitin synthase and chitinase mRNA of whiteleg shrimp Litopenaeus vannamei during the molting cycle. Aquaculture 2012, 330–333, 111–115. [Google Scholar] [CrossRef]
- Nagasawa, H. The crustacean cuticle: Structure, composition and mineralization. Front. Biosci. 2012, 4, 711–720. [Google Scholar] [CrossRef]
- Cohen, E. Chitin synthesis and inhibition: A revisit. Pest Manag. Sci. 2001, 57, 946–950. [Google Scholar] [CrossRef]
- Merzendorfer, H. The cellular basis of chitin synthesis in fungi and insects: Common principles and differences. Eur. J. Cell Biol. 2011, 90, 759–769. [Google Scholar] [CrossRef] [PubMed]
- Dermauw, W.; Van Leeuwen, T. The ABC gene family in arthropods: Comparative genomics and role in insecticide transport and resistance. Insect Biochem. Mol. Biol. 2014, 45, 89–110. [Google Scholar] [CrossRef]
- Glazer, L.; Tom, M.; Weil, S.; Roth, Z.; Khalaila, I.; Mittelman, B.; Sagi, A. Hemocyanin with phenoloxidase activity in the chitin matrix of the crayfish gastrolith. J. Exp. Biol. 2013, 216, 1898–1904. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Glazer, L.; Shechter, A.; Tom, M.; Yudkovski, Y.; Weil, S.; Aflalo, E.; Pamuru, R.R.; Khalaila, I.; Bentov, S.; Berman, A.; et al. A Protein Involved in the Assembly of an Extracellular Calcium Storage Matrix. J. Biol. Chem. 2010, 285, 12831–12839. [Google Scholar] [CrossRef] [Green Version]
- Pachapur, V.L.; Guemiza, K.; Rouissi, T.; Sarma, S.J.; Brar, S.K. Novel biological and chemical methods of chitin extraction from crustacean waste using saline water. J. Chem. Technol. Biotechnol. 2016, 91, 2331–2339. [Google Scholar] [CrossRef] [Green Version]
- Borić, M.; Vicente, F.A.; Jurković, D.L.; Novak, U.; Likozar, B. Chitin isolation from crustacean waste using a hybrid demineralization/DBD plasma process. Carbohydr. Polym. 2020, 246, 116648. [Google Scholar] [CrossRef]
- Olsen, R.L.; Toppe, J.; Karunasagar, I. Challenges and realistic opportunities in the use of by-products from processing of fish and shellfish. Trends Food Sci. Technol. 2014, 36, 144–151. [Google Scholar] [CrossRef]
- Pal, J.; Verma, H.O.; Munka, V.K.; Maurya, S.K.; Roy, D.; Kumar, J. Biological method of chitin extraction from shrimp waste an eco-friendly low cost technology and its advanced application. Int. J. Fish. Aquat. Stud. IJFAS 2014, 1, 104–107. Available online: www.fisheriesjournal.com (accessed on 12 May 2021).
- Hours, R.A.; Gortari, M.C. Biotechnological processes for chitin recovery out of crustacean waste: A mini-review. Electron. J. Biotechnol. 2013, 16, 14. [Google Scholar] [CrossRef]
- Liu, S.; Sun, J.; Yu, L.; Zhang, C.; Bi, J.; Zhu, F.; Qu, M.; Jiang, C.; Yang, Q. Extraction and Characterization of Chitin from the Beetle Holotrichia parallela Motschulsky. Mol. 2012, 17, 4604–4611. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Haga, A.; Sekiguchi, H.; Hirano, S. Structure of insect chitin isolated from beetle larva cuticle and silkworm (Bombyx mori) pupa exuvia. Int. J. Biol. Macromol. 2000, 27, 99–105. [Google Scholar] [CrossRef]
- Yang, T.-L. Chitin-based Materials in Tissue Engineering: Applications in Soft Tissue and Epithelial Organ. Int. J. Mol. Sci. 2011, 12, 1936–1963. [Google Scholar] [CrossRef] [Green Version]
- Wan, A.C.; Tai, B.C. CHITIN—A promising biomaterial for tissue engineering and stem cell technologies. Biotechnol. Adv. 2013, 31, 1776–1785. [Google Scholar] [CrossRef]
- Madhumathi, K.; Kumar, P.S.; Kavya, K.; Furuike, T.; Tamura, H.; Nair, S.; Jayakumar, R. Novel chitin/nanosilica composite scaffolds for bone tissue engineering applications. Int. J. Biol. Macromol. 2009, 45, 289–292. [Google Scholar] [CrossRef]
- Wojtowicz, A.M.; Oliveira, S.; Carlson, M.W.; Zawadzka, A.; Rousseau, C.F.; Baksh, D. The importance of both fibroblasts and keratinocytes in a bilayered living cellular construct used in wound healing. Wound Repair Regen. Off. Publ. Wound Heal. Soc. Eu-Ropean Tissue Repair Soc. 2014, 22, 246–255. [Google Scholar] [CrossRef] [Green Version]
- Souren, J.E.M.; Ponec, M.; van Wijk, R. Contraction of collagen by human fibroblasts and keratinocytes. Vitr. Cell. Dev. Biol.—Anim. 1989, 25, 1039–1045. [Google Scholar] [CrossRef] [PubMed]
- Nettles, D.L.; Elder, S.H.; Gilbert, J.A. Potential Use of Chitosan as a Cell Scaffold Material for Cartilage Tissue Engineering. Tissue Eng. 2002, 8, 1009–1016. [Google Scholar] [CrossRef] [PubMed]
- Kuo, Y.-C.; Ku, I.-N. Cartilage Regeneration by Novel Polyethylene Oxide/Chitin/Chitosan Scaffolds. Biomacromolecules 2008, 9, 2662–2669. [Google Scholar] [CrossRef]
- Freier, T.; Montenegro, R.; Koh, H.S.; Shoichet, M.S. Chitin-based tubes for tissue engineering in the nervous system. Biomaterials 2005, 26, 4624–4632. [Google Scholar] [CrossRef]
- Islam, S.; Bhuiyan, M.A.R.; Islam, M.N. Chitin and Chitosan: Structure, Properties and Applications in Biomedical Engineering. J. Polym. Environ. 2017, 25, 854–866. [Google Scholar] [CrossRef]
- Ahmed, S.; Ikram, S. Chitosan Based Scaffolds and Their Applications in Wound Healing. Achiev. Life Sci. 2016, 10, 27–37. [Google Scholar] [CrossRef] [Green Version]
- Jiménez-Gómez, C.P.; Cecilia, J.A. Chitosan: A Natural Biopolymer with a Wide and Varied Range of Applications. Molecules 2020, 25, 3981. [Google Scholar] [CrossRef]
- Kumar, P.S.; Abhilash, S.; Manzoor, K.; Nair, S.; Tamura, H.; Jayakumar, R. Preparation and characterization of novel β-chitin/nanosilver composite scaffolds for wound dressing applications. Carbohydr. Polym. 2010, 80, 761–767. [Google Scholar] [CrossRef]
- Jayakumar, R.; Menon, D.; Manzoor, K.; Nair, S.; Tamura, H. Biomedical applications of chitin and chitosan based nanomaterials—A short review. Carbohydr. Polym. 2010, 82, 227–232. [Google Scholar] [CrossRef]
- Yusof, N.L.B.M.; Wee, A.; Lim, L.Y.; Khor, E. Flexible chitin films as potential wound-dressing materials: Wound model studies. J. Biomed. Mater. Res.—Part A 2003, 66, 224–232. [Google Scholar] [CrossRef]
- Hu, S.; Bi, S.; Yan, D.; Zhou, Z.; Sun, G.; Cheng, X.; Chen, X. Preparation of composite hydroxybutyl chitosan sponge and its role in promoting wound healing. Carbohydr. Polym. 2018, 184, 154–163. [Google Scholar] [CrossRef]
- Ehterami, A.; Salehi, M.; Farzamfar, S.; Samadian, H.; Vaez, A.; Ghorbani, S.; Ai, J.; Sahrapeyma, H. Chitosan/alginate hydrogels containing Alpha-tocopherol for wound healing in rat model. J. Drug Deliv. Sci. Technol. 2019, 51, 204–213. [Google Scholar] [CrossRef]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rejinold, N.S.; Chennazhi, K.P.; Tamura, H.; Nair, S.V.; Rangasamy, J. Multifunctional Chitin Nanogels for Simultaneous Drug Delivery, Bioimaging, and Biosensing. ACS Appl. Mater. Interfaces 2011, 3, 3654–3665. [Google Scholar] [CrossRef] [PubMed]
- Parhi, R. Drug delivery applications of chitin and chitosan: A review. Environ. Chem. Lett. 2020, 18, 577–594. [Google Scholar] [CrossRef]
- Jayakumar, R.; Prabaharan, M.; Nair, S.; Tokura, S.; Tamura, H.; Selvamurugan, N. Novel carboxymethyl derivatives of chitin and chitosan materials and their biomedical applications. Prog. Mater. Sci. 2010, 55, 675–709. [Google Scholar] [CrossRef]
- Narayanan, D.; Jayakumar, R.; Chennazhi, K.P. Versatile carboxymethyl chitin and chitosan nanomaterials: A review. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2014, 6, 574–598. [Google Scholar] [CrossRef]
- Dev, A.; Binulal, N.; Anitha, A.; Nair, S.; Furuike, T.; Tamura, H.; Jayakumar, R. Preparation of poly(lactic acid)/chitosan nanoparticles for anti-HIV drug delivery applications. Carbohydr. Polym. 2010, 80, 833–838. [Google Scholar] [CrossRef] [Green Version]
- Mi, F.-L.; Shyu, S.-S.; Lin, Y.-M.; Wu, Y.-B.; Peng, C.-K.; Tsai, Y.-H. Chitin/PLGA blend microspheres as a biodegradable drug delivery system: A new delivery system for protein. Biomaterials 2003, 24, 5023–5036. [Google Scholar] [CrossRef]
- Peng, C.-W.; Li, Y. Application of Quantum Dots-Based Biotechnology in Cancer Diagnosis: Current Status and Future Perspectives. J. Nanomater. 2010, 2010, 1–11. [Google Scholar] [CrossRef]
- Karagozlu, M.Z.; Kim, S.-K. Chapter Twelve—Anticancer Effects of Chitin and Chitosan Derivatives. In Advances in Food and Nutrition Research; Kim, S.-K., Ed.; Academic Press: Waltham, MA, USA, 2014; Volume 72, pp. 215–225. [Google Scholar]
- Peter, S.; Lyczko, N.; Gopakumar, D.; Maria, H.J.; Nzihou, A.; Thomas, S. Chitin and Chitosan Based Composites for Energy and Environmental Applications: A Review. Waste Biomass-Valorization 2021, 12, 4777–4804. [Google Scholar] [CrossRef]
- Rameshthangam, P.; Solairaj, D.; Arunachalam, G.; Ramasamy, P.; Veterinary, T.N.; Nadu, T.J. Chitin and Chitinases: Biomedical and Environmental Applications of Chitin and its Derivatives. J. Enzymes 2018, 1, 20–43. [Google Scholar] [CrossRef] [Green Version]
- Xu, R.; Mao, J.; Peng, N.; Luo, X.; Chang, C. Chitin/clay microspheres with hierarchical architecture for highly efficient removal of organic dyes. Carbohydr. Polym. 2018, 188, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.-L.; Pan, Z.-H.; Shi, Q.-X.; Yu, J.-Y. Modification of chitin with high adsorption capacity for methylene blue removal. Int. J. Biol. Macromol. 2018, 114, 392–399. [Google Scholar] [CrossRef]
- Schirmer, R.H.; Adler, H.; Pickhardt, M.; Mandelkow, E. Lest we forget you—Methylene blue. Neurobiol. Aging 2011, 32, 2325.e7–2325.e16. [Google Scholar] [CrossRef]
- Druzian, S.P.; Zanatta, N.P.; Côrtes, L.N.; Streit, A.F.M.; Dotto, G.L. Preparation of chitin nanowhiskers and its application for crystal violet dye removal from wastewaters. Environ. Sci. Pollut. Res. 2019, 26, 28548–28557. [Google Scholar] [CrossRef] [PubMed]
- Meshkat, S.S.; Nezhad, M.N.; Bazmi, M.R. Investigation of Carmine Dye Removal by Green Chitin Nanowhiskers Adsorbent. Emerg. Sci. J. 2019, 3, 187–194. [Google Scholar] [CrossRef]
- González, J.A.; Villanueva, M.E.; Piehl, L.L.; Copello, G. Development of a chitin/graphene oxide hybrid composite for the removal of pollutant dyes: Adsorption and desorption study. Chem. Eng. J. 2015, 280, 41–48. [Google Scholar] [CrossRef]
- Ma, Z.; Liu, D.; Zhu, Y.; Li, Z.; Li, Z.; Tian, H.; Liu, H. Graphene oxide/chitin nanofibril composite foams as column adsorbents for aqueous pollutants. Carbohydr. Polym. 2016, 144, 230–237. [Google Scholar] [CrossRef]
- Nasrollahzadeh, M.; Sajjadi, M.; Iravani, S.; Varma, R.S. Starch, cellulose, pectin, gum, alginate, chitin and chitosan derived (nano)materials for sustainable water treatment: A review. Carbohydr. Polym. 2021, 251, 116986. [Google Scholar] [CrossRef]
- Boulaiche, W.; Hamdi, B.; Trari, M. Removal of heavy metals by chitin: Equilibrium, kinetic and thermodynamic studies. Appl. Water Sci. 2019, 9, 39. [Google Scholar] [CrossRef] [Green Version]
- Saravanan, D.; Gomathi, T.; Sudha, P. Sorption studies on heavy metal removal using chitin/bentonite biocomposite. Int. J. Biol. Macromol. 2013, 53, 67–71. [Google Scholar] [CrossRef]
- Nithya, R.; Sudha, P.N. Removal of heavy metals from tannery effluent using chitosan-g-poly(butyl acrylate)/bentonite nanocomposite as an adsorbent. Text. Cloth. Sustain. 2017, 2, 1. [Google Scholar] [CrossRef] [Green Version]
- Saxena, R.; Saxena, M.; Lochab, A. Recent Progress in Nanomaterials for Adsorptive Removal of Organic Contaminants from Wastewater. ChemistrySelect 2020, 5, 335–353. [Google Scholar] [CrossRef]
- Żółtowska-Aksamitowska, S.; Bartczak, P.; Zembrzuska, J.; Jesionowski, T. Removal of hazardous non-steroidal anti-inflammatory drugs from aqueous solutions by biosorbent based on chitin and lignin. Sci. Total. Environ. 2018, 612, 1223–1233. [Google Scholar] [CrossRef] [PubMed]
- Gwenzi, W.; Chaukura, N. Organic contaminants in African aquatic systems: Current knowledge, health risks, and future research directions. Sci. Total. Environ. 2018, 619–620, 1493–1514. [Google Scholar] [CrossRef] [PubMed]
- Shamshina, J.L.; Kelly, A.; Oldham, T.; Rogers, R.D. Agricultural uses of chitin polymers. Environ. Chem. Lett. 2020, 18, 53–60. [Google Scholar] [CrossRef]
- Ramírez, M.Á.; Rodríguez, A.T.; Alfonso, L.; Peniche, C.; Experimental, E.; Nacional, I.; Agrícolas, D.C. Chitin is a biodegradable polymer widely spread in nature. Biotecnol. Apl. 2010, 27, 270–276. [Google Scholar]
- Wan, J.; Zhang, X.-C.; Stacey, G. Chitin signaling and plant disease resistance. Plant Signal. Behav. 2008, 3, 831–833. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eckardt, N.A. Chitin Signaling in Plants: Insights into the Perception of Fungal Pathogens and Rhizobacterial Symbionts. Plant Cell 2008, 20, 241–243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maximova, S.N.; Marelli, J.-P.; Young, A.; Pishak, S.; Verica, J.A.; Guiltinan, M.J. Over-expression of a cacao class I chitinase gene in Theobroma cacao L. enhances resistance against the pathogen, Colletotrichum gloeosporioides. Planta 2006, 224, 740–749. [Google Scholar] [CrossRef]
- Iqbal, M.M.; Nazir, F.; Ali, S.; Asif, M.A.; Zafar, Y.; Iqbal, J.; Ali, G.M. Over Expression of Rice chitinase Gene in Transgenic Peanut (Arachis hypogaea L.) Improves Resistance Against Leaf Spot. Mol. Biotechnol. 2012, 50, 129–136. [Google Scholar] [CrossRef]
- Yamaguchi, K.; Yamada, K.; Ishikawa, K.; Yoshimura, S.; Hayashi, N.; Uchihashi, K.; Ishihama, N.; Kishi-Kaboshi, M.; Takahashi, A.; Tsuge, S.; et al. A Receptor-like Cytoplasmic Kinase Targeted by a Plant Pathogen Effector Is Directly Phosphorylated by the Chitin Receptor and Mediates Rice Immunity. Cell Host Microbe 2013, 13, 347–357. [Google Scholar] [CrossRef] [Green Version]
- Petutschnig, E.K.; Jones, A.M.; Serazetdinova, L.; Lipka, U.; Lipka, V. The Lysin Motif Receptor-like Kinase (LysM-RLK) CERK1 Is a Major Chitin-binding Protein in Arabidopsis thaliana and Subject to Chitin-induced Phosphorylation. J. Biol. Chem. 2010, 285, 28902–28911. [Google Scholar] [CrossRef] [Green Version]
- Leppyanen, I.V.; Shakhnazarova, V.Y.; Shtark, O.Y.; Vishnevskaya, N.A.; Tikhonovich, I.A.; Dolgikh, E.A. Receptor-Like Kinase LYK9 in Pisum sativum L. Is the CERK1-Like Receptor that Controls Both Plant Immunity and AM Symbiosis Development. Int. J. Mol. Sci. 2018, 19, 8. [Google Scholar] [CrossRef] [Green Version]
- Li, K.; Xing, R.; Liu, S.; Li, P. Chitin and Chitosan Fragments Responsible for Plant Elicitor and Growth Stimulator. J. Agric. Food Chem. 2020, 68, 12203–12211. [Google Scholar] [CrossRef] [PubMed]
- Malerba, M.; Cerana, R. Recent Applications of Chitin- and Chitosan-Based Polymers in Plants. Polymers 2019, 11, 839. [Google Scholar] [CrossRef] [Green Version]
- Malerba, M.; Cerana, R. Chitosan Effects on Plant Systems. Int. J. Mol. Sci. 2016, 17, 996. [Google Scholar] [CrossRef]
- Winkler, A.J.; Nuñez, J.A.D.; Aranaz, I.; Poza-Carrión, C.; Ramonell, K.; Somerville, S.; Berrocal-Lobo, M. Short-Chain Chitin Oligomers: Promoters of Plant Growth. Mar. Drugs 2017, 15, 40. [Google Scholar] [CrossRef] [Green Version]
- Debode, J.; De Tender, C.; Soltaninejad, S.; Van Malderghem, C.; Haegeman, A.; Van Der Linden, I.; Cottyn, B.; Heyndrickx, M.; Maes, M. Chitin Mixed in Potting Soil Alters Lettuce Growth, the Survival of Zoonotic Bacteria on the Leaves and Associated Rhizosphere Microbiology. Front. Microbiol. 2016, 7, 565. [Google Scholar] [CrossRef] [PubMed]
- Hallmann, J.; Rodríguez-Kábana, R.; Kloepper, J. Chitin-mediated changes in bacterial communities of the soil, rhizosphere and within roots of cotton in relation to nematode control. Soil Biol. Biochem. 1999, 31, 551–560. [Google Scholar] [CrossRef]
- Sun, R.; Liu, C.; Zhang, H.; Wang, Q. Benzoylurea Chitin Synthesis Inhibitors. J. Agric. Food Chem. 2015, 63, 6847–6865. [Google Scholar] [CrossRef] [PubMed]
- Lewis, W.J.; van Lenteren, J.C.; Phatak, S.C.; Tumlinson, J.H. A total system approach to sustainable pest management. Proc. Natl. Acad. Sci. USA 1997, 94, 12243–12248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsurkan, M.V.; Voronkina, A.; Khrunyk, Y.; Wysokowski, M.; Petrenko, I.; Ehrlich, H. Progress in chitin analytics. Carbohydr. Polym. 2021, 252, 117204. [Google Scholar] [CrossRef] [PubMed]
- Merzendorfer, H.; Kim, H.S.; Chaudhari, S.S.; Kumari, M.; Specht, C.A.; Butcher, S.; Brown, S.J.; Manak, J.R.; Beeman, R.W.; Kramer, K.J.; et al. Genomic and proteomic studies on the effects of the insect growth regulator diflubenzuron in the model beetle species Tribolium castaneum. Insect Biochem. Mol. Biol. 2012, 42, 264–276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Eck, W. Mode of action of two benzoylphenyl ureas as inhibitors of chitin synthesis in insects. Insect Biochem. 1979, 9, 295–300. [Google Scholar] [CrossRef]
- Nijhout, H.F. Physiological Control of Molting in Insects. Am. Zoöl. 1981, 21, 631–640. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dave, U.; Somanader, E.; Baharlouei, P.; Pham, L.; Rahman, M.A. Applications of Chitin in Medical, Environmental, and Agricultural Industries. J. Mar. Sci. Eng. 2021, 9, 1173. https://doi.org/10.3390/jmse9111173
Dave U, Somanader E, Baharlouei P, Pham L, Rahman MA. Applications of Chitin in Medical, Environmental, and Agricultural Industries. Journal of Marine Science and Engineering. 2021; 9(11):1173. https://doi.org/10.3390/jmse9111173
Chicago/Turabian StyleDave, Uday, Esther Somanader, Parnian Baharlouei, Linh Pham, and M. Azizur Rahman. 2021. "Applications of Chitin in Medical, Environmental, and Agricultural Industries" Journal of Marine Science and Engineering 9, no. 11: 1173. https://doi.org/10.3390/jmse9111173
APA StyleDave, U., Somanader, E., Baharlouei, P., Pham, L., & Rahman, M. A. (2021). Applications of Chitin in Medical, Environmental, and Agricultural Industries. Journal of Marine Science and Engineering, 9(11), 1173. https://doi.org/10.3390/jmse9111173