Lead, Mercury and Cadmium in Fish and Shellfish from the Indian Ocean and Red Sea (African Countries): Public Health Challenges
Abstract
1. Introduction
2. Heavy Metals and Their Effects on Humans
2.1. Cadmium
Cadmium Toxicity
2.2. Mercury
Methyl Mercury Toxicity (from Seafood)
2.3. Lead
2.4. Lead Toxicity
3. Heavy Metals Poisoning Treatment
4. Concentration of Cadmium, Mercury and Lead in Seafood from African Countries of the Indian Ocean and the Red Sea
5. Final Considerations and Recommendations
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Smith, M.D.; Roheim, C.A.; Crowder, L.B.; Halpern, B.S.; Turnipseed, M.; Anderson, J.L.; Asche, F.; Bourillón, L.; Guttormsen, A.G.; Khan, A. Sustainability and Global Seafood. Science 2010, 327, 784–786. [Google Scholar] [CrossRef] [PubMed]
- Saria, J.A. Assessment of Health Risks Associated with Concentrations of Heavy Metals in Fish from the Coast of Tanzania. J Multidiscip. Eng. Sci. Stud. 2016, 2, 1147–1153. [Google Scholar]
- Zalloua, P.; Hsu, Y.-H.; Terwedow, H.; Zang, T.; Wu, D.; Tang, G.; Li, Z.; Hong, X.; Azar, S.; Wang, B.; et al. Impact of seafood and fruit consumption on bone mineral density. Maturitas 2007, 56, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Vogiatzoglou, A.; Smith, A.D.; Nurk, E.; Berstad, P.; A Drevon, C.; Ueland, P.M.; E Vollset, S.; Tell, G.S.; Refsum, H.; Smith, A. Dietary sources of vitamin B-12 and their association with plasma vitamin B-12 concentrations in the general population: The Hordaland Homocysteine Study. Am. J. Clin. Nutr. 2009, 89, 1078–1087. [Google Scholar] [CrossRef]
- Ekweagwu, E.; Agwu, A.; Madukwe, E. The role of micronutrients in child health: A review of the literature. Afr. J. Biotechnol. 2008, 7, 3804–3810. [Google Scholar]
- Weber, D.N.; Connaughton, V.; Dellinger, J.A.; Klemer, D.; Udvadia, A.; Carvan, M.J. Selenomethionine reduces visual deficits due to developmental methylmercury exposures. Physiol. Behav. 2008, 93, 250–260. [Google Scholar] [CrossRef]
- Heaney, R.P. Long-latency deficiency disease: Insights from calcium and vitamin D. Am. J. Clin. Nutr. 2003, 78, 912–919. [Google Scholar] [CrossRef]
- Toyokuni, S. Role of iron in carcinogenesis: Cancer as a ferrotoxic disease. Cancer Sci. 2009, 100, 9–16. [Google Scholar] [CrossRef]
- Millward, D.J.; Layman, D.; Tomé, D.; Schaafsma, G. Protein quality assessment: Impact of expanding understanding of protein and amino acid needs for optimal health. Am. J. Clin. Nutr. 2008, 87, 1576S–1581S. [Google Scholar] [CrossRef]
- Ruxton, C.H.S.; Calder, P.C.; Reed, S.C.; Simpson, M.J.A. The impact of long-chainn-3 polyunsaturated fatty acids on human health. Nutr. Res. Rev. 2005, 18, 113–129. [Google Scholar] [CrossRef]
- Fatoki, O.; Mathabatha, S. An assessment of heavy metal pollution in the East London and Port Elizabeth harbours. Water SA 2004, 27, 233–240. [Google Scholar] [CrossRef]
- Hervé, R.P.; Andriamalala, R.; Yves, M.; Marcellin, R.; Christine, R.; Andriamandimbisoa, N. Assessment of heavy metals concentrations in coastal sediments in north-western cities of Madagascar. Afr. J. Environ. Sci. Technol. 2010, 4. [Google Scholar]
- El Nemr, A.; El-Said, G.F.; Khaled, A.; Ragab, S. Distribution and ecological risk assessment of some heavy metals in coastal surface sediments along the Red Sea, Egypt. Int. J. Sediment Res. 2016, 31, 164–172. [Google Scholar] [CrossRef]
- Madkour, H.A. Distribution and relationships of heavy metals in the giant clam (Tridacna maxima) and associated sediments from different sites in the Egyptian Red Sea coast. Egypt. J. Aquatic Res. 2005, 31, 45–59. [Google Scholar]
- Mamboya, F.A. Heavy Metal Contamination and Toxicity: Studies of Macroalgae from the Tanzanian Coast. Ph.D. Thesis, Botaniska Institutionen, Stockholm University, Stockholm, Sweden, December 2007. [Google Scholar]
- Usman, A.R.A.; Alkredaa, R.S.; Al-Wabel, M. Heavy metal contamination in sediments and mangroves from the coast of Red Sea: Avicennia marina as potential metal bioaccumulator. Ecotoxicol. Environ. Saf. 2013, 97, 263–270. [Google Scholar] [CrossRef]
- Dœlsch, E.; Van De Kerchove, V.; Macary, H.S.; Doelsch, E. Heavy metal content in soils of Réunion (Indian Ocean). Geoderma 2006, 134, 119–134. [Google Scholar] [CrossRef]
- Kamau, J.N. Heavy metal distribution and enrichment at Port-Reitz Creek, Mombasa. West. Indian Ocean J. Mar. Sci. 2002, 1, 65–70. [Google Scholar]
- Hamed, M.A.; Emara, A.M. Marine molluscs as biomonitors for heavy metal levels in the Gulf of Suez, Red Sea. J. Mar. Syst. 2006, 60, 220–234. [Google Scholar] [CrossRef]
- Biney, C.; Amuzu, A.; Calamari, D.; Kaba, N.; Mbome, I.; Naeve, H.; Ochumba, P.; Osibanjo, O.; Radegonde, V.; Saad, M. Review of Heavy Metals in the African Aquatic Environment. Ecotoxicol. Environ. Saf. 1994, 28, 134–159. [Google Scholar] [CrossRef]
- Dœlsch, E.; Macary, H.S.; Van De Kerchove, V.; Doelsch, E. Sources of very high heavy metal content in soils of volcanic island (La Réunion). J. Geochem. Explor. 2006, 88, 194–197. [Google Scholar] [CrossRef]
- Abu-Hilal, A.H.; Badran, M.M. Effect of pollution sources on metal concentration in sediment cores from the Gulf of Aqaba (red sea). Mar. Pollut. Bull. 1990, 21, 190–197. [Google Scholar] [CrossRef]
- Machiwa, J. Coastal Marine Pollution in Dar es Salaam (Tanzania) relative to Recommended Environmental Quality Targets for the Western Indian Ocean. West. Indian Ocean J. Mar. Sci. 2010, 9, 17–30. [Google Scholar]
- Mohammed, S.M. A Review of Water Quality and Pollution Studies in Tanzania. Ambio 2002, 31, 617. [Google Scholar] [CrossRef]
- Ramessur, R.T. Anthropogenic-driven changes with focus on the coastal zone of Mauritius, south-western Indian Ocean. Reg. Environ. Chang. 2002, 3, 99–106. [Google Scholar] [CrossRef]
- Martin, S.; Griswold, W. Human health effects of heavy metals. Environ. Sci. Technol. Briefs Citiz. 2009, 15, 1–6. [Google Scholar]
- Morais, S.; E Costa, F.G.; de Lourdes Pereira, M. Heavy metals and human health. In Environmental Health-Emerging Issues and Practice; IntechOpen: London, UK, 2012. [Google Scholar]
- Kirschvink, N.; Martin, N.; Fievez, L.; Smith, N.; Marlin, D.; Gustin, P. Airway inflammation in cadmium-exposed rats is associated with pulmonary oxidative stress and emphysema. Free. Radic. Res. 2006, 40, 241–250. [Google Scholar] [CrossRef] [PubMed]
- Rana, S.V.S.; Boora, P. Antiperoxidative mechanisms offered by selenium against liver injury caused by cadmium and mercury in rat. Bull. Environ. Contam. Toxicol. 1992, 48, 120–124. [Google Scholar] [CrossRef]
- De Vizcaya-Ruiz, A.; Barbier, O.; Ruiz-Ramos, R.; Cebrián, M.E. Biomarkers of oxidative stress and damage in human populations exposed to arsenic. Mutat. Res. Toxicol. Environ. Mutagen. 2009, 674, 85–92. [Google Scholar] [CrossRef]
- Wardman, P. Fluorescent and luminescent probes for measurement of oxidative and nitrosative species in cells and tissues: Progress, pitfalls, and prospects. Free Radic. Boil. Med. 2007, 43, 995–1022. [Google Scholar] [CrossRef]
- Lee, D.-H.; Lim, J.-S.; Song, K.; Boo, Y.C.; Jacobs, D.R. Graded Associations of Blood Lead and Urinary Cadmium Concentrations with Oxidative-Stress–Related Markers in the U.S. Population: Results from the Third National Health and Nutrition Examination Survey. Environ. Heal. Perspect. 2006, 114, 350–354. [Google Scholar] [CrossRef]
- Carneiro, M.F.H.; Grotto, D.; Barbosa, F. Inorganic and Methylmercury Levels in Plasma are Differentially Associated with Age, Gender, and Oxidative Stress Markers in a Population Exposed to Mercury Through Fish Consumption. J. Toxicol. Environ. Heal. Part A 2014, 77, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, Z.A.; Vu, T.T.; Zaman, K. Oxidative Stress as a Mechanism of Chronic Cadmium-Induced Hepatotoxicity and Renal Toxicity and Protection by Antioxidants. Toxicol. Appl. Pharmacol. 1999, 154, 256–263. [Google Scholar] [CrossRef] [PubMed]
- Lund, B.-O.; Miller, D.M.; Woods, J.S. Studies on Hg(II)-induced H2O2 formation and oxidative stress in vivo and in vitro in rat kidney mitochondria. Biochem. Pharmacol. 1993, 45, 2017–2024. [Google Scholar] [CrossRef]
- Yoshioka, N.; Nakashima, H.; Hosoda, K.; Eitaki, Y.; Shimada, N.; Omae, K. Urinary Excretion of an Oxidative Stress Marker, 8-hydroxyguanine (8-OH-Gua), among Nickel-cadmium Battery Workers. J. Occup. Heal. 2008, 50, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Goering, P.L.; Klaassen, C.D. Zinc-induced tolerance to cadmium hepatotoxicity. Toxicol. Appl. Pharmacol. 1984, 74, 299–307. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, J.; Iszard, M.; Andrews, G.; Palmiter, R.; Klaassen, C. Transgenic Mice That Overexpress Metallothionein-I Are Protected from Cadmium Lethality and Hepatotoxicity. Toxicol. Appl. Pharmacol. 1995, 135, 222–228. [Google Scholar] [CrossRef]
- Singhal, R.K.; Anderson, M.E.; Meister, A. Glutathione, a first line of defense against cadmium toxicity. FASEB J. 1987, 1, 220–223. [Google Scholar] [CrossRef]
- Bagchi, D.; Bagchi, M.; Hassoun, E.A.; Stohs, S.J. Cadmium-induced excretion of urinary lipid metabolites, DNA damage, glutathione depletion, and hepatic lipid peroxidation in sprague-dawley rats. Boil. Trace Element Res. 1996, 52, 143–154. [Google Scholar] [CrossRef]
- Goering, P.L. Lead-protein interactions as a basis for lead toxicity. NeuroToxicology 1993, 14, 45–60. [Google Scholar]
- Castro-González, M.; Méndez-Armenta, M. Heavy metals: Implications associated to fish consumption. Environ. Toxicol. Pharmacol. 2008, 26, 263–271. [Google Scholar] [CrossRef]
- Rahman, M.S.; Molla, A.H.; Saha, N.; Rahman, A. Study on heavy metals levels and its risk assessment in some edible fishes from Bangshi River, Savar, Dhaka, Bangladesh. Food Chem. 2012, 134, 1847–1854. [Google Scholar] [CrossRef] [PubMed]
- Jaishankar, M.; Tseten, T.; Anbalagan, N.; Mathew, B.B.; Beeregowda, K.N. Toxicity, mechanism and health effects of some heavy metals. Interdiscip. Toxicol. 2014, 7, 60–72. [Google Scholar] [CrossRef] [PubMed]
- Hutton, M.L.; Hutchinson, T.C. Lead, Mercury, Cadmium and Arsenic in the Environment; Hutchinson, T.C., Meema, K.M., Eds.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 1987. [Google Scholar]
- Hutton, M. Sources of cadmium in the environment. Ecotoxicol. Environ. Saf. 1983, 7, 9–24. [Google Scholar] [CrossRef]
- Fielder, R.; Dale, E. Cadmium and Its Compounds; HM Stationery Office: Richmond, UK, 1983; Volume 7. [Google Scholar]
- Thornton, I. Sources and pathways of cadmium in the environment. IARC Sci. Publ. 1992, 149–162. [Google Scholar]
- Bernard, A.; Lauwerys, R. Cadmium in human population. Cell. Mol. Life Sci. 1984, 40, 143–152. [Google Scholar] [CrossRef]
- Ros, J.; Slooff, W. Integrated Criteria Document. Cadmium; Technical Report; Rijksinstituut voor Volksgezondheid: Bilthoven, The Netherlands, December 2012. [Google Scholar]
- WHO, C. Environmental Health Criteria; World Health Organization: Geneva, Switzerland, 1992. [Google Scholar]
- Yu, M.-H. Environmental Toxicology. Environ. Toxicol. 2004. [Google Scholar]
- Goldberg, E.D. Baseline Studies of Pollutants in the Marine Environment and Research Recommendations; National Science Foundation: Alexandria, VA, USA, 1972.
- Soliman, Z.I. A study of heavy metals pollution in some aquatic organisms in Suez Canal in Port-Said Harbour. J. Appl. Sci. Res. 2006, 2, 657–663. [Google Scholar]
- Ray, S. Bioaccumulation of cadmium in marine organisms. Cell. Mol. Life Sci. 1984, 40, 14–23. [Google Scholar] [CrossRef]
- Ray, S.; McLeese, D. Factors affecting uptake of cadmium and other trace metals from marine sediments by some bottom-dwelling marine invertebrates. Dredged-Mater. Dispos. Ocean. Ocean 1983, 2. [Google Scholar]
- Nordberg, M. Studies on metallothionein and cadmium. Environ. Res. 1978, 15, 381–404. [Google Scholar] [CrossRef]
- Prozialeck, W.C.; Edwards, J.; Woods, J.M. The vascular endothelium as a target of cadmium toxicity. Life Sci. 2006, 79, 1493–1506. [Google Scholar] [CrossRef] [PubMed]
- Akesson, A.; Lundh, T.; Vahter, M.; Bjellerup, P.; Lidfeldt, J.; Nerbrand, C. Tubular and glomerular kidney effects in Swedish women with low environmental cadmium exposure. Environ. Health Perspect. 2005, 113, 1627–1631. [Google Scholar] [CrossRef] [PubMed]
- Satarug, S.; Nishijo, M.; Ujjin, P.; Vanavanitkun, Y.; Moore, M.R. Cadmium-induced nephropathy in the development of high blood pressure. Toxicol. Lett. 2005, 157, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Jelovcan, S.; Gutschi, A.; Kleinhappl, B.; Sedlmayr, P.; Barth, S.; Marth, E. Effects of low concentrations of cadmium on immunoglobulin E production by human B lymphocytes in vitro. Toxicol. 2003, 188, 35–48. [Google Scholar] [CrossRef]
- Hemdan, N.Y.A.; Emmrich, F.; Sack, U.; Wichmann, G.; Lehmann, J.; Adham, K.; Lehmann, I. The in vitro immune modulation by cadmium depends on the way of cell activation. Toxicol. 2006, 222, 37–45. [Google Scholar] [CrossRef]
- Di Gioacchino, M.; Petrarca, C.; Perrone, A.; Farina, M.; Sabbioni, E.; Hartung, T.; Martino, S.; Esposito, D.L.; Lotti, L.V.; Mariani-Costantini, R. Autophagy as an ultrastructural marker of heavy metal toxicity in human cord blood hematopoietic stem cells. Sci. Total. Environ. 2008, 392, 50–58. [Google Scholar] [CrossRef]
- Thompson, J.; Bannigan, J. Cadmium: Toxic effects on the reproductive system and the embryo. Reprod. Toxicol. 2008, 25, 304–315. [Google Scholar] [CrossRef]
- Friberg, L.; Elinder, C.; Kjellstrom, T.; Nordberg, G.F. Cadmium and Health: A Toxicological and Epidemiological Appraisal Volume II: Effects and Response; CRC Press: Boca Raton, FL, USA, 1985; p. 320. [Google Scholar]
- Johri, N.; Jacquillet, G.; Unwin, R. Heavy metal poisoning: The effects of cadmium on the kidney. BioMetals 2010, 23, 783–792. [Google Scholar] [CrossRef]
- Nordberg, G. Historical perspectives on cadmium toxicology. Toxicol. Appl. Pharmacol. 2009, 238, 192–200. [Google Scholar] [CrossRef]
- Bernard, A.; Stolte, H.; De Broe, M.E.; Mueller, P.W.; Mason, H.; Lash, L.H.; Fowler, B.A. Urinary biomarkers to detect significant effects of environmental and occupational exposure to nephrotoxins. IV. Current information on interpreting the health implications of tests. Ren. Fail. 1997, 19, 553–566. [Google Scholar] [CrossRef]
- Teeyakasem, W.; Nishijo, M.; Honda, R.; Satarug, S.; Swaddiwudhipong, W.; Ruangyuttikarn, W. Monitoring of cadmium toxicity in a Thai population with high-level environmental exposure. Toxicol. Lett. 2007, 169, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Nogawa, K.; Ishizaki, A.; Fukushima, M.; Shibata, I.; Hagino, N. Studies on the women with acquired fanconi syndrome observed in the Ichi River basin polluted by cadmium. Environ. Res. 1975, 10, 280–307. [Google Scholar] [CrossRef]
- Bhattacharyya, M.H.; A Sacco-Gibson, N.; Peterson, D.P. Cadmium-induced bone loss: Increased susceptibility in female beagles after ovariectomy. IARC Sci. Publ. 1992, 279–286. [Google Scholar]
- Goering, P.; Klaassen, C. Hepatotoxicology of copper, iron, and cadmium. Compr. Toxicol. 1997, 9, 389–406. [Google Scholar]
- E Rikans, L.; Yamano, T. Mechanisms of cadmium-mediated acute hepatotoxicity. J. Biochem. Mol. Toxicol. 2000, 14, 110–117. [Google Scholar] [CrossRef]
- Chou, I.N. Distinct cytoskeletal injuries induced by As, Cd, Co, Cr, and Ni compounds. Biomed. Environ. Sci. 1989, 2, 358–365. [Google Scholar] [PubMed]
- Li, W.; Kagan, H.; Chou, I. Alterations in Cytoskeletal Organization and Homeostasis of Cellular Thiols in Cadmium-Resistant Cells. Toxicol. Appl. Pharmacol. 1994, 126, 114–123. [Google Scholar] [CrossRef]
- Hussain, T.; Shukla, G.S.; Chandra, S.V. Effects of cadmium on superoxide dismutase and lipid peroxidation in liver and kidney of growing rats: In vivo and in vitro studies. Pharmacol. Toxicol. 1987, 60, 355–358. [Google Scholar] [CrossRef]
- Stacey, N.H.; Cantilena, L.R.; Klaassen, C.D. Cadmium toxicity and lipid peroxidation in isolated rat hepatocytes. Toxicol. Appl. Pharmacol. 1980, 53, 470–480. [Google Scholar] [CrossRef]
- Müller, L. Consequences of cadmium toxicity in rat hepatocytes: Mitochondrial dysfunction and lipid peroxidation. Toxicol. 1986, 40, 285–295. [Google Scholar] [CrossRef]
- Strubelt, O. Comparative studies on the toxicity of mercury, cadmium, and copper toward the isolated perfused rat liver. J. Toxicol. Environ. Heal. Part A 1996, 47, 267–283. [Google Scholar] [CrossRef] [PubMed]
- Hassoun, E. Cadmium-induced production of superoxide anion and nitric oxide, DNA single strand breaks and lactate dehydrogenase leakage in J774A.1 cell cultures. Toxicology 1996, 112, 219–226. [Google Scholar] [CrossRef]
- Habeebu, S.S.; Liu, J.; Klaassen, C.D. Cadmium-Induced Apoptosis in Mouse Liver. Toxicol. Appl. Pharmacol. 1998, 149, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Lemasters, J.J.V. Necrapoptosis and the mitochondrial permeability transition: Shared pathways to necrosis and apoptosis. Am. J. Physiol. Content 1999, 276, G1–G6. [Google Scholar] [CrossRef] [PubMed]
- Dudley, R.E.; Gammal, L.M.; Klaassen, C.D. Cadmium-induced hepatic and renal injury in chronically exposed rats: Likely role of hepatic cadmium-metallothionein in nephrotoxicity. Toxicol. Appl. Pharmacol. 1985, 77, 414–426. [Google Scholar] [CrossRef]
- Friberg, L. Cadmium and the kidney. Environ. Health Perspect. 1984, 54, 1–11. [Google Scholar] [CrossRef]
- Shaikh, Z.A.; Smith, J. The mechanisms of hepatic and renal metallothionein biosynthesis in cadmium-exposed rats. Chem. Interact. 1977, 19, 161–171. [Google Scholar] [CrossRef]
- Shaikh, Z.A.; Smith, J. The biosynthesis of metallothionein in rat liver and kidney after administration of cadmium. Chem. Interactions 1976, 15, 327–336. [Google Scholar] [CrossRef]
- Shaikh, Z.A.; Lucis, O.J. Cadmium and Zinc Binding in Mammalian Liver and Kidneys. Arch. Environ. Heal. Int. J. 1972, 24, 419–425. [Google Scholar] [CrossRef]
- Webb, M. Role of Metallothionein in Cadmium Metabolism; Springer Science and Business Media LLC.: Berlin/Heidelberg, Germany, 1986; Volume 80, pp. 281–337. [Google Scholar]
- Chan, H.-M.; Zhu, L.; Zhong, R.; Grant, D.; Goyer, R.; Cherian, M. Nephrotoxicity in Rats Following Liver Transplantation from Cadmium-Exposed Rats. Toxicol. Appl. Pharmacol. 1993, 123, 89–96. [Google Scholar] [CrossRef]
- Cherian, M.G.; Shaikh, Z.A. Metabolism of intravenously injected cadmium-binding protein. Biochem. Biophys. Res. Commun. 1975, 65, 863–869. [Google Scholar] [CrossRef]
- Foulkes, E. Renal tubular transport of cadmium-metallothionein. Toxicol. Appl. Pharmacol. 1978, 45, 505–512. [Google Scholar] [CrossRef]
- Squibb, K.S.; Ridlington, J.W.; Carmichael, N.G.; Fowler, B.A. Early cellular effects of circulating cadmium-thionein on kidney proximal tubules. Environ. Health Perspect. 1979, 28, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Nomiyama, K.; Nomiyama, H. Critical concentration of ‘unbound’ cadmium in the rabbit renal cortex. Cell. Mol. Life Sci. 1986, 42, 149. [Google Scholar] [CrossRef] [PubMed]
- Squibb, K.S.; Pritchard, J.B.; A Fowler, B. Cadmium-Metallothionein nephropathy: Relationships between ultrastructural/biochemical alterations and intracellular cadmium binding. J. Pharmacol. Exp. Ther. 1984, 229, 311–321. [Google Scholar] [PubMed]
- Goyer, R.A.; Miller, C.R.; Zhu, S.-Y.; Victery, W. Non-metallothionein-bound cadmium in the pathogenesis of cadmium nephrotoxicity in the rat. Toxicol. Appl. Pharmacol. 1989, 101, 232–244. [Google Scholar] [CrossRef]
- International Agency for Research on Can. IARC Monographs on the Evaluation of the Carcinogenic Risks to Humans: Beryllium, Cadmium, Mercury, and Exposures in the Glass Manufacturing Industry; World Health Organization: Geneva, Switzerland, 1993; Volume 58. [Google Scholar]
- Program, N.T. Ninth Report on Carcinogens; National Toxicology Program: Research Triangle Park, NC, USA, 2000.
- Achanzar, W.E.; Achanzar, K.B.; Lewis, J.G.; Webber, M.M.; Waalkes, M.P. Cadmium Induces c-myc, p53, and c-jun Expression in Normal Human Prostate Epithelial Cells as a Prelude to Apoptosis. Toxicol. Appl. Pharmacol. 2000, 164, 291–300. [Google Scholar] [CrossRef]
- Polla, B.S.; Stubbe, H.; Maridonneau-Parini, I.; Jacquier-Sarlin, M. Differential induction of stress proteins and functional effects of heat shock in human phagocytes. Inflammation 1995, 19, 363–378. [Google Scholar] [CrossRef]
- Somji, S.; Todd, J.H.; Sens, M.A.; Garrett, S.H.; Sens, D.A. Expression of the constitutive and inducible forms of heat shock protein 70 in human proximal tubule cells exposed to heat, sodium arsenite, and CdCl (2). Environ. Health Perspect. 1999, 107, 887–893. [Google Scholar]
- Garcia-Morales, P.; Saceda, M.; Kenney, N.; Kim, N.; Salomon, D.S.; Gottardis, M.M.; Solomon, H.B.; Sholler, P.F.; Jordan, V.C.; Martin, M.B. Effect of cadmium on estrogen receptor levels and estrogen-induced responses in human breast cancer cells. J. Boil. Chem. 1994, 269, 16896–16901. [Google Scholar]
- Hatcher, E.L.; Chen, Y.; Kang, Y. Cadmium resistance in A549 cells correlates with elevated glutathione content but not antioxidant enzymatic activities. Free. Radic. Boil. Med. 1995, 19, 805–812. [Google Scholar] [CrossRef]
- Schwartz, G.G.; Reis, I.M. Is cadmium a cause of human pancreatic cancer? Cancer Epidemiol. Biomarkers Prev. 2000, 9, 139–145. [Google Scholar]
- Pesch, B.; Haerting, J.; Ranft, U.; Klimpel, A.; Oelschlägel, B.; Schill, W. Occupational risk factors for urothelial carcinoma: Agent-specific results from a case-control study in Germany. Int. J. Epidemiol. 2000, 29, 238–247. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Mao, Y.; White, K. The Canadian Cancer Registries Epidemiology Research Group Renal cell carcinoma and occupational exposure to chemicals in Canada. Occup. Med. 2002, 52, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Keeler, G.; Glinsorn, G.; Pirrone, N. Particulate mercury in the atmosphere: Its significance, transport, transformation and sources. Water Air Soil Pollut. 1995, 80, 159–168. [Google Scholar] [CrossRef]
- Mason, R.P. Mercury emissions from natural processes and their importance in the global mercury cycle. In Mercury Fate and Transport in the Global Atmosphere; Springer Science and Business Media LLC.: Berlin/Heidelberg, Germany, 2009; pp. 173–191. [Google Scholar]
- Pirrone, N.; Costa, P.; Pacyna, J.; Ferrara, R. Mercury emissions to the atmosphere from natural and anthropogenic sources in the Mediterranean region. Atmos. Environ. 2001, 35, 2997–3006. [Google Scholar] [CrossRef]
- Pacyna, E.G.; Pacyna, J.M.; Steenhuisen, F.; Wilson, S. Global anthropogenic mercury emission inventory for 2000. Atmos. Environ. 2006, 40, 4048–4063. [Google Scholar] [CrossRef]
- Pirrone, N.; Cinnirella, S.; Feng, X.; Finkelman, R.B.; Friedli, H.R.; Leaner, J.; Mason, R.P.; Mukherjee, A.B.; Stracher, G.B.; Streets, D.G.; et al. Global mercury emissions to the atmosphere from anthropogenic and natural sources. Atmos. Chem. Phys. Discuss. 2010, 10, 5951–5964. [Google Scholar] [CrossRef]
- EFSA Panel on Contaminants in the Food Chain (CONTAM). Scientific Opinion on the risk for public health related to the presence of mercury and methylmercury in food. EFSA J. 2012, 10, 2985. [Google Scholar]
- Kuban, P.; Houserová, P.; Kuban, P.; Hauser, P.C.; Kubáň, V. Mercury speciation by CE: A review. Electrophoresis 2007, 28, 58–68. [Google Scholar] [CrossRef]
- Paquette, K.E.; Helz, G.R. Inorganic Speciation of Mercury in Sulfidic Waters: The Importance of Zero-Valent Sulfur. Environ. Sci. Technol. 1997, 31, 2148–2153. [Google Scholar] [CrossRef]
- FAO/WHO (Food and Agriculture Organization of the United Nations/World Health. Safety evaluation of certain food additives and contaminants. Methylmercury. WHO Food Addit. Ser. 2011, 63, 605–684. [Google Scholar]
- Bloom, N.S. On the Chemical Form of Mercury in Edible Fish and Marine Invertebrate Tissue. Can. J. Fish. Aquat. Sci. 1992, 49, 1010–1017. [Google Scholar] [CrossRef]
- Silbernagel, S.M.; Carpenter, D.O.; Gilbert, S.G.; Gochfeld, M.; Groth, E.; Hightower, J.; Schiavone, F.M. Recognizing and Preventing Overexposure to Methylmercury from Fish and Seafood Consumption: Information for Physicians. J. Toxicol. 2011, 2011, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Harris, H.H. The Chemical Form of Mercury in Fish. Science 2003, 301, 1203. [Google Scholar] [CrossRef] [PubMed]
- Ratcliffe, H.E.; Swanson, G.M.; Fischer, L.J. Human exposure to mercury: A critical assessment of the evidence of adverse health effects. J. Toxicol. Environ. Health 1996, 49, 221–270. [Google Scholar] [CrossRef] [PubMed]
- Franco, J.L.; Braga, H.; Stringari, J.; Missau, F.C.; Posser, T.; Mendes, B.G.; Leal, R.; Dos Santos, A.R.S.; Dafre, A.L.; Pizzolatti, M.G.; et al. Mercurial-Induced Hydrogen Peroxide Generation in Mouse Brain Mitochondria: Protective Effects of Quercetin. Chem. Res. Toxicol. 2007, 20, 1919–1926. [Google Scholar] [CrossRef]
- Baraldi, M.; Zanoli, P.; Tascedda, F.; Blom, J.M.C.; Brunello, N. Cognitive deficits and changes in gene expression of NMDA receptors after prenatal methylmercury exposure. Environ. Heal. Perspect. 2002, 110, 855–858. [Google Scholar] [CrossRef]
- Harada, M. Minamata Disease: Methylmercury Poisoning in Japan Caused by Environmental Pollution. Crit. Rev. Toxicol. 1995, 25, 1–24. [Google Scholar] [CrossRef]
- Castoldi, A.F.; Coccini, T.; Ceccatelli, S.; Manzo, L. Neurotoxicity and molecular effects of methylmercury. Brain Res. Bull. 2001, 55, 197–203. [Google Scholar] [CrossRef]
- Bose-O’Reilly, S.; McCarty, K.M.; Steckling, N.; Lettmeier, B. Mercury exposure and children’s health. Curr. Probl. Pediatric Adolesc. Health Care 2010, 40, 186–215. [Google Scholar] [CrossRef] [PubMed]
- Zalups, R.K. Molecular interactions with mercury in the kidney. Pharmacol. Rev. 2000, 52, 113–144. [Google Scholar] [PubMed]
- Ballatori, N. Transport of toxic metals by molecular mimicry. Environ. Heal. Perspect. 2002, 110, 689–694. [Google Scholar] [CrossRef] [PubMed]
- Bando, I.; Reus, M.I.S.; Andrés, D.; Cascales, M. Endogenous antioxidant defence system in rat liver following mercury chloride oral intoxication. J. Biochem. Mol. Toxicol. 2005, 19, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Rooney, J. The role of thiols, dithiols, nutritional factors and interacting ligands in the toxicology of mercury. Toxicology 2007, 234, 145–156. [Google Scholar] [CrossRef] [PubMed]
- Papp, L.V.; Lu, J.; Holmgren, A.; Khanna, K.K. From Selenium to Selenoproteins: Synthesis, Identity, and Their Role in Human Health. Antioxidants Redox Signal. 2007, 9, 775–806. [Google Scholar] [CrossRef]
- Carvalho, C.M.; Chew, E.-H.; Hashemy, S.I.; Lu, J.; Holmgren, A. Inhibition of the human thioredoxin system a molecular mechanism of mercury toxicity. J. Biol. Chem. 2008, 283, 11913–11923. [Google Scholar] [CrossRef]
- Prati, M.; Gornati, R.; Boracchi, P.; Biganzoli, E.M.; Fortaner, S.; Pietra, R.; Sabbioni, E.; Bernardini, G. A comparative study of the toxicity of mercury dichloride and methylmercury, assayed by the Frog Embryo Teratogenesis Assay--Xenopus (FETAX). Altern. Lab. Anim. 2002, 30, 23–32. [Google Scholar] [CrossRef]
- Counter, S.; Buchanan, L.H. Mercury exposure in children: A review. Toxicol. Appl. Pharmacol. 2004, 198, 209–230. [Google Scholar] [CrossRef]
- Davidson, P.W.; Myers, G.J.; Weiss, B. Mercury exposure and child development outcomes. Pediatr. 2004, 113, 1023–1029. [Google Scholar]
- Boffetta, P.; Merler, E.; Vainio, H. Carcinogenicity of mercury and mercury compounds. Scand. J. Work. Environ. Heal. 1993, 19, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Lopez, M.E.C.; Macêdo, G.L.; Pereira, S.I.; Arrifano, G.D.P.; Picanço-Diniz, D.L.; Nascimento, J.L.M.D.; Herculano, A. Mercury and human genotoxicity: Critical considerations and possible molecular mechanisms. Pharmacol. Res. 2009, 60, 212–220. [Google Scholar] [CrossRef] [PubMed]
- Leonard, A.; Jacquet, P.; Lauwerys, R. Mutagenicity and teratogenicity of mercury compounds. Mutat. Res. Genet. Toxicol. 1983, 114, 1–18. [Google Scholar] [CrossRef]
- E Ariza, M.; Holliday, J.; Williams, M.V. Mutagenic effect of mercury (II) in eukaryotic cells. Vivo 1994, 8, 559–563. [Google Scholar]
- Tchounwou, P.B.; Ayensu, W.K.; Ninashvili, N.; Sutton, D. Review: Environmental exposure to mercury and its toxicopathologic implications for public health. Environ. Toxicol. 2003, 18, 149–175. [Google Scholar] [CrossRef] [PubMed]
- Flegal, A.R. Lead in tropical marine systems: A review. Sci. Total. Environ. 1986, 58, 1–8. [Google Scholar] [CrossRef]
- EFSA Panel on Contaminants in the Food Chain. Scientific Opinion on lead in food. EFSA J. 2010, 8, 1570. [Google Scholar] [CrossRef]
- Tukker, A.; Buijst, H.; van Oers, L.; van der Voet, E. Risks to health and the environment related to the use of lead in products. In TNO Strategy, Technology and Policy; TNO: Delft, The Netherlands, 2001. [Google Scholar]
- Lee, T.-H.; Jiang, S.-J. Speciation of lead compounds in fish by capillary electrophoresis-inductively coupled plasma mass spectrometry. J. Anal. At. Spectrom. 2005, 20, 1270. [Google Scholar] [CrossRef]
- Chen, Y.; Huang, L.; Wu, W.; Ruan, Y.; Wu, Z.; Xue, Z.; Fu, F. Speciation analysis of lead in marine animals by using capillary electrophoresis couple online with inductively coupled plasma mass spectrometry. Electrophoresis 2013, 35, 1346–1352. [Google Scholar] [CrossRef]
- Chang, L.-F.; Jiang, S.-J.; Sahayam, A. Speciation analysis of mercury and lead in fish samples using liquid chromatography–inductively coupled plasma mass spectrometry. J. Chromatogr. A 2007, 1176, 143–148. [Google Scholar] [CrossRef]
- Sakai, T. Biomarkers of Lead Exposure. Ind. Heal. 2000, 38, 127–142. [Google Scholar] [CrossRef]
- O’Flaherty, E.J. Physiologically based models for bone-seeking elements. V. Lead absorption and disposition in childhood. Toxicol. Appl. Pharmacol. 1995, 131, 297–308. [Google Scholar] [CrossRef]
- Lerda, D. Study of sperm characteristics in persons occupationally exposed to lead. Am. J. Ind. Med. 1992, 22, 567–571. [Google Scholar] [CrossRef] [PubMed]
- Braunstein, G.D.; Dahlgren, J.; Loriaux, D.L. Hypogonadism in chronically lead-poisoned men. Infertil. 1978, 1, 33–51. [Google Scholar]
- Assennato, G.; Paci, C.; Baser, M.E.; Molinini, R.; Candela, R.G.; Altamura, B.M.; Giorgino, R. Sperm Count Suppression without Endocrine Dysfunction in Lead-Exposed Men. Arch. Environ. Heal. Int. J. 1987, 42, 124–127. [Google Scholar] [CrossRef] [PubMed]
- Telisman, S.; Cvitković, P.; Jurasović, J.; Pizent, A.; Gavella, M.; Rocić, B. Semen quality and reproductive endocrine function in relation to biomarkers of lead, cadmium, zinc, and copper in men. Environ. Health Perspect. 2000, 108, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Apostoli, P.; Kiss, P.; Porru, S.; Bonde, J.P.; Vanhoorne, M. Male reproductive toxicity of lead in animals and humans. ASCLEPIOS Study Group. Occup. Environ. Med. 1998, 55, 364–374. [Google Scholar] [CrossRef]
- Hogstedt, C.; Hane, M.; Agrell, A.; Bodin, L. Neuropsychological test results and symptoms among workers with well-defined long-term exposure to lead. Occup. Environ. Med. 1983, 40, 99–105. [Google Scholar] [CrossRef]
- Mantere, P.; Hänninen, H.; Hernberg, S.; Luukkonen, R. A prospective follow-up study on psychological effects in workers exposed to low levels of lead. Scand. J. Work. Environ. Heal. 1984, 10, 43–50. [Google Scholar] [CrossRef]
- Campara, P.; D’Andrea, F.; Micciolo, R.; Savonitto, C.; Tansella, M.; Zimmermann-Tansella, C. Psychological performance of workers with blood-lead concentration below the current threshold limit value. Int. Arch. Occup. Environ. Heal. 1984, 53, 233–246. [Google Scholar] [CrossRef]
- Lead, W.I. Environmental Health Criteria 165: International Programme on Chemical Safety; World Health Organization: Geneva, Switzerland, 1995. [Google Scholar]
- Loghman-Adham, M. Renal effects of environmental and occupational lead exposure. Environ. Health Perspect. 1997, 105, 928–939. [Google Scholar] [CrossRef]
- Ehrlich, R.; Robins, T.; Jordaan, E.; Miller, S.; Mbuli, S.; Selby, P.; Wynchank, S.; Cantrell, A.; De Broe, M.; D’Haese, P.; et al. Lead absorption and renal dysfunction in a South African battery factory. Occup. Environ. Med. 1998, 55, 453–460. [Google Scholar] [CrossRef] [PubMed]
- Goyer, R. Mechanisms of lead and cadmium nephrotoxicity. Toxicol. Lett. 1989, 46, 153–162. [Google Scholar] [CrossRef]
- Gerhardsson, L.; Chettle, D.R.; Englyst, V.; Nordberg, G.F.; Nyhlin, H.; Scott, M.C.; Todd, A.C.; Vesterberg, O. Kidney effects in long term exposed lead smelter workers. Occup. Environ. Med. 1992, 49, 186–192. [Google Scholar] [CrossRef] [PubMed]
- Coscia, G.C.; Discalzi, G.; Ponzetti, C. Immunological aspects of occupational lead exposure. La Med. del Lav. 1987, 78, 360. [Google Scholar]
- Ewers, U.; Stiller-Winkler, R.; Idel, H. Serum immunoglobulin, complement C3, and salivary IgA levels in lead workers. Environ. Res. 1982, 29, 351–357. [Google Scholar] [CrossRef]
- Gidlow, D. Lead toxicity. Occup. Med. 2015, 65, 770. [Google Scholar] [CrossRef][Green Version]
- Markowitz, M. Lead Poisoning. Pediatr. Rev. 2000, 21, 327–335. [Google Scholar] [CrossRef]
- Philip, A.T.; Gerson, B. Lead Poisoning-Part I: Incidence, Etiology, and Toxicokinetics. Clin. Lab. Med. 1994, 14, 423–444. [Google Scholar] [CrossRef]
- Rabinowitz, M.B. Toxicokinetics of bone lead. Environ. Health Perspect. 1991, 91, 33–37. [Google Scholar] [CrossRef]
- Skerfving, S.; Nilsson, U.; Schütz, A.; Gerhardsson, L. Biological monitoring of inorganic lead. Scand. J. Work. Environ. Heal. 1993, 19, 59–64. [Google Scholar]
- Roberts, J.R.; Reigart, J.R.; Ebeling, M.; Hulsey, T.C.; Roberts, J. Time Required for Blood Lead Levels to Decline in Nonchelated Children. J. Toxicol. Clin. Toxicol. 2001, 39, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Rabinowitz, M.B.; Wetherill, G.W.; Kopple, J.D. Kinetic analysis of lead metabolism in healthy humans. J. Clin. Investig. 1976, 58, 260–270. [Google Scholar] [CrossRef] [PubMed]
- Papanikolaou, N.C.; Hatzidaki, E.G.; Belivanis, S.; Tzanakakis, G.N.; Tsatsakis, A.M. Lead toxicity update. A brief review. Med Sci. Monit. 2005, 11, 329. [Google Scholar]
- Philip, A.T.; Gerson, B. Lead Poisoning-Part II: Effects and Assay. Clin. Lab. Med. 1994, 14, 651–670. [Google Scholar] [CrossRef]
- Seppäläinen, A.M. Electrophysiological evaluation of central and peripheral neural effects of lead exposure. NeuroToxicology 1984, 5, 43–52. [Google Scholar] [PubMed]
- Needleman, H.L.; Gunnoe, C.; Leviton, A.; Reed, R.; Peresie, H.; Maher, C.; Barrett, P. Deficits in Psychologic and Classroom Performance of Children with Elevated Dentine Lead Levels. N. Engl. J. Med. 1979, 300, 689–695. [Google Scholar] [CrossRef]
- Lidsky, T.I.; Schneider, J.S. Lead neurotoxicity in children: Basic mechanisms and clinical correlates. Brain 2003, 126, 5–19. [Google Scholar] [CrossRef]
- A Otto, D.; A Fox, D. Auditory and visual dysfunction following lead exposure. NeuroToxicology 1993, 14, 191–207. [Google Scholar]
- Holdstein, Y.; Pratt, H.; Goldsher, M.; Rosen, G.; Shenhav, R.; Linn, S.; Mor, A.; Barkai, A. Auditory brainstem evoked potentials in asymptomatic lead-exposed subjects. J. Laryngol. Otol. 1986, 100, 1031–1036. [Google Scholar] [CrossRef]
- Cullen, M.R.; Robins, J.M.; Eskenazi, B. Adult inorganic lead intoxication: Presentation of 31 new cases and a review of recent advances in the literature. Medicine 1983, 62, 221–247. [Google Scholar] [CrossRef] [PubMed]
- Needleman, H.L.; Schell, A.; Bellinger, D.; Leviton, A.; Allred, E.N. The Long-Term Effects of Exposure to Low Doses of Lead in Childhood. N. Engl. J. Med. 1990, 322, 83–88. [Google Scholar] [CrossRef] [PubMed]
- White, R.; Diamond, R.; Proctor, S.; Morey, C.; Hu, H. Residual cognitive deficits 50 years after lead poisoning during childhood. Occup. Environ. Med. 1993, 50, 613–622. [Google Scholar] [CrossRef] [PubMed]
- Feldman, R.G.; White, R. Lead Neurotoxicity and Disorders of Learning. J. Child Neurol. 1992, 7, 354–359. [Google Scholar] [CrossRef] [PubMed]
- National Research Council Measuring Lead Exposure in Infants, Children, and Other Sensitive Populations; The National Academies Press: Washington, DC, USA, 1993.
- Needleman, H.L. Lead-associated intellectual deficit. N. Engl. J. Med. 1982, 306, 367. [Google Scholar]
- Loghman-Adham, M. Aminoaciduria and glycosuria following severe childhood lead poisoning. Pediatr. Nephrol. 1998, 12, 218–221. [Google Scholar] [CrossRef]
- Hu, H. A 50-year follow-up of childhood plumbism. Hypertension, renal function, and hemoglobin levels among survivors. Am. J. Dis. Child. 1991, 145, 681–687. [Google Scholar] [CrossRef]
- A Abdullah, M. Lead poisoning among children in Saudi Arabia. J. Trop. Med. Hyg. 1984, 87, 67–70. [Google Scholar]
- Ponka, P. Cell biology of heme. Am. J. Med. Sci. 1999, 318, 241–256. [Google Scholar] [CrossRef]
- Alvares, A.P.; Fischbein, A.; Sassa, S.; Anderson, K.; Kappas, A. Lead intoxication: Effects on cytochrome P-450-mediated hepatic oxidations. Clin. Pharmacol. Ther. 1976, 19, 183–190. [Google Scholar] [CrossRef]
- Goldman, L.S.; Genel, M.; Bezman, R.J.; Slanetz, P. American Medical Association for the Council on Scientific Affairs Diagnosis and Treatment of Attention-Deficit/Hyperactivity Disorder in Children and Adolescents. JAMA 1998, 279, 1100. [Google Scholar] [CrossRef] [PubMed]
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Inorganic and organic lead compounds. IARC Monogr. Eval. Carcinog. Risks Hum. 2006, 87, 1. [Google Scholar]
- Evis, M.J.; Kane, K.A.; Moore, M.R.; Parratt, J.R. The effects of chronic lead treatment and hypertension on the severity of cardiac arrhythmias induced by coronary artery occlusion or by noradrenaline in anaesthetised rats. Arch. Toxicol. 1987, 59, 336–340. [Google Scholar] [CrossRef] [PubMed]
- Lai, B.; Murthy, R.C.; Anand, M.; Chandra, S.V.; Kumar, R.; Tripathi, O.; Srimal, R.C. Cardiotoxicity and Hypertension in Rats After Oral Lead Exposure. Drug Chem. Toxicol. 1991, 14, 305–318. [Google Scholar] [CrossRef] [PubMed]
- Khalil-Manesh, F.; Gonick, H.C.; Cohen, A.H. Experimental Model of Lead Nephropathy. III. Continuous Low-level Lead Administration. Arch. Environ. Heal. Int. J. 1993, 48, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Dreisbach, R. Handbook of poisoning: Prevention, diagnosis and treatment Lang Medical Publications. Los Altos 1983, 401, 3. [Google Scholar]
- Sheabar, F.Z.; Yannai, S. Extracorporeal Complexation and Haemodialysis for the Treatment of Cadmium Poisoning. I. Effects of Four Chelators on thein VitroElimination of Cadmium from Human Blood. Pharmacol. Toxicol. 1989, 64, 257–261. [Google Scholar] [CrossRef]
- Jones, M.M.; Basinger, M.A.; Topping, R.J.; Gale, G.R.; Jones, S.G.; Holscher, M.A. Meso-2,3-dimercaptosuccinic acid and sodium N-benzyl-N-dithiocarboxy-D-glucamine as antagonists for cadmium intoxication. Arch. Toxicol. 1988, 62, 29–36. [Google Scholar] [CrossRef]
- Gale, G.R.; Smith, A.B.; Walker, E.M. Diethyldithiocarbamate in treatment of acute cadmium poisoning. Ann. Clin. Lab. Sci. 1981, 11, 476–483. [Google Scholar]
- Miller, A.L. Dimercaptosuccinic acid (DMSA), a non-toxic, water-soluble treatment for heavy metal toxicity. Altern. Med. Rev. A J. Clin. Ther. 1998, 3, 199–207. [Google Scholar]
- Cotter, L.H. Treatment of cadmium poisoning with edathamil calcium disodium. J. Am. Med Assoc. 1958, 166, 735. [Google Scholar] [CrossRef] [PubMed]
- Baum, C.R. Treatment of mercury intoxication. Curr. Opin. Pediatr. 1999, 11, 265–268. [Google Scholar] [CrossRef] [PubMed]
- Toet, A.; Van Dijk, A.; Savelkoul, T.; Meulenbelt, J. Mercury Kinetics in a Case of Severe Mercuric Chloride Poisoning Treated with Dimercapto-1-propane Sulphonate (DMPS). Hum. Exp. Toxicol. 1994, 13, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Pfab, R.; Mückter, H.; Roider, G.; Zilker, T. Clinical Course of Severe Poisoning with Thiomersal. J. Toxicol. Clin. Toxicol. 1996, 34, 453–460. [Google Scholar] [CrossRef]
- Longcope, W.T.; Luetscher, J.A.; Calkins, E.; Grob, D.; Bush, S.W.; Eisenberg, H. CLINICAL USES OF 2,3-DIMERCAPTOPROPANOL (BAL). XI. THE TREATMENT OF ACUTE MERCURY POISONING BY BAL 1. J. Clin. Investig. 1946, 25, 557–567. [Google Scholar] [CrossRef]
- Berlin, C.M.; Gorman, R.L.; May, D.G.; Notterman, D.A.; Weismann, D.N.; Wilson, S.G.; Wilson, J.T.; Bennett, D.R.; Mulinare, J.; Kaufman, P.; et al. Treatment guidelines for lead exposure in children. Pediatrics 1995, 96, 155–160. [Google Scholar]
- Garçon, G.; Leleu, B.; Marez, T.; Zerimech, F.; Haguenoer, J.-M.; Furon, D.; Shirali, P. Biomonitoring of the adverse effects induced by the chronic exposure to lead and cadmium on kidney function: Usefulness of alpha-glutathione S-transferase. Sci. Total. Environ. 2007, 377, 165–172. [Google Scholar] [CrossRef]
- Uchida, M.; Teranishi, H.; Aoshima, K.; Katoh, T.; Kasuya, M.; Inadera, H. Elevated urinary levels of vitamin D-binding protein in the inhabitants of a cadmium polluted area, Jinzu River basin, Japan. Tohoku J. Exp. Med. 2007, 211, 269–274. [Google Scholar] [CrossRef]
- Yamagami, T.; Suna, T.; Fukui, Y.; Ohashi, F.; Takada, S.; Sakurai, H.; Aoshima, K.; Ikeda, M. Biological variations in cadmium, α1-microglobulin, β2-microglobulin and N-acetyl-β-d-glucosaminidase in adult women in a non-polluted area. Int. Arch. Occup. Environ. Heal. 2007, 81, 263–271. [Google Scholar] [CrossRef]
- Prozialeck, W.; Vaidya, V.; Liu, J.; Waalkes, M.; Edwards, J.; Lamar, P.; Bernard, A.; Dumont, X.; Bonventre, J. Kidney injury molecule-1 is an early biomarker of cadmium nephrotoxicity. Kidney Int. 2007, 72, 985–993. [Google Scholar] [CrossRef]
- UN Food and Agriculture Organization. Legal Notice No 66/2003 Heavy Metals Regulation, in 66; FAO: Rome, Italy, 2003. [Google Scholar]
- Commission, E. Commission Regulation (EC) No 1881/2006 of 19 December 2006 Setting maximum levels for certain contaminants in foodstuffs. Off. J. Eur. Union 2006, 364. [Google Scholar]
- South African Department of Health (DOH). Foodstuffs, Cosmetics and Disinfectants Act, 1972 (Act. 54 of 1972); South African Department of Health (DOH): Pretoria, South Africa, 2004.
- Djedjibegovic, J.; Larssen, T.; Skrbo, A.; Marjanovic, A.; Sober, M. Contents of cadmium, copper, mercury and lead in fish from the Neretva river (Bosnia and Herzegovina) determined by inductively coupled plasma mass spectrometry (ICP-MS). Food Chem. 2012, 131, 469–476. [Google Scholar] [CrossRef]
- Miedico, O.; Iammarino, M.; Pompa, C.; Tarallo, M.; Chiaravalle, A.E. Assessment of lead, cadmium and mercury in seafood marketed in Puglia and Basilicata (Italy) by inductively coupled plasma mass spectrometry. Food Addit. Contam. Part B 2015, 8, 1–8. [Google Scholar] [CrossRef]
- Schmitt, C.; Brumbaugh, W.G.; Linder, G.L.; Hinck, J.E. A Screening-Level Assessment of Lead, Cadmium, and Zinc in Fish and Crayfish from Northeastern Oklahoma, USA. Environ. Geochem. Heal. 2006, 28, 445–471. [Google Scholar] [CrossRef] [PubMed]
- Dural, M.; Genc, E.; Yemenicioğlu, S.; Sangun, M.K. Accumulation of Some Heavy Metals Seasonally in Hysterotylacium aduncum (Nematoda) and Its Host Red Sea Bream, Pagellus erythrinus (Sparidae) from Gulf of Iskenderun (North-Eastern Mediterranean). Bull. Environ. Contam. Toxicol. 2009, 84, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Andrew, A.S.; Warren, A.J.; Barchowsky, A.; A Temple, K.; Klei, L.; Soucy, N.V.; A O’Hara, K.; Hamilton, J.W. Genomic and proteomic profiling of responses to toxic metals in human lung cells. Environ. Heal. Perspect. 2003, 111, 825–835. [Google Scholar] [CrossRef]
- Shimoda, R.; Achanzar, W.E.; Qu, W.; Nagamine, T.; Takagi, H.; Mori, M.; Waalkes, M.P. Metallothionein Is a Potential Negative Regulator of Apoptosis. Toxicol. Sci. 2003, 73, 294–300. [Google Scholar] [CrossRef]
- Voegborlo, R.; El-Methnani, A.; Abedin, M. Mercury, cadmium and lead content of canned tuna fish. Food Chem. 1999, 67, 341–345. [Google Scholar] [CrossRef]
- Çelik, U.; Oehlenschläger, J. High contents of cadmium, lead, zinc and copper in popular fishery products sold in Turkish supermarkets. Food Control. 2007, 18, 258–261. [Google Scholar] [CrossRef]
- Santos, L.F.P.; Trigueiro, I.N.S.; Lemos, V.A.; Furtunato, D.M.D.N.; Cardoso, R.D.C.V. Assessment of cadmium and lead in commercially important seafood from São Francisco do Conde, Bahia, Brazil. Food Control. 2013, 33, 193–199. [Google Scholar] [CrossRef]
- Geier, D.A.; Geier, M.R. A Prospective Study of Mercury Toxicity Biomarkers in Autistic Spectrum Disorders∗. J. Toxicol. Environ. Heal. Part A 2007, 70, 1723–1730. [Google Scholar] [CrossRef] [PubMed]
- Sigel, A.; Sigel, H. Metal Ions in Biological Systems: Mercury and Its Effects on Environment and Biology; CRC Press: Boca Raton, FL, USA, 1997; Volume 34. [Google Scholar]
- Piotrowski, J.; Trojanowska, B.; Wiśniewska-Knypl, J.; Bolanowska, W. Mercury binding in the kidney and liver of rats repeatedly exposed to mercuric chloride: Induction of metallothionein by mercury and cadmium. Toxicol. Appl. Pharmacol. 1974, 27, 11–19. [Google Scholar] [CrossRef]
- Branco, V.; Caito, S.; Farina, M.; Da Rocha, J.T.; Aschner, M.; Carvalho, C.; Da Rocha, J.B.T. Biomarkers of mercury toxicity: Past, present, and future trends. J. Toxicol. Environ. Heal. Part B 2017, 20, 119–154. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, A.C.; Boischio, A.A.; East, G.A.; Ferrari, I.; Gonçalves, A.; Silva, P.R.M.; Da Cruz, T.M.E. Mercury contamination in the Brazilian Amazon. Environmental and occupational aspects. Water Air Soil Pollut. 1995, 80, 109–121. [Google Scholar] [CrossRef]
- Dickman, M.; Leung, K. Mercury and organochlorine exposure from fish consumption in Hong Kong. Chemosphere 1998, 37, 991–1015. [Google Scholar] [CrossRef]
- Nakagawa, R.; Yumita, Y.; Hiromoto, M. Total mercury intake from fish and shellfish by Japanese people. Chemosphere 1997, 35, 2909–2913. [Google Scholar] [CrossRef]
- Vieira, C.; Morais, S.; Ramos, S.; Delerue-Matos, C.; Oliveira, M.B.P.P. Mercury, cadmium, lead and arsenic levels in three pelagic fish species from the Atlantic Ocean: Intra- and inter-specific variability and human health risks for consumption. Food Chem. Toxicol. 2011, 49, 923–932. [Google Scholar] [CrossRef]
- Burger, J.; Gochfeld, M. Heavy metals in commercial fish in New Jersey. Environ. Res. 2005, 99, 403–412. [Google Scholar] [CrossRef]
- Hight, S.C.; Cheng, J. Determination of total mercury in seafood by cold vapor-atomic absorption spectroscopy (CVAAS) after microwave decomposition. Food Chem. 2005, 91, 557–570. [Google Scholar] [CrossRef]
- Plessi, M.; Bertelli, D.; Monzani, A. Mercury and Selenium Content in Selected Seafood. J. Food Compos. Anal. 2001, 14, 461–467. [Google Scholar] [CrossRef]
- Pastore, A.; Federici, G.; Bertini, E.; Piemonte, F. Analysis of glutathione: Implication in redox and detoxification. Clin. Chim. Acta 2003, 333, 19–39. [Google Scholar] [CrossRef]
- Gerson, R.J.; Shaikh, Z.A. Uptake and binding of cadmium and mercury to metallothionein in rat hepatocyte primary cultures. Biochem. J. 1982, 208, 465–472. [Google Scholar] [CrossRef] [PubMed]
- Batista, B.L.; Rodrigues, J.L.; De Souza, S.S.; Souza, V.C.O.; Barbosa, F.; Barbosa, F. Mercury speciation in seafood samples by LC–ICP-MS with a rapid ultrasound-assisted extraction procedure: Application to the determination of mercury in Brazilian seafood samples. Food Chem. 2011, 126, 2000–2004. [Google Scholar] [CrossRef] [PubMed]
- Hight, S.C.; Cheng, J. Determination of methylmercury and estimation of total mercury in seafood using high performance liquid chromatography (HPLC) and inductively coupled plasma-mass spectrometry (ICP-MS): Method development and validation. Anal. Chim. Acta 2006, 567, 160–172. [Google Scholar] [CrossRef]
- Liu, Q. Determination of mercury and methylmercury in seafood by ion chromatography using photo-induced chemical vapor generation atomic fluorescence spectrometric detection. Microchem. J. 2010, 95, 255–258. [Google Scholar] [CrossRef]
- Zmozinski, A.V.; Carneado, S.; Ibáñez-Palomino, C.; Sahuquillo, À.; López-Sánchez, F.; Da Silva, M.M. Method development for the simultaneous determination of methylmercury and inorganic mercury in seafood. Food Control. 2014, 46, 351–359. [Google Scholar] [CrossRef]
- Berglund, M.; Lind, B.; Björnberg, K.A.; Palm, B.; Einarsson, O.; Vahter, M. Inter-individual variations of human mercury exposure biomarkers: A cross-sectional assessment. Environ. Heal. 2005, 4, 20. [Google Scholar] [CrossRef]
- Haut, M.; Morrow, L.A.; Pool, D.; Callahan, T.S.; Haut, J.S.; Franzen, M.D. Neurobehavioral Effects of Acute Exposure to Inorganic Mercury Vapor. Appl. Neuropsychol. 1999, 6, 193–200. [Google Scholar] [CrossRef]
- Torres, D.P.; Martins-Teixeira, M.; Silva, E.; Queiroz, H. Method development for the control determination of mercury in seafood by solid-sampling thermal decomposition amalgamation atomic absorption spectrometry (TDA AAS). Food Addit. Contam. Part A 2012, 29, 625–632. [Google Scholar] [CrossRef]
- Motts, J.A.; Shirley, D.L.; Silbergeld, E.K.; Nyland, J.F. Novel biomarkers of mercury-induced autoimmune dysfunction: A cross-sectional study in Amazonian Brazil. Environ. Res. 2014, 132, 12–18. [Google Scholar] [CrossRef]
- Nevado, J.J.B.; Martín-Doimeadiós, R.C.R.; Bernardo, F.J.G.; Moreno, M.J. Determination of mercury species in fish reference materials by gas chromatography-atomic fluorescence detection after closed-vessel microwave-assisted extraction. J. Chromatogr. A 2005, 1093, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Gerbersmann, C.; Heisterkamp, M.; Adams, F.C.; Broekaert, J. Two methods for the speciation analysis of mercury in fish involving microwave-assisted digestion and gas chromatography-atomic emission spectrometry. Anal. Chim. Acta 1997, 350, 273–285. [Google Scholar] [CrossRef]
- Pereiro, I.R.; Wasik, A.; Łobiński, R.; ?obi?ski, R. Determination of mercury species in fish reference materials by isothermal multicapillary gas chromatography with atomic emission detection after microwave-assisted solubilization and solvent extraction. J. Anal. At. Spectrom. 1998, 13, 743–747. [Google Scholar] [CrossRef]
- Ebdon, L.; Foulkes, M.E.; Le Roux, S.; Mu??oz-Olivas, R.; Muñoz-Olivas, R. Cold vapour atomic fluorescence spectrometry and gas chromatography-pyrolysis-atomic fluorescence spectrometry for routine determination of total and organometallic mercury in food samples. Analyst 2002, 127, 1108–1114. [Google Scholar] [CrossRef] [PubMed]
- Grinberg, P.; Campos, R.C.; Mester, Z.; Sturgeon, R. Solid phase microextraction capillary gas chromatography combined with furnace atomization plasma emission spectrometry for speciation of mercury in fish tissues. Spectrochim. Acta Part B: At. Spectrosc. 2003, 58, 427–441. [Google Scholar] [CrossRef]
- E Desilva, P. Determination of lead in plasma and studies on its relationship to lead in erythrocytes. Occup. Environ. Med. 1981, 38, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Sakai, T.; Morita, Y.; Araki, T. Determination of lead in plasma, whole blood, and urine by icp-ms and the relationships among the three exposure indices. J. Occup. Med. Tox. 2003, 51, 50–57. [Google Scholar]
- Schütz, A.; Bergdahl, I.; Ekholm, A.; Skerfving, S. Measurement by ICP-MS of lead in plasma and whole blood of lead workers and controls. Occup. Environ. Med. 1996, 53, 736–740. [Google Scholar] [CrossRef]
- Schramel, P.; Wendler, I.; Angerer, J. The determination of metals (antimony, bismuth, lead, cadmium, mercury, palladium, platinum, tellurium, thallium, tin and tungsten) in urine samples by inductively coupled plasma-mass spectrometry. Int. Arch. Occup. Environ. Heal. 1997, 69, 219–223. [Google Scholar] [CrossRef]
- Osman, K.; Zejda, J.E.; Schütz, A.; Mielzynska, D.; Elinder, C.G.; Vahter, M. Exposure to lead and other metals in children from Katowice district, Poland. Int. Arch. Occup. Environ. Heal. 1998, 71, 180–186. [Google Scholar] [CrossRef]
- Mauras, Y.; Premel-Cabic, A.; Berre, S.; Allain, P. Simulataneous determination of lead, bismuth and thallium in plasma and urine by inductively coupled plasma mass spectrometry. Clin. Chim. Acta 1993, 218, 201–205. [Google Scholar] [CrossRef]
- Berlin, A.; Schaller, K.H. European standardized method for the determination of delta-aminolevulinic acid dehydratase activity in blood. Z. klin. Chem. Klin. Biochem. 1974, 12, 389–390. [Google Scholar] [PubMed]
- Vergnano, C.; Cartasegna, C.; Ardoino, V. Meccanismi di inhibizione dell’ativita’δ-aminolevulinico deidratasica eritrocitaria nell’intossicazione da piombo umana e sperimentale. Med. Lav. 1969, 60, 505–516. [Google Scholar] [PubMed]
- Meredith, P.; Moore, M.; Goldberg, A. Effects of Aluminium, Lead and Zinc on δ-Aminolaevulinic Acid Dehydratase. Enzym. 1977, 22, 22–27. [Google Scholar] [CrossRef]
- Evans, N.; Games, D.; Jackson, A.; Matlin, S. Applications of high-pressure liquid chromatography and field desorption mass spectrometry in studies of natural porphyrins and chlorophyll derivatives. J. Chromatogr. A 1975, 115, 325–333. [Google Scholar] [CrossRef]
- Sakai, T.; Niinuma, Y.; Yanagihara, S.; Ushio, K. Liquid-chromatographic separation and determination of coproporphyrins I and III in urine. Clin. Chem. 1983, 29, 350–353. [Google Scholar] [CrossRef]
- Salmi, M.; Tenhunen, R. New method for liquid-chromatographic measurement of erythrocyte protoporphyrin and coproporphyrin. Clin. Chem. 1980, 26, 1832–1835. [Google Scholar] [CrossRef]
- Morgano, M.; Rabonato, L.C.; Milani, R.F.; Miyagusku, L.; Quintaes, K.D. As, Cd, Cr, Pb and Hg in seafood species used for sashimi and evaluation of dietary exposure. Food Control. 2014, 36, 24–29. [Google Scholar] [CrossRef]
- Piomelli, S. A micromethod for free erythrocyte porphyrins: The FEP test. J. Lab. Clin. Med. 1973, 81, 932–940. [Google Scholar]
- Niinuma, Y.; Sakai, T.; Yanagihara, S.; Ushio, K. [A modified FEP (free erythrocyte protoporphyrin) test (author’s transl)]. Sangyo Igaku 1981, 23, 254–259. [Google Scholar] [CrossRef]
- A Lamola, A.; Joselow, O.; Yamane, T. Zinc Protoporphyrin (ZPP): A Simple, Sensitive, Fluorometric Screening Test for Lead Poisioning. Clin. Chem. 1975, 21, 93–97. [Google Scholar] [CrossRef] [PubMed]
- A Lamola, A.; Eisinger, J.; E Blumberg, W.; Kometani, T.; Burnham, B.F. Quantitative determination of erythrocyte zinc protoporphyrin. J. Lab. Clin. Med. 1977, 89, 881–890. [Google Scholar] [PubMed]
- Hanna, T.L.; Dietzler, D.N.; Smith, C.H.; Gupta, S.; Zarkowsky, H.S. Erythrocyte porphyrin analysis in the detection of lead poisoning in children: Evaluation of four micromethods. Clin. Chem. 1976, 22, 161–168. [Google Scholar] [CrossRef]
- Hart, D.; Piomelli, S. Simultaneous quantitation of zinc protoporphyrin and free protoporphyrin in erythrocytes by acetone extraction. Clin. Chem. 1981, 27, 220–222. [Google Scholar] [CrossRef] [PubMed]
- E Blumberg, W.; Eisinger, J.; A Lamola, A.; Zuckerman, D.M. The hematofluorometer. Clin. Chem. 1977, 23, 270–274. [Google Scholar] [CrossRef]
- Niinuma, Y.; Sakai, T.; Yanagihara, S.; Ushio, K. Relationship between ZP levels by hematofluorometer and the other biological parameters of lead exposure. Jpn. J. Traumatol. Occup. Med. 1982, 30, 816–824. [Google Scholar]
- Sakai, T.; Takeuchi, Y.; Ikeya, Y.; Araki, T.; Ushio, K. Automated HPLC method for determining zinc protoporphyrin ix and protoporphyrin ix in erythrocytes of workers exposed to lead. Sangyo Igaku 1988, 30, 467–474. [Google Scholar] [CrossRef][Green Version]
- Sakai, T.; Takeuchi, Y.; Araki, T.; Ushio, K. Determination of erythrocyte porphyrins by reversed-phase high-performance liquid chromatography using capsule-type silica gels coated with silicone polymer. J. Chromatogr. B Biomed. Sci. Appl. 1988, 433, 73–79. [Google Scholar] [CrossRef]
- A Lemos, V.; Ferreira, S.L. On-line preconcentration system for lead determination in seafood samples by flame atomic absorption spectrometry using polyurethane foam loaded with 2-(2-benzothiazolylazo)-2-p-cresol. Anal. Chim. Acta 2001, 441, 281–289. [Google Scholar] [CrossRef]
- Okayama, A.; Fujii, S.; Miura, R. Optimized fluorometric determination of urinary delta-aminolevulinic acid by using pre-column derivatization, and identification of the derivative. Clin. Chem. 1990, 36, 1494–1497. [Google Scholar] [CrossRef]
- Valentine, W.N.; Fink, K.; Paglia, D.E.; Harris, S.R.; Adams, W.S. Hereditary hemolytic anemia with human erythrocyte pyrimidine 5′-nucleotidase deficiency. J. Clin. Invest. 1974, 54, 866–879. [Google Scholar] [CrossRef] [PubMed]
- Baloh, R.W. Laboratory Diagnosis of Increased Lead Absorption. Arch. Environ. Heal. Int. J. 1974, 28, 198–208. [Google Scholar] [CrossRef] [PubMed]
- Sakai, T.; Yanagihara, S.; Ushio, K. Determination of 5′-nucleotidase activity in human erythrocytes and plasma using high-performance liquid chromatography. J. Chromatogr. A 1982, 239, 717–721. [Google Scholar] [CrossRef]
- Sakai, T.; Ushio, K. A simplified method for determining erythrocyte pyrimidine 5’-nucleotidase (P5N) activity by HPLC and its value in monitoring lead exposure. Occup. Environ. Med. 1986, 43, 839–844. [Google Scholar] [CrossRef]
- Zerez, C.R.; Tanaka, K.R. Impaired nicotinamide adenine dinucleotide synthesis in pyruvate kinase-deficient human erythrocytes: A mechanism for decreased total NAD content and a possible secondary cause of hemolysis. Blood 1987, 69, 999–1005. [Google Scholar] [CrossRef]
- Sakai, T.; Morita, Y.; Araki, T.; Masuyama, Y. Simple and rapid method for determining nicotinamide adenine dinucleotide synthetase activity by high-performance liquid chromatography. J. Chromatogr. B Biomed. Sci. Appl. 1997, 704, 77–81. [Google Scholar] [CrossRef]
- Khaled, A.; El Nemr, A.; El Sikaily, A. Contamination of coral reef by heavy metals along the Egyptian Red Sea coast. Bull. Environ. Contam. Toxicol. 2003, 71, 577–584. [Google Scholar] [CrossRef]
- Khaled, A.; El Nemr, A.; El Sikaily, A. An assessment of heavy-metal contamination in surface sediments of the Suez Gulf using geoaccumulation indexes and statistical analysis. Chem. Ecol. 2006, 22, 239–252. [Google Scholar] [CrossRef]
- El-Sikaily, A.; Khaled, A.; El Nemr, A. Heavy metals monitoring using bivalves from Mediterranean Sea and Red Sea. Environ. Monit. Assess. 2004, 98, 41–58. [Google Scholar] [CrossRef]
- El Sikaily, A.; Khaled, A.; El Nemr, A. Leachable and total heavy metals in muddy and sandy sediments collected from Suez Gulf. Egypt. J. Aquat. Res. 2005, 31, 99–119. [Google Scholar]
- El Nemr, A.; Khaled, A.; El Sikaily, A. Distribution and Statistical Analysis of Leachable and Total Heavy Metals in the Sediments of the Suez Gulf. Environ. Monit. Assess. 2006, 118, 89–112. [Google Scholar] [CrossRef] [PubMed]
- Youssef, D.H.; El-Said, G.F. Assessment of some heavy metals in surface sediments of the Aqaba Gulf, Egypt. Environ. Monit. Assess. 2011, 180, 229–242. [Google Scholar] [CrossRef] [PubMed]
- Gabr, H.R.; Gab-Alla, A.F. Effect of transplantation on heavy metal concentrations in commercial clams of Lake Timsah, Suez Canal, Egypt. Oceanologia 2008, 50, 83–93. [Google Scholar]
- Idris, A.M.; Eltayeb, M.; Potgieter-Vermaak, S.; Van Grieken, R.; Potgieter, J. Assessment of heavy metals pollution in Sudanese harbours along the Red Sea Coast. Microchem. J. 2007, 87, 104–112. [Google Scholar] [CrossRef]
- Awaleh, M.O.; Bouraleh, H.F.; Soubaneh, Y.D.; Badran, M.; Bahga, H.O.; Samaleh, A.I. Impact of Human Activity on Marine and Coastal Environment in the Gulf of Tadjourah. J. Mar. Sci. Res. Dev. 2015, 5. [Google Scholar] [CrossRef]
- Mwashote, B.M. Levels of Cadmium and Lead in Water, Sediments and Selected Fish Species in Mombasa, Kenya. West. Indian Ocean J. Mar. Sci. 2004, 2, 25–34. [Google Scholar] [CrossRef]
- Maritim, P.K.; Gachanja, A.; Munyao, T.M. Speciation of Trace Metals Pb, Zn, Cu and Cd in Surficial Sediment from Makupa Creek Mombasa, Coastal Kenya. OALib 2016, 3, 1–10. [Google Scholar] [CrossRef]
- Mshana, J.G.; Sekadende, B. Assessment of Heavy Metal Pollution in Octopus cyanea in the Coastal Waters of Tanzania. J. Heal. Pollut. 2014, 4, 10–17. [Google Scholar] [CrossRef]
- Fatoki, O.S.; Okoro, H.; Adekola, F.; Ximba, B.; Snyman, R.G. Bioaccumulation of metals in black mussels (Mytilus galloprovincialis) in Cape Town Harbour, South Africa. Environmentalist 2011, 32, 48–57. [Google Scholar] [CrossRef]
- Boldrocchi, G.; Monticelli, D.; Omar, Y.M.; Bettinetti, R. Trace elements and POPs in two commercial shark species from Djibouti: Implications for human exposure. Sci. Total. Environ. 2019, 669, 637–648. [Google Scholar] [CrossRef]
- El Nemr, A.; El-Said, G.F.; Ragab, S.; Khaled, A.; El-Sikaily, A. The distribution, contamination and risk assessment of heavy metals in sediment and shellfish from the Red Sea coast, Egypt. Chemosphere 2016, 165, 369–380. [Google Scholar] [CrossRef] [PubMed]
- El-Moselhy, K.M.; Othman, A.; El-Azem, H.A.; El-Metwally, M. Bioaccumulation of heavy metals in some tissues of fish in the Red Sea, Egypt. Egypt. J. Basic Appl. Sci. 2014, 1, 97–105. [Google Scholar] [CrossRef]
- Shilla, D.A. Distribution of Pb, Cr, Cu and Zn in the marine-coastal region of Zanzibar (Tanzanian archipelago, East Africa). Chem. Ecol. 2016, 32, 1–12. [Google Scholar] [CrossRef]
- Kojadinovic, J.; Potier, M.; Le Corre, M.; Cosson, R.P.; Bustamante, P. Bioaccumulation of trace elements in pelagic fish from the Western Indian Ocean. Environ. Pollut. 2007, 146, 548–566. [Google Scholar] [CrossRef] [PubMed]
- Gomaa, M.; Abou-Arab, A.A.K.; Badawy, A.; Khayria, N. Distribution pattern of some heavy metals in Egyptian fish organs. Food Chem. 1995, 53, 385–389. [Google Scholar] [CrossRef]
- Salam, H.A. Heavy Metals Monitoring Using Commercially Important Crustacean and Mollusks collected from Egyptian and Saudi Arabia Coasts. Anim. Veter- Sci. 2014, 2, 49. [Google Scholar] [CrossRef][Green Version]
- Mziray, P.; Kimirei, I.A. Bioaccumulation of heavy metals in marine fishes (Siganus sutor, Lethrinus harak, and Rastrelliger kanagurta) from Dar es Salaam Tanzania. Reg. Stud. Mar. Sci. 2016, 7, 72–80. [Google Scholar] [CrossRef]
- Authman, M.M. Use of Fish as Bio-indicator of the Effects of Heavy Metals Pollution. J. Aquac. Res. Dev. 2015, 6, 1–13. [Google Scholar] [CrossRef]
- Authman, M.M.; Abbas, W.; Gaafar, A.Y. Metals concentrations in Nile tilapia Oreochromis niloticus () from illegal fish farm in Al-Minufiya Province, Egypt, and their effects on some tissues structures. Ecotoxicol. Environ. Saf. 2012, 84, 163–172. [Google Scholar] [CrossRef]
- Whitfield, A.; Elliott, M. Fishes as indicators of environmental and ecological changes within estuaries: A review of progress and some suggestions for the future. J. Fish Biol. 2002, 61, 229–250. [Google Scholar] [CrossRef]
- Khallaf, E.A.; Galal, M.; Authman, M. Assessment of heavy metals pollution and their effects on Oreochromis niloticus in aquatic drainage canals. J. Egypt. Ger. Soc. Zool. 1998, 26, 39–74. [Google Scholar]
- Al-Yousuf, M.; El-Shahawi, M.; Al-Ghais, S. Trace metals in liver, skin and muscle of Lethrinus lentjan fish species in relation to body length and sex. Sci. Total. Environ. 2000, 256, 87–94. [Google Scholar] [CrossRef]
- Çavaş, T.; Garanko, N.N.; Arkhipchuk, V.V. Induction of micronuclei and binuclei in blood, gill and liver cells of fishes subchronically exposed to cadmium chloride and copper sulphate. Food Chem. Toxicol. 2005, 43, 569–574. [Google Scholar] [CrossRef] [PubMed]
- Van Dyk, J.; Pieterse, G.; Van Vuren, J.; Van Vuren, J. Histological changes in the liver of Oreochromis mossambicus (Cichlidae) after exposure to cadmium and zinc. Ecotoxicol. Environ. Saf. 2007, 66, 432–440. [Google Scholar] [CrossRef]
- Giari, L.; Manera, M.; Simoni, E.; Dezfuli, B.S. Cellular alterations in different organs of European sea bass Dicentrarchus labrax (L.) exposed to cadmium. Chemosphere 2007, 67, 1171–1181. [Google Scholar] [CrossRef]
- Omer, S.A.; Elobeid, M.A.; Fouad, D.; Daghestani, M.; Al-Olayan, E.M.; Elamin, M.H.; Virk, P.; El-Mahassn, A. Cadmium Bioaccumulation and Toxicity in Tilapia Fish (Oreochromis niloticus). J. Anim. Veter- Adv. 2012, 11, 1601–1606. [Google Scholar] [CrossRef]
- Vetillard, A.; Bailhache, T. Cadmium: An Endocrine Disrupter That Affects Gene Expression in the Liver and Brain of Juvenile Rainbow Trout1. Boil. Reprod. 2005, 72, 119–126. [Google Scholar] [CrossRef]
- Mukherjee, D.; Kumar, V.; Chakraborti, P. Effect of mercuric chloride and cadmium chloride on gonadal function and its regulation in sexually mature common carp Cyprinus carpio. Biomed. Environ. Sci. 1994, 7, 13–24. [Google Scholar]
- Vijayram, K.; Geraldine, P.; Varadarajan, T.S.; John, G.; Loganathan, P. Cadmium induced changes in the biochemistry of an air breathing fish Anabas testudineus. J. Ecobiol. 1989, 4, 245–251. [Google Scholar]
- Sanchez-Galan, S.; Linde, A.; Ayllon, F.; Vazquez, E.G. Induction of Micronuclei in Eel (Anguilla anguilla L.) by Heavy Metals. Ecotoxicol. Environ. Saf. 2001, 49, 139–143. [Google Scholar] [CrossRef]
- Jia, X.; Zhang, H.; Liu, X. Low levels of cadmium exposure induce DNA damage and oxidative stress in the liver of Oujiang colored common carp Cyprinus carpio var. color. Fish Physiol. Biochem. 2010, 37, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Souid, G.; Souayed, N.; Yaktiti, F.; Maaroufi, K. Effect of acute cadmium exposure on metal accumulation and oxidative stress biomarkers of Sparus aurata. Ecotoxicol. Environ. Saf. 2013, 89, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Witeska, M.; Sarnowski, P.; Ługowska, K.; Kowal, E. The effects of cadmium and copper on embryonic and larval development of ide Leuciscus idus L. Fish Physiol. Biochem. 2013, 40, 151–163. [Google Scholar] [CrossRef] [PubMed]
- Low, J.; Higgs, D.M. Sublethal effects of cadmium on auditory structure and function in fathead minnows (Pimephales promelas). Fish Physiol. Biochem. 2014, 41, 357–369. [Google Scholar] [CrossRef] [PubMed]
- Katti, S.; Sathyanesan, A. Lead nitrate induced changes in lipid and cholesterol levels in the freshwater fish Clarias batrachus. Toxicol. Lett. 1983, 19, 93–96. [Google Scholar] [CrossRef]
- Olojo, E.A.A.; Olurin, K.B.; Mbaka, G.; Oluwemimo, A.D. Histopathology of the gill and liver tissues of the African catfish Clarias gariepinus exposed to lead. Afr. J. Biotechnol. 2005, 4, 117–122. [Google Scholar]
- Hou, J.L.; Zhuang, P.; Zhang, L.Z.; Feng, L.; Zhang, T.; Liu, J.Y.; Feng, G.P. Morphological deformities and recovery, accumulation and elimination of lead in body tissues of Chinese sturgeon, Acipenser sinensis, early life stages: A laboratory study. J. Appl. Ichthyol. 2011, 27, 514–519. [Google Scholar] [CrossRef]
- Kirubagaran, R.; Joy, K. Toxic effects of three mercurial compounds on survival, and histology of the kidney of the catfish Clarias batrachus (L.). Ecotoxicol. Environ. Saf. 1988, 15, 171–179. [Google Scholar] [CrossRef]
- Mona, S.Z.; Nabila, E.; Fawzi, O.M.; Awad, I.; Nagwa, S.A. Effect of mercuric oxide toxicity on some biochemical parameters on African cat fish Clarias gariepinus present in the River Nile. Life Sci. J. 2011, 8, 363–368. [Google Scholar]
| Structure | R1 | R2 | R3 | R4 | Name |
|---|---|---|---|---|---|
 | CH3CH2 | CH3CH2 | CH3CH2 | CH3CH2 | TtEL |
| X | CH3CH2 | CH3CH2 | CH3CH2 | TEL | |
| X | CH3 | CH3 | CH3 | TML | |
| PbSO4 | - | - | - | - | Lead sulphate |
| Pb3(PO4)2 | - | - | - | - | Lead phosphate |
| PbS | - | - | - | - | Lead sulfide |
| PbCO3 | - | - | - | - | Lead carbonate |
| Metal | Biomarkers in Humans | Biomarkers Detection | Limit in Seafood, mgKg−1 | Detection Method in Seafood | |||
|---|---|---|---|---|---|---|---|
| Tissue | Value | Technique | LOD, µgKg−1 | LOQ, µgKg−1 | |||
| Cd | LMWP (V) [68], β2-MG (V) [68], NAG (V) [68,69] and RBP(V) [68], α-GST [202], VDBP [203], α1-MG [204], 8-OH-G [36] and KIM-1 [205] in urine | CA [36,68,69,202,203,204,205] and EIA [36,68,69,202,203,204,205] | Fish muscle | 0.1 (FAOUN) (a) [206], (EU) (b) [207]; 0.15 (c) (EU) [207]; 0.25 (d) (EU) [207]; 0.05 (e) (EU) [207]; 0.3 (f) (EU) [207] and 1 (SA) [208] | ICP-MS [209,210,211] | 1-30 [209,210,211] | 4-10.1 [209,210] |
| Oxidative stress biomarker in blood [32] | HPLC -UVD and –ECD [32] | Crustacean muscle and abdomen (excluding brown meat of crab) | 0.5 (FAOUN) [206] and (EU) [207] | ICP-AES [212] | |||
| Genomic/proteomic (hsp90) responses in bronchiolar lavage materials cells [213] and Cd-induction of MT in human tumor cell line HepG2 [214] | cDNA microarray analysis [213] and Cd-HRA [214] | FAAS [14,19,54,215] | |||||
| Bivalve mollusks and Cephalopods (without viscera) | 1 (FAOUN) [206] and (EU) [207] | DPSAV [216] | |||||
| Seaweed or dried bivalve mollusks | 3 (EU) [207] | ETAAS [217] | 0.045 [217] | 0.015 [217] | |||
| Autophagy in human CD34+ hematopoietic progenitor cells [63] | CF [63] | ||||||
| Hg | Porphyrins [218]; Hg level(exposure to Hgo) [219]; GSH depletion [35]; MT induction [220]; Renal dysfunction (presence of NAG, RBP, α1,β1-MG, Kim-1) [221] | HPLC-FD [218]; CV-AAS [222]; SM [35]; RGSS [220] | Fish muscle | 1 (FAOUN) [206], (SA) [208] and (UE) (g) [207] 0.3 (China) [223]; 0.5 (UE) [207]; 0.4 (Japan) [224] | CVAAS (tHg) [215,224,225,226,227,228] | 0.2-4600 (tHg) [215,224,225,226,227,228] | 2-9.9 (tHg) [215,224,225,226,227,228] |
| GSH depletion [229]; MT induction [230] | SM [229]; RGSS [230] | Crustaceans | 0.5(h) (EU) [207] | LC-ICP-MS (MHg and EHg) [231]; HPLC-ICP-MS (iHg and MHg) [232]; ICP-MS (tHg) [209,210,231]; IC-PICVGAFS (iHg and MHg) [233]; ICP-AES (tHg) [212]; LC-UV-CV- AFS (iHg, tHg and MHg) [234] | 5.30 [209]; 2 [210]; 0.25 (tHg) [231]; 0.1 (MHg) [231]; 0.2 (EHg) [231]; 0.02 (iHg) [232]; 0.03 (MHg) [232]; 80,000 (MHg MHg) [233]; 100,000 (iHg) [233]; 0.4 (iHg) [234]; 1 (tHg) [234]; 0.3 (MHg) [234] | 16.2 [209], 6 [210]; 1.2 (iHg) [234]; 3 (tHg) [234]; 1 (MHg) [234] | |
| Hg level in hair [219,235] (exposure to MHg) [235]; GSH depletion [119]; Oxidative stress [236]; | CVAFS [235]; SM [119] | Fishery products | 0.5 (EU) [207] | AAS(tHg) [54]; TDA-AAS(tHg) [237] | 3 (tHg) [237] | ||
| Hg level in blood (exposure to MeHg) [123]; GASTA1 increasing [238] in serum | ICP-MS [33]; PPA [238] | CGC-pyro-AFS (iHg and MMHg) [239]; GC-AES (MHg and iHg) [240]; IMGC-AE [241]; CGC-AAS [242]; SPME-GC-FAPES (iHg and MHg) [243] | 1 pg (iHg) [239]; 2 pg (MMHg) [239]; 0.003 (MHg) [240]; 0.0125 (iHg) [240]; 20 (iHg) [241]; 80 (MHg) [241]; 0.1 ng [242]; 1.5 (MHg) [243]; 0.7 (iHg) [243] | ||||
| Pb | Pb-B, Pb-U, Pb-P MPb-U and MPb-P [244,245,246,247,248,249] | GFAAS [244] and ICP-MS [245,246,247,248,249] | Fish muscle | 0.3 (EU) [207]; 0.4 (SA) and FAOUN [206] | GFASS [225,226] | 1.8 [225]; 2 [226] | 6.2 [225] |
| ALAD inhibition in the blood [250,251,252] | PM [250] | Bivalve mollusks | 1.5 (EU) [207] and 1 (FAOUN) [206] | XRFS | |||
| CP-U and CP-B increasing activity [253,254,255] | HPLC-MS [253,254,255] | Crustaceans | 0.5 (EU) (g) [207] and FAOUN [206] | ICP-MS [209,210,211]; ICP-AES [212] | 2.70 [209]; 4 [210]; 10 – 240 [211]; 18 [256] | 8.1 [209]; 12 [210]; 62 [256] | |
| ZP alteration in blood [257,258,259,260,261,262,263,264,265,266] | AE [257,258], DD [259,260], NSE [261,262], HF [261,263,264] and HPLC [265,266] | Cephalopods (without viscera) | 1 (EU) [207] and SA [208] | FAAS [19,215,267]; AAS [14] | 1 [267]; | ||
| ALA-U, ALA-B and ALA-P alteration [268] | HPLC-UVD [268] | ETAAS [217] | 0.0732 [217] | 0.0244 [217] | |||
| P5′N alteration in blood [269,270,271,272] | CM [269], RCMP [270] and HPLC [271,272] | DPSAV [216] | |||||
| NADS alteration in blood [273,274] | HPLC [274] and EIA [273] | ||||||
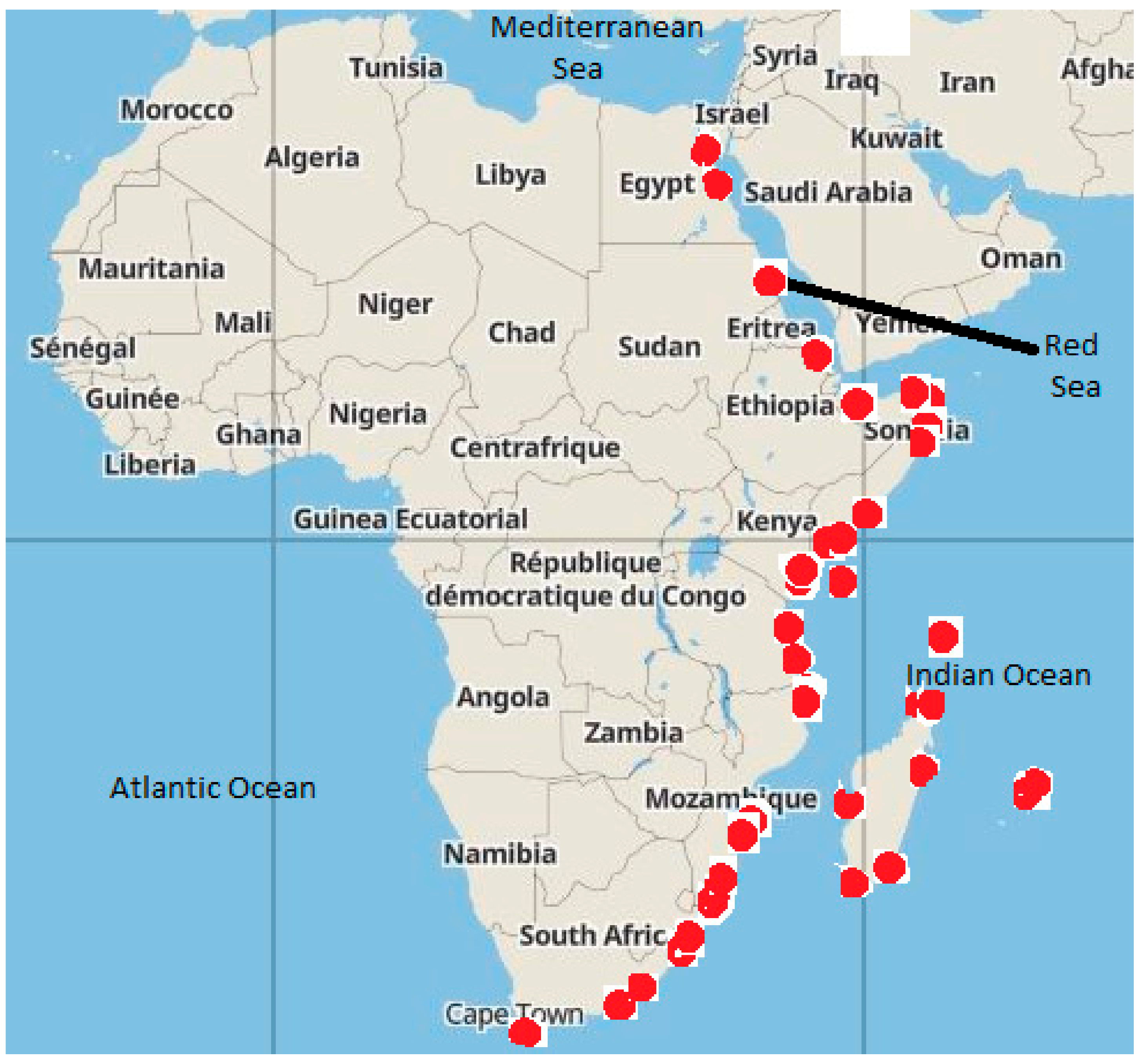
| Country | Local | HM Source |
|---|---|---|
| Egypt | Gulf of Suez [14] and Red Sea [14,275,276,277,278,279,280,281] | Drilling fluids in oil fields, tourism and port activities [14,275,276,277,278,279,280,281] |
| Sudan | Sawaki and Port-Sudan [282] | Port activities [282] |
| Eritrea | Assed and Massawa Ports | Port activities |
| Djibouti | Gulf of Tadjourah [283] | Port activities [283] |
| Somalia | Barawa, Berbera, Bosaso, Eyl, El-Mana, Eyl, Hobyo, Kismaayo, Las Khorey, Mareeg, Merca, Mogadishu, Qandala, Ras Kamboni and Zeila | Port activities |
| Kenya | Watamu, Mombassa and Kilindini harbors | Port and tourism activities [284,285] |
| Tanzania | Mwamba Nyama, Mwamba Wamba, Tanga, Zanzibar, Mbegani and the Dar es Salaam harbors | Port activities [286] and storm water runoff from the city roads and garages as a result of the previous usage of leaded petrol [23] |
| Madagascar | Antsiranana, Mahajanga, Morondava, Toamasina, Tolagnaroand Toliara Ports | Port activities |
| Mozambique | Maputo and Nacala Ports | Port activities |
| Rovuma River Basin | Gas production activities | |
| South Africa | Durban, Elisabeth, Richards Bay, Mossel Bay, Ngqura, East London and Cape Town harbors | Port activities [287] |
| Country | Local | Date | Seafood | Heavy Metal | Concentration, mgKg−1 | Detection | Reference |
|---|---|---|---|---|---|---|---|
| Egypt | Hourghada, Suez and Ismaila | 1986–1989 | Unknown fish species | Cd | 1.24 | AAS | [293] |
| Pb | 0.82 | ||||||
| Gulf of Suez | 2004 | Tridacna Maxima shell (bivalve mollusk) | Cd | 35.83 | AAS | [14] | |
| Pb | 1.79 | ||||||
| Hurghada harbor | Cd | 44.65 | |||||
| Pb | 1.76 | ||||||
| El-Esh | Cd | 39.45 | |||||
| Pb | 1.65 | ||||||
| Suez | 2014 | Erugosquilla massavensis muscle (crustacean) | Hg | 4.91 | AAS | [294] | |
| Pb | 11.49 | ||||||
| Cd | 0.57 | ||||||
| Ismailia | Hg | 4.00 | |||||
| Pb | 9.70 | ||||||
| Cd | 0.44 | ||||||
| Lake Timsah | 2006–2007 | Ruditapes decussatus soft tissue (mollusk) | Cd | 5.00 | FAAS | [281] | |
| Pb | 16.50 | ||||||
| Venerupis pullastra soft tissue (mollusk) | Cd | 9.30 | |||||
| Pb | 19.00 | ||||||
| Gulf of Suez | 2009 | Patella nigrolineata soft tissue (bivalve mollusk) | Pb | 16.89 | FAAS | [289] | |
| Cd | 0.46 | ||||||
| Hg | 0.11 | ||||||
| Ostrea crestata soft tissue (bivalve mollusk) | Pb | 1.03 | |||||
| Cd | 0.31 | ||||||
| Hg | 0.03 | ||||||
| Tridacna squamosa soft tissue (bivalve mollusk) | Pb | 0.35 | |||||
| Cd | 0.36 | ||||||
| Hg | 0.01 | ||||||
| Nerita waigiensis soft tissue (bivalve mollusk) | Pb | 14.48 | |||||
| Cd | 0.37 | ||||||
| Hg | 0.03 | ||||||
| Lepidochiton cinereus soft tissue (bivalve mollusk) | Pb | 1.50 | |||||
| Cd | 0.35 | ||||||
| Hg | 0.02 | ||||||
| Morula squamosa soft tissue (bivalve mollusk) | Pb | 1.16 | |||||
| Cd | 0.47 | ||||||
| Hg | 0.11 | ||||||
| Brachidontes sp. soft tissue (bivalve mollusk) | Pb | 2.35 | |||||
| Cd | 0.72 | ||||||
| Hg | 0.11 | ||||||
| Dinocardum robustum vanhyningi soft tissue (bivalve mollusk) | Pb | 0.88 | |||||
| Cd | 0.04 | ||||||
| Hg | 0.01 | ||||||
| Brachidontes sp. soft tissue (bivalve mollusk) | Pb | 0.75 | |||||
| Cd | 0.05 | ||||||
| Hg | 0.05 | ||||||
| Nassarius clathratus soft tissue (bivalve mollusk) | Pb | 1.03 | |||||
| Cd | 0.10 | ||||||
| Hg | 0.02 | ||||||
| Patella testudinária soft tissue (bivalve mollusk) | Pb | 1.05 | |||||
| Cd | 0.06 | ||||||
| Hg | 0.01 | ||||||
| Hurghada Sheraton | Nerita waigiensis soft tissue (bivalve mollusk) | Pb | 1.55 | ||||
| Cd | 0.11 | ||||||
| Hg | 0.01 | ||||||
| Safaga | Lepidochiton cinereus soft tissue (bivalve mollusk) | Pb | 1.62 | ||||
| Cd | 0.19 | ||||||
| Hg | 0.03 | ||||||
| Morula squamosa soft tissue (bivalve mollusk) | Pb | 11.26 | |||||
| Cd | 0.36 | ||||||
| Hg | 0.15 | ||||||
| Tridacna squamosa soft tissue (bivalve mollusk) | Pb | 3.19 | |||||
| Cd | 0.29 | ||||||
| Hg | 0.21 | ||||||
| Quseir | Psammobia depressa soft tissue (bivalve mollusk) | Pb | 1.03 | ||||
| Cd | 1.72 | ||||||
| Hg | 0 | ||||||
| Nerita peloronta soft tissue (bivalve mollusk) | Pb | 14.3 | |||||
| Cd | 0.58 | ||||||
| Hg | 0.01 | ||||||
| Marsa Alam | Nerita peloronta soft tissue (bivalve mollusk) | Pb | 0.24 | ||||
| Cd | 0.12 | ||||||
| Hg | 0.4 | ||||||
| Morula squamosa soft tissue (bivalve mollusk) | Pb | 1.84 | |||||
| Cd | 0.63 | ||||||
| Hg | 0.15 | ||||||
| Lepidochiton cinereus soft tissue (bivalve mollusk) | Pb | 1.87 | |||||
| Cd | 0.78 | ||||||
| Hg | 0.1 | ||||||
| Shalatin | Nerita undata soft tissue (bivalve mollusk) | Pb | 0.25 | ||||
| Cd | 0.05 | ||||||
| Hg | 0 | ||||||
| Gulf of Suez | 2003 | Barbatus barbatus soft tissue (mollusk) | Pb | 126.74 | FAAS | [19] | |
| Cd | 3.02 | ||||||
| Patella caerulea soft tissue (mollusk) | Pb | 147.55 | |||||
| Cd | 3.74 | ||||||
| Hurghada | Barbatus barbatus soft tissue (mollusk) | Pb | 9.8 | ||||
| Cd | 1.06 | ||||||
| Patella caerulea soft tissue (mollusk) | Pb | 16.78 | |||||
| Cd | 1.33 | ||||||
| Gulf of Suez | 2010–2011 | Epinephelus sp. muscle (fish) | Pb | 0.45 | AAS | [290] | |
| Cd | 0.20 | ||||||
| Synodus sp. Muscle (fish) | Pb | 0.28 | |||||
| Cd | 0.04 | ||||||
| Nemipterus japonicus muscle (fish) | Pb | 0.28 | |||||
| Cd | 0.04 | ||||||
| Sardinella sp. muscle (fish) | Pb | 0.50 | |||||
| Cd | 0.38 | ||||||
| Trachurus mediterraneus muscle (fish) | Pb | 0.40 | |||||
| Cd | 0.20 | ||||||
| Lethrinus sp. muscle (fish) | Pb | 0.25 | |||||
| Cd | 0.23 | ||||||
| Shalateen | Epinephelus sp. muscle (fish) | Pb | 0.88 | ||||
| Cd | 0.12 | ||||||
| Caranx sp. muscle (fish) | Pb | 0.28 | |||||
| Cd | 0.07 | ||||||
| Scarus gibbus muscle (fish) | Pb | 0.21 | |||||
| Cd | 0.03 | ||||||
| Synodus sp. muscle (fish) | Pb | 0.51 | |||||
| Cd | 0.07 | ||||||
| Nemipterus japonicus muscle (fish) | Pb | 0.46 | |||||
| Cd | 0.06 | ||||||
| Carangoides bajad muscle (fish) | Pb | 0.52 | |||||
| Cd | 0.08 | ||||||
| Lutjanus bohar muscle (fish) | Pb | 0.51 | |||||
| Cd | 0.08 | ||||||
| Thunnus albacares muscle (fish) | Pb | 0.32 | |||||
| Cd | 0.06 | ||||||
| Gerres oyena muscle (fish) | Pb | 0.41 | |||||
| Cd | 0.11 | ||||||
| Sargocentron spiniferum muscle (fish) | Pb | 0.28 | |||||
| Cd | 0.06 | ||||||
| Hurghada | Epinephelus sp. muscle (fish) | Pb | 0.45 | ||||
| Cd | 0.05 | ||||||
| Caranx sp. muscle (fish) | Pb | 0.25 | |||||
| Cd | 0.05 | ||||||
| Scarus gibbus muscle (fish) | Pb | 0.24 | |||||
| Cd | 0.03 | ||||||
| Sardinella sp. muscle (fish) | Pb | 0.25 | |||||
| Cd | 0.07 | ||||||
| Siganus rivulatus muscle (fish) | Pb | 0.04 | |||||
| Cd | 0.05 | ||||||
| Djibouti | Gulf of Tadjoura | 2016–2018 | Sphyrna lewini (shark) | Cd | 0.48 | ICP - MS | [288] |
| Hg | 12.51 | ||||||
| Pb | 0.08 | ||||||
| Rhizoprionodon acutus (shark) | Cd | 14.50 | |||||
| Hg | 0.68 | ||||||
| Pb | 0.16 | ||||||
| Kenya | Mombasa | 1997–1998 | Sardinella gibbosa n muscle (fish) | Cd | 4.8 | FAAS | [284] |
| Pb | 3.4 | ||||||
| Leiognathus equula muscle (fish) | Cd | 18.5 | |||||
| Pb | 0.76 | ||||||
| Upeneus spp muscle (fish) | Cd | 6.0 | |||||
| Pb | 0.4 | ||||||
| Lutjanus fulviflamma muscle (fish) | Cd | 2.0 | |||||
| Pb | 0.2 | ||||||
| Sphraena jello muscle (fish) | Cd | 9.8 | |||||
| Pb | 0.09 | ||||||
| Monodactylus argenteus muscle (fish) | Cd | 1.6 | |||||
| Pb | 0.2 | ||||||
| Secutor insidiator muscle (fish) | Cd | 1.2 | |||||
| Pb | 0.2 | ||||||
| Mugil múgil muscle (fish) | Cd | 5.0 | |||||
| Pb | 0.1 | ||||||
| Carangoides gymnostethus muscle (fish) | Cd | 1.6 | |||||
| Pb | 0.2 | ||||||
| Geres oyena muscle (fish) | Cd | 1.0 | |||||
| Pb | nd | ||||||
| Crenidens crenidens muscle (fish) | Cd | 1.4 | |||||
| Pb | 0.2 | ||||||
| Chorinemus tol muscle (fish) | Cd | 2.2 | |||||
| Pb | 0.4 | ||||||
| Leptoscarus vaigiensis muscle (fish) | Cd | 5.8 | |||||
| Pb | 0.2 | ||||||
| Spilotichthys pictus muscle (fish) | Cd | 4.8 | |||||
| Pb | 0.15 | ||||||
| Siganus sutor muscle (fish) | Cd | 6.0 | |||||
| Pb | 0.2 | ||||||
| Lenthrinus sp. muscle (fish) | Cd | 1.0 | |||||
| Pb | 0.2 | ||||||
| Therapon jarbu muscle (fish) | Cd | 0.6 | |||||
| Pb | 0.04 | ||||||
| Tanzania | Dar es Salaam | 2015 | Siganus sutor muscle (fish) | Cd | 0.04 | HR-ICP-MS | [295] |
| Pb | 0.045 | ||||||
| Lethrinus harak muscle (fish) | Cd | 0.14 | |||||
| Pb | 0.144 | ||||||
| Rastrelliger Kanagurta muscle (fish) | Cd | 0.13 | |||||
| Pb | 0.067 | ||||||
| Dar es Salaam | 2013 | Octopus cyanea muscle (octopus) | Pb | 7.22 | ICP-AES | [286] | |
| Tanga | Pb | 3.24 | |||||
| Dar es Salaam | 2007–2008 | Saccostrea Cucullate soft tissue (oyster) | Cd | 1.00 | ICP-OES | [23] | |
| Pb | 2.00 | ||||||
| Hg | 0.081 | SAHgA | |||||
| Dar es Salaam | 2016 | Rastrellieger kanagurta muscle (fish) | Pb | 0.03 | FAAS | [2] | |
| Cd | 0.06 | ||||||
| Lutjanus fulvus muscle (fish) | Pb | 0.14 | |||||
| Cd | 0.16 | ||||||
| Fenneropenaeus indicus muscle (fish) | Pb | 0.06 | |||||
| Cd | 0.01 | ||||||
| Zanzibar | 2011 | Anadara antiquata muscle (fish) | Pb | 3.5 | FAAS | [291] | |
| Mozambique Channel | East of Madagascar | 2004 | Xiphias gladius muscle (fish) | Cd | 0.60 | ICP-AES | [292] |
| Pb | 0.01 | ||||||
| Hg | 3.97 | AHgA | |||||
| Thunnus albacares muscle (fish) | Cd | 0.26 | ICP-AES | ||||
| Pb | 0.02 | ||||||
| Hg | 1.15 | AHgA | |||||
| Katsuwonus pelamis muscle (fish) | Cd | 0.61 | ICP-AES | ||||
| Pb | 0.07 | ||||||
| Hg | 0.67 | AHgA | |||||
| Coryphaena hippurus muscle (fish) | Cd | 0.13 | ICP-AES | ||||
| Pb | 0.06 | ||||||
| Hg | 0.21 | AHgA | |||||
| Northern part | Xiphias gladius muscle (fish) | Cd | 1.04 | ICP-AES | |||
| Pb | 0.12 | ||||||
| Hg | 1.61 | AHgA | |||||
| Thunnus albacares muscle (fish) | Cd | 0.25 | ICP-AES | ||||
| Pb | 0.09 | ||||||
| Hg | 0.56 | AHgA | |||||
| Coryphaena hippurus muscle (fish) | Cd | 0.12 | ICP-AES | ||||
| Pb | 0.14 | ||||||
| Hg | 0.98 | AHgA | |||||
| South Africa | Cape Town | 2011 | Mytilus galloprovincialis (bivalve mollusk) | Cd | 1.99 | ICP - MS | [287] |
| Pb | 7.30 | ||||||
| Hg | 4.93 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tamele, I.J.; Vázquez Loureiro, P. Lead, Mercury and Cadmium in Fish and Shellfish from the Indian Ocean and Red Sea (African Countries): Public Health Challenges. J. Mar. Sci. Eng. 2020, 8, 344. https://doi.org/10.3390/jmse8050344
Tamele IJ, Vázquez Loureiro P. Lead, Mercury and Cadmium in Fish and Shellfish from the Indian Ocean and Red Sea (African Countries): Public Health Challenges. Journal of Marine Science and Engineering. 2020; 8(5):344. https://doi.org/10.3390/jmse8050344
Chicago/Turabian StyleTamele, Isidro José, and Patricia Vázquez Loureiro. 2020. "Lead, Mercury and Cadmium in Fish and Shellfish from the Indian Ocean and Red Sea (African Countries): Public Health Challenges" Journal of Marine Science and Engineering 8, no. 5: 344. https://doi.org/10.3390/jmse8050344
APA StyleTamele, I. J., & Vázquez Loureiro, P. (2020). Lead, Mercury and Cadmium in Fish and Shellfish from the Indian Ocean and Red Sea (African Countries): Public Health Challenges. Journal of Marine Science and Engineering, 8(5), 344. https://doi.org/10.3390/jmse8050344





