Phytoplankton Dynamics in the Middle Adriatic Estuary, with a Focus on the Potentially Toxic Genus Pseudo-nitzschia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area and Sampling
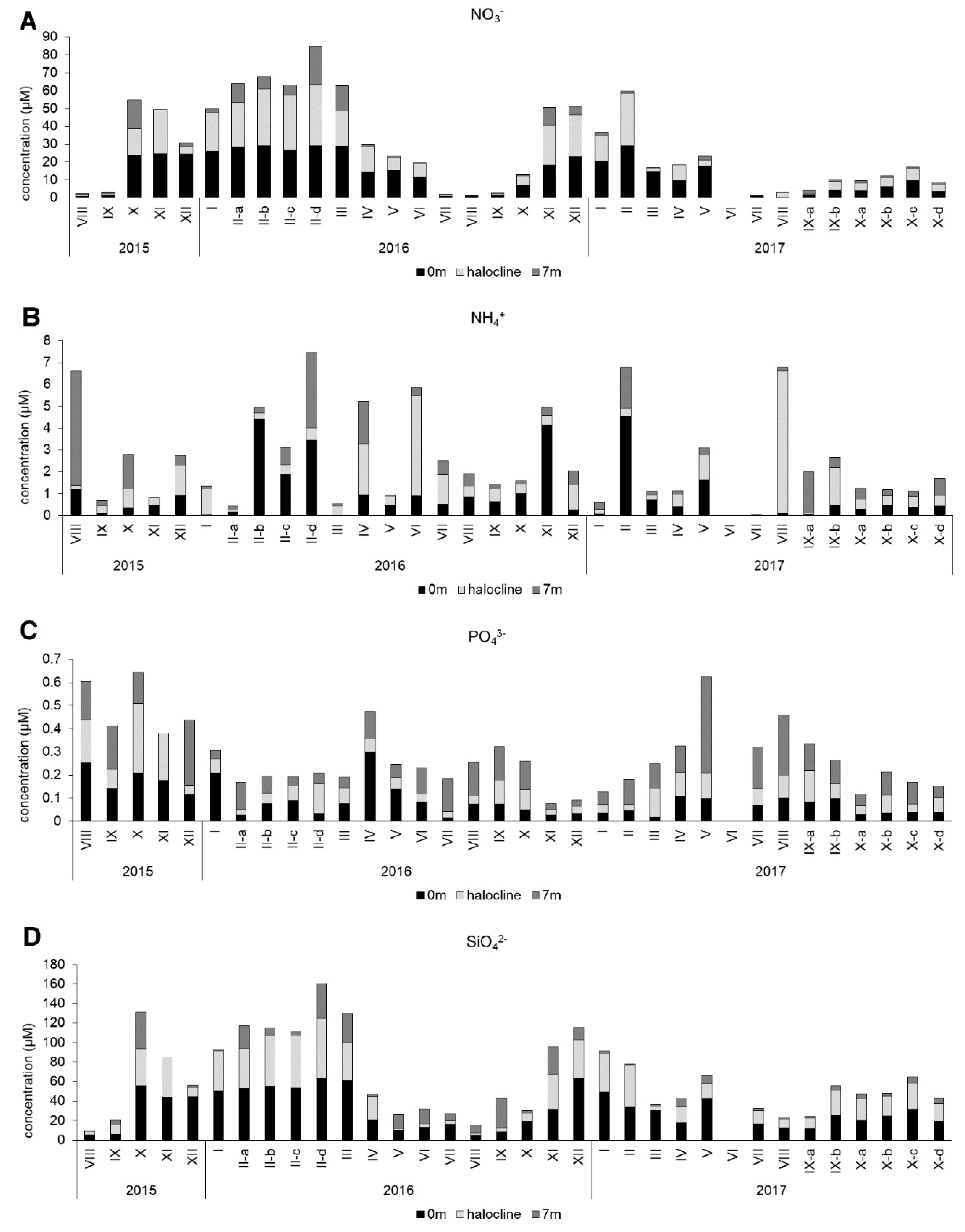
2.2. Environmental Parameters and Shellfish Toxin Analyses
2.3. Phytoplankton Analyses (LM and SEM)
2.4. Statistical Analyses
3. Results
3.1. Phytoplankton Community Structure
3.2. Pseudo-nitzschia Assemblage Seasonality and Diversity
3.3. Shellfish Toxicity
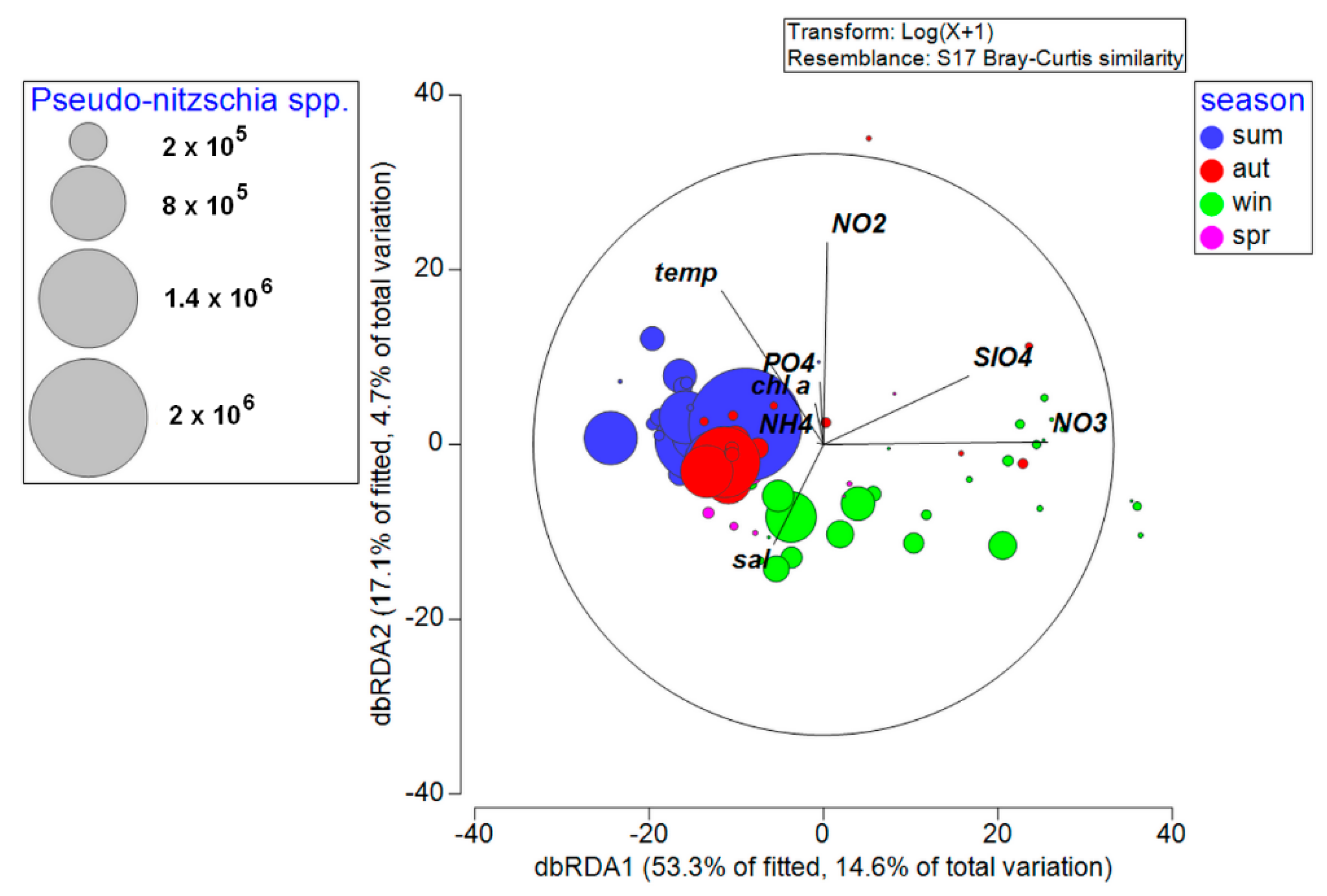
3.4. Environmental Factors Associated with Phytoplankton Community
4. Discussion
4.1. Phytoplankton Community
4.2. Pseudo-nitzschia Assemblage
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lancelot, C.; Muylaert, K. Trends in Estuarine Phytoplankton Ecology; Elsevier Inc.: Amsterdam, The Netherlands, 2012; Volume 7. [Google Scholar] [CrossRef]
- Denant, V.; Saliot, A.; Mantoura, R.F.C. Distribution of Algal Chlorophyll and Carotenoid Pigments in a Stratified Estuary: The Krka River, Adriatic Sea. Mar. Chem. 1991, 32, 285–297. [Google Scholar] [CrossRef]
- Viličić, D.; Legović, T.; Žutić, V. Vertical Distribution of Phytoplankton in a Stratified Estuary. Aquat. Sci. 1989, 51, 31–46. [Google Scholar] [CrossRef]
- Cetinić, I.; Viličić, D.; Burić, Z.; Olujić, G. Phytoplankton Seasonality in a Highly Stratified Karstic Estuary (Krka, Adriatic Sea). Hydrobiologia 2006, 555, 31–40. [Google Scholar] [CrossRef]
- Svensen, C.; Viličić, D.; Wassmann, P.; Arashkevich, E.; Ratkova, T. Plankton Distribution and Vertical Flux of Biogenic Matter during High Summer Stratification in the Krka Estuary (Eastern Adriatic). Estuar. Coast. Shelf Sci. 2007, 71, 381–390. [Google Scholar] [CrossRef]
- Ninčević-Gladan, Ž.; Skejić, S.; Bužančić, M.; Marasović, I.; Arapov, J.; Ujević, I.; Bojanić, N.; Grbec, B.; Kušpilić, G.; Vidjak, O. Seasonal Variability in Dinophysis spp. Abundances and Diarrhetic Shellfish Poisoning Outbreaks along the Eastern Adriatic Coast. Bot. Mar. 2008, 51, 449–463. [Google Scholar] [CrossRef] [Green Version]
- Arapov, J.; Ujević, I.; Ninčević Gladan, Ž.; Skejić, S.; Ceredi, A.; Milandri, A.; Pigozzi, S.; Riccardi, E.; Vilar-González, A.; Rodríguez-Velasco, M.L.; et al. Shellfish Lipophilic Toxin Profile and Toxic Phytoplankton Species along Eastern Adriatic Coast. Fresenius Environ. Bull. 2015, 24, 4799–4806. [Google Scholar]
- Ujević, I.; Ninčević-Gladan, Ž.; Roje, R.; Skejić, S.; Arapov, J.; Marasović, I. Domoic Acid-A New Toxin in the Croatian Adriatic Shellfish Toxin Profile. Molecules 2010, 15, 6835–6849. [Google Scholar] [CrossRef] [Green Version]
- Arapov, J.; Ujević, I.; Pfannkuchen, D.M.; Godrijan, J.; Bakrač, A.; Ninčević Gladan, Ž.; Marasović, I. Domoic Acid in Phytoplankton Net Samples and Shellfish from the Krka River Estuary in the Central Adriatic Sea. Mediterr. Mar. Sci. 2016, 17, 340–350. [Google Scholar] [CrossRef]
- Viličić, D.; Djakovac, T.; Burić, Z.; Bosak, S. Composition and Annual Cycle of Phytoplankton Assemblages in the Northeastern Adriatic Sea. Bot. Mar. 2009, 52, 291–305. [Google Scholar] [CrossRef]
- Bužančić, M.; Ninčević Gladan, Ž.; Marasović, I.; Kušpilić, G.; Grbec, B.; Matijević, S. Population Structure and Abundance of Phytoplankton in Three Bays on the Eastern Adriatic Coast: Šibenik Bay, Kaštela Bay and Mali Ston Bay. Acta Adriat. 2012, 53, 413–435. [Google Scholar]
- Marić, D.; Kraus, R.; Godrijan, J.; Supić, N.; Djakovac, T.; Precali, R. Phytoplankton Response to Climatic and Anthropogenic Influences in the North-Eastern Adriatic during the Last Four Decades. Estuar. Coast. Shelf Sci. 2012, 115, 98–112. [Google Scholar] [CrossRef]
- Ninčević Gladan, Ž.; Matić, F.; Arapov, J.; Skejić, S.; Bužančić, M.; Bakrač, A.; Straka, M.; Dekneudt, Q.; Grbec, B.; Garber, R.; et al. The Relationship between Toxic Phytoplankton Species Occurrence and Environmental and Meteorological Factors along the Eastern Adriatic Coast. Harmful Algae 2020, 92, 101745. [Google Scholar] [CrossRef] [PubMed]
- Turk Dermastia, T.; Cerino, F.; Stanković, D.; Francé, J.; Ramšak, A.; Žnidarič Tušek, M.; Beran, A.; Natali, V.; Cabrini, M.; Mozetič, P. Ecological Time Series and Integrative Taxonomy Unveil Seasonality and Diversity of the Toxic Diatom Pseudo-nitzschia H. Peragallo in the Northern Adriatic Sea. Harmful Algae 2020, 93. [Google Scholar] [CrossRef] [PubMed]
- Zingone, A.; Escalera, L.; Aligizaki, K.; Fernández-Tejedor, M.; Ismael, A.; Montresor, M.; Mozetič, P.; Taş, S.; Totti, C. Toxic Marine Microalgae and Noxious Blooms in the Mediterranean Sea: A Contribution to the Global HAB Status Report. Harmful Algae 2020, 101843. [Google Scholar] [CrossRef]
- Lelong, A.; Hégaret, H.; Soudant, P.; Bates, S.S. Pseudo-nitzschia (Bacillariophyceae) Species, Domoic Acid and Amnesic Shellfish Poisoning: Revisiting Previous Paradigms. Phycologia 2012, 51, 168–216. [Google Scholar] [CrossRef] [Green Version]
- Gržetić, Z.; Precali, R.; Degobbis, D.; Škrivanić, A. Nutrient Enrichment and Phytoplankton Response in an Adriatic Karstic Estuary. Mar. Chem. 1991, 32, 313–331. [Google Scholar] [CrossRef]
- Vidjak, O.; Bojanić, N.; Matijević, S.; Kušpilić, G.; Ninčević Gladan, Z.; Skejić, S.; Grbec, B.; Brautović, I. Environmental Drivers of Zooplankton Variability in the Coastal Eastern Adriatic (Mediterranean Sea). Acta Adriat. 2012, 53, 243–261. [Google Scholar]
- Legović, T.; Žutić, V.; Gržetić, Z.; Cauwet, G.; Precali, R.; Viličić, D. Eutrophication in the Krka Estuary. Mar. Chem. 1994, 46, 203–215. [Google Scholar] [CrossRef]
- Schlitzer, R. Ocean Data View. 2018. Available online: https://odv.awi.de (accessed on 18 June 2020).
- Strickland, J.D.H.; Parsons, T.R. A Practical Handbook of Seawater Analyses; Bulletin Fisheries Research Board of Canada 167; Fisheries Reasearch Board of Canada: Ottawa, ON, Canada, 1972; p. 311. [Google Scholar]
- Grasshoff, K.; Kremling, K.; Ehrhardt, M. Methods of Seawater Analysis; Wiley-VCH: Weinheim, Germany, 1999; pp. 159–228. [Google Scholar]
- Quilliam, M.A. A Rapid Extraction and Cleanup Procedure for the Liquid Chromatographic Determination of Domoic Acid in Unsalted Seafood. J. AOAC Int. 1995, 78, 543–554. [Google Scholar] [CrossRef]
- Edler, L.; Elbrächter, M. The Utermöhl Method for Quantitative Phytoplankton Analysis. In Microscopic and Molecular Methods for Quantitative Phytoplankton Analysis; Karlson, B., Cusack, C., Bresnan, E., Eds.; Intergovernmental Oceanographic Commission of UNESCO: Paris, France, 2010; pp. 13–20. [Google Scholar]
- Arapov, J.; Skejić, S.; Bužančić, M.; Bakrač, A.; Vidjak, O.; Bojanić, N.; Ujević, I.; Ninčević Gladan, Ž. Taxonomical Diversity of Pseudo-nitzschia from the Central Adriatic Sea. Phycol. Res. 2017, 65. [Google Scholar] [CrossRef]
- Skejić, S.; Arapov, J.; Bužančić, M.; Ninčević-Gladan, Ž.; Bakrač, A.; Straka, M.; Mandić, J. First Evidence of Intensive Bloom of Coccolithophore Syracosphaera halldalii in a Highly Variable Estuarine Environment. 2020; In Review. [Google Scholar]
- Arapov, J.; Bužančić, M.; Penna, A.; Casabianca, S.; Capellacci, S.; Andreoni, F.; Skejić, S.; Bakrač, A.; Straka, M.; Mandić, J.; et al. High Proliferation of Pseudo-nitzschia cf. arenysensis in the Adriatic Sea: Ecological and Morphological Characterisation. 2020; In Review. [Google Scholar]
- Šupraha, L.; Ljubešić, Z.; Mihanović, H.; Henderiks, J. Coccolithophore Life-Cycle Dynamics in a Coastal Mediterranean Ecosystem: Seasonality and Species-Specific Patterns. J. Plankton Res. 2016, 38, 1178–1193. [Google Scholar] [CrossRef] [Green Version]
- Cerino, F.; Fornasaro, D.; Kralj, M.; Giani, M.; Cabrini, M. Phytoplankton Temporal Dynamics in the Coastal Waters of the North-Eastern Adriatic Sea (Mediterranean Sea) from 2010 to 2017. Nat. Conserv. 2019, 34, 343–372. [Google Scholar] [CrossRef]
- Ljubešić, Z.; Bosak, S.; Viličić, D.; Borojević, K.K.; Marić, D.; Godrijan, J.; Ujević, I.; Peharec, P.; Dakovac, T. Ecology and Taxonomy of Potentially Toxic Pseudo-nitzschia Species in Lim Bay (North-Eastern Adriatic Sea). Harmful Algae 2011, 10, 713–722. [Google Scholar] [CrossRef]
- Giani, M.; Djakovac, T.; Degobbis, D.; Cozzi, S.; Solidoro, C.; Umani, S.F. Recent Changes in the Marine Ecosystems of the Northern Adriatic Sea. Estuar. Coast. Shelf Sci. 2012, 115, 1–13. [Google Scholar] [CrossRef]
- Skejić, S.; Bojanić, N.; Matijević, S.; Vidjak, O.; Grbeć, B.; Ninčević, G.; Šestanović, S.; Šantić, D. Analysis of the Phytoplankton Community in the Vicinity of Domestic Sewage Outflow during Stratified Conditions. Mediterr. Mar. Sci. 2014, 15, 574–586. [Google Scholar] [CrossRef] [Green Version]
- Totti, C.; Romagnoli, T.; Accoroni, S.; Coluccelli, A.; Pellegrini, M.; Campanelli, A.; Grilli, F.; Marini, M. Phytoplankton Communities in the Northwestern Adriatic Sea: Interdecadal Variability over a 30-Years Period (1988–2016) and Relationships with Meteoclimatic Drivers. J. Mar. Syst. 2019, 193, 137–153. [Google Scholar] [CrossRef]
- Mozetič, P.; Fonda Umani, S.; Cataletto, B.; Malej, A. Seasonal and Inter-Annual Plankton Variability in the Gulf of Trieste (Northern Adriatic). ICES J. Mar. Sci. 1998, 55, 711–722. [Google Scholar] [CrossRef] [Green Version]
- Caroppo, C.; Fiocca, A.; Sammarco, P.; Magazzu, G. Seasonal Variations of Nutrients and Phytoplankton in the Coastal SW Adriatic Sea (1995–1997). Bot. Mar. 1999, 42, 389–400. [Google Scholar] [CrossRef]
- Houdan, A.; Probert, I.; Zatylny, C.; Véron, B.; Billard, C. Ecology of Oceanic Coccolithophores. I. Nutritional Preferences of the Two Stages in the Life Cycle of Coccolithus braarudii and Calcidiscus leptoporus. Aquat. Microb. Ecol. 2006, 44, 291–301. [Google Scholar] [CrossRef] [Green Version]
- Marasović, I. Occurence of Prorocentrum minimum in the Adriatic Sea. In Rapports et Proces-Verbaux des Reunions; Commission International pour l’exploration Scientifique de la mer Méditerranée: Monaco, 1986; p. 186. [Google Scholar]
- Marasović, I.; Pucher-Petković, T.; Petrova-Karadjova, V. Prorocentrum minimum (Dinophyceae) in the Adriatic and Black Sea. J. Mar. Biol. Assoc. UK 1990, 70, 473–476. [Google Scholar] [CrossRef]
- Giacobbe, M.G.; Oliva, F.; La Ferla, R.; Puglisi, A.; Crisafi, E.; Maimone, G. Potentially Toxic Dinoflagellates in Mediterranean Waters (Sicily) and Related Hydrobiological Conditions. Aquat. Microb. Ecol. 1995, 9, 63–68. [Google Scholar] [CrossRef]
- Burić, Z.; Viličić, D.; Caput Mihalić, K.; Carić, M.; Kralj, K.; Ljubešić, N. Pseudo-nitzschia Blooms in the Zrmanja River Estuary (Eastern Adriatic Sea). Diatom Res. 2008, 23, 51–63. [Google Scholar] [CrossRef]
- Godrijan, J.; Marić, D.; Tomažić, I.; Precali, R.; Pfannkuchen, M. Seasonal Phytoplankton Dynamics in the Coastal Waters of the North-Eastern Adriatic Sea. J. Sea Res. 2013, 77, 32–44. [Google Scholar] [CrossRef] [Green Version]
- Penna, A.; Ingarao, C.; Ercolessi, M.; Rocchi, M.; Penna, N. Potentially Harmful Microalgal Distribution in an Area of the NW Adriatic Coastline: Sampling Procedure and Correlations with Environmental Factors. Estuar. Coast. Shelf Sci. 2006, 70, 307–316. [Google Scholar] [CrossRef]
- Cabrini, M.; Fornasaro, D.; Cossarini, G.; Lipizer, M.; Virgilio, D. Phytoplankton Temporal Changes in a Coastal Northern Adriatic Site during the Last 25 Years. Estuar. Coast. Shelf Sci. 2012, 115, 113–124. [Google Scholar] [CrossRef]
- Penna, A.; Casabianca, S.; Perini, F.; Bastianini, M.; Riccardi, E.; Pigozzi, S.; Scardi, M. Toxic Pseudo-nitzschia spp. in the Northwestern Adriatic Sea: Characterisation of Species Composition by Genetic and Molecular Quantitative Analyses. J. Plankton Res. 2013, 35, 352–366. [Google Scholar] [CrossRef] [Green Version]
- Rijal Leblad, B.; Lundholm, N.; Goux, D.; Veron, B.; Sagou, R.; Taleb, H.; Nhhala, H.; Er-Raioui, H. Pseudo-nitzschia Peragallo (Bacillariophyceae) Diversity and Domoic Acid Accumulation in Tuberculate Cockles and Sweet Clams in M’diq Bay, Morocco. Acta Bot. Croat. 2013, 72, 35–47. [Google Scholar] [CrossRef] [Green Version]
- Marić Phannkuchen, D. Potentially Toxic Diatom Genus Pseudo-nitzschia in the Northern Adriatic Sea: Ecological, Taxonomical and Molecular Features. Ph.D. Thesis, University of Zagreb, Faculty of Science, Zagreb, Croatia, 2013; p. 195. [Google Scholar]
- Quiroga, I. Pseudo-nitzschia Blooms in the Bay of Banyuls-Sur-Mer, Northwestern Mediterranean Sea. Diatom Res. 2006, 21, 91–104. [Google Scholar] [CrossRef]
- Marić, D.; Ljubešić, Z.; Godrijan, J.; Viličić, D.; Ujević, I.; Precali, R. Blooms of the Potentially Toxic Diatom Pseudo-nitzschia calliantha Lundholm, Moestrup & Hasle in Coastal Waters of the Northern Adriatic Sea (Croatia). Estuar. Coast. Shelf Sci. 2011, 92, 323–331. [Google Scholar] [CrossRef]
- Sahraoui, I.; Hlaili, A.S.; Mabrouk, H.H.; Léger, C.; Bates, S.S. Blooms of the Diatom Genus Pseudo-nitzschia H. Peragallo in Bizerte Lagoon (Tunisia, Sw Mediterranean). Diatom Res. 2009, 24, 175–190. [Google Scholar] [CrossRef]
- Skejić, S.; Marasović, I.; Ninčević-Gladan, Ž. Phytoplankton Assemblages at the Fish Farm in Maslinova Bay (Thr Island of Brač). Croat. J. Fish. 2012, 70, 41–52. [Google Scholar]
- Skejić, S.; Vilibić, I.; Matijević, S.; Jozić, S.; Gladan, Ž.N.; Morović, M.; Marasović, I.; Prelesnik, H. Long-Term Regulating Mechanisms of Phytoplankton Biomass in a Traditional Shellfish Aquaculture Area. Fresenius Environ. Bull. 2015, 24, 3001–3013. [Google Scholar]
- Sahraoui, I.; Grami, B.; Bates, S.S.; Bouchouicha, D.; Chikhaoui, M.A.; Mabrouk, H.H.; Hlaili, A.S. Response of Potentially Toxic Pseudo-nitzschia (Bacillariophyceae) Populations and Domoic Acid to Environmental Conditions in a Eutrophied, SW Mediterranean Coastal Lagoon (Tunisia). Estuar. Coast. Shelf Sci. 2012, 102, 95–104. [Google Scholar] [CrossRef]
- Quijano-Scheggia, S.; Garcés, E.; Sampedro, N.; van Lenning, K.; Flo, E.; Andree, K.; Fortuño, J.M.; Camp, J. Identification and Characterisation of the Dominant Pseudo-nitzschia Species (Bacillariophyceae) along the NE Spanish Coast (Catalonia, NW Mediterranean). Sci. Mar. 2008, 72, 343–359. [Google Scholar] [CrossRef] [Green Version]
- Moschandreou, K.K.; Nikolaidis, G. The Genus Pseudo-nitzschia (Bacillariophyceae) in Greek Coastal Waters. Bot. Mar. 2010, 53, 159–172. [Google Scholar] [CrossRef]
- Sahraoui, I.; Bates, S.S.; Bouchouicha, D.; Mabrouk, H.H.; Sakka Hlaili, A. Toxicity of Pseudo-nitzschia Populations from Bizerte Lagoon, Tunisia, Southwest Mediterranean, and First Report of Domoic Acid Production by P. Brasiliana. Diatom Res. 2011, 26, 293–303. [Google Scholar] [CrossRef]
- Ruggiero, M.V.; Sarno, D.; Barra, L.; Kooistra, W.H.C.F.; Montresor, M.; Zingone, A. Diversity and Temporal Pattern of Pseudo-nitzschia Species (Bacillariophyceae) through the Molecular Lens. Harmful Algae 2015, 42, 15–24. [Google Scholar] [CrossRef]
- Zingone, A.; Siano, R.; D’Alelio, D.; Sarno, D. Potentially Toxic and Harmful Microalgae from Coastal Waters of the Campania Region (Tyrrhenian Sea, Mediterranean Sea). Harmful Algae 2006, 5, 321–337. [Google Scholar] [CrossRef]
- Caroppo, C.; Congestri, R.; Bracchini, L.; Albertano, P. On the Presence of Pseudo-nitzschia calliantha Lundholm, Moestrup et Hasle and Pseudo-nitzschia delicatissima (Cleve) Heiden in the Southern Adriatic Sea (Mediterranean Sea, Italy). J. Plankton Res. 2005, 27, 763–774. [Google Scholar] [CrossRef]
- Quijano-Scheggia, S.; Garcés, E.; Andree, K.B.; De la Iglesia, P.; Diogène, J.; Fortuño, J.M.; Camp, J. Pseudo-nitzschia Species on the Catalan Coast: Characterisation and Contribution to the Current Knowledge of the Distribution of This Genus in the Mediterranean Sea. Sci. Mar. 2010, 74, 395–410. [Google Scholar] [CrossRef]
- Pistocchi, R.; Guerrini, F.; Pezzolesi, L.; Riccardi, M.; Vanucci, S.; Ciminiello, P.; Dell’Aversano, C.; Forino, M.; Fattorusso, E.; Tartaglione, L.; et al. Toxin Levels and Profiles in Microalgae from the North-Western Adriatic Sea 15 Years of Studies on Cultured Species. Mar. Drugs 2012, 10, 140–162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arapov, J.; Ujević, I.; Straka, M.; Skejić, S.; Bužančić, M.; Bakrač, A.; Ninčević-Gladan, Ž. First Evidence of Domoic Acid Production in Pseudo-nitzschia calliantha Cultures from the Central Adriatic Sea. 2020; In Review. [Google Scholar]
- Thorel, M.; Claquin, P.; Schapira, M.; Le Gendre, R.; Riou, P.; Goux, D.; Le Roy, B.; Raimbault, V.; Deton-Cabanillas, A.F.; Bazin, P.; et al. Nutrient Ratios Influence Variability in Pseudo-nitzschia Species Diversity and Particulate Domoic Acid Production in the Bay of Seine (France). Harmful Algae 2017, 68, 192–205. [Google Scholar] [CrossRef] [PubMed]
- Fehling, J.; Davidson, K.; Bolch, C.J.; Bates, S.S. Growth and Domoic Acid Production by Pseudo-nitzschia seriata (Bacillariophyceae) under Phosphate and Silicate Limitation. J. Phycol. 2004, 40, 674–683. [Google Scholar] [CrossRef]
- Macintyre, H.L.; Stutes, A.L.; Smith, W.L.; Dorsey, C.P.; Annabraham, A.; Dickey, R.W. Environmental Correlates of Community Composition and Toxicity during a Bloom of Pseudo-nitzschia spp. in the Northern Gulf of Mexico. J. Plankton Res. 2011, 33, 273–295. [Google Scholar] [CrossRef]
- Ninčević-Gladan, Ž.; Bužančić, M.; Kušpilić, G.; Grbec, B.; Matijević, S.; Skejić, S.; Marasović, I.; Morović, M. The Response of Phytoplankton Community to Anthropogenic Pressure Gradient in the Coastal Waters of the Eastern Adriatic Sea. Ecol. Indic. 2015, 56, 106–115. [Google Scholar] [CrossRef] [Green Version]
- Spatharis, S.; Danielidis, D.B.; Tsirtsis, G. Recurrent Pseudo-nitzschia calliantha (Bacillariophyceae) and Alexandrium insuetum (Dinophyceae) Winter Blooms Induced by Agricultural Runoff. Harmful Algae 2007, 6, 811–822. [Google Scholar] [CrossRef]
- Rezultati Sustavnog Istraživanja Kakvoće Prijelaznih i Priobalnih Voda u 2016. i 2017; Instititut za Oceanografiju i Ribarstvo: Split, Croatia; Institut Ruđer Bošković: Zagreb, Croatia; Nacionalni Laboratorij za Zdravlje, okolje in hrano: Maribor, Slovenia, 2017; p. 318. (In Croatian)

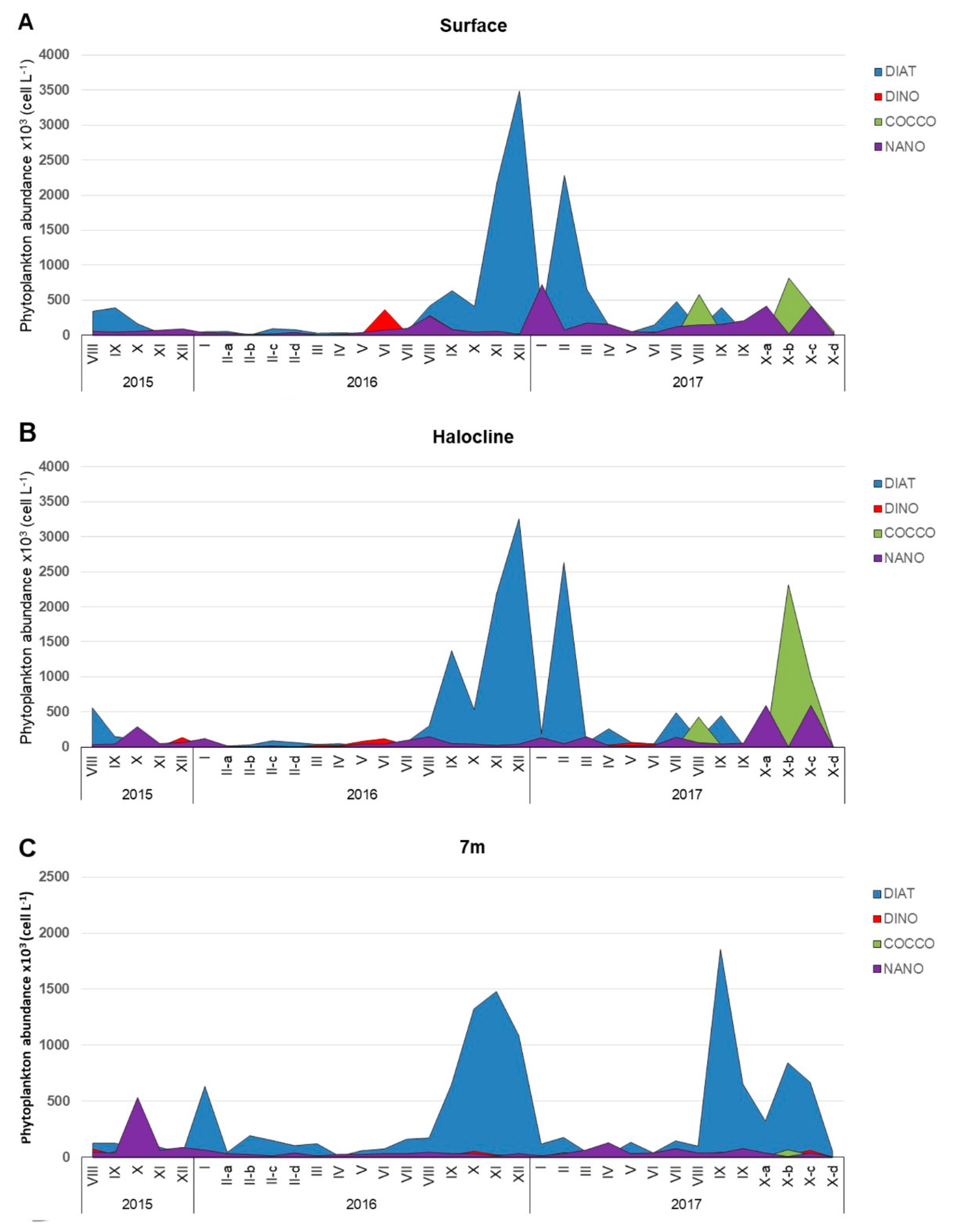
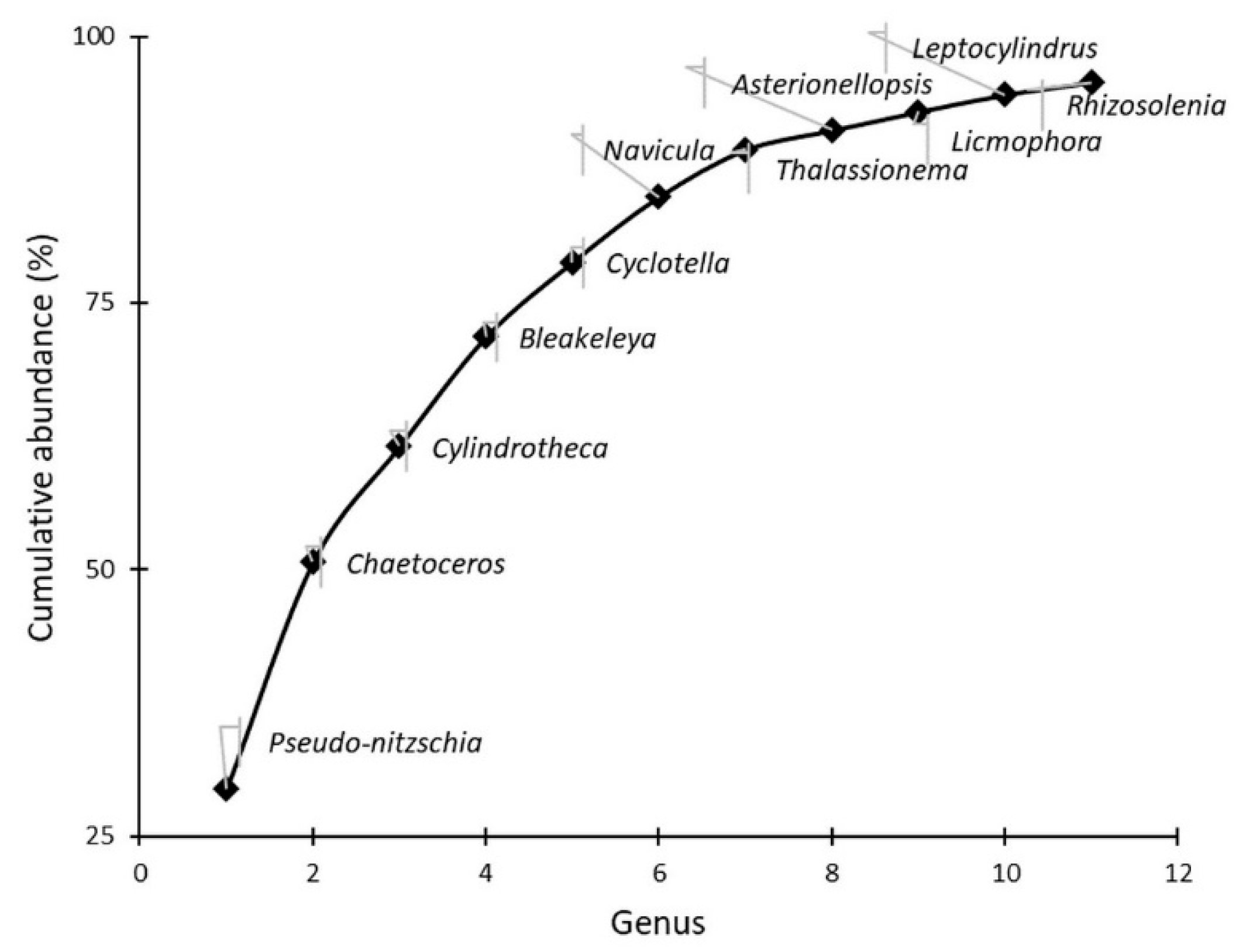

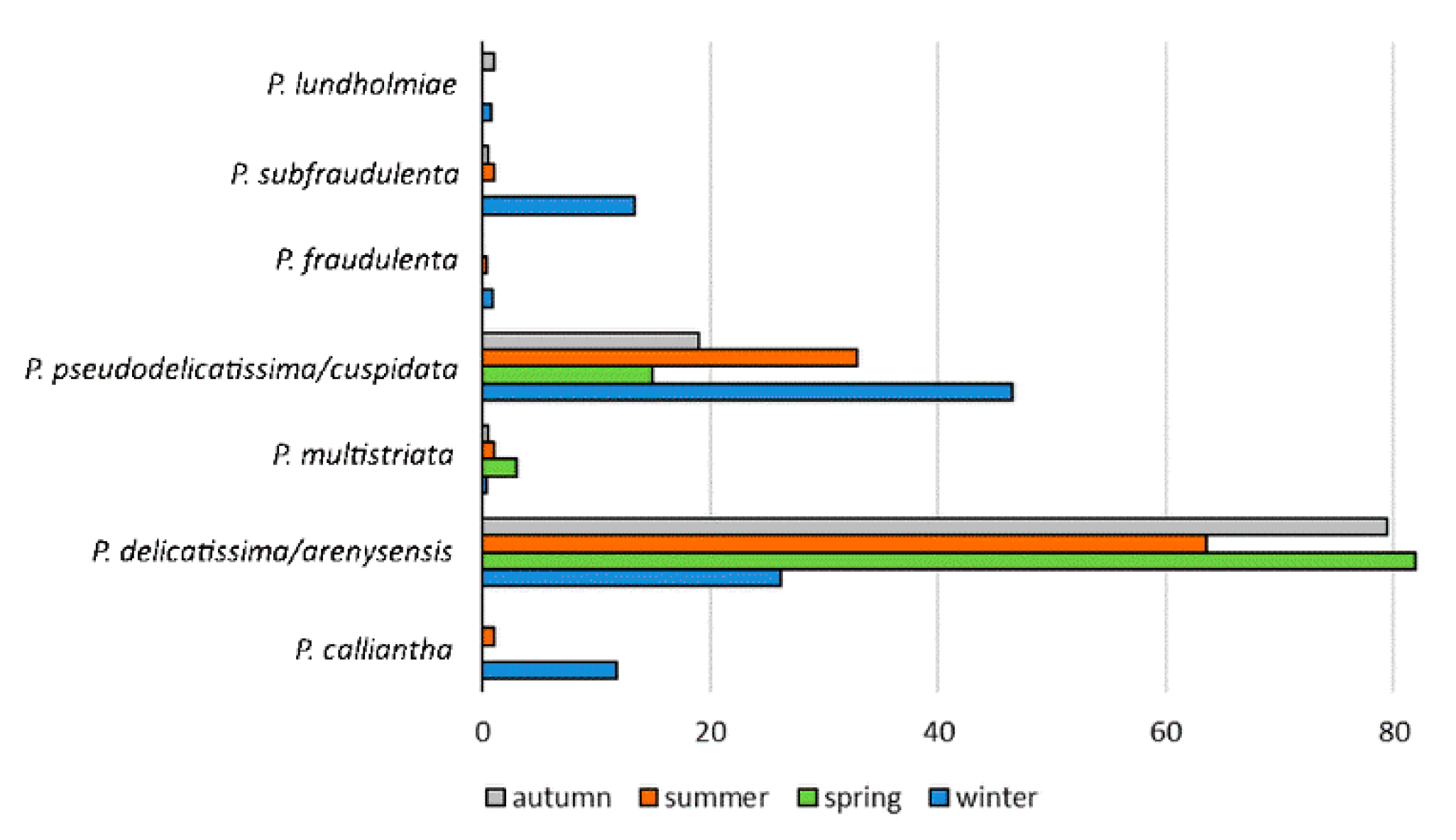


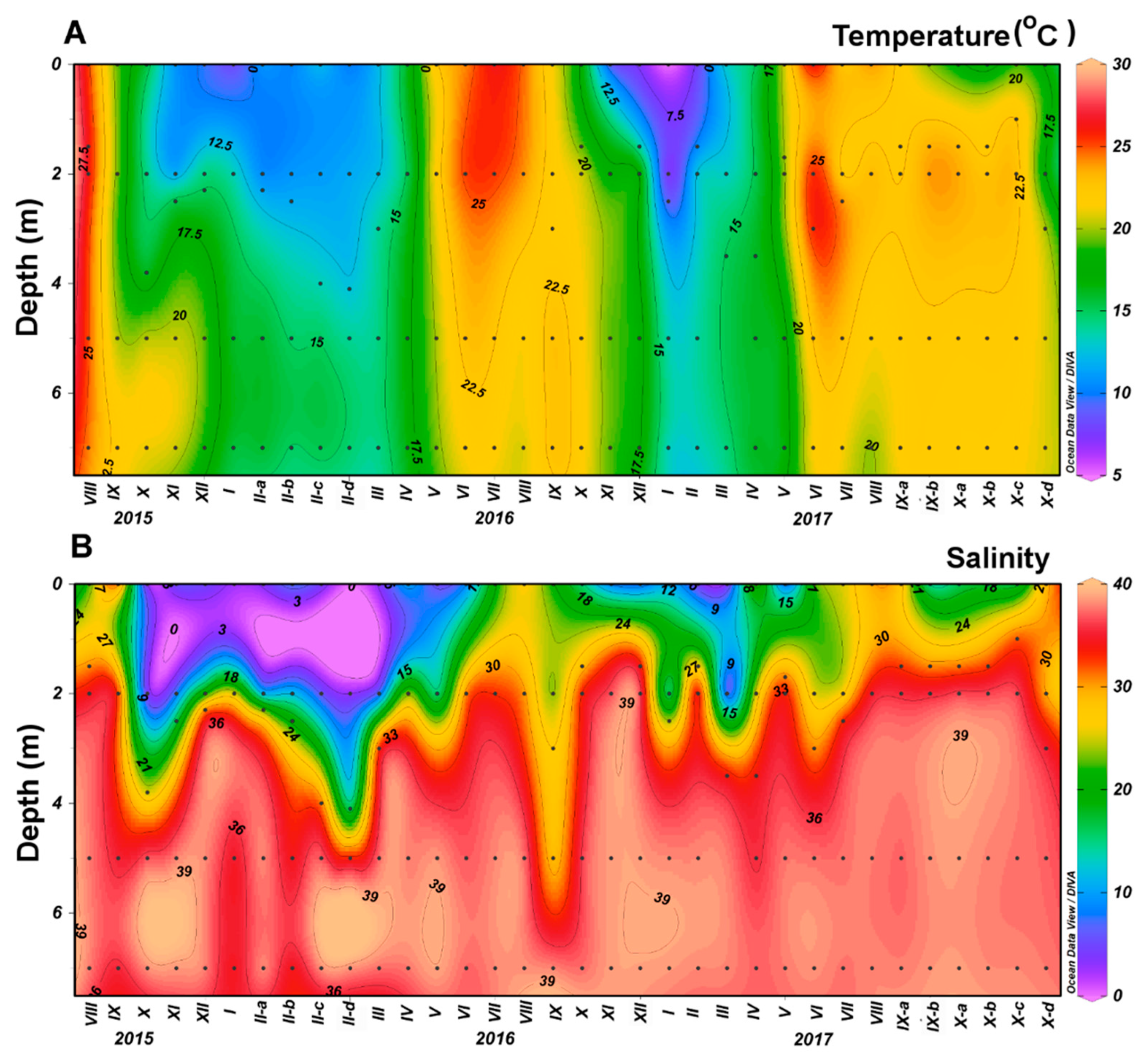
| Species (n) | Width (μm) | Length (μm) | Central Nodule | Fibulae (10 μm) | Interstriae (10 μm) | Poroid Rows | Poroids (1 μm) |
|---|---|---|---|---|---|---|---|
| P. delicatissima/arenysensis (384) | 0.90–1.78 | 40.50–95.49 | + | 20–27 | 37–42 | 2 | 8–13 |
| 1.21 ± 0.16 | 57.49 ± 8.94 | 22.98 ± 1.41 | 39.33 ± 1.44 | - | - | ||
| P. calliantha (32) | 1.29–1.68 | 69.69–112.18 | + | 15–22 | 30–38 | 1 | 4–6 |
| 1.51 ± 0.11 | 95.58 ± 12.91 | 18.16 ± 1.57 | 33.72 ± 2.58 | - | - | ||
| P. lundholmiae (10) | 1.62–2.17 | 51.13–68.76 | + | 16–19 | 32–35 | 1–2 | 5–6 |
| 1.93 ± 0.19 | 58.70 ± 7.81 | 17.10 ± 0.99 | 33.50 ± 0.85 | - | - | ||
| P. multistriata (13) | 1.97–2.30 | 63.89–77.80 | − | 24–25 | 37–41 | 1–3 | 10–12 |
| 2.14 ± 0.10 | 71.67 ± 4.59 | 24.69 ± 0.48 | 39.00 ± 1.15 | - | - | ||
| P. pseudodelicatissima/cuspidata (298) | 1.01–1.60 | 48.29–140.11 | + | 16–25 | 32–42 | 1 | 4–7 |
| 1.26 ± 0.10 | 91.29 ± 20.26 | 20.89 ± 2.04 | 37.57 ± 2.43 | - | - | ||
| P. fraudulenta (9) | 4.22–5.52 | 80.81–111.3 | + | 21–23 | 23–24 | 2–3 | 5–7 |
| 4.77 ± 0.39 | 99.36 ± 9.80 | 22.11 ± 0.93 | 23.33 ± 0.50 | - | - | ||
| P. subfraudulenta (47) | 3.16–4.40 | 92.13–139.40 | + | 13–17 | 23–26 | 1–3 | 5–7 |
| 3.68 ± 0.27 | 111.32 ± 13.24 | 14.72 ± 0.88 | 24.63 ± 0.73 | - | - |
| Parameter | Temp. | Salinity | Chl a | NO3− | NO2− | NH4+ | PO43− | SiO42− | N:P | Si:N | Si:P |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Salinity | 0.298 * | 1 | −0.379 ** | −0.560 ** | −0.213 | −0.130 | 0.127 | −0.586 ** | −0.484 ** | 0.139 | −0.478 |
| Chl a | −0.206 | −0.379 ** | 1 | 0.422 ** | 0.253 | 0.262 * | −0.192 | 0.321 * | 0.407 ** | −0.259 | 0.328 ** |
| Diatoms | 0.203 | −0.060 | 0.182 | 0.066 | 0.239 | −0.033 | 0.070 | −0.025 | 0.038 | −0.152 | −0.030 |
| Dinoflagellates | −0.031 | 0.112 | −0.141 | −0.165 | −0.313 * | −0.003 | 0.049 | −0.079 | −0.170 | 0.215 | −0.103 |
| Coccolithophores | −0.192 | −0.031 | −0.110 | 0.072 | −0.042 | −0.012 | −0.119 | 0.103 | 0.083 | 0.054 | 0.140 |
| Nanoflagellates | 0.118 | −0.127 | 0.089 | −0.096 | 0.085 | 0.101 | 0.070 | −0.006 | −0.090 | 0.171 | −0.006 |
| Total phytoplankton | 0.230 | 0.080 | 0.170 | −0.204 | −0.021 | 0.035 | −0.155 | −0.161 | −0.076 | 0.129 | −0.021 |
| Pseudo-nitzschia spp. | 0.343 ** | 0.478 ** | −0.254 | −0.573 ** | −0.210 | −0.257 | 0.144 | −0.525 ** | −0.519 ** | 0.334 ** | −0.462 ** |
| Pseudo-nitzschia spp. (7 m layer) | 0.379 | −0.011 | 0.306 | −0.061 | 0.103 | −0.080 | −0.065 | −0.305 | −0.031 | −0.088 | −0.211 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arapov, J.; Bužančić, M.; Skejić, S.; Mandić, J.; Bakrač, A.; Straka, M.; Ninčević Gladan, Ž. Phytoplankton Dynamics in the Middle Adriatic Estuary, with a Focus on the Potentially Toxic Genus Pseudo-nitzschia. J. Mar. Sci. Eng. 2020, 8, 608. https://doi.org/10.3390/jmse8080608
Arapov J, Bužančić M, Skejić S, Mandić J, Bakrač A, Straka M, Ninčević Gladan Ž. Phytoplankton Dynamics in the Middle Adriatic Estuary, with a Focus on the Potentially Toxic Genus Pseudo-nitzschia. Journal of Marine Science and Engineering. 2020; 8(8):608. https://doi.org/10.3390/jmse8080608
Chicago/Turabian StyleArapov, Jasna, Mia Bužančić, Sanda Skejić, Jelena Mandić, Ana Bakrač, Maja Straka, and Živana Ninčević Gladan. 2020. "Phytoplankton Dynamics in the Middle Adriatic Estuary, with a Focus on the Potentially Toxic Genus Pseudo-nitzschia" Journal of Marine Science and Engineering 8, no. 8: 608. https://doi.org/10.3390/jmse8080608
APA StyleArapov, J., Bužančić, M., Skejić, S., Mandić, J., Bakrač, A., Straka, M., & Ninčević Gladan, Ž. (2020). Phytoplankton Dynamics in the Middle Adriatic Estuary, with a Focus on the Potentially Toxic Genus Pseudo-nitzschia. Journal of Marine Science and Engineering, 8(8), 608. https://doi.org/10.3390/jmse8080608





