Long-Term Strength of Alkali-Activated Mortars with Steel Fibres Cured in Various Conditions
Abstract
1. Introduction
2. Experimental Program
2.1. Materials
2.2. Mix Composition and Mixing Procedure
2.3. Curing Conditions
2.4. Testing Procedure
3. Results and Discussions
3.1. Fibre Distribution
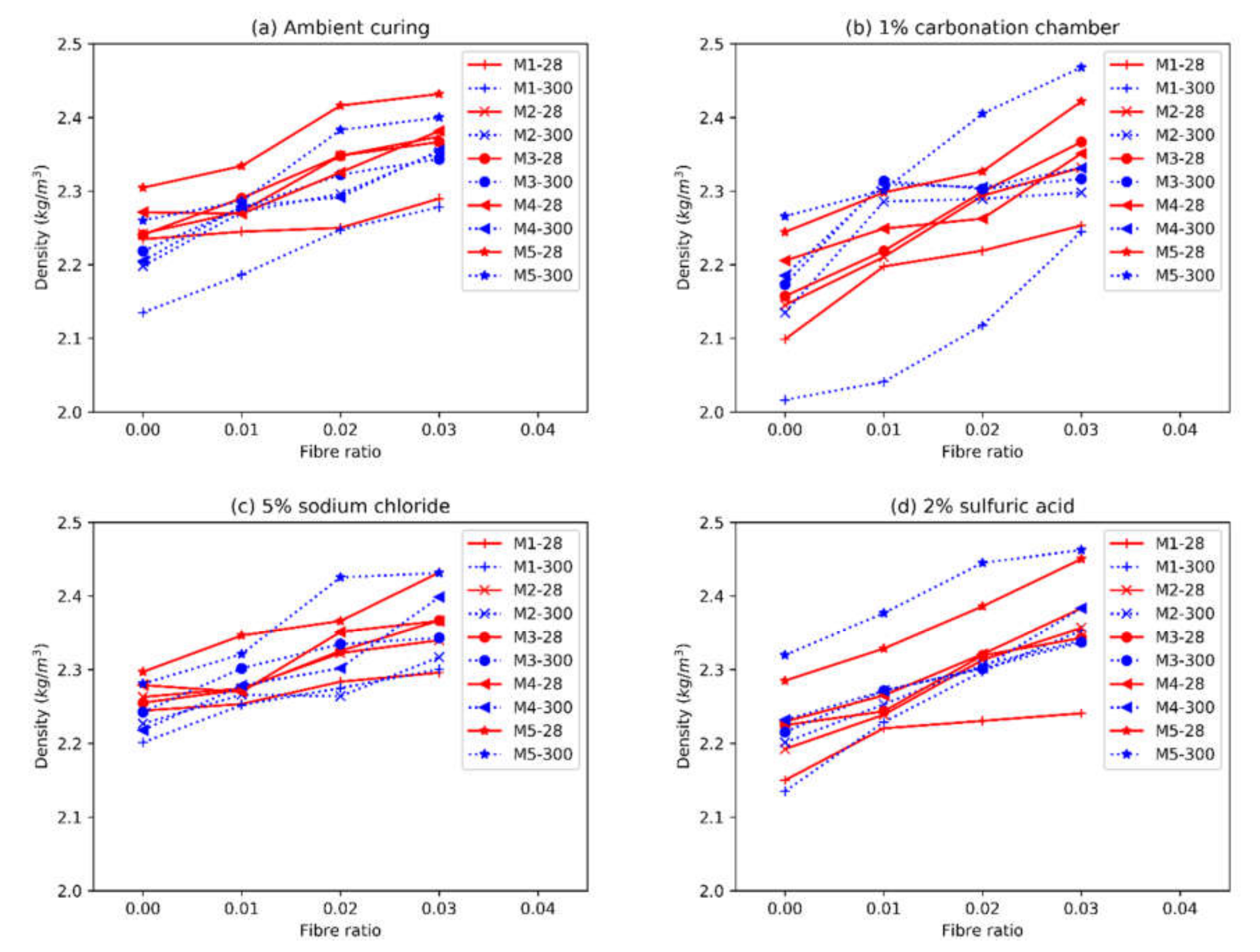
3.2. Density
3.3. Compressive Strengths
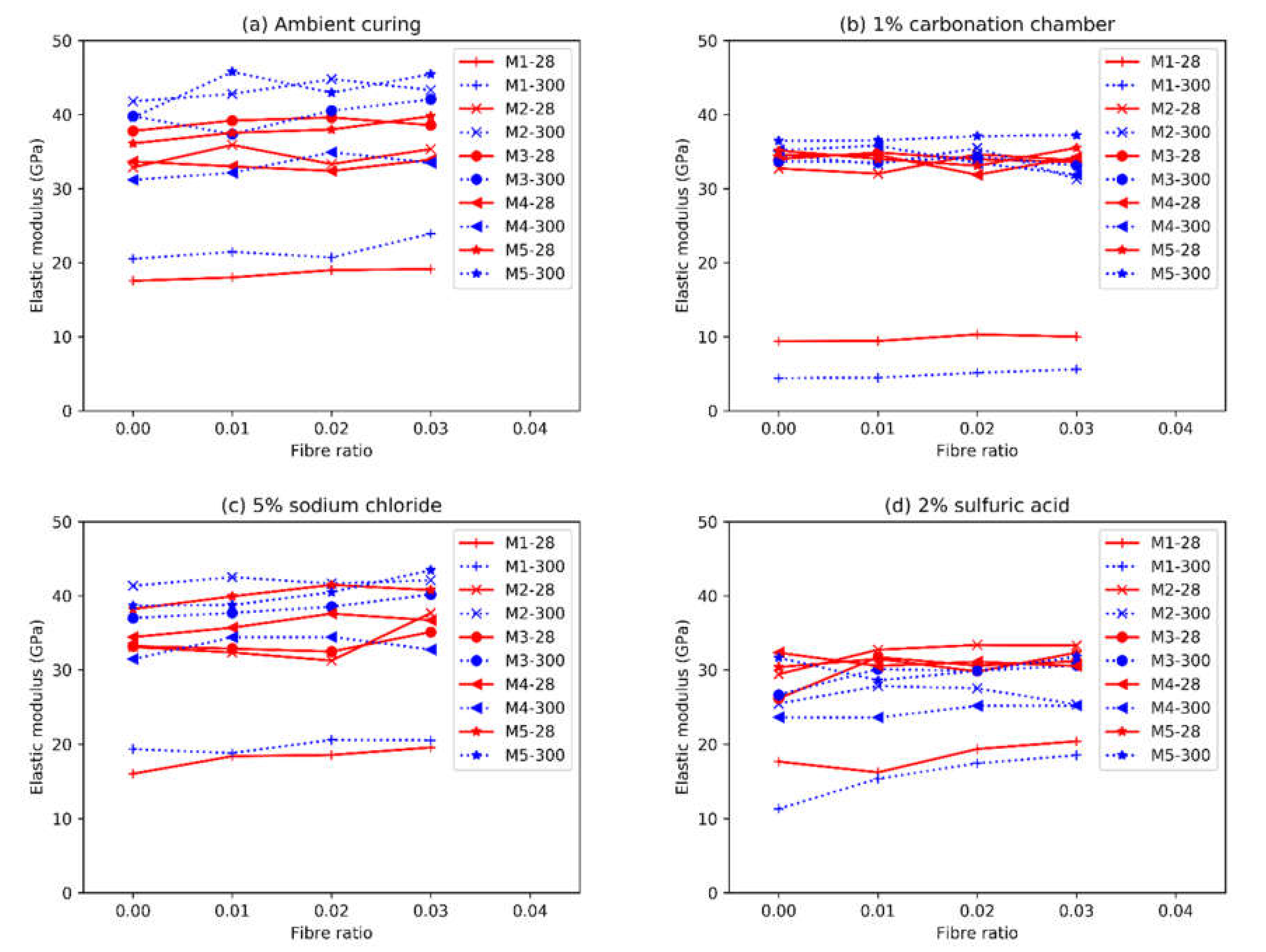
3.4. Elastic Modulus
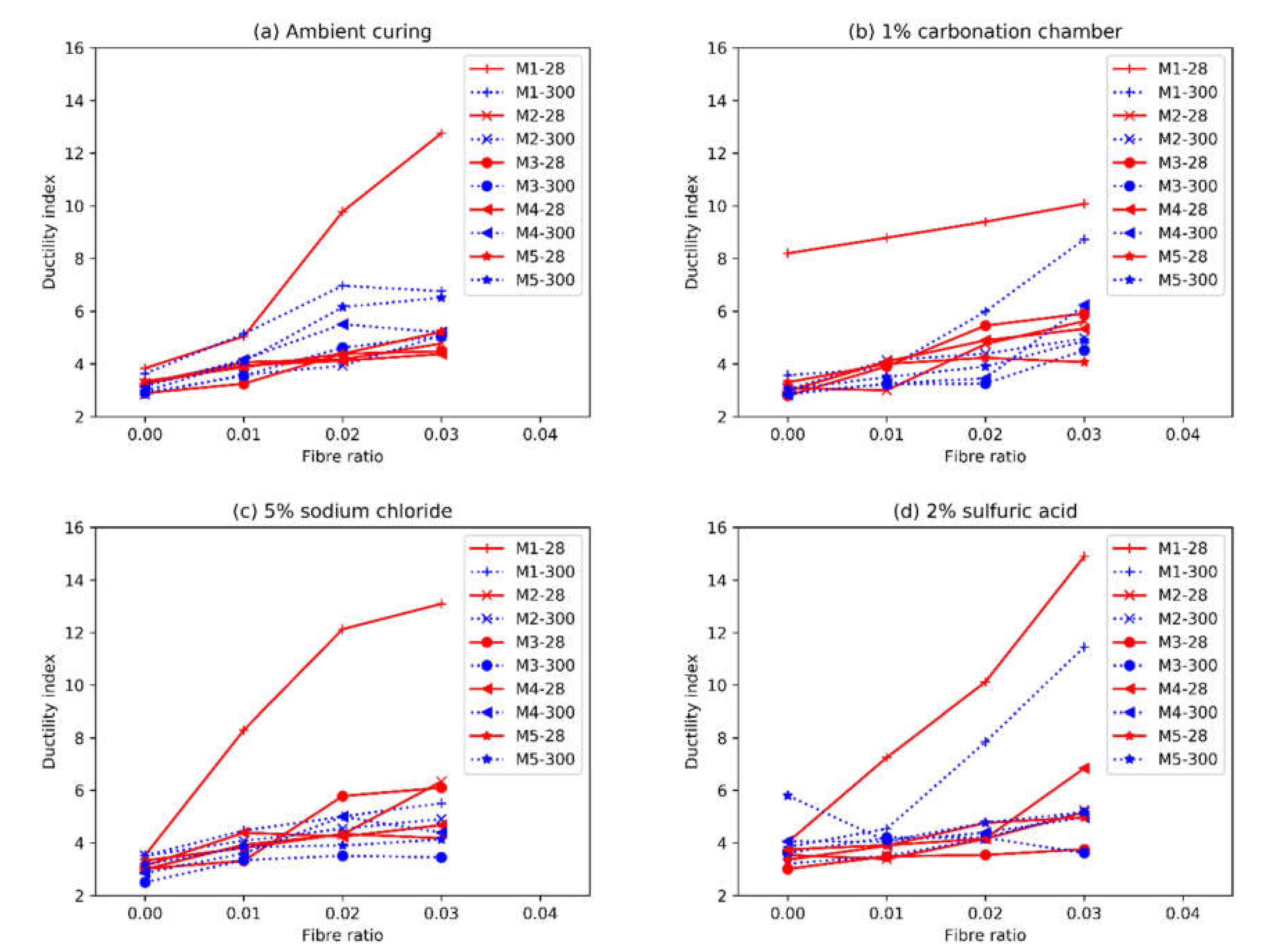
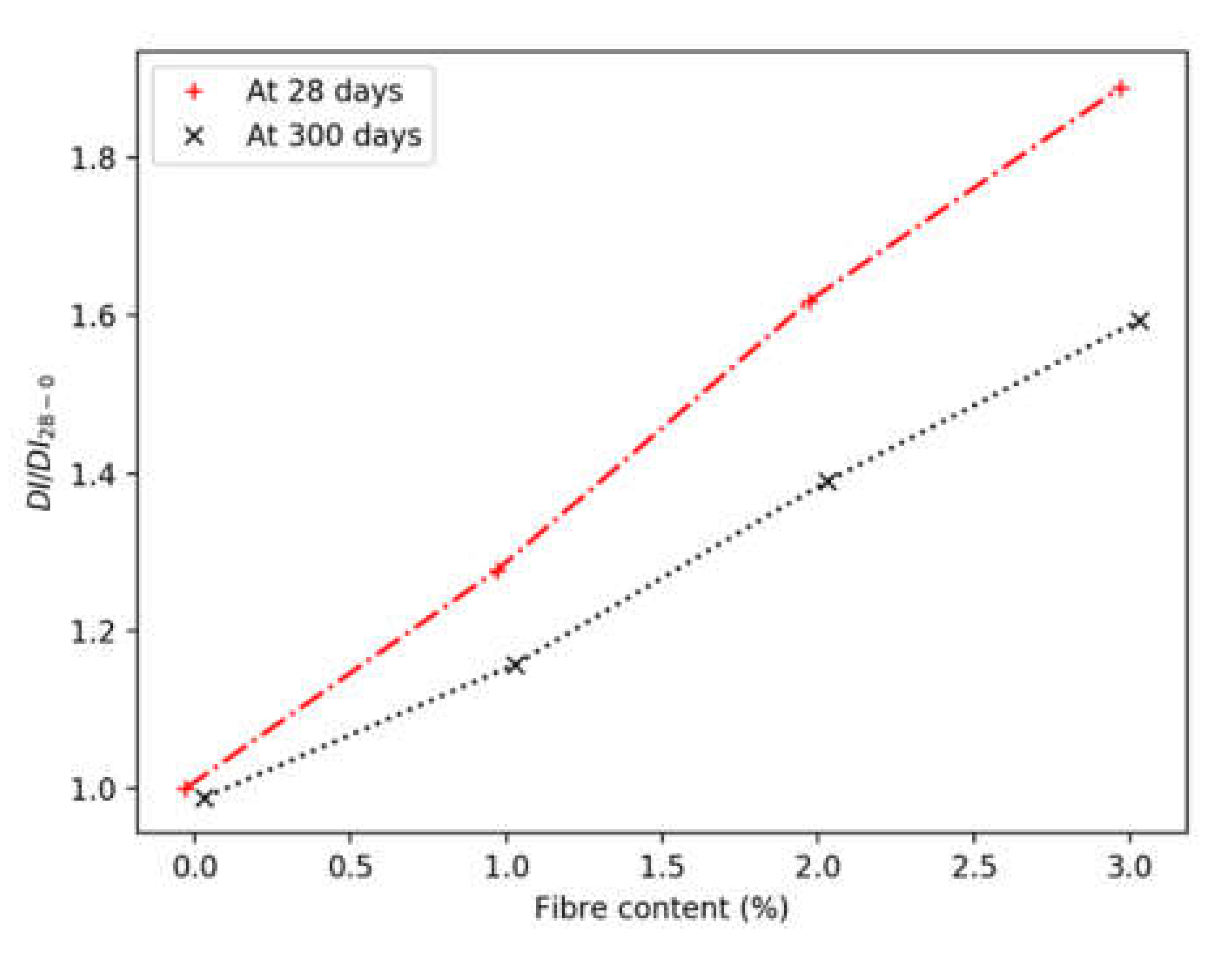
3.5. Ductility Indices
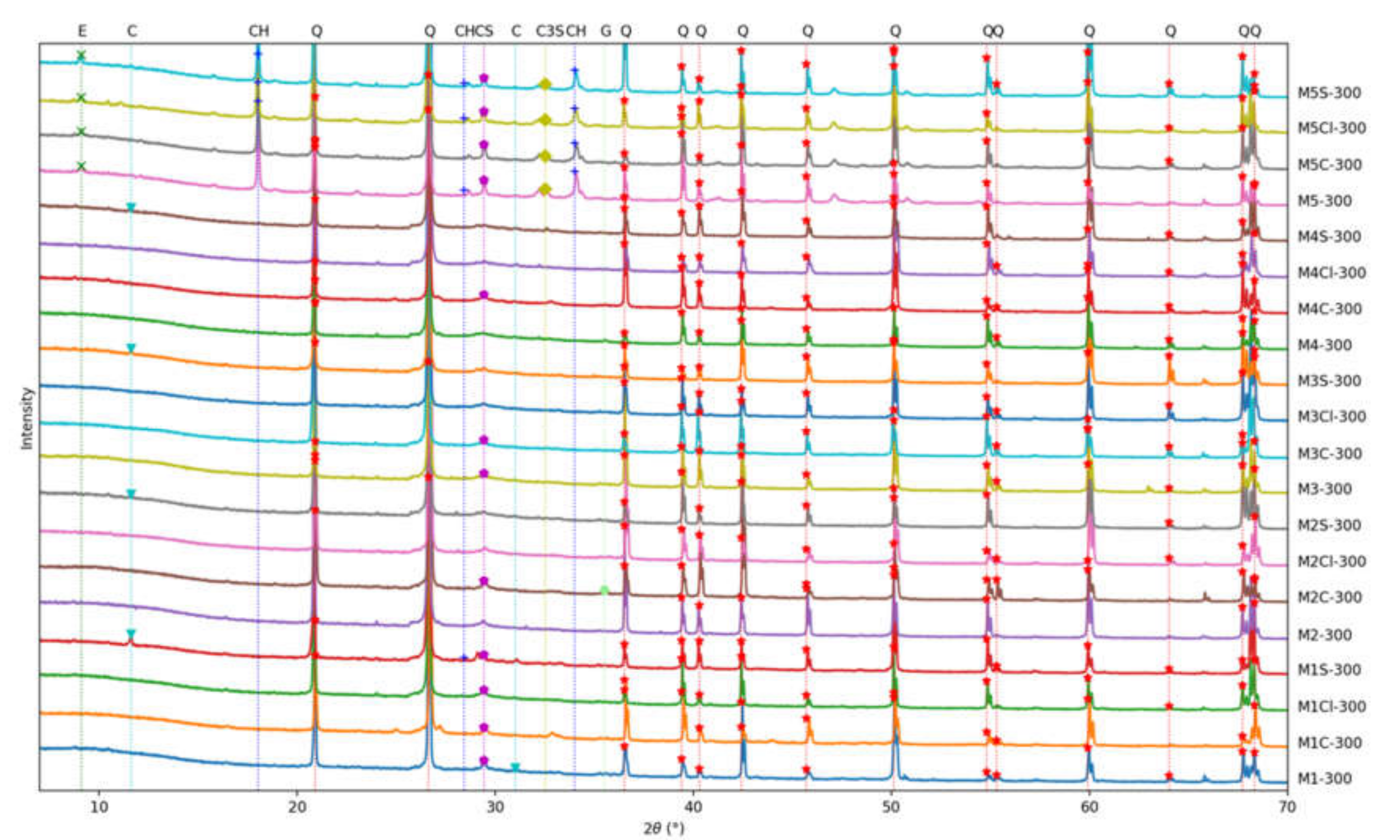
3.6. XRD Results
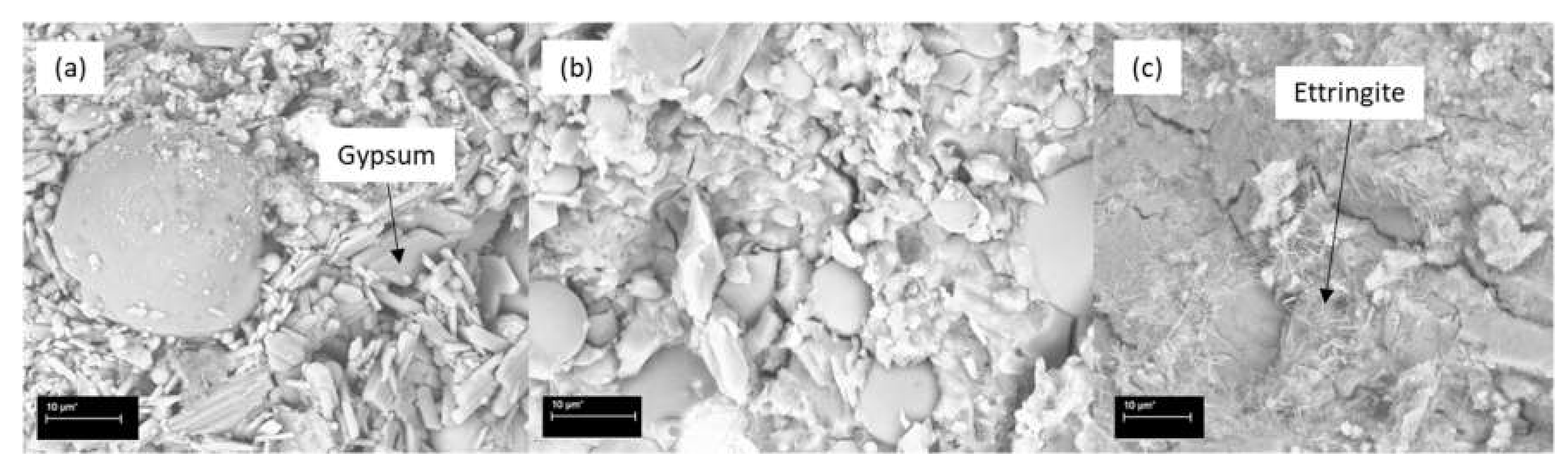
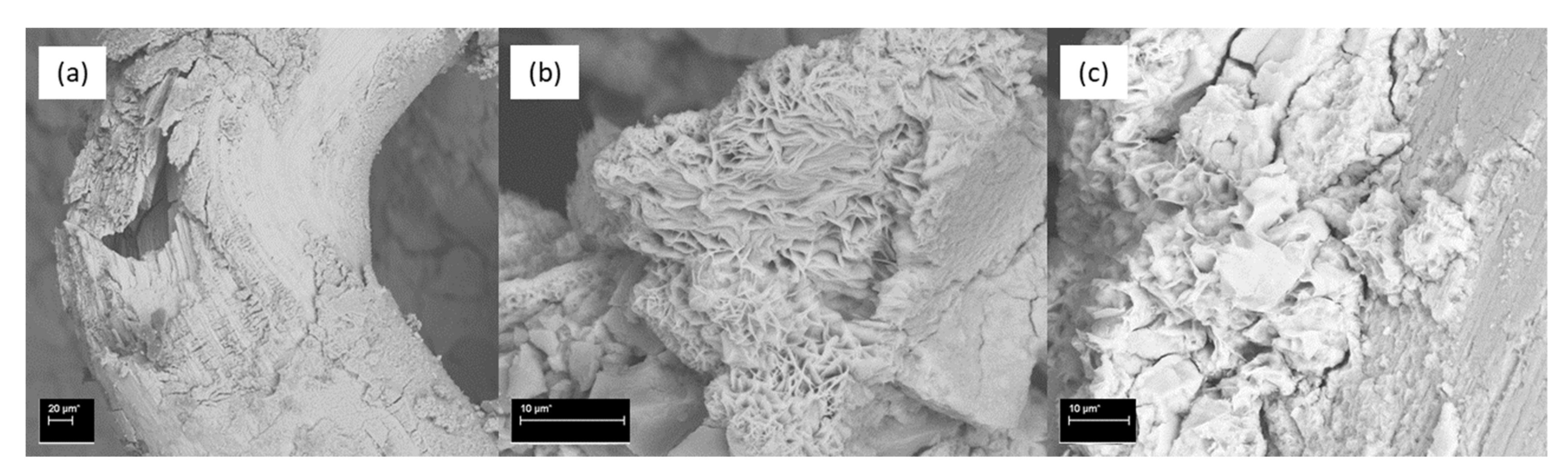
3.7. SEM Results
4. Conclusions
- A high activator content above 6% Na2O did not lead to improvements in the compressive strength, but increased the resistance of AAFS mortars to the ingress of deleterious materials, such as CO2 and sulfuric acid. A high activator content can also provide protection for the embedded fibres.
- The addition of fibre had a low impact on the compressive strength of the mortar specimens. A low fibre content could slightly improve the strength while a large fibre content could lead to disruptions in the matrix and reduce the strength.
- The most damaging environment for both the OPC and AAFS was the 2% sulfuric acid. The sulfuric acid caused spalling in the OPC mortar, while the density of the unaffected part remained relatively unchanged. For AAFS mortar, this leached out the pore fluids, and caused the detachment of the sodium cations, causing a reduction in density. The addition of short fibres could limit the development of cracking, thus reducing the negative effects of the aforementioned processes. The ettringite peak in the XRD spectrum of the OPC specimen in sulfuric acid was more profound, indicating that delayed ettringite formation had occurred. In AAFS, small gypsum peaks were identified, with no ettringite formation. The SEM showed that the regions on the fibre exposed to the air voids were prone to rust. Both chloride and acid attacks could significantly impact the performance of the fibres.
- The elastic moduli of both AAFS and OPC mortars were strongly correlated to the square root of the compressive strength, regardless of the curing condition. AAFS composites had lower elastic moduli than OPC.
- The ductility increased linearly with the increase of fibre content, even at a 3% volumetric ratio. At 28 days, each percent increase in fibre content resulted in a 30% increment in ductility. This reduced to about 20% after 300 days of curing, due to the higher mortar strength.
- The inter-fibre distance was not significantly reduced by tripling the fibre content from 1% by volume to 3%. Based on the results presented in this study, the combination of 1%–2% volumetric ratio of short steel fibres and 9% Na2O in the AAFS was the most suitable combination against the tested extreme environments. The option to use fibre reinforced AAFS to extend the design life of the marine structures could be also explored.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Provis, J.L.; van Deventer, J.S.J. Alkali Activated Materials; Springer: Dordrecht, The Netherlands, 2014; Volume 13. [Google Scholar] [CrossRef]
- Chen, C.; Gong, W.; Lutze, W.; Pegg, I.L.; Zhai, J. Kinetics of fly ash leaching in strongly alkaline solutions. J. Mater. Sci. 2011, 46, 590–597. [Google Scholar] [CrossRef]
- Mustafa Al Bakria, A.M.; Kamarudin, H.; Bin Hussain, M.; Khairul Nizar, I.; Zarina, Y.; Rafiza, A.R. The effect of curing temperature on physical and chemical properties of geopolymers. Phys. Procedia 2011, 22, 286–291. [Google Scholar] [CrossRef]
- Li, X.; Wang, Z.; Jiao, Z. Influence of curing on the strength development of calcium-containing geopolymer mortar. Materials 2013, 6, 5069–5076. [Google Scholar] [CrossRef] [PubMed]
- Komnitsas, K.; Yurramendi, L.; Bartzas, G.; Karmali, V.; Petrakis, E. Factors affecting co-valorization of fayalitic and ferronickel slags for the production of alkali activated materials. Sci. Total Environ. 2020, 721. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.G.; Lee, N.K.; Lee, H.K. Fresh and hardened properties of alkali-activated fly ash/slag pastes with superplasticizers. Constr. Build. Mater. 2014, 50, 169–176. [Google Scholar] [CrossRef]
- Dong, M.; Feng, W.; Elchalakani, M.; Li, G.; Karrech, A.; May, E.F. Development of a high strength geopolymer by novel solar curing. Ceram. Int. 2017, 43, 11233–11243. [Google Scholar] [CrossRef]
- Khan, M.Z.N.; Shaikh, F.A.; Hao, Y.; Hao, H. Synthesis of high strength ambient cured geopolymer composite by using low calcium fly ash. Constr. Build. Mater. 2016, 125, 809–820. [Google Scholar] [CrossRef]
- Al-Majidi, M.H.; Lampropoulos, A.; Cundy, A.B. Steel fibre reinforced geopolymer concrete (SFRGC) with improved microstructure and enhanced fibre-matrix interfacial properties. Constr. Build. Mater. 2017, 139, 286–307. [Google Scholar] [CrossRef]
- Chen, W.; Brouwers, H.J.H. The hydration of slag, part 1: Reaction models for alkali-activated slag. J. Mater. Sci. 2007, 42, 428–443. [Google Scholar] [CrossRef]
- Brough, A.R.; Holloway, M.; Sykes, J.; Atkinson, A. Sodium silicate-based alkali-activated slag mortars. Part II. The retarding effect of additions of sodium chloride or malic acid. Cem. Concr. Res. 2000, 30, 1375–1379. [Google Scholar] [CrossRef]
- Dong, M.; Elchalakani, M.; Karrech, A. Curing conditions of alkali-activated fly ash and slag mortar. J. Mater. Civ. Eng. 2019. [Google Scholar] [CrossRef]
- Olivia, M. Durability Related Properties of Low Calcium Fly Ash Based Geopolymer Concrete; Curtin University of Technology: Bentley, Australia, 2011. [Google Scholar]
- Komljenović, M.; Baščarević, Z.; Marjanović, N.; Nikolić, V. External sulfate attack on alkali-activated slag. Constr. Build. Mater. 2013, 49, 31–39. [Google Scholar] [CrossRef]
- Allaby, M. A Dictionary of Geology and Earth Sciences, 4th ed.; Oxford University Press: Oxford, UK, 2013. [Google Scholar]
- Albitar, M.; Mohamed Ali, M.S.; Visintin, P.; Drechsler, M. Durability evaluation of geopolymer and conventional concretes. Constr. Build. Mater. 2017, 136, 374–385. [Google Scholar] [CrossRef]
- Iwahiro, T.; Komatsu, R.; Ikeda, K. Chemical Compositions of Gels Prepared from Sodium Metasilicate and Aluminum Nitrate Solutions. In Proceedings of the International Conferences on Geopolymers 2002—Turn Potential into Profit, Melbourne, Australia, 28–29 October 2002; pp. 1–8. [Google Scholar]
- Shayan, A.; Tennakoon, C.; Xu, A. Specification and Use of Geopolymer Concrete in the Manufacture of Structural and Non-Structural Components: Experimental Work; Austroads Ltd.: Sydney, Australia, 2016. [Google Scholar]
- Yao, X.; Yang, T.; Zhang, Z. Compressive strength development and shrinkage of alkali-activated fly ash–slag blends associated with efflorescence. Mater. Struct. 2016, 49, 2907–2918. [Google Scholar] [CrossRef]
- Dong, M.; Elchalakani, M.; Karrech, A. Development of high strength one-part geopolymer mortar using sodium metasilicate. Constr. Build. Mater. 2020, 236. [Google Scholar] [CrossRef]
- Ganesan, N.; Abraham, R.; Deepa Raj, S. Durability characteristics of steel fibre reinforced geopolymer concrete. Constr. Build. Mater. 2015, 93, 471–476. [Google Scholar] [CrossRef]
- Rekha, K.; Hazeena, R. Strength and durability of fibre reinforced geopolymer concrete. Int. J. Sci. Eng. Res. 2014, 5, 412–416. [Google Scholar]
- Rivera, F.J.M. Strength and Durability of Fly Ash-Based Fiber-Reinforced Geopolymer Concrete in a Simulated Marine Environment; Florida Atlantic University: Boca Raton, FL, USA, 2013. [Google Scholar]
- Standards Australia. Supplementary Cementitious Materials Part 1: Fly Ash; AS/NZS 3582.1:2016; Standards Australia: Sydney, Australia, 2016. [Google Scholar]
- Wang, S.D.; Scrivener, K.L.; Pratt, P.L. Factors affecting the strength of alkali-activated slag. Cem. Concr. Res. 1994, 24, 1033–1043. [Google Scholar] [CrossRef]
- Standards Australia. Methods of Testing Concrete; AS 1012; Standards Australia: Sydney, Australia, 2014; pp. 1–63. [Google Scholar]
- Elchalakani, M.; Dong, M.; Karrech, A.; Li, G.; Mohamed Ali, M.S.; Manalo, A. Behaviour and design of air cured GFRP-reinforced geopolymer concrete square columns. Mag. Concr. Res. 2018, 1–63. [Google Scholar] [CrossRef]
- OpenCV. OpenCV n.d. Available online: https://opencv.org (accessed on 3 January 2020).
- Gao, X.; Yu, Q.L.; Brouwers, H.J.H. Properties of alkali activated slag-fly ash blends with limestone addition. Cem. Concr. Compos. 2015, 59, 119–128. [Google Scholar] [CrossRef]
- Melo Neto, A.A.; Cincotto, M.A.; Repette, W. Drying and autogenous shrinkage of pastes and mortars with activated slag cement. Cem. Concr. Res. 2008, 38, 565–574. [Google Scholar] [CrossRef]
- Sayyad, A.S.; Patankar, S.V. Effect of Steel Fibres and Low Calcium Fly Ash on Mechanical and Elastic Properties of Geopolymer Concrete Composites. Indian J. Mater. Sci. 2013, 2013. [Google Scholar] [CrossRef]
- Warner, R.F.; Rangan, B.B.; Hall, A.S.; Faulkes, K.A. Concrete Structures; Longman: South Melbourne, Australia, 1998. [Google Scholar]
- Sturm, P.; Gluth, G.J.G.; Jäger, C.; Brouwers, H.J.H.; Kühne, H.C. Sulfuric acid resistance of one-part alkali-activated mortars. Cem. Concr. Res. 2018, 109, 54–63. [Google Scholar] [CrossRef]
- Allahverdi, A.; Škvára, F. Sulfuric acid attack on hardened paste of geopolymer cements part 1. Mechanism of corrosion at relatively high concentrations. Ceram. Silik. 2005, 49, 225–229. [Google Scholar]
- Wang, Z.; Zhao, X.-L.; Xian, G.; Wu, G.; Raman, S.R.K.; Al-Saadi, S.; Haque, A. Long-term durability of basalt- and glass-fibre reinforced polymer (BFRP/GFRP) bars in seawater and sea sand concrete environment. Constr. Build. Mater. 2017, 139, 467–489. [Google Scholar] [CrossRef]
- Panossian, Z.; De Almeida, N.L.; De Sousa, R.M.F.; Pimenta, G.D.S.; Marques, L.B.S. Corrosion of carbon steel pipes and tanks by concentrated sulfuric acid: A review. Corros. Sci. 2012, 58, 1–11. [Google Scholar] [CrossRef]
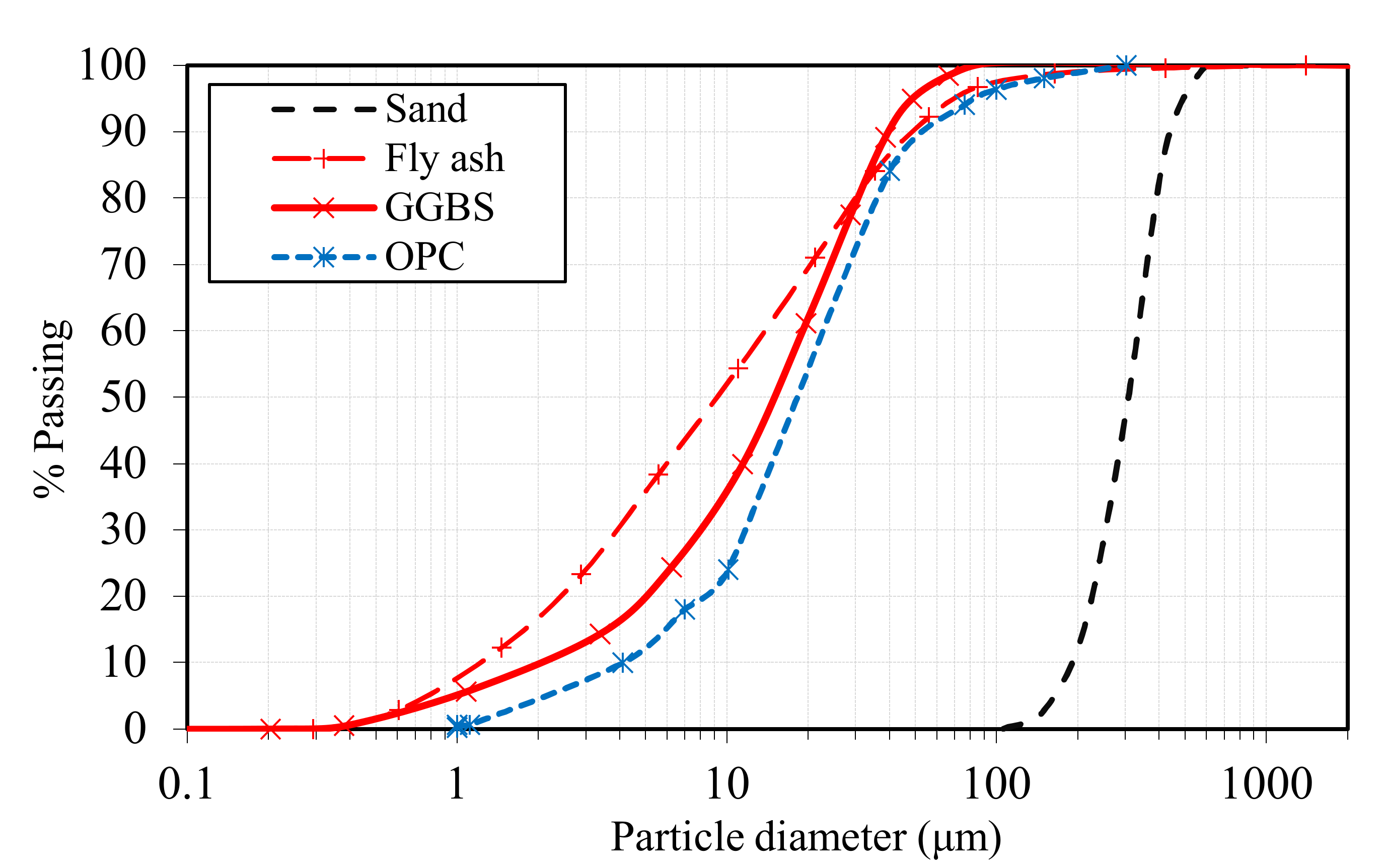





| Material | Al2O3 | CaO | Fe2O3 | K2O | MgO | MnO | Na2O | P2O5 | SiO2 | SO3 | TiO2 | LOI 1 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| % | % | % | % | % | % | % | % | % | % | % | % | |
| Fly ash | 23.9 | 7.0 | 7.9 | 1.0 | 1.3 | 0.1 | 0.4 | 0.5 | 55.9 | 0.3 | 1.3 | 0.4 |
| GGBS | 13.1 | 43.2 | 0.8 | 0.3 | 5.5 | 0.2 | 0.3 | 0.0 | 31.4 | 4.0 | 0.6 | 0.6 |
| OPC | 5.3 | 65.1 | 2.6 | 0.4 | 1.4 | 0.1 | 0.4 | 0.0 | 21.0 | 0.0 | 0.3 | 3.4 |
| Mix | M1 | M2 | M3 | M4 | M5OPC |
|---|---|---|---|---|---|
| Ms | 1 | 1 | 1 | 1 | - |
| w/b | 0.35 | 0.35 | 0.35 | 0.40 | 0.35 |
| Na2O% | 3.00% | 6.00% | 9.00% | 6.00% | - |
| Fly ash (%) | 14% | 13% | 12% | 13% | - |
| Slag (%) | 14% | 13% | 12% | 13% | - |
| OPC (%) | - | - | - | - | 30% |
| Activator (%) | 5% | 10% | 15% | 10% | - |
| Sand (%) | 58% | 59% | 59% | 58% | 59.7% |
| Free water (%) | 9% | 5% | 2% | 6% | 10% |
| Superplasticiser (%) | - | - | - | - | 0.3% |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, M.; Elchalakani, M.; Karrech, A.; Yang, B. Long-Term Strength of Alkali-Activated Mortars with Steel Fibres Cured in Various Conditions. J. Mar. Sci. Eng. 2020, 8, 278. https://doi.org/10.3390/jmse8040278
Dong M, Elchalakani M, Karrech A, Yang B. Long-Term Strength of Alkali-Activated Mortars with Steel Fibres Cured in Various Conditions. Journal of Marine Science and Engineering. 2020; 8(4):278. https://doi.org/10.3390/jmse8040278
Chicago/Turabian StyleDong, Minhao, Mohamed Elchalakani, Ali Karrech, and Bo Yang. 2020. "Long-Term Strength of Alkali-Activated Mortars with Steel Fibres Cured in Various Conditions" Journal of Marine Science and Engineering 8, no. 4: 278. https://doi.org/10.3390/jmse8040278
APA StyleDong, M., Elchalakani, M., Karrech, A., & Yang, B. (2020). Long-Term Strength of Alkali-Activated Mortars with Steel Fibres Cured in Various Conditions. Journal of Marine Science and Engineering, 8(4), 278. https://doi.org/10.3390/jmse8040278








