Exposure to Decreased pH and Caffeine Affects Hemocyte Parameters in the Mussel Mytilus galloprovincialis
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
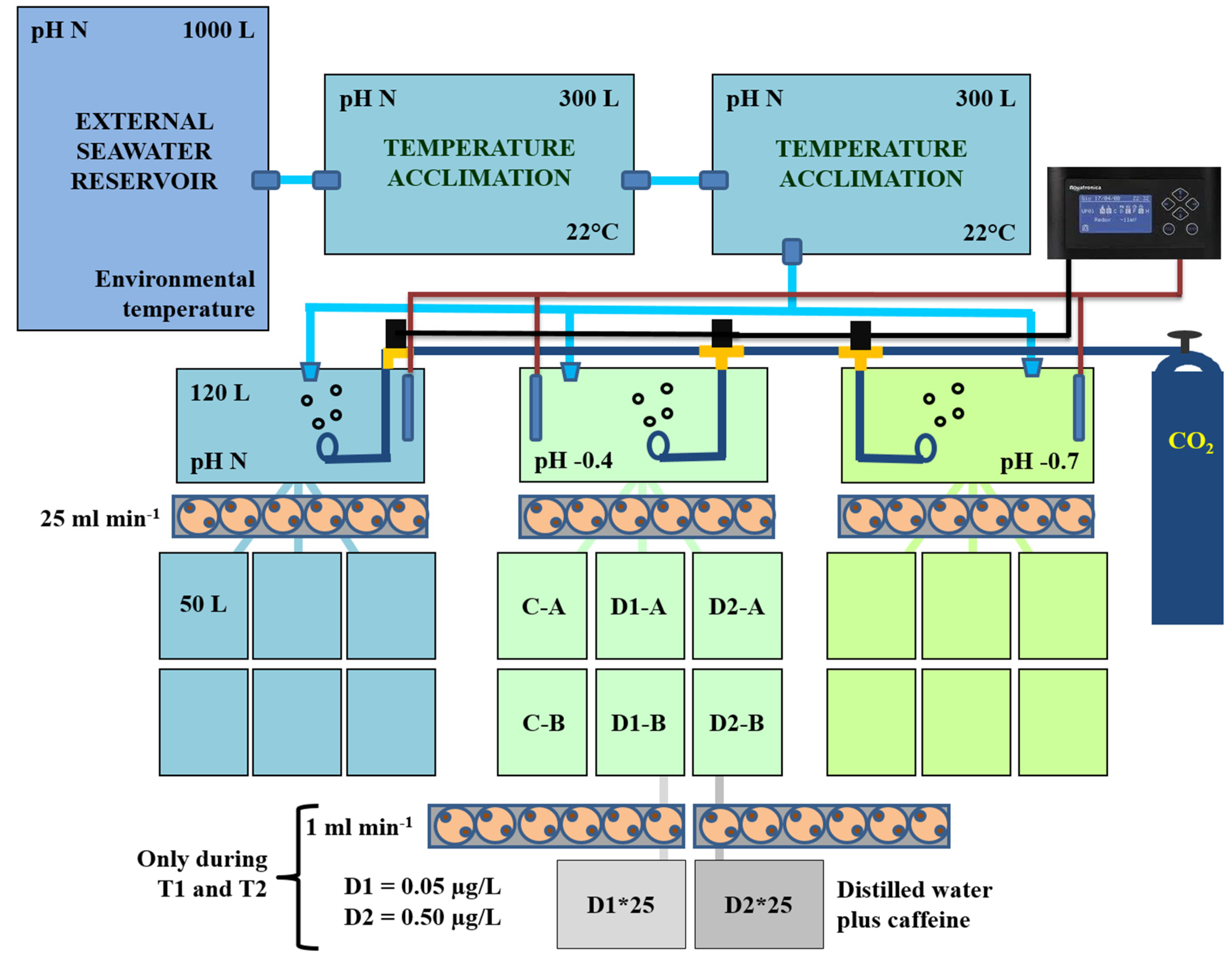
2.2. Experimental Set-Up for Mussel Exposure
2.3. Hemolymph Parameters
2.4. Statistical Analysis
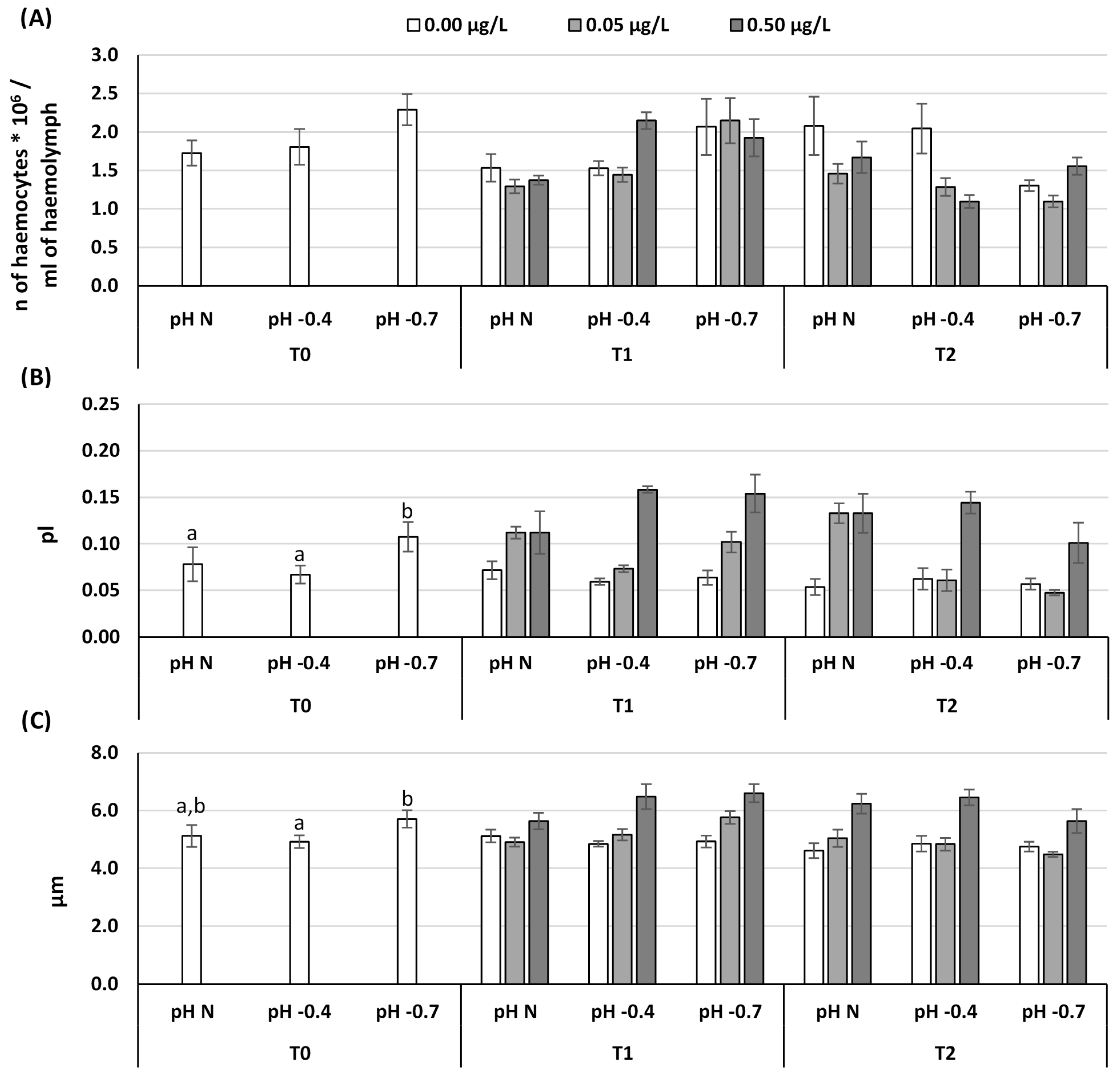
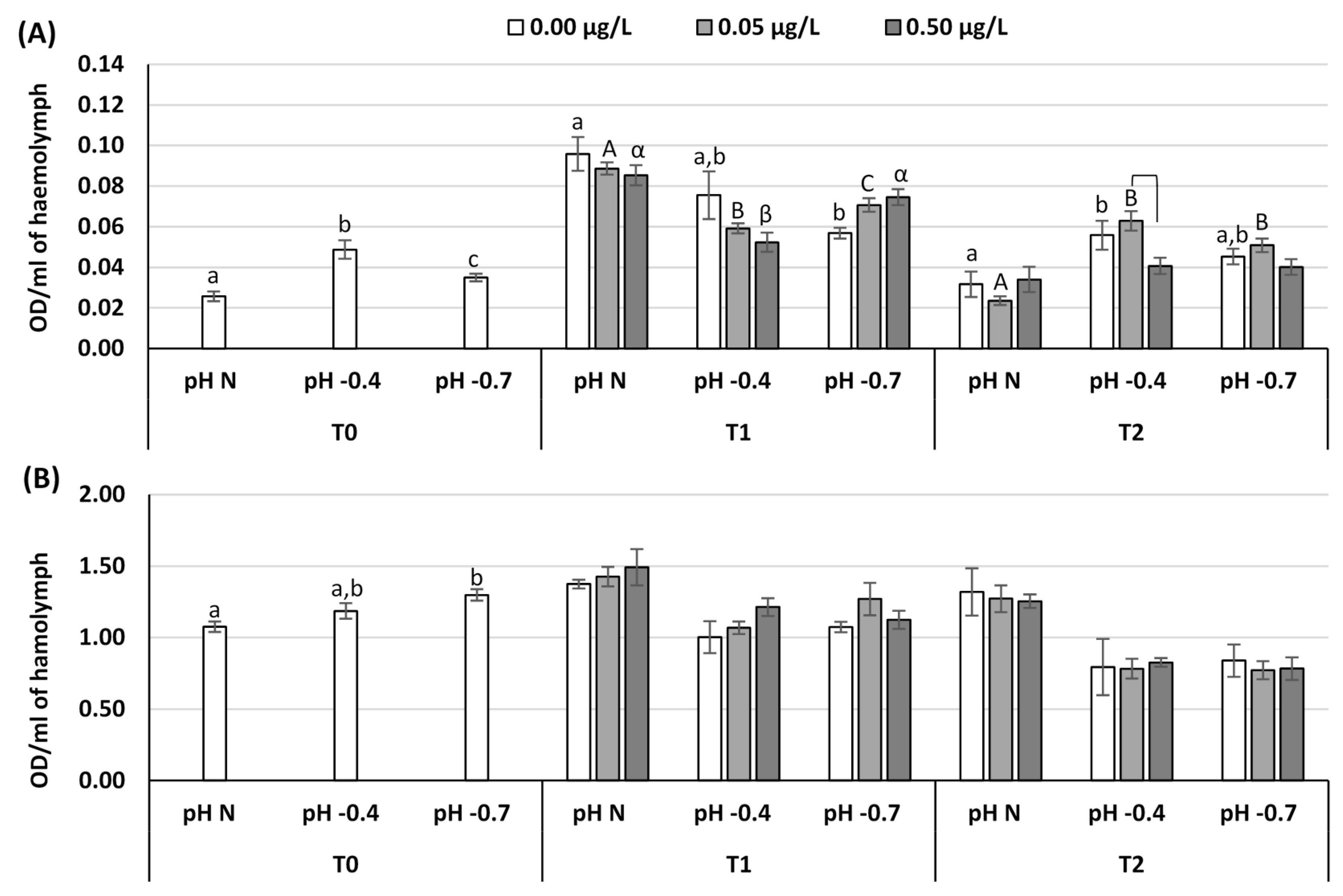
3. Results
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Gaw, S.; Thomas, K.V.; Hutchinson, T.H. Sources, impacts and trends of pharmaceuticals in the marine and coastal environment. Phil. Trans. R. Soc. B 2014, 369, 20130572. [Google Scholar] [CrossRef] [PubMed]
- Mezzelani, M.; Gorbi, S.; Regoli, F. Pharmaceuticals in the aquatic environments: Evidence of emerged threat and future challenges for marine organisms. Mar. Environ. Res. 2018, 140, 41–60. [Google Scholar] [CrossRef] [PubMed]
- Fekadu, S.; Alemayehu, E.; Dewil, R.; Van der Bruggen, B. Pharmaceuticals in freshwater aquatic environments: A comparison of the African and European challenge. Sci. Total Environ. 2019, 654, 324–337. [Google Scholar] [CrossRef] [PubMed]
- Comeau, F.; Surette, C.; Brun, G.L.; Losier, R. The occurrence of acidic drugs and caffeine in sewage effluents and receiving waters from three coastal watersheds in Atlantic Canada. Sci. Total Environ. 2008, 396, 132–146. [Google Scholar] [CrossRef] [PubMed]
- Loos, R.; Gawlik, B.M.; Locoro, G.; Rimaviciute, E.; Contini, S.; Bidoglio, G. EU-wide survey of polar organic persistent pollutants in European river waters. Environ. Pollut. 2009, 157, 561–568. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez del Rey, Z.; Granek, E.F.; Sylvester, S. Occurrence and concentration of caffeine in Oregon coastal waters. Mar. Pollut. Bull. 2012, 64, 1417–1424. [Google Scholar] [CrossRef]
- Alygizakis, N.A.; Gago-Ferrero, P.; Borova, V.L.; Pavlidou, A.; Hatzianestis, I.; Thomaidis, N.S. Occurrence and spatial distribution of 158 pharmaceuticals, drugs of abuse and related metabolites in offshore seawater. Sci. Total Environ. 2016, 541, 1097–1105. [Google Scholar] [CrossRef]
- Brumovský, M.; Bečanová, J.; Kohoutek, J.; Borghini, M.; Nizzetto, L. Contaminants of emerging concern in the open sea waters of the Western Mediterranean. Environ. Pollut. 2017, 229, 976–983. [Google Scholar] [CrossRef]
- Nawrot, P.; Jordan, S.; Eastwood, J.; Rotstein, J.; Hugenholtz, A.; Feeley, M. Effects of caffeine on human health. Food Addit. Contam. 2003, 20, 1–30. [Google Scholar] [CrossRef]
- Fulgoni, V.L.; Keast, D.R.; Lieberman, H.R. Trends in intake and sources of caffeine in the diets of US adults: 2001–20101–4. Am. J. Clin. Nutr. 2015, 101, 1081–1087. [Google Scholar] [CrossRef]
- Buerge, I.J.; Poiger, T.; Müller, M.D.; Buser, H.R. Caffeine, an anthropogenic marker for wastewater contamination of surface waters. Environ. Sci. Technol. 2003, 37, 691–700. [Google Scholar] [CrossRef] [PubMed]
- Quadra, G.R.; Paranaíba, J.R.; Vilas-Boas, J.; Roland, F.; Amado, A.M.; Barros, N.; Dias, R.J.P.; Cardoso, S.J. A global trend of caffeine consumption over time and related environmental impacts. Environ. Pollut. 2020, 256, 113343. [Google Scholar] [CrossRef] [PubMed]
- Kolpin, D.W.; Furlong, E.T.; Meyer, M.T.; Thurman, E.M.; Zaugg, S.D.; Barber, L.B.; Buxton, H.T. Pharmaceuticals, hormones, and other organic wastewater contaminants in US streams, 1999-2000: A national reconnaissance. Environ. Sci. Technol. 2002, 36, 1202–1211. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, P.J.; Bernot, M.J.; Doll, J.C.; Lauer, T.E. Detection of pharmaceuticals and personal care products (PPCPs) in near-shore habitats of southern Lake Michigan. Sci. Total Environ. 2013, 458, 187–196. [Google Scholar] [CrossRef]
- Siegener, R.; Chen, R.F. Caffeine in Boston Harbor seawater. Mar. Pollut. Bull. 2002, 44, 383–387. [Google Scholar] [CrossRef]
- Rodríguez-Gil, J.; Cáceres, N.; Dafouz, R.; Valcárcel, Y. Caffeine and paraxanthine in aquatic systems: Global exposure distributions and probabilistic risk assessment. Sci. Total Environ. 2018, 612, 1058–1071. [Google Scholar] [CrossRef]
- Benotti, M.J.; Brownawell, B.J. Distributions of pharmaceuticals in an urban estuary during both dry- and wet-weather conditions. Environ. Sci. Technol. 2007, 41, 5795–5802. [Google Scholar] [CrossRef]
- French, V.A.; King, S.C.; Kumar, A.; Northcott, G.; McGuinness, K.; Parry, D. Characterisation of microcontaminants in Darwin Harbour, a tropical estuary of northern Australia undergoing rapid development. Sci. Total Environ. 2015, 536, 639–647. [Google Scholar] [CrossRef]
- IPCC. Summary for policymakers. In Climate Change 2007: The Physical Science Basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change; Solomon, S., Qin, D., Manning, M., Chen, Z., Marquis, M., Averyt, K.B., Tignor, M., Miller, H.L., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2007; p. 996. [Google Scholar]
- Orr, J.C.; Fabry, V.I.; Aumont, O.; Bopp, L.; Doney, S.C.; Feely, R.A.; Gnanadesikan, A.; Gruber, N.; Ishida, A.; Joos, F.; et al. Anthropogenic ocean acidification over the twenty-first century and its impact on calcifying organisms. Nature 2005, 437, 681–686. [Google Scholar] [CrossRef]
- Williamson, P.; Turley, C. Ocean acidification in a geoengineering context. Phil. Trans. R. Soc. A 2012, 370, 4317–4342. [Google Scholar] [CrossRef]
- Hartin, C.A.; Bond-Lamberti, B.; Patel, P.; Mundra, A. Ocean acidification over the next three centuries using a simple global climate carbon-cycle model: Projections and sensitivities. Biogeosciences 2016, 13, 4329–4342. [Google Scholar] [CrossRef]
- IPCC. Summary for Policymakers. In IPCC Special Report on the Ocean and Cryosphere in a Changing Climate; Pörtner, H.O., Roberts, D.C., Masson-Delmotte, V., Zhai, P., Tignor, M., Poloczanska, E., Mintenbeck, K., Alegría, A., Nicolai, M., Okem, A., et al., Eds.; Available online: https://report.ipcc.ch/srocc/pdf/SROCC_FinalDraft_FullReport.pdf (accessed on 10 February 2020).
- Cheng, T.C. Hemocytes: Forms and functions. In The Eastern Oyster Crassostrea Virginica; Kennedy, V.S., Newell, R.I.E., Eble, A.F., Eds.; Maryland Sea Grant Book: College Park, MD, USA, 1996; pp. 299–333. [Google Scholar]
- Cheng, T.C. Cellular defense mechanisms in oysters. In Recent Advances in Marine Biotechnology: Immunobiology and Pathology; Fingerman, N., Nagabhushanam, R., Eds.; Science Publishers: Enfield, NH, USA, 2000; pp. 43–83. [Google Scholar]
- Chu, F.L.E. Defense mechanisms of marine bivalves. In Recent Advances in Marine Biotechnology: Immunobiology and Pathology; Fingerman, N., Nagabhushanam, R., Eds.; Science Publishers: Enfield, NH, USA, 2000; pp. 1–42. [Google Scholar]
- Munari, M.; Matozzo, V.; Marin, M.G. Combined effects of temperature and salinity on functional responses of hemocytes and survival in air of the clam Ruditapes philippinarum. Fish Shellfish Immun. 2011, 30, 1024–1030. [Google Scholar] [CrossRef] [PubMed]
- Matozzo, V.; Chinellato, A.; Munari, M.; Finos, L.; Bressan, M.; Marin, M.G. First evidence of immunomodulation in bivalves under seawater acidification and increased temperature. PLoS ONE 2012, 7, e33820. [Google Scholar] [CrossRef] [PubMed]
- Monari, M.; Matozzo, V.; Foschi, J.; Cattani, O.; Serrazanetti, G.P.; Marin, M.G. Effects of high temperatures on functional responses of hemocytes in the clam Chamelea gallina. Fish Shellfish Immun. 2007, 22, 98–114. [Google Scholar] [CrossRef] [PubMed]
- Noyes, P.D.; Mcelwee, M.K.; Miller, H.D.; Clark, B.W.; Van Tiem, L.A.; Walcott, K.C.; Erwin, K.N.; Levin, E.D. The toxicology of climate change: Environmental contaminants in a warming world. Environ. Int. 2009, 35, 971–986. [Google Scholar] [CrossRef] [PubMed]
- Millero, F.J.; Woosley, R.; Ditrolio, B.; Waters, J. Effect of ocean acidification on the speciation of metals in seawater. Oceanography 2009, 22, 72–85. [Google Scholar] [CrossRef]
- Munari, M.; Matozzo, V.; Chemello, G.; Riedl, V.; Pastore, P.; Badocco, D.; Marin, M.G. Seawater acidification and emerging contaminants: A dangerous marriage for hemocytes of marine bivalves. Environ. Res. 2019, 175, 11–21. [Google Scholar] [CrossRef]
- Gagné, F.; Burgeot, T. Bivalves in Ecotoxicology. In Encyclopedia of Aquatic Ecotoxicology; Férard, J.F., Blaise, C., Eds.; Springer: Dordrecht, The Netherlands, 2013. [Google Scholar]
- Matozzo, V.; Gagné, F. Immunotoxicology approaches in ecotoxicology: Lessons from mollusks. In Lessons in Immunity: From Single-Cell Organisms to Mammals, 1st ed.; Ballarin, L., Cammarata, M., Eds.; Academic Press-Elsevier: London, UK, 2016; pp. 29–51. [Google Scholar]
- Munari, M.; Matozzo, V.; Gagné, F.; Chemello, G.; Riedl, V.; Finos, L.; Pastore, P.; Badocco, D.; Marin, M.G. Does exposure to reduced pH and diclofenac induce oxidative stress in marine bivalves? A comparative study with the mussel Mytilus galloprovincialis and the clam Ruditapes philippinarum. Environ. Pollut. 2018, 240, 925–937. [Google Scholar]
- Cajaraville, M.P.; Olabarrieta, I.; Marigomez, I. In vitro activities in mussel hemocytes as biomarkers of environmental quality: A case study in the Abra Estuary (Biscay Bay). Ecotox. Environ. Safe. 1996, 35, 253–260. [Google Scholar] [CrossRef]
- Matozzo, V.; Ballarin, L.; Marin, M.G. In vitro effects of tributyltin on functional responses of hemocytes in the clam Tapes philippinarum. Appl. Organomet. Chem. 2002, 16, 169–174. [Google Scholar] [CrossRef]
- Matozzo, V.; Ballarin, L.; Pampanin, D.M.; Marin, M.G. Effects of copper and cadmium exposure on functional responses of hemocytes in the clam, Tapes philippinarum. Arch. Environ. Con. Tox. 2001, 41, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Sauve, S.; Brousseau, P.; Pellerin, J.; Morin, Y.; Senecal, L.; Goudreau, P.; Fournier, M. Phagocytic activity of marine and freshwater bivalves: In vitro exposure of hemocytes to metals (Ag, Cd, Hg and Zn). Aquat. Toxicol. 2002, 58, 189–200. [Google Scholar] [CrossRef]
- Duchemin, M.B.; Auffret, M.; Wessel, N.; Fortier, M.; Morin, Y.; Pellerin, J.; Fournier, M. Multiple experimental approaches of immunotoxic effects of mercury chloride in the blue mussel, Mytilus edulis, through in vivo, in tubo and in vitro exposures. Environ. Pollut. 2008, 153, 416–423. [Google Scholar] [CrossRef] [PubMed]
- Guerra-Rivas, G.; Gomez-Gutierrez, C.M.; Marquez-Rocha, F.J. Effect of polycyclic aromatic hydrocarbons on the pallial fluid buffering capacity of the marine mussel, Mytilus galloprovincialis. Comp. Biochem. Phys. C 2002, 132, 171–179. [Google Scholar] [CrossRef]
- Giannapas, M.; Karnis, L.; Dailianis, S. Generation of free radicals in hemocytes of mussels after exposure to low molecular weight PAH components: Immune activation, oxidative and genotoxic effects. Comp. Biochem. Phys. C 2012, 155, 182–189. [Google Scholar]
- Parolini, M.; Binelli, A.; Cogni, D.; Riva, C.; Provini, A. An in vitro biomarker approach for the evaluation of the ecotoxicity of non-steroidal anti-inflammatory drugs (NSAIDs). Toxicol. In Vitro 2009, 23, 935–942. [Google Scholar] [CrossRef] [PubMed]
- Matozzo, V.; Bertin, V.; Battistara, M.; Guidolin, A.; Masiero, L.; Marisa, I.; Orsetti, A. Does the antibiotic amoxicillin affect hemocyte parameters in non-target aquatic invertebrates? The clam Ruditapes philippinarum and the mussel Mytilus galloprovincialis as model organisms. Mar. Environ. Res. 2016, 119, 51–58. [Google Scholar] [CrossRef]
- Matozzo, V.; Rova, S.; Marin, M.G. The nonsteroidal anti-inflammatory drug, ibuprofen, affects the immune parameters in the clam Ruditapes philippinarum. Mar. Environ. Res. 2012, 79, 116–121. [Google Scholar] [CrossRef]
- Matozzo, V.; Costa Devoti, A.; Marin, M.G. Immunotoxic effects of triclosan in the clam Ruditapes philippinarum. Ecotoxicology 2012, 21, 66–74. [Google Scholar] [CrossRef]
- Luna-Acosta, A.; Renault, T.; Thomas-Guyon, H.; Faury, N.; Saulnier, D.; Budzinski, H.; Le Menach, K.; Pardon, P.; Fruitier-Arnaudin, I.; Bustamante, P. Detection of early effects of a single herbicide (Diuron) and a mix of herbicides and pharmaceuticals (Diuron, Isoproturon, Ibuprofen) on immunological parameters of pacific oyster (Crassostrea gigas) spat. Chemosphere 2012, 87, 1335–1340. [Google Scholar] [CrossRef][Green Version]
- Canesi, L.; Ciacci, C.; Betti, M.; Fabbri, R.; Canonico, B.; Fantinati, A.; Marcornini, A.; Pojana, G. Immunotoxicity of carbon black nanoparticles to blue mussel hemocytes. Environ. Int. 2008, 34, 1114–1119. [Google Scholar] [CrossRef] [PubMed]
- Marisa, I.; Matozzo, V.; Munari, M.; Binelli, A.; Parolini, M.; Martucci, A.; Franceschinis, E.; Brianese, N.; Marin, M.G. In vivo exposure of the marine clam Ruditapes philippinarum to zinc oxide nanoparticles: Responses in gills, digestive gland and haemolymph. Environ. Sci. Pollut. Res. 2016, 23, 15275–15293. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Han, Y.; Guo, C.; Zhao, X.; Liu, S.; Su, W.; Zha, S.; Wang, Y.; Liu, G. Immunotoxicity of nanoparticle nTiO2 to a commercial marine bivalve species, Tegillarca granosa. Fish Shellfish Immun. 2017, 66, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez del Rey, Z.; Granek, E.F.; Buckley, B.A. Expression of Hsp70 in Mytilus californianus following exposure to caffeine. Ecotoxicology 2011, 20, 855–861. [Google Scholar] [CrossRef] [PubMed]
- Capolupo, M.; Valbonesi, P.; Kiwan, A.; Buratti, S.; Franzellitti, S.; Fabbri, E. Use of an integrated biomarker-based strategy to evaluate physiological stress responses induced by environmental concentrations of caffeine in the Mediterranean mussel Mytilus galloprovincialis. Sci. Total Environ. 2016, 563, 538–548. [Google Scholar] [CrossRef] [PubMed]
- Aguirre-Martínez, G.V.; DelValls, T.A.; Martín-Díaz, M.L. General stress, detoxification pathways, neurotoxicity and genotoxicity evaluated in Ruditapes philippinarum exposed to human pharmaceuticals. Ecotoxicol. Environ. Safe. 2016, 124, 18–31. [Google Scholar] [CrossRef]
- Aguirre-Martínez, G.V.; DelValls, A.T.; Martín-Díaz, M.L. Yes, caffeine, ibuprofen, carbamazepine, novobiocin and tamoxifen have an effect on Corbicula fluminea (Muller, 1774). Ecotoxicol. Environ. Safe. 2015, 120, 142–154. [Google Scholar] [CrossRef]
- Aguirre-Martínez, G.V.; Buratti, S.; Fabbri, E.; DelValls, A.T.; Martín-Díaz, M.L. Using lysosomal membrane stability of hemocytes in Ruditapes philippinarum as a biomarker of cellular stress to assess contamination by caffeine, ibuprofen, carbamazepine and novobiocin. J. Environ. Sci. 2013, 25, 1408–1418. [Google Scholar] [CrossRef]
- Al Reef, T.; Ghanem, E. Caffeine: Well-known as psychotropic substance, but little as immunomodulatory. Immunobiology 2018, 223, 818–825. [Google Scholar] [CrossRef]
- Chen, M.; Yang, H.; Delaporte, M.; Zhao, S. Immune condition of Chlamys farreri in response to acute temperature challenge. Aquaculture 2007, 271, 479–487. [Google Scholar] [CrossRef]
- Perrigault, M.; Dahl, S.F.; Espinosa, E.P.; Gambino, L.; Allam, B. Effects of temperature on hard clam (Mercenaria mercenaria) immunity and QPX (Quahog Parasite Unknown) disease development: II. Defense parameters. J. Invertebr. Pathol. 2011, 106, 322–332. [Google Scholar] [CrossRef] [PubMed]
- Matozzo, V.; Monari, M.; Foschi, J.; Serrazanetti, G.P.; Cattani, O.; Marin, M.G. Effects of salinity on the clam Chamelea gallina. Part I: Alterations in immune responses. Mar. Biol. 2007, 151, 1051–1058. [Google Scholar] [CrossRef]
- Bamber, S.D. Does sustained tolerance of reduced salinity seawater alter phagocytosis efficiency in hemocytes of the blue mussel Mytilus edulis (L.)? J. Exp. Mar. Biol. Ecol. 2018, 500, 132–139. [Google Scholar] [CrossRef]
- Bibby, R.; Widdicombe, S.; Parry, H.; Spicer, J.; Pipe, R. Effects of ocean acidification on the immune response of the blue mussel Mytilus edulis. Aquat. Biol. 2008, 2, 67–74. [Google Scholar] [CrossRef]
- Wang, Q.; Cao, R.; Ning, X.; You, L.; Mu, C.; Wang, C.; Wei, L.; Cong, M.; Wu, H.; Zhao, J. Effects of ocean acidification on immune responses of the Pacific oyster Crassostrea gigas. Fish Shellfish Immun. 2016, 49, 24–33. [Google Scholar] [CrossRef]
- Su, W.; Rong, J.; Zha, S.; Yan, M.; Fang, J.; Liu, G. Ocean acidification affects the cytoskeleton, lysozymes, and nitric oxide of hemocytes: A possible explanation for the hampered phagocytosis in blood clams, Tegillarca granosa. Front. Physiol. 2018, 9, 619. [Google Scholar] [CrossRef]
- Oliver, L.M.; Fisher, W.S. Appraisal of prospective bivalve immunomarkers. Biomarkers 1999, 4, 510–530. [Google Scholar]
- Pipe, R.K.; Coles, J.A. Environmental contaminants influencing immune function in marine bivalve molluscs. Fish Shellfish Immun. 1995, 5, 581–595. [Google Scholar] [CrossRef]
- Parry, H.E.; Pipe, R.K. Interactive effects of temperature and copper on immunocompetence and disease susceptibility in mussels (Mytilus edulis). Aquat. Toxicol. 2004, 69, 311–325. [Google Scholar] [CrossRef]
- Matozzo, V.; Marin, M.G.; Cima, F.; Ballarin, L. First evidence of cell division in circulating hemocytes from the Manila clam Tapes philippinarum. Cell Biol. Int. 2008, 32, 865–868. [Google Scholar] [CrossRef]
- Renwrantz, L.; Siegmund, E.; Woldmann, M. Variations in hemocyte counts in the mussel, Mytilus edulis: Similar reaction patterns occur in disappearance and return of molluscan hemocytes and vertebrate leukocytes. Comp. Biochem. Phys. A 2013, 164, 629–637. [Google Scholar] [CrossRef] [PubMed]
- Mayrand, E.; St-Jean, S.D.; Courtenay, S.C. Hemocyte responses of blue mussels (Mytilus edulis L.) transferred from a contaminated site to a reference site: Can the immune system recuperate? Aquac. Res. 2005, 36, 962–971. [Google Scholar] [CrossRef]
- Hauton, C.; Hawkins, L.E.; Hutchinson, S. The use of the neutral red retention assay to examine the effects of temperature and salinity on hemocytes of the European flat oyster Ostrea edulis (L). Comp. Biochem. Phys. B 1998, 119, 619–623. [Google Scholar] [CrossRef]
- Canesi, L.; Lorusso, L.C.; Ciacci, C.; Betti, M.; Regoli, F.; Pojana, G.; Gallo, G. Effects of blood lipid lowering pharmaceuticals (bezafibrate and gemfibrozil) on immune and digestive gland functions of the bivalve mollusc, Mytilus galloprovincialis. Chemosphere 2007, 69, 994–1002. [Google Scholar] [CrossRef]
- Binelli, A.; Cogni, D.; Parolini, M.; Riva, C.; Provini, A. Cytotoxic and genotoxic effects of in vitro exposure to triclosan and trimethoprim on zebra mussel (Dreissena polymorpha) hemocytes. Comp. Biochem. Phys. C 2009, 150, 50–56. [Google Scholar] [CrossRef]
- Coles, J.A.; Farley, S.R.; Pipe, R.K. Alteration of the immune response of the common marine mussel Mytilus edulis resulting from exposure to cadmium. Dis. Aquat. Org. 1995, 22, 59–65. [Google Scholar] [CrossRef]
| Sampling Time | Conditions (pH and Diclofenac) | pHT | TA | DIC | pCO2 | Ωcal | Ωarg | |
|---|---|---|---|---|---|---|---|---|
| T0 | N pH | 8.07 ± 0.01 | 2884.42 ± 32.08 | 2665.50 ± 30.58 | 631.47 ± 14.39 | 5.72 ± 0.09 | 3.76 ± 0.06 | |
| pH −0.4 | 7.76 ± 0.01 | 2800.33 ± 21.06 | 2681.52 ± 17.34 | 1080.65 ± 31.21 | 3.62 ± 0.10 | 2.38 ± 0.06 | ||
| pH −0.7 | 7.34 ± 0.03 | 2894.53 ± 30.64 | 2923.77 ± 25.36 | 3248.09 ± 214.45 | 1.56 ± 0.11 | 1.02 ± 0.07 | ||
| T1–T2 | N pH | 0.00 µg/L | 8.14 ± 0.03 | 2848.91 ± 10.38 | 2539.97 ± 12.27 | 399.19 ± 39.00 | 7.71 ± 0.34 | 5.07 ± 0.22 |
| 0.05 µg/L | 8.14 ± 0.04 | 2840.80 ± 6.34 | 2529.65 ± 13.70 | 391.60 ± 37.96 | 7.76 ± 0.38 | 5.10 ± 0.25 | ||
| 0.50 µg/L | 8.14 ± 0.04 | 2841.81 ± 8.03 | 2531.97 ± 15.17 | 395.75 ± 31.85 | 7.73 ± 0.31 | 5.09 ± 0.20 | ||
| pH −0.4 | 0.00 µg/L | 7.71 ± 0.04 | 2837.30 ± 10.42 | 2734.99 ± 19.68 | 1262.87 ± 122.78 | 3.34 ± 0.25 | 2.20 ± 0.17 | |
| 0.05 µg/L | 7.73 ± 0.03 | 2825.60 ± 10.79 | 2713.87 ± 13.89 | 1167.10 ± 87.11 | 3.49 ± 0.18 | 2.30 ± 0.12 | ||
| 0.50 µg/L | 7.74 ± 0.04 | 2820.23 ± 9.65 | 2708.33 ± 19.47 | 1143.96 ± 152.52 | 3.49 ± 0.19 | 2.29 ± 0.13 | ||
| pH −0.7 | 0.00 µg/L | 7.39 ± 0.01 | 2879.65 ± 18.05 | 2886.92 ± 19.91 | 2734.47 ± 156.34 | 1.72 ± 0.12 | 1.13 ± 0.08 | |
| 0.05 µg/L | 7.42 ± 0.02 | 2883.54 ± 19.37 | 2882.98 ± 26.64 | 2566.18 ± 162.27 | 1.81 ± 0.12 | 1.19 ± 0.08 | ||
| 0.50 µg/L | 7.43 ± 0.02 | 2878.18 ± 16.18 | 2872.24 ± 24.44 | 2504.41 ± 181.90 | 1.87 ± 0.12 | 1.23 ± 0.08 | ||
| Sampling Time | Factors | All Variables in | THC | Haemocytes Volume (pl) | Haemocytes Diameter (µm) | NRU | Cell Proliferation |
|---|---|---|---|---|---|---|---|
| T0 | pH | F(2,89) = 3.181 p(MC) = 0.047 | F(2,89) = 2.281 p(MC) = 0.106 | F(2,89) = 5.301 p(MC) = 0.006 | F(2,89) = 4.562 p(MC) = 0.013 | F(2,89) = 13.403 p(MC) < 0.001 | F(2,89) = 6.320 p(MC) < 0.001 |
| T1 | pH | F(2,53) = 8.968 p(MC) < 0.001 | F(2,53) = 8.285 p(MC) < 0.005 | F(2,53) = 1.825 p(MC) = 0.175 | F(2,53) = 3.363 p(MC) = 0.046 | F(2,53) = 19.306 p(MC) < 0.001 | F(2,53) = 14.833 p(MC) < 0.001 |
| caffeine | F(2,53) = 0.416 p(MC) = 0.6624 | F(2,53) = 0.275 p(MC) = 0.758 | F(2,53) = 19.994 p(MC) < 0.001 | F(2,53) = 20.295 p(MC) < 0.005 | F(2,53) = 0.643 p(MC) = 0.522 | F(2,53) = 2.125 p(MC) = 0.132 | |
| pH*caffeine | F(4,53) = 1.268 p(MC) = 0.291 | F(4,53) = 1.108 p(MC) = 0.360 | F(4,53) = 1.209 p(MC) = 0.283 | F(4,53) = 2.051 p(MC) = 0.102 | F(4,53) = 3.497 p(MC) = 0.014 | F(4,53) = 0.914 p(MC) = 0.459 | |
| T2 | pH | F(2,53) = 3.712 p(MC) = 0.032 | F(2,53) = 3.621 p(MC) = 0.033 | F(2,53) = 1.997 p(MC) = 0.150 | F(2,53) = 2.019 p(MC) = 0.142 | F(2,53) = 17.974 p(MC) < 0.001 | F(2,53) = 20.194 p(MC) < 0.001 |
| caffeine | F(2,53) = 5.684 p(MC) = 0.007 | F(2,53) = 5.489 p(MC) = 0.008 | F(2,53) = 25.008 p(MC) < 0.001 | F(2,53) = 41.010 p(MC) < 0.001 | F(2,53) = 1.970 p(MC) = 0.158 | F(2,53) = 0.144 p(MC) = 0.869 | |
| pH*caffeine | F(4,53) = 1.079 p(MC) = 0.375 | F(4,53) = 1.138 p(MC) = 0.355 | F(4,53) = 0.772 p(MC) = 0.553 | F(4,53) = 0.802 p(MC) = 0.529 | F(4,53) = 2.960 p(MC) = 0.028 | F(4,53) = 0.0548 p(MC) = 0.992 |
| T1 | pH N−0.7pH −0.4 | pH N−0.7pH −0.7 | pH −0.4−0.7pH −0.7 | |
| THC | 0.013 | 0.003 | 0.035 | |
| Haemocytes Diameter (µm) | 0.200 | 0.009 | 0.225 | |
| NRU | <0.001 | <0.001 | 0.052 | |
| Cell proliferation | <0.001 | <0.001 | 0.348 | |
| 0.00 µg/L−0.70.05 µg/L | 0.00 µg/L−0.70.50 µg/L | 0.05 µg/L−0.70.50 µg/L | ||
| Haemocytes Volume (pl) | 0.042 | <0.001 | <0.001 | |
| Haemocytes Diameter (µm) | 0.052 | <0.001 | <0.001 | |
| T2 | pH N−0.7pH −0.4 | pH N−0.7pH −0.7 | pH −0.4−0.7pH −0.7 | |
| THC | 0.566 | 0.015 | 0.021 | |
| NRU | <0.001 | <0.001 | 0.052 | |
| Cell Proliferation | <0.001 | <0.001 | 0.938 | |
| 0.00 µg/L−0.70.05 µg/L | 0.00 µg/L−0.70.50 µg/L | 0.05 µg/L−0.70.50 µg/L | ||
| THC | 0.005 | 0.262 | 0.005 | |
| Haemocytes Volume (pl) | 0.796 | <0.001 | <0.001 | |
| Haemocytes Diameter (µm) | 0.790 | <0.001 | <0.001 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Munari, M.; Matozzo, V.; Benetello, G.; Riedl, V.; Pastore, P.; Badocco, D.; Marin, M.G. Exposure to Decreased pH and Caffeine Affects Hemocyte Parameters in the Mussel Mytilus galloprovincialis. J. Mar. Sci. Eng. 2020, 8, 238. https://doi.org/10.3390/jmse8040238
Munari M, Matozzo V, Benetello G, Riedl V, Pastore P, Badocco D, Marin MG. Exposure to Decreased pH and Caffeine Affects Hemocyte Parameters in the Mussel Mytilus galloprovincialis. Journal of Marine Science and Engineering. 2020; 8(4):238. https://doi.org/10.3390/jmse8040238
Chicago/Turabian StyleMunari, Marco, Valerio Matozzo, Giuditta Benetello, Verena Riedl, Paolo Pastore, Denis Badocco, and Maria Gabriella Marin. 2020. "Exposure to Decreased pH and Caffeine Affects Hemocyte Parameters in the Mussel Mytilus galloprovincialis" Journal of Marine Science and Engineering 8, no. 4: 238. https://doi.org/10.3390/jmse8040238
APA StyleMunari, M., Matozzo, V., Benetello, G., Riedl, V., Pastore, P., Badocco, D., & Marin, M. G. (2020). Exposure to Decreased pH and Caffeine Affects Hemocyte Parameters in the Mussel Mytilus galloprovincialis. Journal of Marine Science and Engineering, 8(4), 238. https://doi.org/10.3390/jmse8040238








