Toxicity of Deltamethrin to Zebrafish Gonads Revealed by Cellular Biomarkers
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Animals
2.2. Experimental Design
2.3. Histological Analyses, Immunohistochemistry, Immunofluorescence and Tunel Assay for Detection of Oxidative Stress, Apoptosis, and Proliferation in Gonadal Tissue
2.4. Measurements and Analysis of Histological Modifications
3. Results
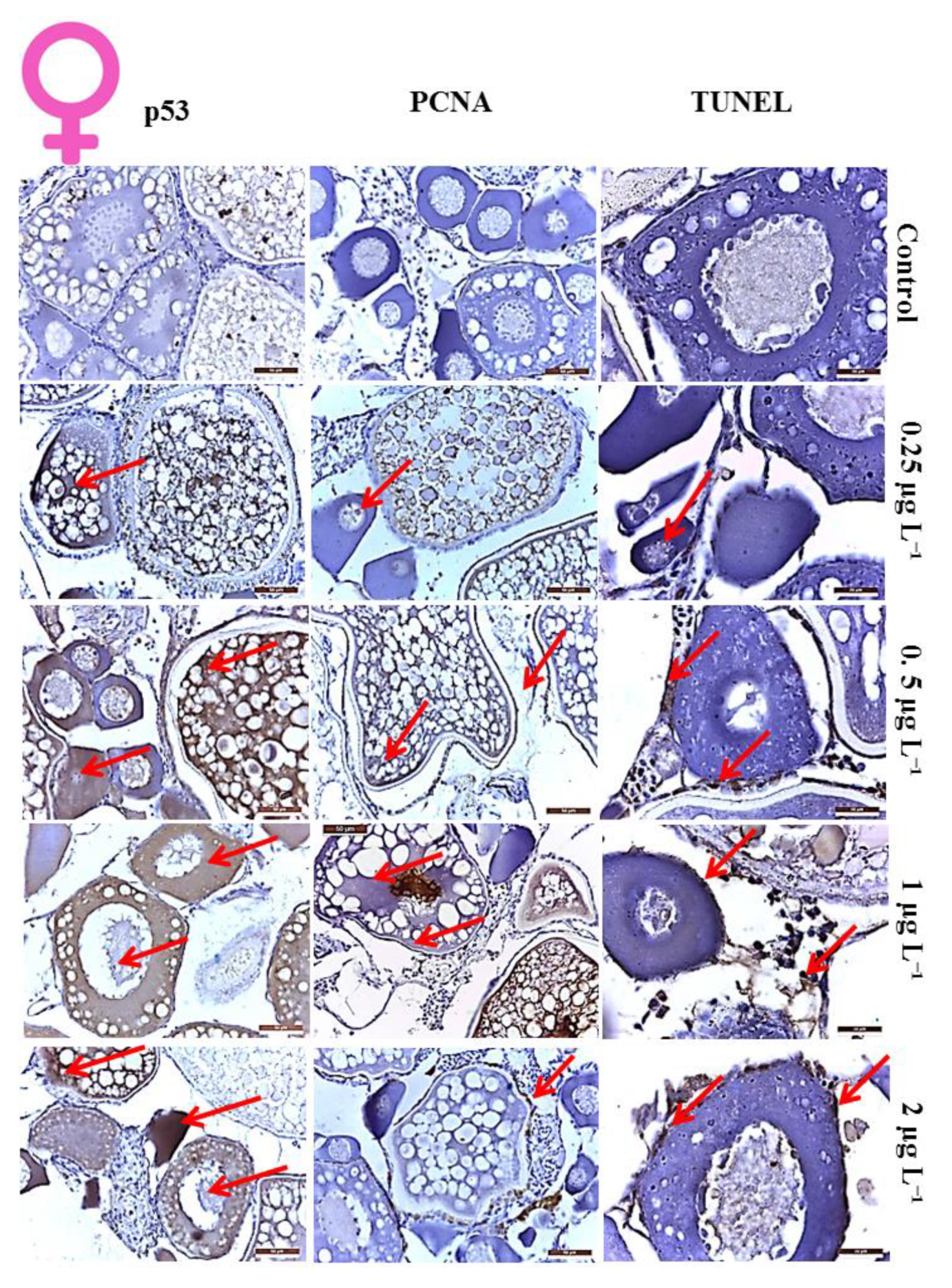
3.1. Histological Analysis of DM Toxicity on Ovary
3.2. Proliferation Index of the Cells in Ovaries
3.3. Apoptotic Index of the Cells in Ovaries
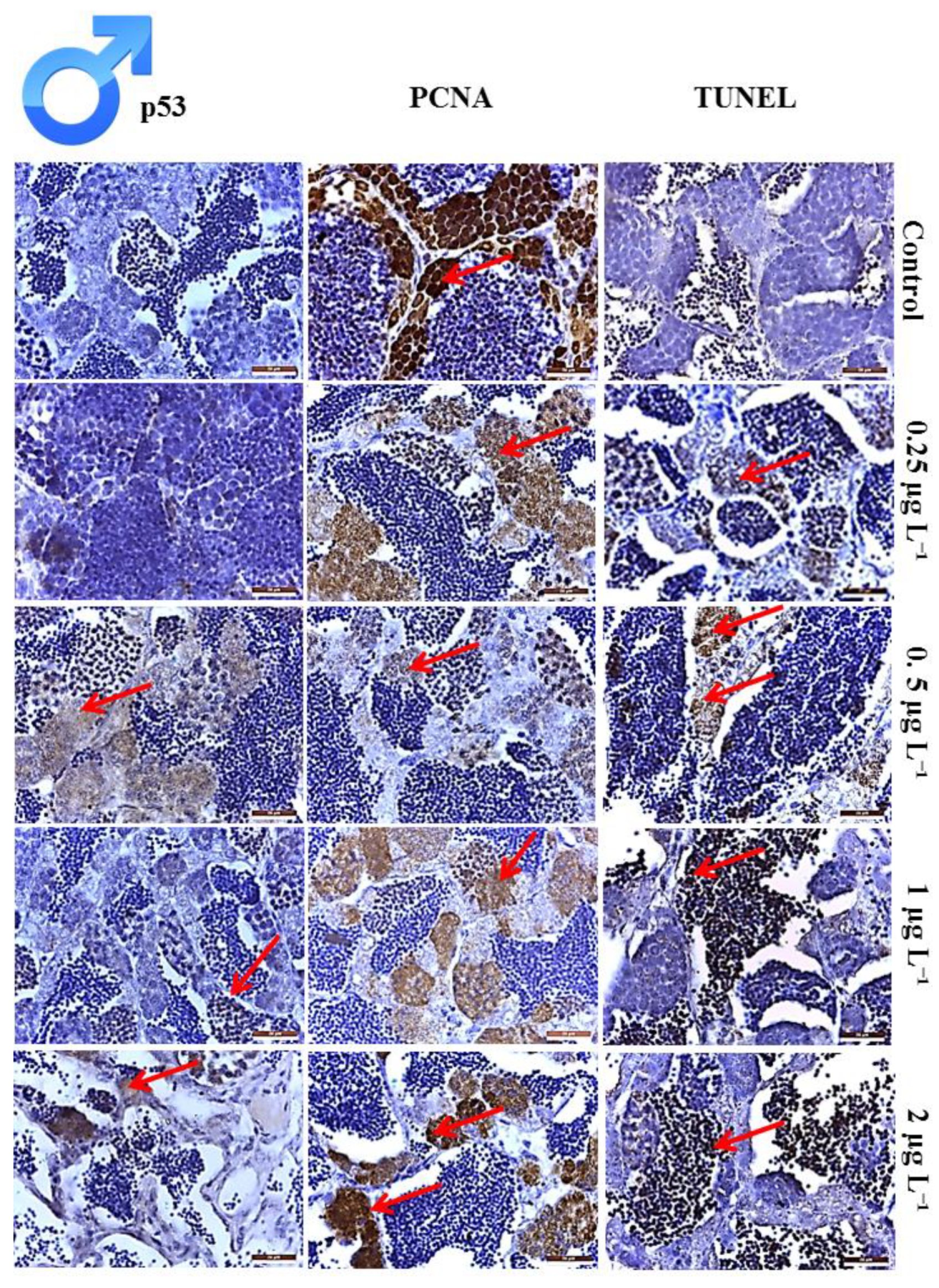
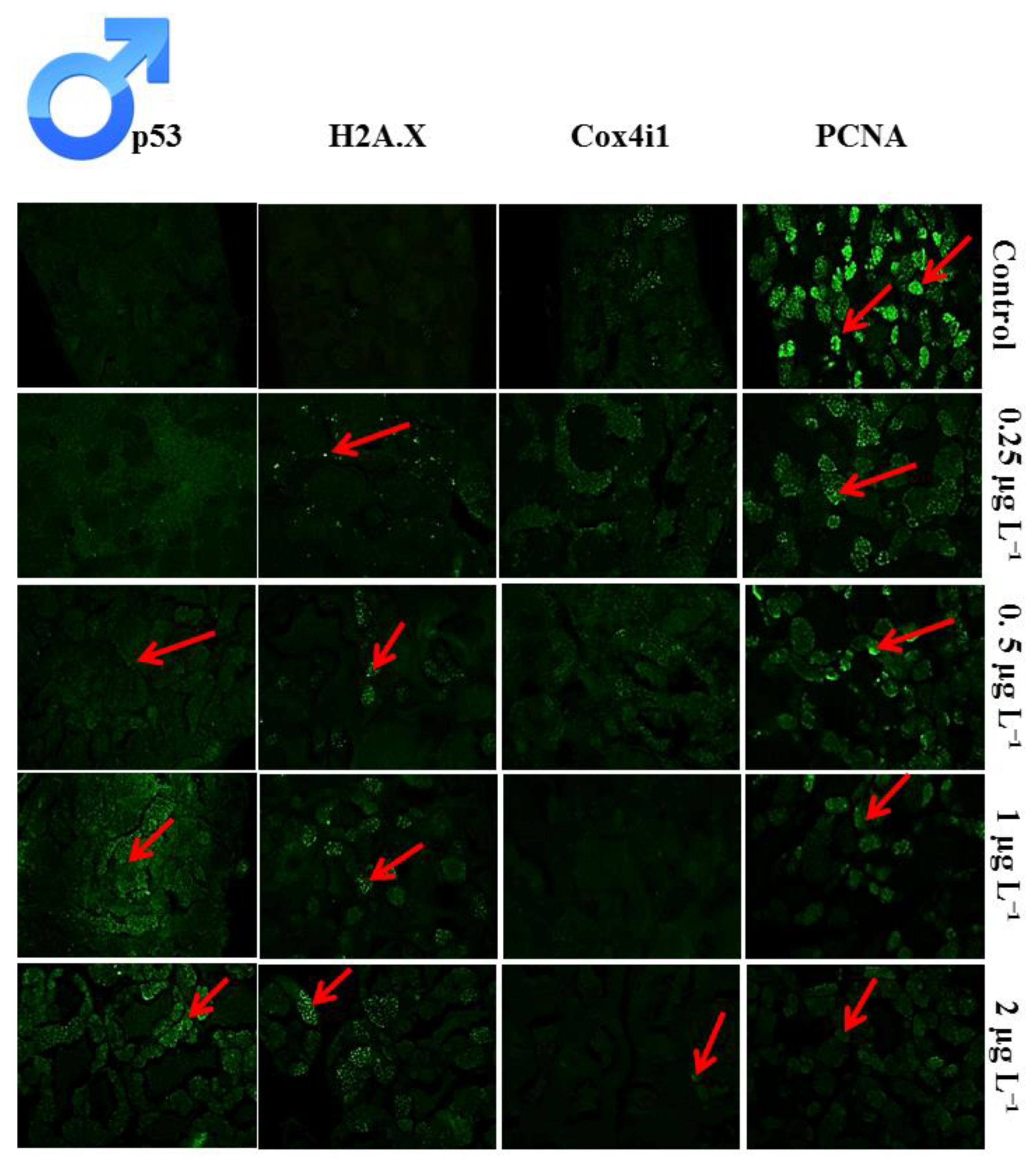
3.4. Histological Analysis of Toxicity on Testis
3.5. Proliferation Index of the Cells in Testis
3.6. Apoptotic Index of the Cells in Testis
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Ethical Approval
References
- Plhalova, L.; Blahova, J.; Divisova, L.; Enevova, V.; Casuscelli Di Tocco, F.; Faggio, C.; Tichy, F.; Vecerek, V.; Svobodova, Z. The effects of subchronic exposure to NeemAzal T/S on zebrafish (Danio rerio). Chem. Ecol. 2017, 21, 302–307. [Google Scholar] [CrossRef]
- Chromcova, L.; Blahova, J.; Zivna, D.; Plhalova, L.; Casuscelli Di Tocco, F.; Divisova, L.; Prokes, M.; Faggio, C.; Tichy, F.; Svobodova, Z. NeemAzal T/S—Toxicity to early-life stages of common carp (Cyprinus carpio L.). Vet. Med. 2015, 60, 23–30. [Google Scholar]
- Fiorino, E.; Sehonova, P.; Plhalova, L.; Blahova, J.; Svobodova, Z.; Faggio, C. Effect of glyphosate on early life stages: Comparison between Cyprinus carpio and Danio rerio. Environ. Sci. Pollut. Res. 2018, 25, 8542–8549. [Google Scholar] [CrossRef]
- Stara, A.; Bellinvia, R.; Velisek, J.; Strouhova, A.; Kouba, A.; Faggio, C. Acute exposure of neonicotinoid pesticide on common yabby (Cherax destructor). Sci. Total Environ. 2019, 665, 718–723. [Google Scholar] [CrossRef]
- Stara, A.; Kubec, J.; Zuskova, E.; Buric, M.; Faggio, C.; Kouba, A.; Velisek, J. Effects of S-metolachlor and its degradation product metolachlor OA on marbled crayfish (Procambarus virginalis). Chemospere 2019, 224, 616–625. [Google Scholar] [CrossRef]
- Stara, A.; Pagano, M.; Capillo, G.; Fabrello, J.; Sandova, M.; Vazzana, I.; Zuskova, E.; Velisek, J.; Matozzo, V.; Faggio, C. Assessing the effects of neonicotinoid insecticide on the bivalve mollusc Mytilus galloprovincialis. Sci. Total Environ. 2020, 700, 134914. [Google Scholar] [CrossRef]
- Burgos-Aceves, M.A.; Cohen, A.; Smith, Y.; Faggio, C. MicroRNAs and their role on fish oxidative stress during xenobiotic environmental exposures. Ecotoxicol. Environ. Saf. 2018, 148, 995–1000. [Google Scholar] [CrossRef]
- Burgos Aceves, M.A.; Lionetti, L.; Faggio, C. Multidisciplinary hematology as prognostic device in environmental and xenobiotic stress-induced response in fish. Sci. Total Environ. 2019, 670, 1170–1183. [Google Scholar] [CrossRef]
- Sehonova, P.; Tokanova, N.; Hodkovicova, N.; Kocour Kroupova, H.; Tumova, J.; Blahova, J.; Marsalek, P.; Plhalova, L.; Doubkova, V.; Dobsikova, R.; et al. Oxidative stress induced by fluoroquinolone enrofloxacin in zebrafish (Danio rerio) can be ameliorated after a prolonged exposure. Env. Toxicol. Pharmacol. 2019, 67, 87–93. [Google Scholar] [CrossRef]
- Kim, K.H.; Kabir, E.; Jahan, S.A. Exposure to pesticides and the associated human health effects. Sci. Total Environ. 2017, 575, 525–535. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Wang, D.; Wang, J.; Wu, Z.; Li, L.; Huang, M.; Xu, S.; Yan, D. Pyrethroid pesticide residues in the global environment: An overview. Chemosphere 2017, 191, 990–1007. [Google Scholar] [CrossRef] [PubMed]
- Barata, C.; Baird, D.J.; Nogueira, A.J.A.; Soares, A.M.V.M.; Riva, M.C. Toxicity of binary mixtures of metals and pyrethroid insecticides to Daphnia magna Straus. Implications for multi-substance risks assessment. Aquat. Toxicol. 2006, 78, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Toumi, H.; Boumaiza, M.; Immel, F.; Sohm, B.; Felten, V.; Férard, J.F. Effect of deltamethrin (pyrethroid insecticide) on two clones of Daphnia magna (Crustacea, Cladocera): A proteomic investigation. Aquat. Toxicol. 2014, 148, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Kunce, W.; Stoks, R.; Johansson, F. Single and mixture impacts of two pyrethroids on damselfly predatory behavior and physiological biomarkers. Aquat. Toxicol. 2017, 190, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Kung, T.S.; Richardson, J.R.; Cooper, K.R.; White, L.A. Developmental deltamethrin exposure causes persistent changes in dopaminergic gene expression, neurochemistry, and locomotor activity in zebrafish. Toxicol. Sci. 2015, 146, 235–243. [Google Scholar] [CrossRef]
- Song, J.; Qiao, L.; Ji, L.; Ren, B.; Hu, Y.; Zhao, R.; Ren, Z. Toxic responses of zebrafish (Danio rerio) to thallium and deltamethrin characterized in the electrocardiogram. Chemosphere 2018, 212, 1085–1094. [Google Scholar] [CrossRef]
- Awoyemi, O.M.; Kumar, N.; Schmitt, C.; Subbiah, S.; Crago, J. Behavioral, molecular and physiological responses of embryo-larval zebrafish exposed to types I and II pyrethroids. Chemosphere 2019, 219, 526–537. [Google Scholar] [CrossRef]
- Haverinen, J.; Vornanen, M. Deltamethrin is toxic to the fish (crucian carp, Carassius carassius) heart. Pestic. Biochem. Phys. 2016, 129, 36–42. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, Q.; Pang, Y.; Song, X.; Zhou, N.; Wang, J.; He, L.; Lv, J.; Song, Y.; Cheng, Y.; et al. The protective effects of melatonin on oxidative damage and the immune system of the Chinese mitten crab (Eriocheir sinensis) exposed to deltamethrin. Sci. Total Environ. 2019, 25, 1426–1434. [Google Scholar] [CrossRef]
- Ullah, S.; Li, Z.; Ul Arifeen, M.Z.; Khan, S.U.; Fahad, S. Multiple biomarkers based appraisal of deltamethrin induced toxicity in silver carp (Hypophthalmichthys molitrix). Chemosphere 2019, 214, 519–533. [Google Scholar] [CrossRef]
- Karatas, T.; Yildirim, S.; Arslan, H.; Aggul, A.G. The effects on brown trout (Salmo trutta fario) of different concentrations of deltamethrin. Comp. Biochem. Physiol. C Toxicol Pharmacol. 2019, 15. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Yang, M.; Pan, H.; Li, S.; Ren, B.; Ren, Z.; Xing, N.; Qi, L.; Ren, Q.; Xu, S.; et al. Does time difference of the acetylcholinesterase (AChE) inhibition in different tissues exist? A case study of zebra fish (Danio rerio) exposed to cadmium chloride and deltamethrin. Chemosphere 2017, 168, 908–916. [Google Scholar] [CrossRef] [PubMed]
- Ren, Q.; Zhang, T.; Li, S.; Ren, Z.; Yang, M.; Pan, H.; Xu, S.; Qi, L.; Chon, T.S. Integrative characterization of toxic response of zebra fish (Danio rerio) to deltamethrin dased on AChE activity and behavior strength. Biomed. Res. Int. 2016, 2016. [Google Scholar] [CrossRef]
- Liu, X.Y.; Zhang, Q.P.; Li, S.B.; Mi, P.; Chen, D.Y.; Zhao, X.; Feng, X.Z. Developmental toxicity and neurotoxicity of synthetic organic insecticides in zebrafish (Danio rerio): A comparative study of deltamethrin, acephate, and thiamethoxam. Chemosphere 2018, 199, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Koç, N.D.; Muşlu, M.N.; Kayhan, F.E.; Çolak, S. Histopathological changes in ovaries of zebrafish (Danio rerio) following administration of deltamethrin. Fresenius Environ. Bull. 2009, 18, 1872–1878. [Google Scholar]
- Huang, Y.; Jin, S.Z.; Han, X.; Huang, T. The use of zebrafish (Danio rerio) behavioral responses in identifying sublethal exposures to deltamethrin. Int. J. Environ. Res. Public Health 2014, 11, 3650–3660. [Google Scholar] [CrossRef] [PubMed]
- DeMicco, A.; Cooper, K.R.; Richardson, J.R.; White, L.A. Developmental neurotoxicity of pyrethroid Insecticides in zebrafish embryos. Toxicol. Sci. 2010, 113, 177–186. [Google Scholar] [CrossRef]
- Dinu, D.; Marinescu, D.; Munteanu, M.C.; Staicu, A.C.; Costache, M.; Dinischiotu, A. Modulatory effects of deltamethrin on antioxidant defense mechanisms and lipid peroxidation in Carassius auratus gibelio liver and Intestine. Arch. Environ. Contam. Toxicol. 2010, 58, 757–764. [Google Scholar] [CrossRef]
- Cengiz, E.I. Gill and kidney histopathology in the freshwater fish Cyprinus carpio after acute exposure to deltamethrin. Environ. Toxicol. Pharmacol. 2006, 22, 200–204. [Google Scholar] [CrossRef]
- Svobodová, Z.; Lusková, V.; Drastichová, J.; Svoboda, M.; Îlábek, V. Effect of deltamethrin on haematological indices of common carp (Cyprinus carpio L.). Acta Vet. Brno 2003, 72, 79–85. [Google Scholar]
- Sarasamma, S.; Varikkodan, M.M.; Liang, S.T.; Lin, Y.C.; Wang, W.P.; Hsiao, C.D. Zebrafish: A premier vertebrate model for biomedical research in indian scenario. Zebrafish 2017, 14, 589–605. [Google Scholar] [CrossRef] [PubMed]
- Lam, P.Y.; Peterson, R.T. Developing zebrafish disease models for in vivo small molecule screens. Curr. Opin. Chem. Biol. 2019, 50, 37–44. [Google Scholar] [CrossRef]
- Zang, L.; Maddison, L.A.; Chen, W. Zebrafish as a Model for Obesity and Diabetes. Front. Cell Dev. Biol. 2018, 6, 91. [Google Scholar]
- Commission Recommendation. 2007/526/EC of 18 June 2007 on Guidelines for the Accommodation and Care of Animals Used for Experimental and Other Scientific Purposes (Notified Under Document Number C(2007) 2525). Official Journal of the European Union; Volume 50. Available online: http://eur-lex.europa.eu/legalcontent/RO/TXT/?Uri=CELEX%3A32007H0526 (accessed on 30 July 2007).
- Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010 on the Protection of Animals Used for Scientific Purposes. Official Journal of the European Union; Volume 53. Available online: http://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex:32010L0063 (accessed on 20 October 2010).
- Strungaru, S.; Radojković, P.; Dumitru, G.; Nicoara, M.; Plavan, G.; Todirascu-Ciornea, E. Oxidative stress and changes in swimming performances at zebrafish model (Danio Rerio, H. 1822) produced by acute exposure to deltamethrin. Surv. Fish. Sci. 2019, 5, 121–137. [Google Scholar]
- Strungaru, S.A.; Plavan, G.; Ciobica, A.; Nicoara, M.; Robea, M.A.; Solcan, C.; Petrovici, A. Toxicity and chronic effects of deltamethrin exposure on zebrafish (Danio rerio) as a reference model for freshwaterfish community. Ecotoxicol. Environ. Saf. 2019, 171, 854–862. [Google Scholar] [CrossRef] [PubMed]
- Jijie, R.; Solcan, G.; Nicoara, M.; Strungaru, S.A. Antagonistic effects in zebrafish (Danio rerio) behavior and oxidativestress induced by toxic metals and deltamethrin acute exposure. Sci. Total Environ. 2020, 698, 134299. [Google Scholar] [CrossRef] [PubMed]
- Klibansky, N.; Juanes, F. Procedures for efficiently producing high-quality fecundity data on a small budget. Fish. Res. 2008, 89, 84–89. [Google Scholar] [CrossRef]
- Douiev, L.; Abu-Libdeh, B.; Saada, A. Cytochrome c oxidase deficiency, oxidative stress, possible antioxidant therapy and link to nuclear DNA damage. Eur. J. Hum. Genet. 2018, 26, 579–581. [Google Scholar] [CrossRef]
- Kadiri, P.; Gundala, H.P. Reproductive performance of zebrafish (Danio rerio) exposed to deltamethrin: Fecundity, histological and hormonal end points. J. Exp. Appl. Anim. Sci. 2014, 1, 253–268. [Google Scholar]
- Korfsmeier, K.H. PCNA in the ovary of zebrafish (Brachydanio rerio, Ham. Buch.). Acta Histochem. 2002, 104, 73–76. [Google Scholar] [CrossRef]
- D’Andrea, M.R.; Lawrence, D.; Nagele, R.G.; Wang, C.Y.; Damiano, B.P. PCNA indexing as a preclinical immunohistochemical biomarker for testicular toxicity. Biotech. Histochem. 2008, 83, 211–220. [Google Scholar] [CrossRef] [PubMed]
- D’Andrea, M.R.; Alicknavitch, M.; Nagele, R.G.; Damiano, B.P. Simultaneous PCNA and TUNEL labeling for testicular toxicity evaluation suggests that detection of apoptosis may be more sensitive than proliferation. Biotech. Histochem. 2010, 85, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Storer, N.Y.; Zon, L.I. Zebrafish Models of p53 Functions, Cold Spring Harb Perspect. Cold Spring Harb. Perspect. Biol. 2010, 2, a001123. [Google Scholar] [CrossRef] [PubMed]
- González-Rojo, S.; Lombó, M.; Fernández-Díez, C.; Herráez, M.P. Male exposure to bisphenol a impairs spermatogenesis and triggers histone hyperacetylation in zebrafish testes. Environ. Pollut. 2019, 248, 368–369. [Google Scholar] [CrossRef] [PubMed]
- Jaamaa, S.; af Hallstrom, T.M.; Sankila, A.; Rantanen, V.; Koistinen, H.; Stenman, U.H.; Laiho, M. DNA Damage Recognition via Activated ATM and p53 Pathway in Nonproliferating Human Prostate Tissue. Cancer Res. 2010, 70, 8630–8641. [Google Scholar] [CrossRef] [PubMed]
- Maalej, A.; Mahmoudi, A.; Bouallagui, Z.; Fki, I.; Marrekchi, R.; Sayadi, S. Olive phenolic compounds attenuate deltamethrin-induced liver and kidney toxicity through regulating oxidative stress, inflammation and apoptosis. Food Chem. Toxicol. 2017, 106, 455–465. [Google Scholar] [CrossRef]

| Oocytes Types Percentages | |||
|---|---|---|---|
| Previtellogenic (%) | Vitellogenic (%) | Atretic (%) | |
| Control | 78 ± 1.4 | 9.5 ± 1.6 | 12.5 ± 1.1 |
| 0.25 μg L−1 | 66.8 ± 1.7 | 8.6 ± 1.07 | 24.5 ± 1.5 |
| 0.5 μg L−1 | 55.7 ± 1 | 5.2 ± 0.96 | 39 ± 0.7 |
| 1 μg L−1 | 52.4 ± 2.4 | 3.5 ± 1 | 44 ± 1.4 |
| 2 μg L−1 | 46.3 ± 1.4 | 3.1 ± 1.1 | 50.5 ± 1.2 |
| one-way ANOVA | |||
| F statistics | 1401.7 | 150.5 | 3876.3 |
| variance | 3804.3 | 205.8 | 5698.2 |
| p value | <0.001 | <0.001 | <0.001 |
| Tukey HSD test without significant results | Control vs. 0.25 μg L−1 p = 0.065 | ||
| 1 μg L−1 vs. 2 μg L−1 p = 0.759 | |||
| Mature Oocytes | Vitellogenin Oocytes | Pre-Vitellogenic Oocytes | Atretic Follicles | Lipofuscin | Follicular Cells Hypertrophy | Membrane Invagination | Extravascular Proteic Fluid | |
|---|---|---|---|---|---|---|---|---|
| Control | + | + | + | ± | ± | − | − | − |
| 0.25 μg L−1 | ± | ++ | ++ | + | + | + | + | + |
| 0.5 μg L−1 | ++ | ± | + | ++ | ++ | + | + | + |
| 1 μg L−1 | +++ | ± | ++ | ++ | ++ | ++ | ± | + |
| 2 μg L−1 | +++ | ± | ++ | +++ | +++ | ++ | + | ± |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petrovici, A.; Strungaru, S.-A.; Nicoara, M.; Robea, M.A.; Solcan, C.; Faggio, C. Toxicity of Deltamethrin to Zebrafish Gonads Revealed by Cellular Biomarkers. J. Mar. Sci. Eng. 2020, 8, 73. https://doi.org/10.3390/jmse8020073
Petrovici A, Strungaru S-A, Nicoara M, Robea MA, Solcan C, Faggio C. Toxicity of Deltamethrin to Zebrafish Gonads Revealed by Cellular Biomarkers. Journal of Marine Science and Engineering. 2020; 8(2):73. https://doi.org/10.3390/jmse8020073
Chicago/Turabian StylePetrovici, Adriana, Stefan-Adrian Strungaru, Mircea Nicoara, Madalina Andreea Robea, Carmen Solcan, and Caterina Faggio. 2020. "Toxicity of Deltamethrin to Zebrafish Gonads Revealed by Cellular Biomarkers" Journal of Marine Science and Engineering 8, no. 2: 73. https://doi.org/10.3390/jmse8020073
APA StylePetrovici, A., Strungaru, S.-A., Nicoara, M., Robea, M. A., Solcan, C., & Faggio, C. (2020). Toxicity of Deltamethrin to Zebrafish Gonads Revealed by Cellular Biomarkers. Journal of Marine Science and Engineering, 8(2), 73. https://doi.org/10.3390/jmse8020073






