Effects of Fine Sediment on Seagrass Meadows: A Case Study of Zostera muelleri in Pāuatahanui Inlet, New Zealand
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Location
2.2. Field Sampling
2.3. Laboratory Analysis
2.4. Hydrodynamic Model Results and Background Information for Study Sites
2.5. Data Analysis
3. Results
3.1. Substrate Conditions
3.2. Substrate Bulk Density, Organic Matter and Redox Potential
3.3. Porewater Chemistry
3.4. Photosynthetically Available Radiation (PAR)
3.5. Relationship of Habitat to Substrate Characteristics and PAR
3.6. Seagrass Traits
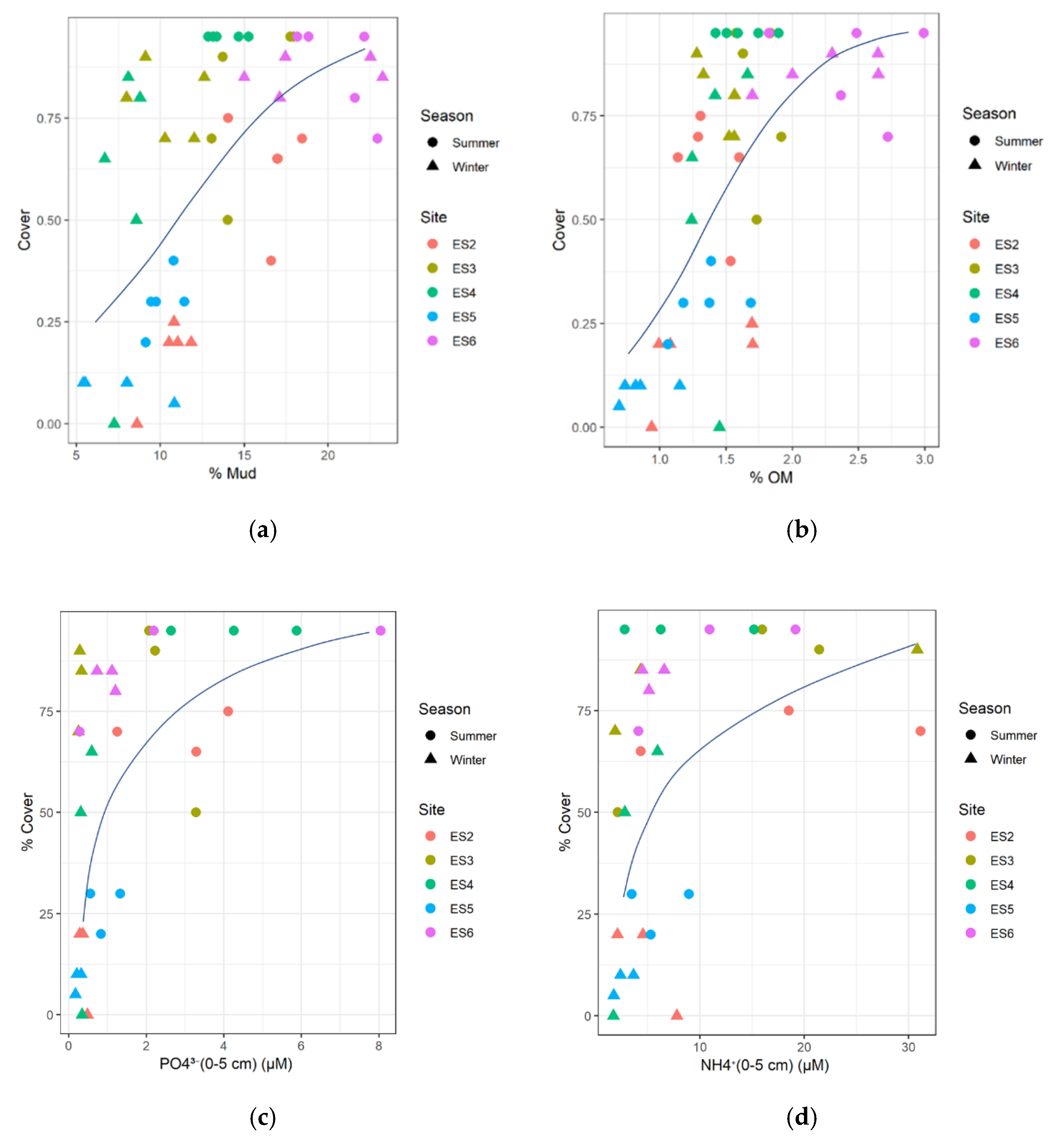
3.7. Seagrass Relationships with Substrate Conditions
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Waycott, M.; Duarte, C.M.; Carruthers, T.J.B.; Orth, R.J.; Dennison, W.C.; Olyarnik, S.; Calladine, A.; Fourqurean, J.W.; Heck, K.L.; Hughes, A.R.; et al. Accelerating loss of seagrasses across the globe threatens coastal ecosystems. Proc. Natl. Acad. Sci. USA 2009, 106, 12377–12381. [Google Scholar] [CrossRef]
- Orth, R.J.; Carruthers, T.J.B.; Dennison, W.C.; Duarte, C.M.; Fourqurean, J.W.; Heck, K.L.; Hughes, A.R.; Kendrick, G.A.; Kenworthy, W.J.; Olyarnik, S.; et al. A Global Crisis for Seagrass Ecosystems. Bioscience 2006, 56, 987–996. [Google Scholar] [CrossRef]
- Burkholder, J.M.; Tomasko, D.A.; Touchette, B.W. Seagrasses and eutrophication. J. Exp. Mar. Biol. Ecol. 2007, 350, 46–72. [Google Scholar] [CrossRef]
- Cunha, A.H.; Marbà, N.; Van Katwijk, M.M.; Pickerell, C.; Henriques, M.; Bernard, G.; Ferreira, M.A.; García, S.; Garmendia, J.M.; Manent, P. Changing Paradigms in Seagrass Restoration. Restor. Ecol. 2012, 20, 427–430. [Google Scholar] [CrossRef]
- Short, F.T.; Coles, R.G. Global Seagrass Research Methods; Elsevier: Amsterdam, The Netherlands, 2001. [Google Scholar]
- Duarte, C.M. Seagrass depth limits. Aquat. Bot. 1991, 40, 363–377. [Google Scholar] [CrossRef]
- Dennison, W.C.; Orth, R.J.; Moore, K.A.; Stevenson, J.C.; Carter, V.; Kollar, S.; Bergstrom, P.W.; Batiuk, R.A. Assessing Water Quality with Submersed Aquatic Vegetation. Bioscience 1993, 43, 86–94. [Google Scholar] [CrossRef]
- Duarte, C.M.; Marbà, N.; Santos, R. What may cause loss of seagrasses. In European Seagrasses: An Introduction to Monitoring and Management; Borum, J., Duarte, C.M., Krause-Jensen, D., Greve, T.M., Eds.; The M&MS Project: Copenhagen, Denmark, 2004; Available online: http://www.seagrasses.org (accessed on 18 May 2020).
- Ralph, P.J.; Gademann, R. Rapid light curves: A powerful tool to assess photosynthetic activity. Aquat. Bot. 2005, 82, 222–237. [Google Scholar] [CrossRef]
- Adams, M.P.; Hovey, R.K.; Hipsey, M.R.; Bruce, L.C.; Ghisalberti, M.; Lowe, R.; Gruber, R.K.; Ruiz-Montoya, L.; Maxwell, P.S.; Callaghan, D.P.; et al. Feedback between sediment and light for seagrass: Where is it important? Limnol. Oceanogr. 2016, 61, 1937–1955. [Google Scholar] [CrossRef]
- Chartrand, K.M.; Bryant, C.; Carter, A.B.; Ralph, P.J.; Rasheed, M.A. Light Thresholds to Prevent Dredging Impacts on the Great Barrier Reef Seagrass, Zostera muelleri ssp. capricorni. Front. Mar. Sci. 2016, 3, 106. [Google Scholar] [CrossRef]
- Collier, C.J.; Adams, M.P.; Langlois, L.; Waycott, M.; O’Brien, K.R.; Maxwell, P.; McKenzie, L.J. Thresholds for morphological response to light reduction for four tropical seagrass species. Ecol. Indic. 2016, 67, 358–366. [Google Scholar] [CrossRef]
- Kirkman, H.; Kuo, J. Pattern and process in southern Western Australian seagrasses. Aquat. Bot. 1990, 37, 367–382. [Google Scholar] [CrossRef]
- Fourqurean, J.W.; Rutten, L.M. The impact of Hurricane Georges on soft-bottom, back reef communities: Site-and species-specific effects in south Florida seagrass beds. Bull. Mar. Sci. 2004, 75, 239–257. [Google Scholar]
- Suykerbuyk, W.; Bouma, T.J.; Govers, L.L.; Giesen, K.; De Jong, D.J.; Herman, P.; Hendriks, A.J.; Van Katwijk, M.M. Surviving in Changing Seascapes: Sediment Dynamics as Bottleneck for Long-Term Seagrass Presence. Ecosystems 2015, 19, 296–310. [Google Scholar] [CrossRef]
- Short, F.T.; Wyllie-Echeverria, S. Natural and human-induced disturbance of seagrasses. Environ. Conserv. 1996, 23, 17–27. [Google Scholar] [CrossRef]
- Erftemeijer, P.L.A.; Lewis, R.R.R. Environmental impacts of dredging on seagrasses: A review. Mar. Pollut. Bull. 2006, 52, 1553–1572. [Google Scholar] [CrossRef] [PubMed]
- Cabaco, S.; Santos, R.; Duarte, C.M. The impact of sediment burial and erosion on seagrasses: A review. Estuar. Coast. Shelf Sci. 2008, 79, 354–366. [Google Scholar] [CrossRef]
- Benham, C.F.; Jackson, E.L.; Beavis, S.G. Tolerance of tropical seagrasses Zostera muelleri and Halophila ovalis to burial: Toward an understanding of threshold effects. Estuar. Coast. Shelf Sci. 2019, 218, 131–138. [Google Scholar] [CrossRef]
- Ralph, P.; Durako, M.; Enríquez, S.; Collier, C.J.; Doblin, M.A. Impact of light limitation on seagrasses. J. Exp. Mar. Biol. Ecol. 2007, 350, 176–193. [Google Scholar] [CrossRef]
- Moore, K.A.; Wetzel, R.L.; Orth, R.J. Seasonal pulses of turbidity and their relations to eelgrass (Zostera marina L.) survival in an estuary. J. Exp. Mar. Biol. Ecol. 1997, 215, 115–134. [Google Scholar] [CrossRef]
- Pedersen, T.M.; Gallegos, C.; Nielsen, S.L. Influence of near-bottom re-suspended sediment on benthic light availability. Estuar. Coast. Shelf Sci. 2012, 106, 93–101. [Google Scholar] [CrossRef]
- Bainbridge, Z.; Lewis, S.; Bartley, R.; Fabricius, K.; Collier, C.J.; Waterhouse, J.; Garzon-Garcia, A.; Robson, B.; Burton, J.; Wenger, A.S.; et al. Fine sediment and particulate organic matter: A review and case study on ridge-to-reef transport, transformations, fates, and impacts on marine ecosystems. Mar. Pollut. Bull. 2018, 135, 1205–1220. [Google Scholar] [CrossRef] [PubMed]
- Brodersen, K.E.; Hammer, K.J.; Schrameyer, V.; Floytrup, A.; Rasheed, M.A.; Ralph, P.J.; Kühl, M.; Pedersen, O. Sediment Resuspension and Deposition on Seagrass Leaves Impedes Internal Plant Aeration and Promotes Phytotoxic H2S Intrusion. Front. Plant Sci. 2017, 8, 657. [Google Scholar] [CrossRef] [PubMed]
- Terrados, J.; Duarte, C.M.; Kamp-Nielsen, L.; Agawin, N.; Gacia, E.; Lacap, D.; Fortes, M.; Borum, J.; Lubanski, M.; Greve, T. Are seagrass growth and survival constrained by the reducing conditions of the sediment? Aquat. Bot. 1999, 65, 175–197. [Google Scholar]
- Borum, J.; Pedersen, O.; Greve, T.M.; Frankovich, T.A.; Zieman, J.C.; Fourqurean, J.W.; Madden, C.J. The potential role of plant oxygen and sulphide dynamics in die-off events of the tropical seagrass, Thalassia testudinum. J. Ecol. 2005, 93, 148–158. [Google Scholar] [CrossRef]
- Koch, M.; Schopmeyer, S.; Kyhn-Hansen, C.; Madden, C. Synergistic effects of high temperature and sulfide on tropical seagrass. J. Exp. Mar. Biol. Ecol. 2007, 341, 91–101. [Google Scholar] [CrossRef]
- Brodersen, K.E.; Nielsen, D.A.; Ralph, P.J.; Kühl, M. Oxic microshield and local pH enhancement protects Zostera muelleri from sediment derived hydrogen sulphide. New Phytol. 2014, 205, 1264–1276. [Google Scholar] [CrossRef]
- Green, E.P.; Short, F.T. World Atlas of Seagrasses; University of California Press: Berkeley, CA, USA, 2003. [Google Scholar]
- Thrush, S.; Hewitt, J.; Cummings, V.; Ellis, J.; Hatton, C.; Lohrer, A.; Norkko, A. Muddy waters: Elevating sediment input to coastal and estuarine habitats. Front. Ecol. Environ. 2004, 2, 299–306. [Google Scholar] [CrossRef]
- Matheson, F.E.; Schwarz, A.-M. Growth responses of Zostera capricorni to estuarine sediment conditions. Aquat. Bot. 2007, 87, 299–306. [Google Scholar] [CrossRef]
- Morrison, M.A.; Lowe, M.; Parsons, D.; Usmar, N.; McLeod, I. A Review of Land-Based Effects on Coastal Fisheries and Supporting Biodiversity in New Zealand; New Zealand Aquatic Environment and Biodiversity Report No. 37; Ministry of Fisheries: Wellington, New Zealand, 2009; p. 100. Available online: http://fs.fish.govt.nz/Page.aspx?pk=113&dk=22003 (accessed on 6 July 2020).
- Inglis, G. Seagrasses of New Zealand. In World Atlas of Seagrasses; Green, E.P., Short, F.T., Eds.; University of California Press: Berkeley, CA, USA, 2003; pp. 148–157. [Google Scholar]
- Matheson, F.E.; Lundquist, C.; Gemmill, C.E.; Pilditch, C. New Zealand seagrass—More threatened than IUCN review indicates. Biol. Conserv. 2011, 144, 2749–2750. [Google Scholar] [CrossRef]
- Healy, W.B. Pāuatahanui Inlet: An environmental study; DSIR Information Series 141; Science Information Division: Wellington, New Zealand, 1980. [Google Scholar]
- Matheson, F.E.; Wadhwa, S. Seagrass in Porirua Harbour. In Preliminary Assessment of Restoration Potential. Report No. HAM2012-037; Prepared for Greater Wellington Regional Council; National Institute of Water and Atmospheric Research: Hamilton, New Zealand, 2012; p. 35. Available online: http://www.gw.govt.nz/assets/Seagrass-restoration-in-Porirua-Harbour.pdf (accessed on 8 March 2019).
- Blaschke, P.; Woods, J.; Forsyth, F. The Porirua Harbour and Its Catchment: A Literature Summary and Review; Porirua City Council: Porirua, New Zealand, 2010; p. 99.
- Milne, J.R.; Warr, S. Annual Coastal Monitoring Report for the Wellington Region; Report No GW/EMI-T-07/224; Environmental Monitoring and Investigation Department, Greater Wellington Regional Council: Wellington, New Zealand, 2007.
- Stevens, L.; Robertson, B. Porirua Harbour: Sediment Plate Monitoring 2015/16. In Report Prepared for Greater Wellington Regional Council; Wriggle Coastal Management: Nelson, New Zealand, 2016; p. 16. Available online: http://www.gw.govt.nz/assets/council-publications/Porirua-Harbour-Sediment-plate-monitoring-2016.pdf (accessed on 8 March 2018).
- Gibb, J.G.; Cox, G.J. Patterns and Rates of Sedimentation within Porirua Harbour; Porirua City Council: Porirua, New Zealand, 2009.
- Swales, A.; Bentley, S.; McGlone, M.; Ovenden, R.; Hermanspahn, N.; Budd, R.; Hill, A.; Pickmere, S.; Haskew, R.; Okey, M. Pauatahanui Estuary: Effects of Historical Catchment Landcover Changes on Estuary Sedimentation; National Institute of Water & Atmospheric Research Ltd.: Auckland, New Zealand, 2005. [Google Scholar]
- Townsend, M.; Lohrer, D. ANZECC Guidance for Estuary Sedimentation; Prepared for the Ministry for the Environment by NIWA; NIWA: Hamilton, New Zealand, 2015. [Google Scholar]
- Waycott, M.; Longstaff, B.J.; Mellors, J. Seagrass population dynamics and water quality in the Great Barrier Reef region: A review and future research directions. Mar. Pollut. Bull. 2005, 51, 343–350. [Google Scholar] [CrossRef]
- Duarte, C.M.; Fourqurean, J.W.; Krause-Jensen, D.; Olesen, B. Dynamics of Seagrass Stability and Change. In Seagrasses: Biology, Ecologyand Conservation; Larkum, A.W., Orth, R.J., Duarte, C.M., Eds.; Springer: Dordrecht, The Netherlands, 2007; pp. 271–294. Available online: https://doi.org/10.1007/978-1-4020-2983-7_11 (accessed on 1 May 2019).
- McGlathery, K.J.; Berg, P.; Marino, R. Using porewater profiles to assess nutrient availability in seagrass-vegetated carbonate sediments. Biogeochemistry 2001, 56, 239–263. [Google Scholar] [CrossRef]
- Thimijan, R.W.; Heins, R.D. Photometric, radiometric, and quantum light units of measure: A review of procedures for interconversion. HortScience 1993, 18, 818–822. [Google Scholar]
- Matheson, F.E.; Zabarte-Maeztu, I.; Mackay, G.; Middleton, C.; Oliver, M.; Griffiths, R.; Ovenden, R.; Davies-Colley, R.J. Estuary muddiness and seasonally low light constrains seagrass restoration. Mar. Pollut. Bull. (Under Review).
- Mook, D.H.; Hoskin, C.M. Organic determinations by ignition: Caution advised. Estuar. Coast. Shelf Sci. 1982, 15, 697–699. [Google Scholar] [CrossRef]
- Singer, J.; Anderson, J.; Ledbetter, M.; McCave, I.; Jones, K.; Wright, R. An assessment of analytical techniques for the size analysis of fine-grained sediments. J. Sediment. Res. 1988, 58, 534–543. [Google Scholar]
- Folk, R.L. Petrology of Sedimentary Rocks; Hemphill’s: Austin, TX, USA, 1968; p. 85. [Google Scholar]
- Porirua Harbour-Modelling for Whaitua Collaborative Modelling Group. Report 44800943/01 Prepared for GWRC for the Development of the Te Awarua-o’Porirua Whaitua Implementation Programme; DHI: Wellington, New Zealand, 2019. [Google Scholar]
- Zar, J.H. Biostatistical Analysis; Prentice-Hall: Englewood Cliffs, NY, USA, 1984. [Google Scholar]
- Flanigan, Y.S.; Critchley, C. Light response of D1 turnover and photosystem II efficiency in the seagrass Zostera capricorni. Planta 1996, 198, 319–323. [Google Scholar] [CrossRef]
- Enríquez, S.; Marbà, N.; Duarte, C.M.; Van Tussenbroek, B.I.; Reyes-Zavala, G. Effects of seagrass Thalassia testudinum on sediment redox. Mar. Ecol. Prog. Ser. 2001, 219, 149–158. [Google Scholar] [CrossRef][Green Version]
- Romero, J.; Perez, M.; Mateo-Pérez, M.A.; Sala, E. The belowground organs of the Mediterranean seagrass Posidonia oceanica as a biogeochemical sink. Aquat. Bot. 1994, 47, 13–19. [Google Scholar] [CrossRef]
- Gacia, E.; Duarte, C.M. Sediment Retention by a Mediterranean Posidonia oceanica Meadow: The Balance between Deposition and Resuspension. Estuar. Coast. Shelf Sci. 2001, 52, 505–514. [Google Scholar] [CrossRef]
- Holmer, M.; Andersen, F.Ø.; Nielsen, S.L.; Boschker, H.T. The importance of mineralization based on sulfate reduction for nutrient regeneration in tropical seagrass sediments. Aquat. Bot. 2001, 71, 1–17. [Google Scholar] [CrossRef]
- Gacia, E.; Duarte, C.M.; Marbà, N.; Terrados, J.; Kennedy, H.; Fortes, M.; Tri, N. Sediment deposition and production in SE-Asia seagrass meadows. Estuar. Coast. Shelf Sci. 2003, 56, 909–919. [Google Scholar] [CrossRef]
- Larkum, A.W.; Orth, R.J.; Duarte, C.M. Seagrasses; Springer: Dordrecht, The Netherlands, 2006; p. 691. [Google Scholar]
- Fourqurean, J.W.; Duarte, C.M.; Kennedy, H.; Marbà, N.; Holmer, M.; Mateo-Pérez, M.A.; Apostolaki, E.T.; Kendrick, G.A.; Krause-Jensen, D.; McGlathery, K.J.; et al. Seagrass ecosystems as a globally significant carbon stock. Nat. Geosci. 2012, 5, 505–509. [Google Scholar] [CrossRef]
- Brodersen, K.E.; Kühl, M.; Nielsen, D.A.; Pedersen, O.; Larkum, A.W.D. Rhizome, Root/Sediment Interactions, Aerenchyma and Internal Pressure Changes in Seagrasses. In Seagrasses of Australia; Larkum, A.W.D., Kendrick., G.A., Ralph, P.J., Eds.; Springer Cham: Dordrecht, The Netherlands, 2018; pp. 393–418. [Google Scholar]
- Lohrer, A.M.; Thrush, S.F.; Gibbs, M.M. Bioturbators enhance ecosystem function through complex biogeochemical interactions. Nature 2004, 431, 1092–1095. [Google Scholar] [CrossRef] [PubMed]
- Lohrer, A.M.; Halliday, N.; Thrush, S.F.; Hewitt, J.E.; Rodil, I.F. Ecosystem functioning in a disturbance-recovery context: Contribution of macrofauna to primary production and nutrient release on intertidal sandflats. J. Exp. Mar. Biol. Ecol. 2010, 390, 6–13. [Google Scholar] [CrossRef]
- Lohrer, A.M.; Townsend, M.; Hailes, S.F.; Rodil, I.F.; Cartner, K.; Pratt, D.; Hewitt, J.E. Influence of New Zealand cockles (Austrovenus stutchburyi) on primary productivity in sandflat-seagrass (Zostera muelleri) ecotones. Estuar. Coast. Shelf Sci. 2016, 181, 238–248. [Google Scholar] [CrossRef]
- Pérez, M.; Invers, O.; Ruiz, J.M.; Frederiksen, M.S.; Holmer, M. Physiological responses of the seagrass Posidonia oceanica to elevated organic matter content in sediments: An experimental assessment. J. Exp. Mar. Biol. Ecol. 2007, 344, 149–160. [Google Scholar] [CrossRef]
- Calleja, M.L.; Marbà, N.; Duarte, C.M. The relationship between seagrass (Posidonia oceanica) decline and sulfide porewater concentration in carbonate sediments. Estuar. Coast. Shelf Sci. 2007, 73, 583–588. [Google Scholar] [CrossRef]
- Krause-Jensen, D.; Carstensen, J.; Nielsen, S.L.; Dalsgaard, T.; Christensen, P.; Fossing, H.; Rasmussen, M. Sea bottom characteristics affect depth limits of eelgrass Zostera marina. Mar. Ecol. Prog. Ser. 2011, 425, 91–102. [Google Scholar] [CrossRef]
- Nielsen, A.H.; Lens, P.N.L.; Vollertsen, J.; Hvitvedjacobsen, T. Sulfide–iron interactions in domestic wastewater from a gravity sewer. Water Res. 2005, 39, 2747–2755. [Google Scholar] [CrossRef]
- Ruiz-Halpern, S.; Macko, S.A.; Fourqurean, J.W. The effects of manipulation of sedimentary iron and organic matter on sediment biogeochemistry and seagrasses in a subtropical carbonate environment. Biogeochemistry 2008, 87, 113–126. [Google Scholar] [CrossRef]
- Stoffers, P.; Glasby, G.P.; Plüger, W.L.; Walter, P. Reconnaissance survey of the mineralogy and geochemistry of some New Zealand lake and nearshore sediments. N. Z. J. Mar. Freshw. Res. 1983, 17, 461–480. [Google Scholar] [CrossRef]
- Glasby, G.P.; Moss, R.L.; Stoppers, P. Heavy metal pollution in Porirua Harbour, New Zealand. N. Z. J. Mar. Freshw. Res. 1990, 24, 233–237. [Google Scholar] [CrossRef]
- Macinnis-Ng, C.M.; Ralph, P.J. In situ impact of multiple pulses of metal and herbicide on the seagrass, Zostera capricorni. Aquat. Toxicol. 2004, 67, 227–237. [Google Scholar] [CrossRef] [PubMed]
- Hooper, K.L. The Impact of Stormwater Discharges on Freshwater, Marine Water and Marine Sediments and the Implications for Environmental Management of the Pauatahanui Inlet, Porirua, New Zealand: A Thesis Prepared in Partial Fulfilment of Master of Applied Science (Natural Resource Management). Master’s Thesis, Massey University, Palmerston North, New Zealand, 2002. [Google Scholar]
- Tay, H.; Bryan, K.R.; De Lange, W.; Pilditch, C.; De Lange, W. The hydrodynamics of the southern basin of Tauranga Harbour. N. Z. J. Mar. Freshw. Res. 2013, 47, 249–274. [Google Scholar] [CrossRef]
- Brun, F.G.; Hernández, I.; Vergara, J.; Peralta, G.; Perez-Llorens, J.L. Assessing the toxicity of ammonium pulses to the survival and growth of Zostera noltii. Mar. Ecol. Prog. Ser. 2002, 225, 177–187. [Google Scholar] [CrossRef]
- Govers, L.L.; De Brouwer, J.H.; Suykerbuyk, W.; Bouma, T.J.; Lamers, L.P.; Smolders, A.J.; Van Katwijk, M.M. Toxic effects of increased sediment nutrient and organic matter loading on the seagrass Zostera noltii. Aquat. Toxicol. 2014, 155, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Longstaff, B.J. Investigations into the Light Requirements of Seagrasses in Northeast Australia. Ph.D. Thesis, University of Queensland, Brisbane, Australia, 2003. [Google Scholar]
- Bulmer, R.H.; Kelly, S.; Jeffs, A.G. Light requirements of the seagrass, Zostera muelleri, determined by observations at the maximum depth limit in a temperate estuary, New Zealand. N. Z. J. Mar. Freshw. Res. 2016, 50, 183–194. [Google Scholar] [CrossRef]
- De Boer, W.F. Seagrass–sediment interactions, positive feedbacks and critical thresholds for occurrence: A review. Hydrobiologia 2007, 591, 5–24. [Google Scholar] [CrossRef]
- Vermaat, J.E.; Agawin, N.; Fortes, M.; Uri, J.; Duarte, C.; Marba, N.; Enriquez, S.; Van Vierssen, W. The capacity of seagrasses to survive increased turbidity and siltation: The significance of growth form and light use. Ambio 1997, 26, 499–504. [Google Scholar]
- Schwarz, A.-M. Contribution of photosynthetic gains during tidal emersion to production of Zostera capricorni in a North Island, New Zealand estuary. N. Z. J. Mar. Freshw. Res. 2004, 38, 809–818. [Google Scholar] [CrossRef]
- Drylie, T.; Lohrer, A.M.; Needham, H.R.; Bulmer, R.H.; Pilditch, C. Benthic primary production in emerged intertidal habitats provides resilience to high water column turbidity. J. Sea Res. 2018, 142, 101–112. [Google Scholar] [CrossRef]
- Marbà, N.; Cebrián, J.; Enríquez, S.; Duarte, C.M. Growth patterns of Western Mediterranean seagrasses: Species-specific responses to seasonal forcing. Mar. Ecol. Prog. Ser. 1996, 133, 203–215. [Google Scholar] [CrossRef]
- Walker, D.; McComb, A. Seagrass degradation in Australian coastal waters. Mar. Pollut. Bull. 1992, 25, 191–195. [Google Scholar] [CrossRef]
- Gladstone-Gallagher, R.V.; Hughes, R.W.; Douglas, E.J.; Pilditch, C. Biomass-dependent seagrass resilience to sediment eutrophication. J. Exp. Mar. Biol. Ecol. 2018, 501, 54–64. [Google Scholar] [CrossRef]
- Kenworthy, W.; Fonseca, M. Reciprocal transplant of the seagrass Zostera marina L. Effect of substrate on growth. Aquaculture 1977, 12, 197–213. [Google Scholar] [CrossRef]
- Short, F.T. Effects of sediment nutrients on seagrasses: Literature review and mesocosm experiment. Aquat. Bot. 1987, 27, 41–57. [Google Scholar] [CrossRef]
- Koch, E.W. Beyond Light: Physical, Geological, and Geochemical Parameters as Possible Submersed Aquatic Vegetation Habitat Requirements. Estuaries 2001, 24, 1–17. [Google Scholar] [CrossRef]
- Leschen, A.S.; Ford, K.H.; Evans, N.T. Successful Eelgrass (Zostera marina) Restoration in a Formerly Eutrophic Estuary (Boston Harbor) Supports the Use of a Multifaceted Watershed Approach to Mitigating Eelgrass Loss. Chesap. Sci. 2010, 33, 1340–1354. [Google Scholar] [CrossRef]
- Moksnes, P.-O.; Eriander, L.; Infantes, E.; Holmer, M. Local Regime Shifts Prevent Natural Recovery and Restoration of Lost Eelgrass Beds Along the Swedish West Coast. Chesap. Sci. 2018, 41, 1712–1731. [Google Scholar] [CrossRef]
- Edgar, G.; Shaw, C. The production and trophic ecology of shallow-water fish assemblages in southern Australia III. General relationships between sediments, seagrasses, invertebrates and fishes. J. Exp. Mar. Biol. Ecol. 1995, 194, 107–131. [Google Scholar] [CrossRef]
- Park, S.; Donald, R. Environment Bay of Plenty Tauranga Harbour Regional Plan Environmental Investigations; Ecology of Tauranga Harbour. Environment Bay of Plenty Environmental Report 94/8; Environment BOP: Whakatane, New Zealand, 1994; p. 177. [Google Scholar]
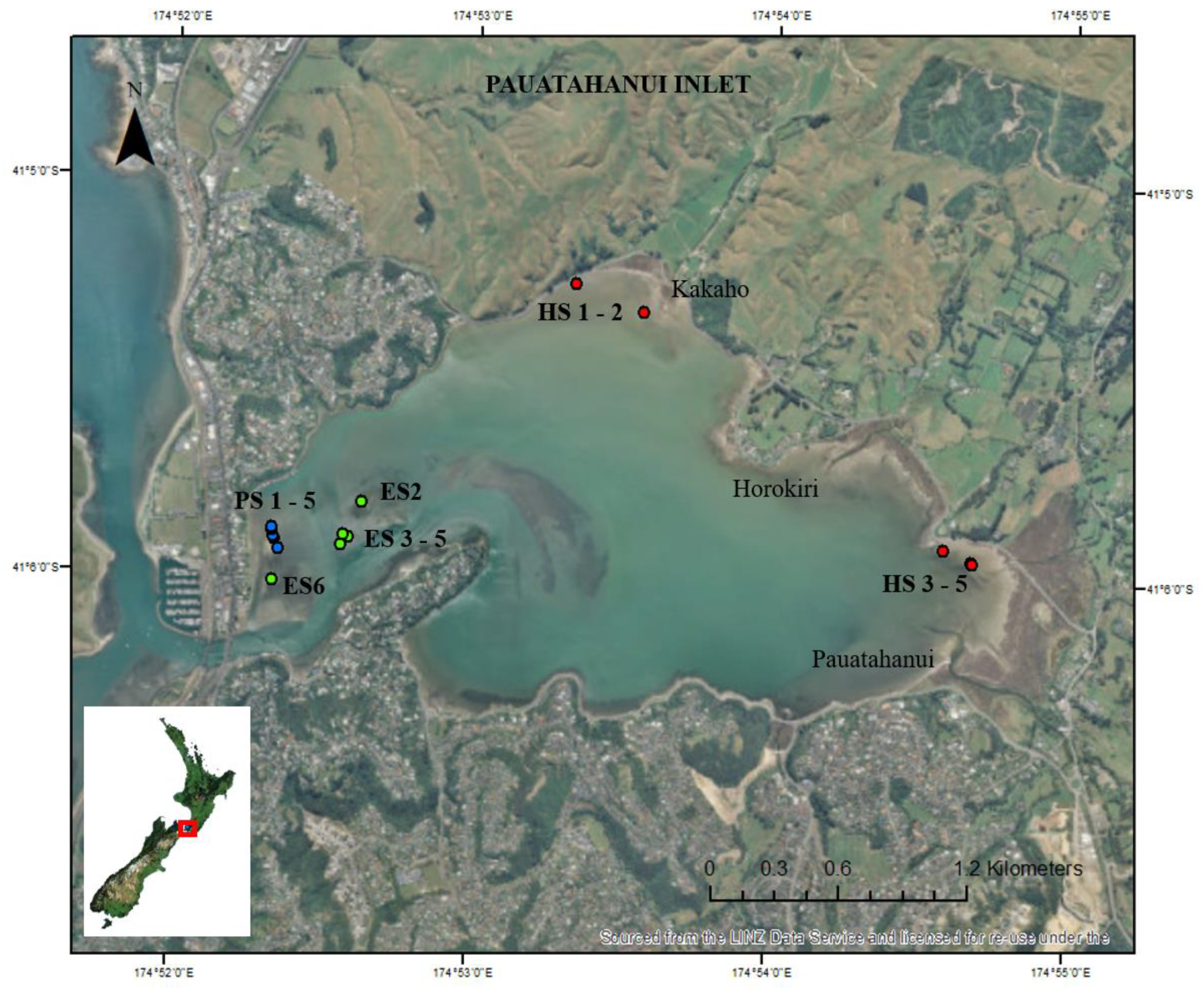
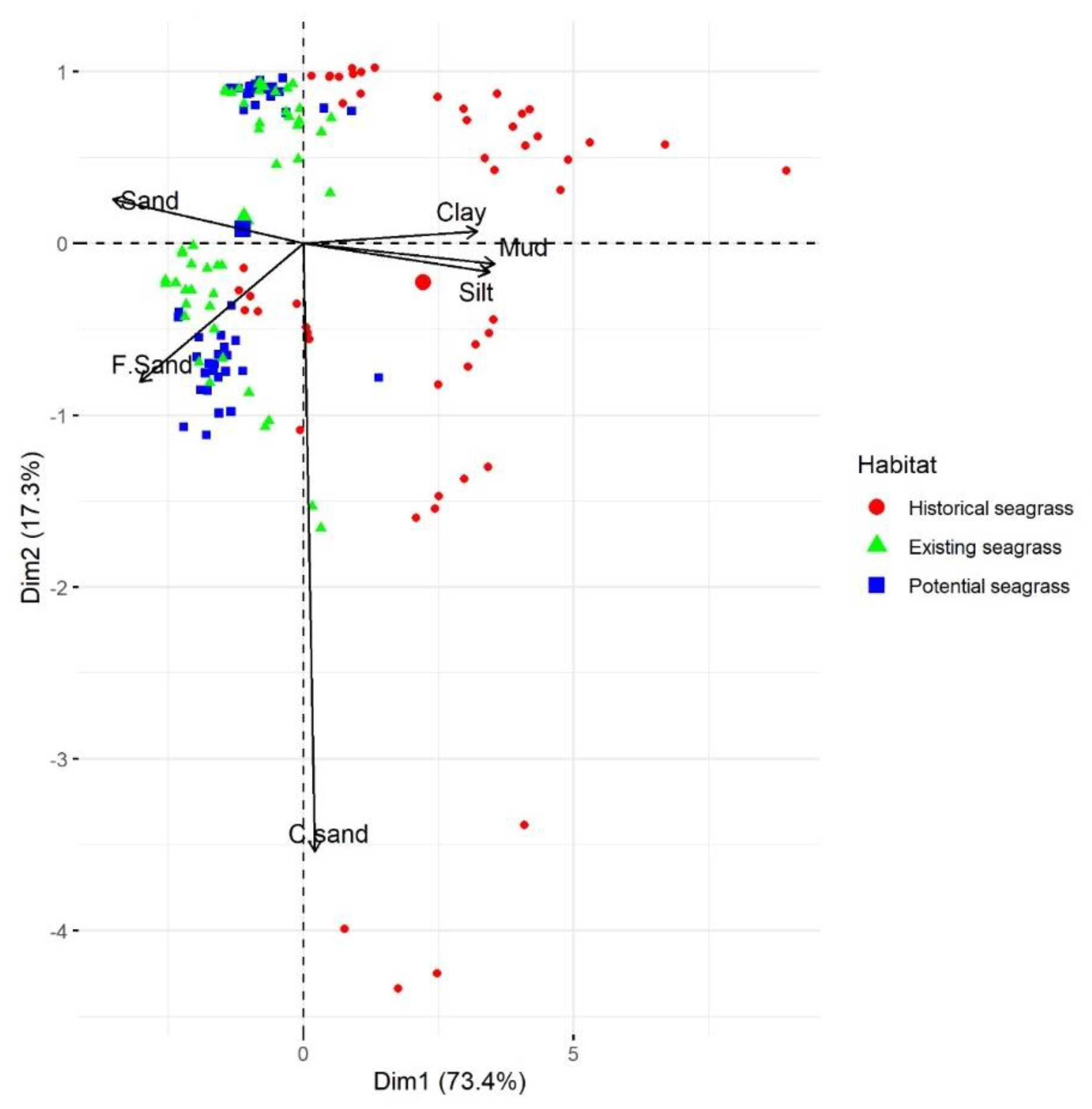
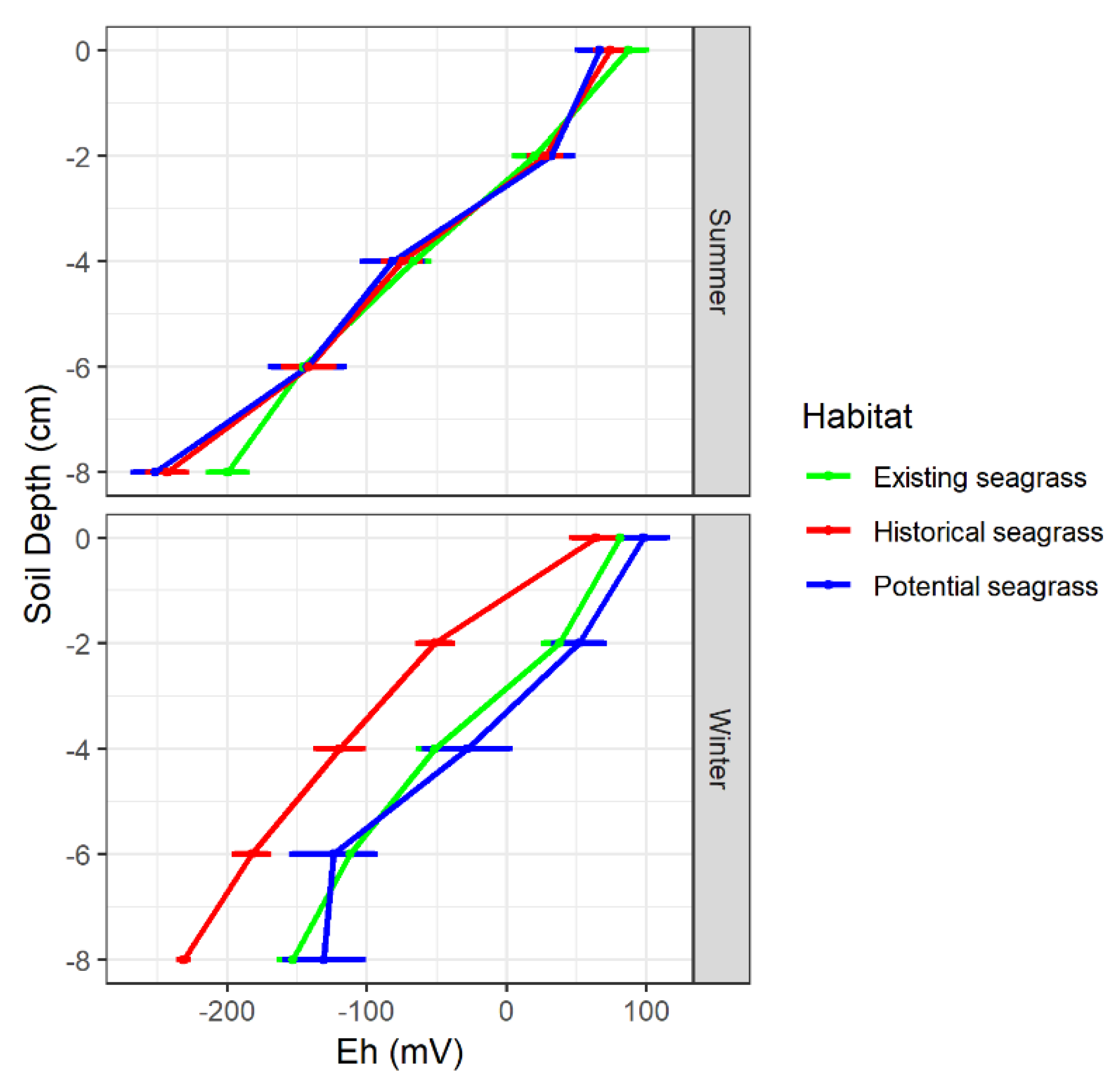
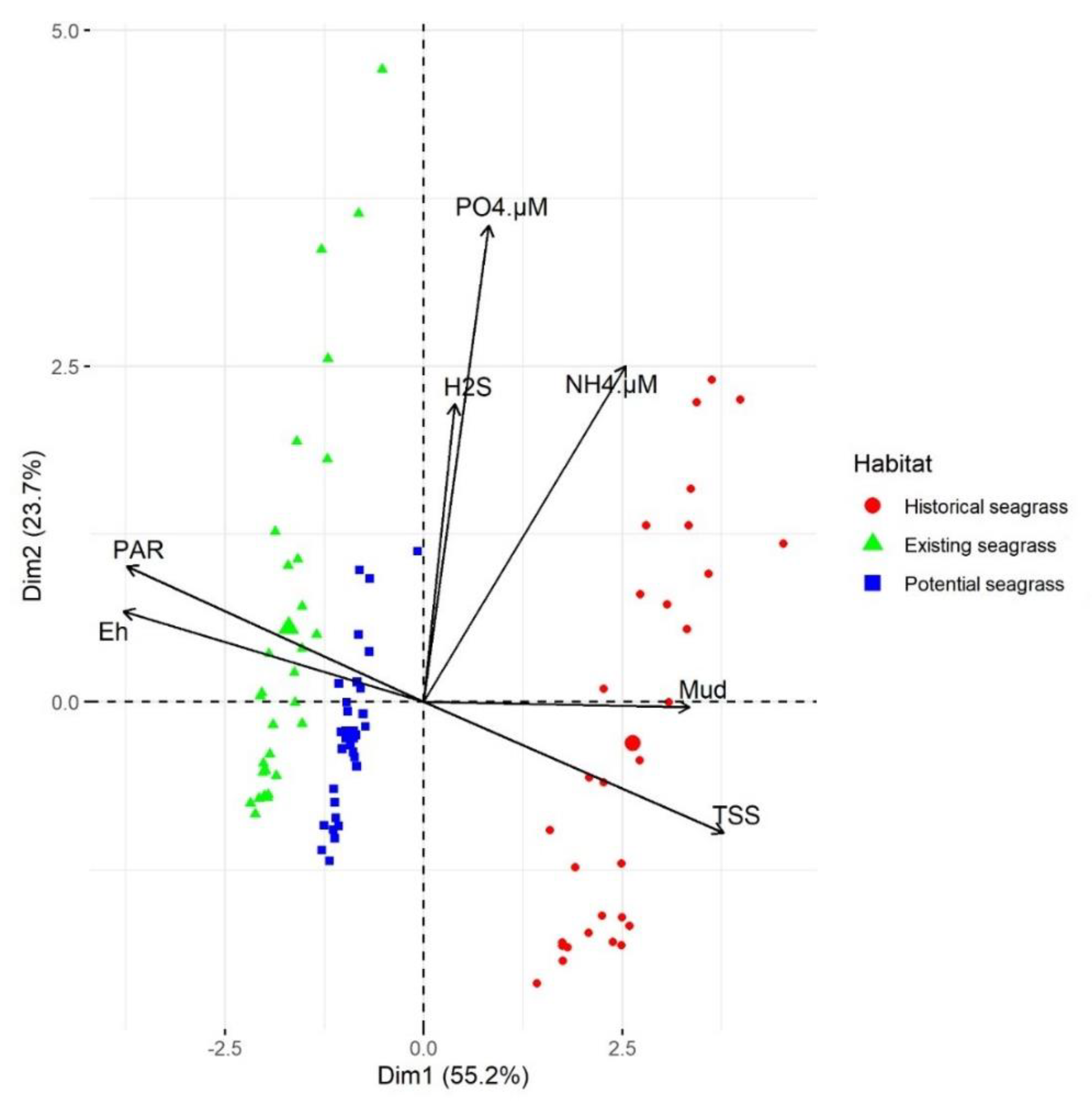
| Habitat | Current Velocity (m s−1) | Wave Period (s) | Salinity (PSU) | SSC (mg L−1) | Deposition (mm yr−1) |
|---|---|---|---|---|---|
| HS | 0.05 ± 0.003 a | 0.59 ± 0.010 a | 18.67 ± 0.76 a | 10.72 ± 0.570 b | 6.4 ± 2.10 c |
| ES | 0.15 ± 0.010 c | 0.66 ± 0.010 c | 25.63 ± 0.24 b | 2.35 ± 0.095 a | 3.6 ± 0.90 b |
| PS | 0.08 ± 0.005 b | 0.61 ± 0.001 b | 25.99 ± 0.01 b | 2.17 ± 0.020 a | 1.22 ± 0.02 a |
| Habitat | Season | Clay (%) | Silt (%) | Mud (%) | Fine Sand (%) | Coarse Sand (%) | Sand (%) | Bulk Density (g cm−3) | % OM 1 | Eh 2 (mV) Mean (0–10 cm) RZ 3 (0–4 cm) |
|---|---|---|---|---|---|---|---|---|---|---|
| HS | Winter | 6.3 ± 0.8 e | 27.5 ± 2.5 c | 33.7 ± 5.8 b | 48.1 ± 4.1 a | 17.8 ± 2.7 d | 65.9 ± 3.2 a | 1.32 ± 0.05 b | 1.79 ± 0.13 d | −79.8 ± 13.1 a Eh (RZ) −35.7 a’ |
| Summer | 8.0 ± 0.9 e | 30.8 ± 1.9 c | 38.8 ± 1.9 b | 49.9 ± 3.2 a | 11.3 ± 0.9 b | 61.2 ± 2.6 a | 1.63 ± 0.07 c | 1.60 ± 0.09 c | −71.6 ± 17.3 a Eh (RZ) 8.9 b’ | |
| ES | Winter | 2.0 ± 0.2 a | 9.1 ± 0.7 a | 11.1 ± 2.1 a | 78.5 ± 1.8 b | 10.1 ± 0.9 b | 88.7 ± 0.9 c | 1.01 ± 0.03 a | 1.53 ± 0.14 c | −33.8 ± 11.4 b Eh (RZ) 22.6 c’ |
| Summer | 3.3 ± 0.1 c | 12.1 ± 0.7 b | 15.3 ± 1.7 a | 73.7 ± 1.4 b | 10.5 ± 0.4 b | 84.7 ± 0.8 b | 1.28 ± 0.05 b | 1.71 ± 0.09 d | −22.3 ± 21.5 b Eh (RZ) 13.6 c’ | |
| PS | Winter | 2.6 ± 0.2 b | 7.7 ± 0.7 a | 10.3 ± 0.8 a | 74.8 ± 1.2 b | 12.6 ± 0.4 c | 87.4 ± 1 c | 1.09 ± 0.05 a | 1.39 ± 0.04 b | −14.4 ± 14.8 c Eh (RZ) 40.6 d’ |
| Summer | 4.7 ± 0.4 d | 8.9 ± 0.8 a | 13.6 ± 1.0 a | 76.9 ± 0.8 b | 9.5 ± 0.3 a | 86.4 ± 0.7 c | 1.16 ± 0.05 a | 1.14 ± 0.02 a | −41.6 ± 22.2 b Eh (RZ) 5.6 b’ |
| Habitat | Season | Sampling Depth | PO4³− (µM) | NH4+ (µM) | H₂S (µM) |
|---|---|---|---|---|---|
| HS | Winter | (0–5 cm) | 0.04 ± 0.004 a | 12.47 ± 3.49 b | 1.11± 0.29 b |
| (5–10 cm) | 0.27 ± 0.03 d | 61.43 ± 5.93 c | 2.69 ± 0.53 d | ||
| Summer | (0–5 cm) | 0.40 ± 0.09 e | 51.72 ± 21.23 c | 2.89 ± 0.18 d | |
| (5–10 cm) | 0.24 ± 0.07 d | 71.52 ± 15.14 c | 3.22 ± 0.12 e | ||
| ES | Winter | (0–5 cm) | 0.05 ± 0.01 b | 5.71 ± 1.89 a | 0.83 ± 0.22 a |
| (5–10 cm) | 0.29 ± 0.06 d | 22.12 ± 4.93 b | 3.23 ± 0.71 e | ||
| Summer | (0–5 cm) | 0.29 ± 0.04 d | 12.70 ± 3.05 b | 2.10 ± 0.19 c | |
| (5–10 cm) | 0.25 ± 0.04 d | 14.97 ± 5.21 b | 2.61 ± 0.23 d | ||
| PS | Winter | (0–5 cm) | 0.07 ± 0.01 b | 8.76 ± 1.33 a | 1.55 ± 0.41 b |
| (5–10 cm) | 0.12 ± 0.01 c | 21.84 ± 1.44 b | 1.62 ± 0.47 b | ||
| Summer | (0–5 cm) | 0.17 ± 0.04 c | 14.41 ± 2.77 b | 1.58 ± 0.21 b | |
| (5–10 cm) | 0.16 ± 0.01 c | 14.53 ± 3.16 b | 1.46 ± 0.14 b |
| Habitat | Season | PAR When Submerged (mol m−2 d−1) | PAR When Emerged (mol m−2 d−1) | Number of Days Where Total Mean PAR Was Below Compensation Irradiance (Days) |
|---|---|---|---|---|
| HS | Winter | 2.2 ± 0.2 a | 30.9 ± 2 a | 6 (2) b |
| Summer | 5.2 ± 0.2 d* | 60 ± 2.5 d* | 3 (1) b* | |
| ES | Winter | 4.4 ± 0.3 c | 44.3 ± 2 b | 3 (1) a |
| Summer | 4.0 ± 0.2 c | 46.1 ± 2 b | 1 (0) a* | |
| PS | Winter | 3.5 ± 0.2 b | 33.0 ± 2 a | 5 (0) a |
| Summer | 4.6 ± 0.2 c* | 53.1 ± 2.4 c* | 0 (0) a* |
| Trait | Season | Mean ± SE | Max | Min | Median |
|---|---|---|---|---|---|
| % Cover | Winter | 49.4 ± 7.1 a | 90 | 0 | 65 |
| Summer | 71.0 ± 5.2 b | 95 | 0 | 75 | |
| Shoot density (m−2) | Winter | 444 ± 51.7 a | 828 | 0 | 414 |
| Summer | 670 ± 51.7 b | 1401 | 159 | 700 | |
| AGB (g m−2) | Winter | 9.4 ± 1.6 a | 22.7 | 0.13 | 6.1 |
| Summer | 10.2 ± 1.0 a | 22.7 | 2.1 | 11.3 | |
| BGB (g m−2) | Winter | 87.8 ± 15.9 a | 302.5 | 1.9 | 46.9 |
| Summer | 150.7 ± 24.2 b | 488.8 | 4.6 | 111.5 | |
| BGB/AGB | Winter | 17.9 ± 4.5 a | 95.1 | 2.04 | 11.5 |
| Summer | 15.9 ±2.3 a | 44.8 | 0.7 | 13.4 | |
| Biomass (g m−2) | Winter | 97.2 ± 17 a | 321.6 | 2.5 | 64.5 |
| Summer | 161 ± 24.6 b | 500 | 10.9 | 122.9 |
| Study Location | Seagrass Species | Depth of Substrate Sampled | Means of Determination | Substrate Mud (%) Where Seagrass Thrives or Recommended for Transplanting | References |
|---|---|---|---|---|---|
| New Bedford Harbour, USA | Z. marina | 0–10 cm | x | <70% | [87] |
| Multiple locations, Review | Z. marina | x | x | 2.3–56.3% <20% | [88] |
| Boston Harbour, USA | Z. marina | 0–10 cm | x | <35% | [89] |
| At >57% transplant failed | |||||
| NW coast, Sweden | Z. marina | 0–9 cm | Wet sieving | Survival at 35% | [90] |
| no survival where >60% | |||||
| Western Port, Australia | Z. muelleri | x | x | 0.5–72% | [91] |
| Tauranga Harbour, New Zealand | Z. muelleri | 0–2 cm | x | <13 % silt threshold | [92] |
| Porirua Harbour, New Zealand | Z. muelleri | 0–10 cm | Laser analyzer | 8–23% | This study |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zabarte-Maeztu, I.; Matheson, F.E.; Manley-Harris, M.; Davies-Colley, R.J.; Oliver, M.; Hawes, I. Effects of Fine Sediment on Seagrass Meadows: A Case Study of Zostera muelleri in Pāuatahanui Inlet, New Zealand. J. Mar. Sci. Eng. 2020, 8, 645. https://doi.org/10.3390/jmse8090645
Zabarte-Maeztu I, Matheson FE, Manley-Harris M, Davies-Colley RJ, Oliver M, Hawes I. Effects of Fine Sediment on Seagrass Meadows: A Case Study of Zostera muelleri in Pāuatahanui Inlet, New Zealand. Journal of Marine Science and Engineering. 2020; 8(9):645. https://doi.org/10.3390/jmse8090645
Chicago/Turabian StyleZabarte-Maeztu, Iñigo, Fleur E. Matheson, Merilyn Manley-Harris, Robert J. Davies-Colley, Megan Oliver, and Ian Hawes. 2020. "Effects of Fine Sediment on Seagrass Meadows: A Case Study of Zostera muelleri in Pāuatahanui Inlet, New Zealand" Journal of Marine Science and Engineering 8, no. 9: 645. https://doi.org/10.3390/jmse8090645
APA StyleZabarte-Maeztu, I., Matheson, F. E., Manley-Harris, M., Davies-Colley, R. J., Oliver, M., & Hawes, I. (2020). Effects of Fine Sediment on Seagrass Meadows: A Case Study of Zostera muelleri in Pāuatahanui Inlet, New Zealand. Journal of Marine Science and Engineering, 8(9), 645. https://doi.org/10.3390/jmse8090645






