Utilization of Industrial Waste in Cement in a Marine Environment with a Tropical Climate
Abstract
1. Introduction
2. Experimental Details
2.1. Materials
2.2. Test Procedures
2.2.1. Water Demand
2.2.2. Chloride Ion and SO3 Permeability Tests
Method of Determining the Amount of SO3
- Use dilute HCl solution to extract the dissolved sulfate in the concrete sample into the solution.
- Precipitate the sulfate ions in the acidic medium by using barium chloride, forming barium sulfate.
- Filter, wash, and precipitate the precipitate at (850 ± 25) °C and weigh the sample.
- The sulfate content of SO3 is calculated as a percentage (%) of the cement sample weight, according to the formula in Vietnam Standard 9336:2012, which corresponds to ASTM C114:where, m1 is the mass of the precipitate in grams (g), m2 is the weight of the beaker in grams (g), m is the mass of sample taken for analysis in grams (g), and 0.343 is the conversion ratio from BaSO4 to SO3.
Method of Determining the Amount of Chlorine
- Cast the paste samples and soak them in saline for some time as determined by the experimental plan.
- Drill the paste samples.
- Use electronic scales to determine a 3 g sample.
- Slowly pour the weighed sample into the prepared 20 mL of acid solution (with the machine) and shake to dissolve the powder into the solution.
- Release the corrected electrode into a jar of acid solution and cement paste.
- The results represent the chloride ion concentration (%) in the cement sample.
2.2.3. Microstructural and Mineralogical Studies
Thermogravimetric Analysis (TGA) Tests
X-ray Diffraction (XRD) Tests
Scanning Electron Microscope (SEM) Analysis
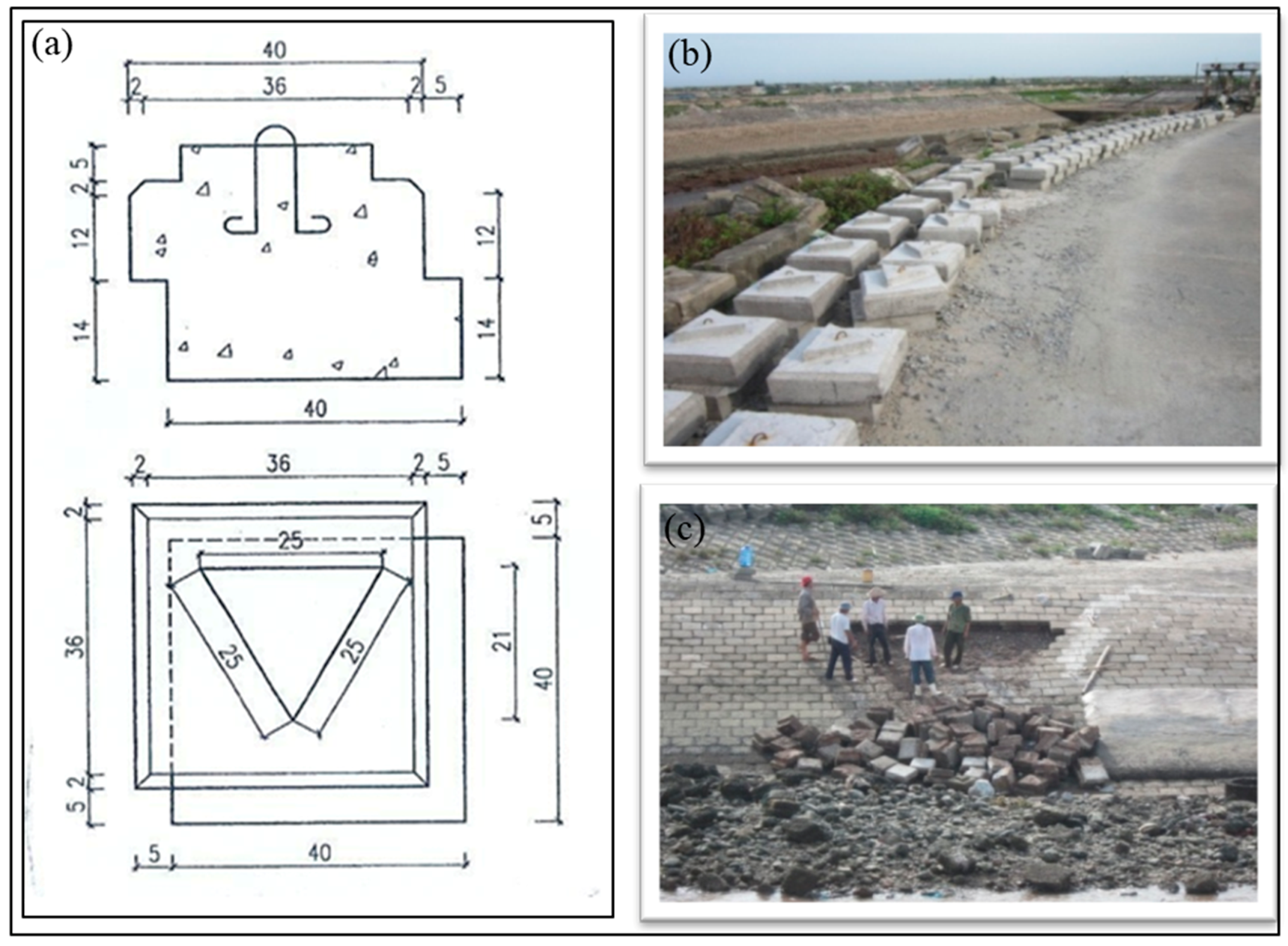
2.2.4. Field Experiments
3. Results and Discussion
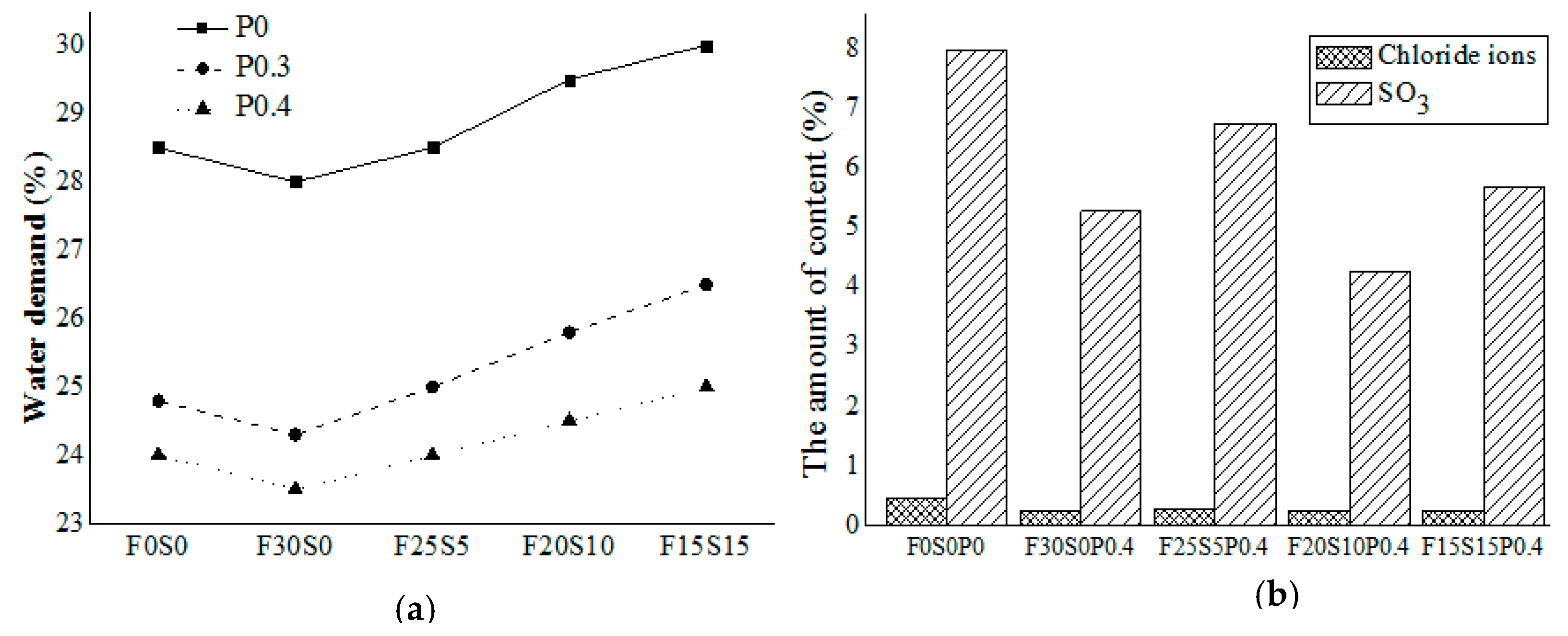
3.1. Water Demand
3.2. Chloride Ion and Sulfate Ion Permeability
3.3. Experimental Results Using Microstructural Analysis
3.3.1. TGA Analysis
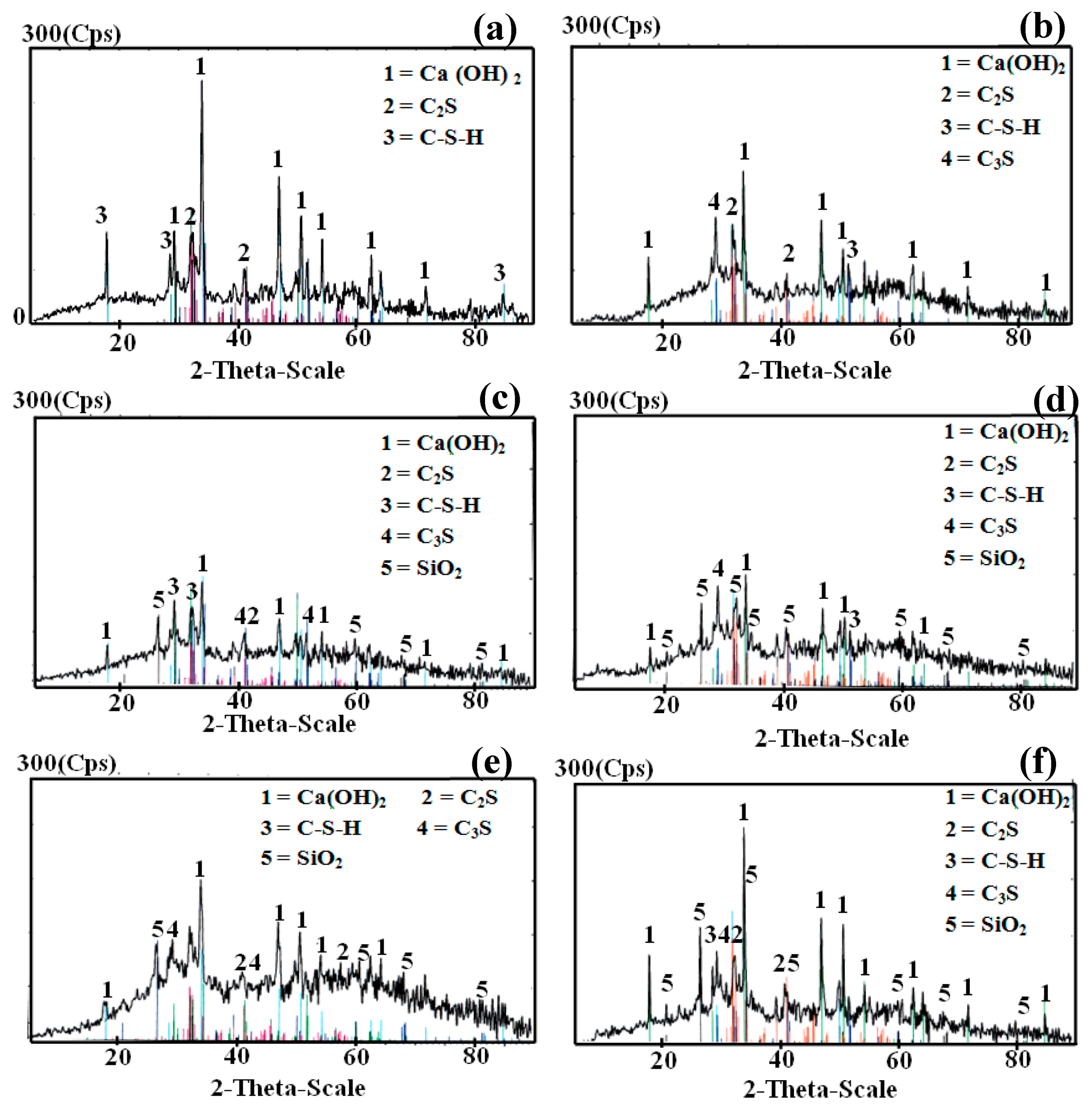
3.3.2. X-ray Diffraction Analysis
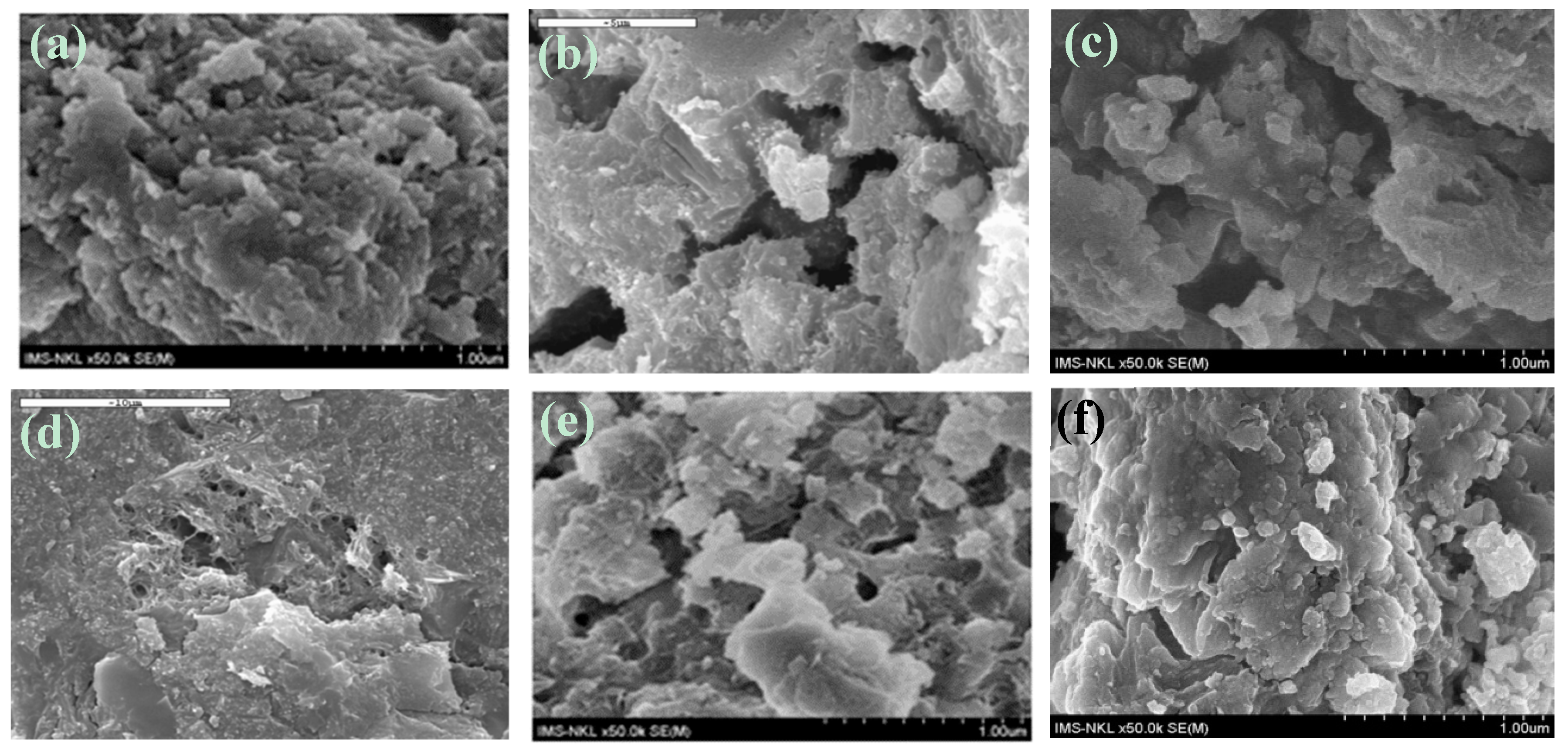
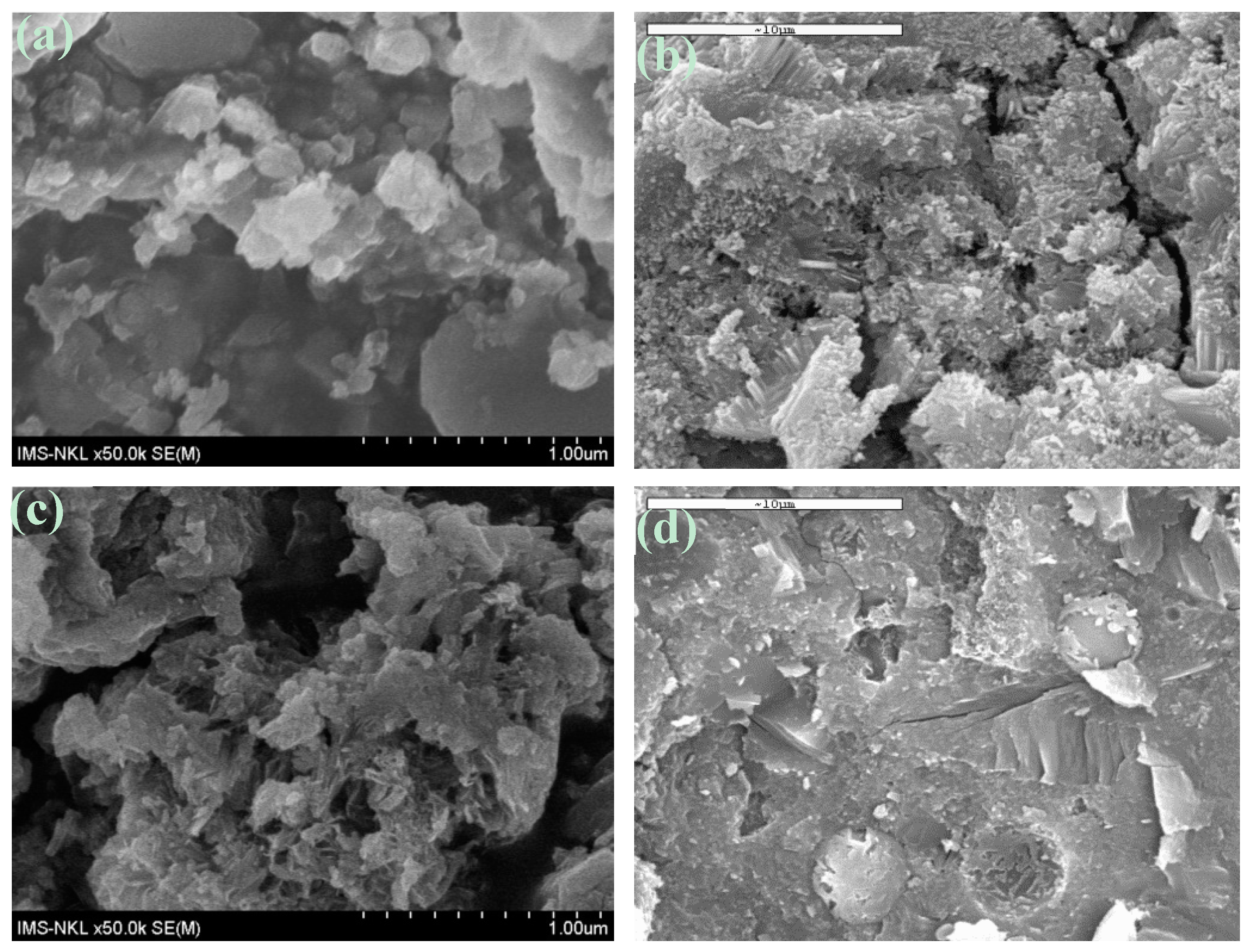
3.3.3. SEM Results
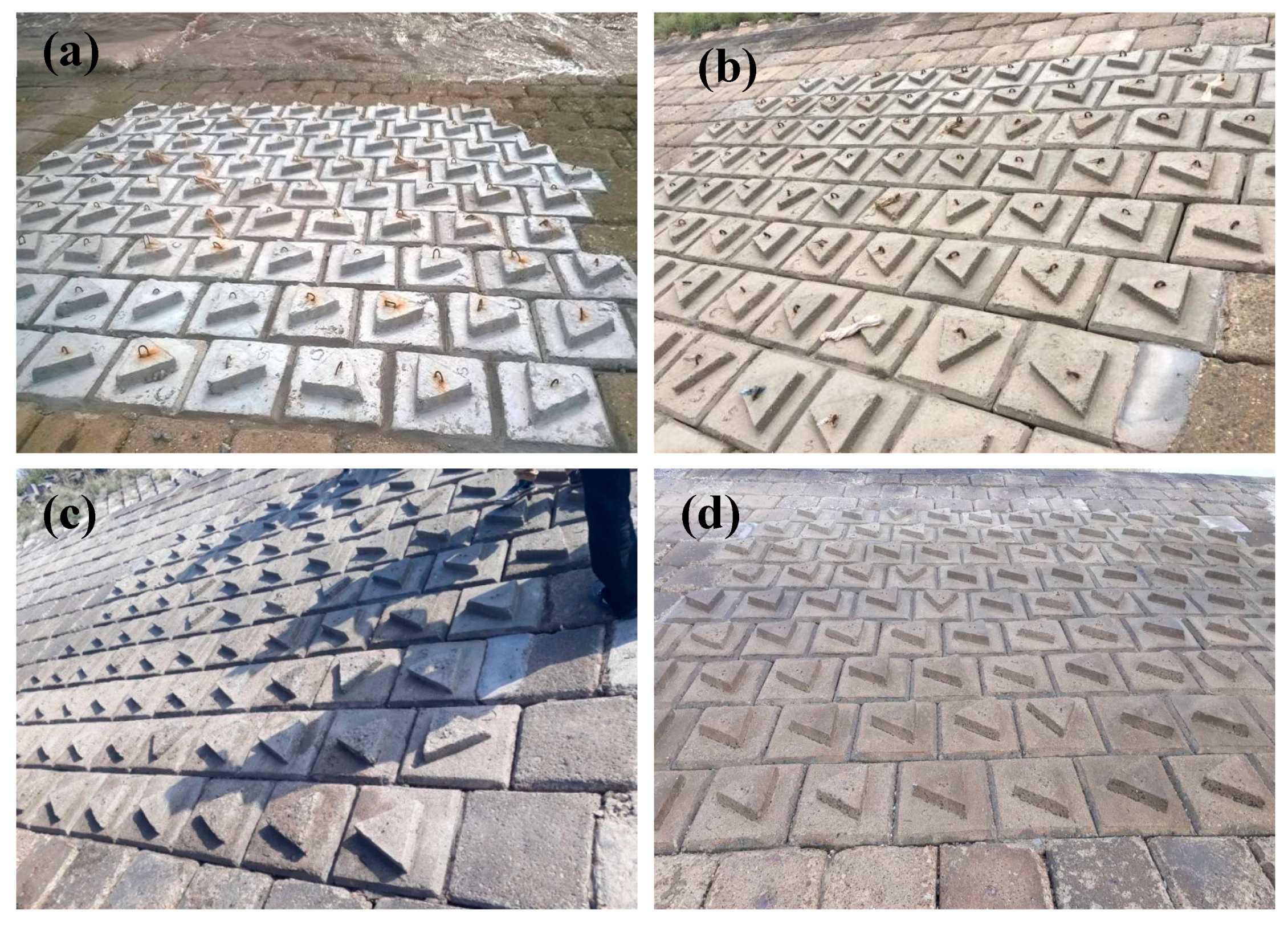
3.4. Results of Field Experiments
4. Conclusions
- (1)
- Using plasticizer, as well as partial replacement of ordinary Portland cement with fly ash and silica fume, led to an improvement of the physical-micro-structural properties and durability of the hardened cement pastes.
- (2)
- The water demand increased as the content of silica fume increased and decreased with the increasing fly ash content.
- (3)
- The incorporation of fly ash and silica fume was found to decrease the chloride ion and sulfate ion concentration.
- (4)
- The microstructure analysis shows that the total porosity values of the hardened OPC–FA–SF blended cement pastes were higher than those of the neat cement paste at the early and intermediate periods of hydration (up to 56 days) and became close to, or even lower than (in the case of some cement blends), the neat OPC paste. In general, the total porosity of all of the blended cement pastes decreased with the length of time of hydration.
- (5)
- The optimal mixture composition was that with 20% fly ash, 10% silica fume, and 0.4% plasticizer.
- (6)
- The results of field tests in Vietnam in a tropical climate (high heat and humidity) after 4 years of operation showed that the components using admixture had better mechanical properties and less degradation than the control components.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Data Availability
References
- Hanh, D.K.; Hien, D.T. Corrosion status of reinforced concrete and resistance corrosion solution for reinforced concrete structures in the Vietnam marine environment. J. Sci. Technol. Water Resour. Environ. Vietnam 2011, 32, 44–49. [Google Scholar]
- Bakharev, T. Geopolymeric materials prepared using Class F fly ash and elevated temperature curing. Cem. Concr. Res. 2005, 35, 1224–1232. [Google Scholar] [CrossRef]
- Rashad, A.M.; Khalil, M.H. A preliminary study of alkali-activated slag blended with silica fume under the effect of thermal loads and thermal shock cycles. Constr. Build. Mater. 2013, 40, 522–532. [Google Scholar] [CrossRef]
- Rashad, A.M. An exploratory study on high-volume fly ash concrete incorporating silica fume subjected to thermal loads. J. Clean. Prod. 2015, 87, 735–744. [Google Scholar] [CrossRef]
- Anilkumar, P.M.; Raghavendra, M.; Sudhakumar, J. Effects of silica fume and fly ash on durability characteristics of high-performance concrete. Int. J. Emerg. Technol. Adv. Eng. 2014, 4, 298–303. [Google Scholar]
- García-taengua, E.; Sonebi, M.; Hossain, K.M.A.; Lachemi, M.; Khatib, J. Effects of the addition of nanosilica on the rheology, hydration and development of the compressive strength of cement mortars. Compos. Part B 2015, 81, 120–129. [Google Scholar] [CrossRef]
- Jalal, M.; Pouladkhan, A.; Harandi, O.F.; Jafari, D. Comparative study on effects of Class F fly ash, nano silica and silica fume on properties of high-performance self-compacting concrete. Construct. Build. Mater. 2015, 94, 90–104. [Google Scholar] [CrossRef]
- Sabet, F.A.; Libre, N.A.; Shekarchi, M. Mechanical and durability properties of self-consolidating high-performance concrete incorporating natural zeolite, silica fume and fly ash. Construct. Build. Mater. 2013, 44, 175–184. [Google Scholar] [CrossRef]
- Neville, A. Properties of Concrete, 4th ed.; Addison Wesley Longman Limited: London, UK, 1997. [Google Scholar]
- Thomas, M.D.A.; Hooton, R.D.; Scott, A.; Zibara, H. The effect of supplementary cementitious materials on chloride binding in hardened cement paste. Cem. Concr. Res. 2012, 42, 1–7. [Google Scholar] [CrossRef]
- Florea, M.V.A.; Brouwers, H.J.H. Chloride binding related to hydration products: Part I: Ordinary Portland Cement. Cem. Concr. Res. 2012, 42, 282–290. [Google Scholar] [CrossRef]
- Chandra, L.; Hardjito, D. The impact of using fly ash, silica fume and calcium carbonate on the workability and compressive strength of mortar. Procedia Eng. 2015, 125, 773–779. [Google Scholar]
- Leung, H.Y.; Kim, J.; Nadeem, A.; Jagannathan, J.; Anwar, M.P. Sorptivity of self-compacting concrete containing fly ash and silica fume. Construct. Build. Mater. 2016, 113, 369–375. [Google Scholar] [CrossRef]
- Benli, A.; Karataş, M.; Gurses, E. Effect of seawater and MgSO4 solution on the mechanical properties and durability of self-compacting mortars with fly ash/silica fume. Construct. Build. Mater. 2017, 146, 464–474. [Google Scholar] [CrossRef]
- Shekarchi, M.; Rafiee, A.; Layssi, H. Long-term chloride diffusion in silica fume concrete in harsh marine climates. Cem. Concr. Res. 2009, 31, 769–775. [Google Scholar] [CrossRef]
- Chalee, W.; Ausapanit, P.; Jaturapitakkul, C. Utilization of fly ash concrete in the marine environment for long term design life analysis. Mater. Design 2010, 31, 1242–1249. [Google Scholar] [CrossRef]
- Taylor, H.F.W.; Turner, A.B. Reactions of tricalcium silicate paste with organic liquids. Cem. Concr. Res. 1987, 17, 613–623. [Google Scholar] [CrossRef]
- Abo-El-Enein, S.A.; El-kady, G.; El-Sokkary, T.M.; Gharieb, M. Physico-mechanical properties of composite cement pastes containing silica fume and fly ash. Hbrc J. 2015, 11, 7–15. [Google Scholar] [CrossRef]
- Alonso, J.; Wesche, K. Characterization of Fly Ash. Fly Ash in Concrete; Taylor and Francis: London, UK, 1991; pp. 3–23. [Google Scholar]
- Helmuth, R.A. Fly ash in cement and concrete, Portland cement association. Cem. Concr. Res. 1987, 30, 201–204. [Google Scholar]
- Nochaiya, T.; Wongkeo, W.; Chaipanich, A. Utilization of fly ash with silica fume and properties of Portland cement—Fly ash–silica fume concrete. Fuel 2010, 89, 768–774. [Google Scholar] [CrossRef]
- Dinakar, P.; Babu, K.G.; Santhanam, M. Durability properties of high volume fly ash self-compacting concrete. Cem. Concr. Compos. 2008, 30, 880–886. [Google Scholar] [CrossRef]
- Yerramala, A.; Ganesh Babu, K. Transport properties of high volume fly ash roller compacted concrete. Cem. Concr. Compos. 2011, 33, 1057–1062. [Google Scholar] [CrossRef]
- Uysal, M.; Akyuncu, V. Durability performance of concrete incorporating Class F and Class C fly ashes. Construct. Build. Mater. 2012, 34, 170–178. [Google Scholar] [CrossRef]
- Aitcin, P.; Pigeon, M.; Pleau, R.; Malier, R.; Gagne, Y. Durability of high performance concretes. High-Performance Concrete. In From Material to Structure; E&FN Spon: London, UK, 1992; pp. 239–251. [Google Scholar]
- EI-Didamony, H.; Helmy, I.; Mostafa, K. Durability of sulfate resisting slag blended cement and mortars in sea water. Indian J. Eng. Mater. Sci. 1996, 3, 35–40. [Google Scholar]
- Duval, R.; Kadri, E. Influence of Silica Fume on the Workability and the Compressive Strength of High-Performance Concretes. Cem. Concr. Res. 1998, 28, 533–547. [Google Scholar] [CrossRef]
- Amarkhail, N. Effects of silica fume on properties of high-strength concrete. Int. J. Tech. Res. Appl. 2015, 32, 13–19. [Google Scholar]


| Properties | Cement | Fly Ash | Silica Fume | Fine Aggregate | Coarse Aggregate |
|---|---|---|---|---|---|
| Specific density (g/cm3) | 3.13 | 2.30 | 2.10 | 2.68 | 2.76 |
| Bulk density (kg/m3) | - | 1084.00 | 925.00 | 1580.00 | 1450.00 |
| Percentage of bulking (%) | - | - | - | 41.00 | 46.90 |
| Humidity (%) | - | - | - | 3.80 | 0.70 |
| Percentage of lumps (%) | - | - | - | 0.87 | 0.45 |
| Fineness modulus (%) | 0.20 | 2.42 | - |
| Components | Cement | Fly Ash | Silica Fume |
|---|---|---|---|
| (%) | (%) | (%) | |
| SiO2 | 20.59 | 85.10 | 93.45 |
| Fe2O3 | 1.09 | 1.75 | 0.52 |
| Al2O3 | 3.15 | 9.87 | 0.92 |
| CaO | 67.44 | 1.09 | 1.57 |
| SO3 | 1.79 | 0.10 | 0.63 |
| Sample Code | P | C | FA | SF | W | Sample Code | P | C | FA | SF | W | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (g) | (g) | (g) | (g) | (g) | (g) | (g) | (g) | (g) | (g) | (g) | (g) | |
| P-F0S0P0 | 0 | 500 | 0 | 0 | 142.5 | P-F20S10P0.3 | 1.5 | 350 | 100 | 50 | 129.0 | |
| P-F30S0P0 | 0 | 350 | 150 | 0 | 140.0 | P-F15S15P0.3 | 1.5 | 350 | 75 | 75 | 132.5 | |
| P-F25S5P0 | 0 | 350 | 125 | 25 | 142.5 | P-F0S0P0.4 | 2.0 | 500 | 0 | 0 | 120.0 | |
| P-F20S10P0 | 0 | 350 | 100 | 50 | 147.5 | P-F30S0P0.4 | 2.0 | 350 | 150 | 0 | 117.5 | |
| P-F15S15P0 | 0 | 350 | 75 | 75 | 150.0 | P-F25S5P0.4 | 2.0 | 350 | 125 | 25 | 120.0 | |
| P-F0S0P0.3 | 1.5 | 500 | 0 | 0 | 124.0 | P-F20S10P0.4 | 2.0 | 350 | 100 | 50 | 122.5 | |
| P-F30S0P0.3 | 1.5 | 350 | 150 | 0 | 121.5 | P-F15S15P0.4 | 2.0 | 350 | 75 | 75 | 125.0 | |
| P-F25S5P0.3 | 1.5 | 350 | 125 | 25 | 125.0 |
| Sample Symbols | Quantity of Materials for Concrete (kg/m3) | w/b | |||||||
|---|---|---|---|---|---|---|---|---|---|
| FA | SF | P | C | Fine Aggregate | Coarse Aggregate | B | W | ||
| C-F0S0P0 | 0 | 0 | 0 | 298 | 684 | 1236 | 298 | 180 | 0.6 |
| C-F20S10P0.4 | 63 | 32 | 1.27 | 221 | 654 | 1219 | 316 | 155 | 0.49 |
| Sample Code | 400–600 °C (Ca(OH)2) | 600–800 °C (C–S–H) | ||
|---|---|---|---|---|
| 28 Days | 56 Days | 28 Days | 56 Days | |
| P-F0S0P0 | 4.09 | 3.56 | 2.55 | 2.56 |
| P-F30S0P0.4 | 2.36 | 1.76 | 3.70 | 3.78 |
| P-F25S5P0.4 | 2.42 | 2.26 | 3.34 | 3.39 |
| P-F20S10P0.4 | 2.03 | 1.46 | 3.62 | 3.85 |
| P-F15S15P0.4 | 2.56 | 1.98 | 3.55 | 3.73 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chu, T.X.H.; Zheng, J.; Chen, D.; Nguyen, T.T.H.; Elbashiry, E.; Tang, V.T. Utilization of Industrial Waste in Cement in a Marine Environment with a Tropical Climate. J. Mar. Sci. Eng. 2019, 7, 245. https://doi.org/10.3390/jmse7080245
Chu TXH, Zheng J, Chen D, Nguyen TTH, Elbashiry E, Tang VT. Utilization of Industrial Waste in Cement in a Marine Environment with a Tropical Climate. Journal of Marine Science and Engineering. 2019; 7(8):245. https://doi.org/10.3390/jmse7080245
Chicago/Turabian StyleChu, Thi Xuan Hoa, Jinhai Zheng, Da Chen, Thi Thu Huong Nguyen, Elsafi Elbashiry, and Van Tai Tang. 2019. "Utilization of Industrial Waste in Cement in a Marine Environment with a Tropical Climate" Journal of Marine Science and Engineering 7, no. 8: 245. https://doi.org/10.3390/jmse7080245
APA StyleChu, T. X. H., Zheng, J., Chen, D., Nguyen, T. T. H., Elbashiry, E., & Tang, V. T. (2019). Utilization of Industrial Waste in Cement in a Marine Environment with a Tropical Climate. Journal of Marine Science and Engineering, 7(8), 245. https://doi.org/10.3390/jmse7080245






