Feeding Whole Thraustochytrid Biomass to Cultured Atlantic Salmon (Salmo salar) Fingerlings: Culture Performance and Fatty Acid Incorporation
Abstract
1. Introduction
2. Materials and Methods
2.1. Thraustochytrid Biomass Production
2.2. Salmon Fingerlings Feeding Trial
2.2.1. Diets
2.2.2. Experimental Setup, Fish and Feeding
2.3. Whole Carcass Composition Analyses
Fatty Acid Analyses
2.4. Statistical Analyses
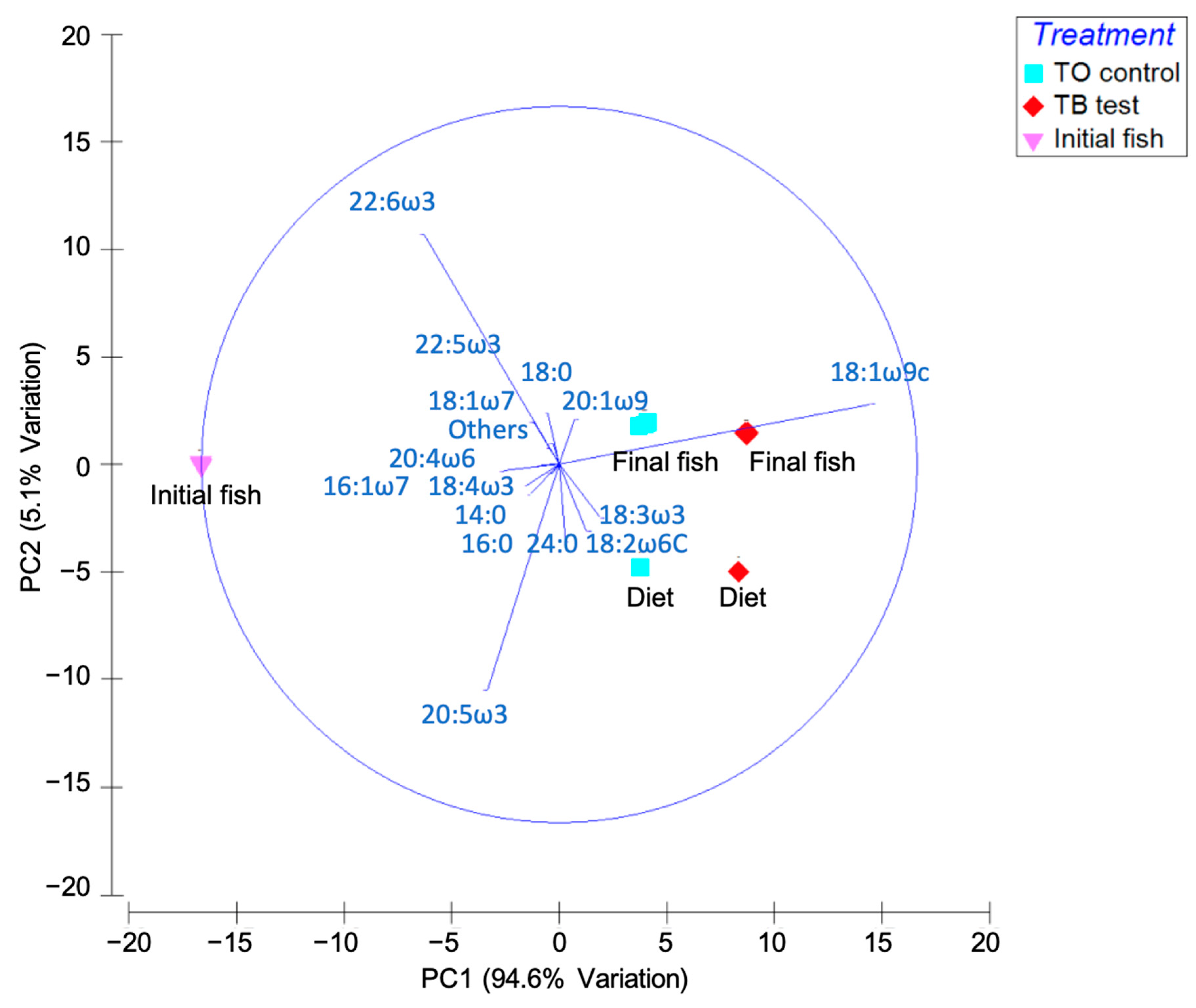
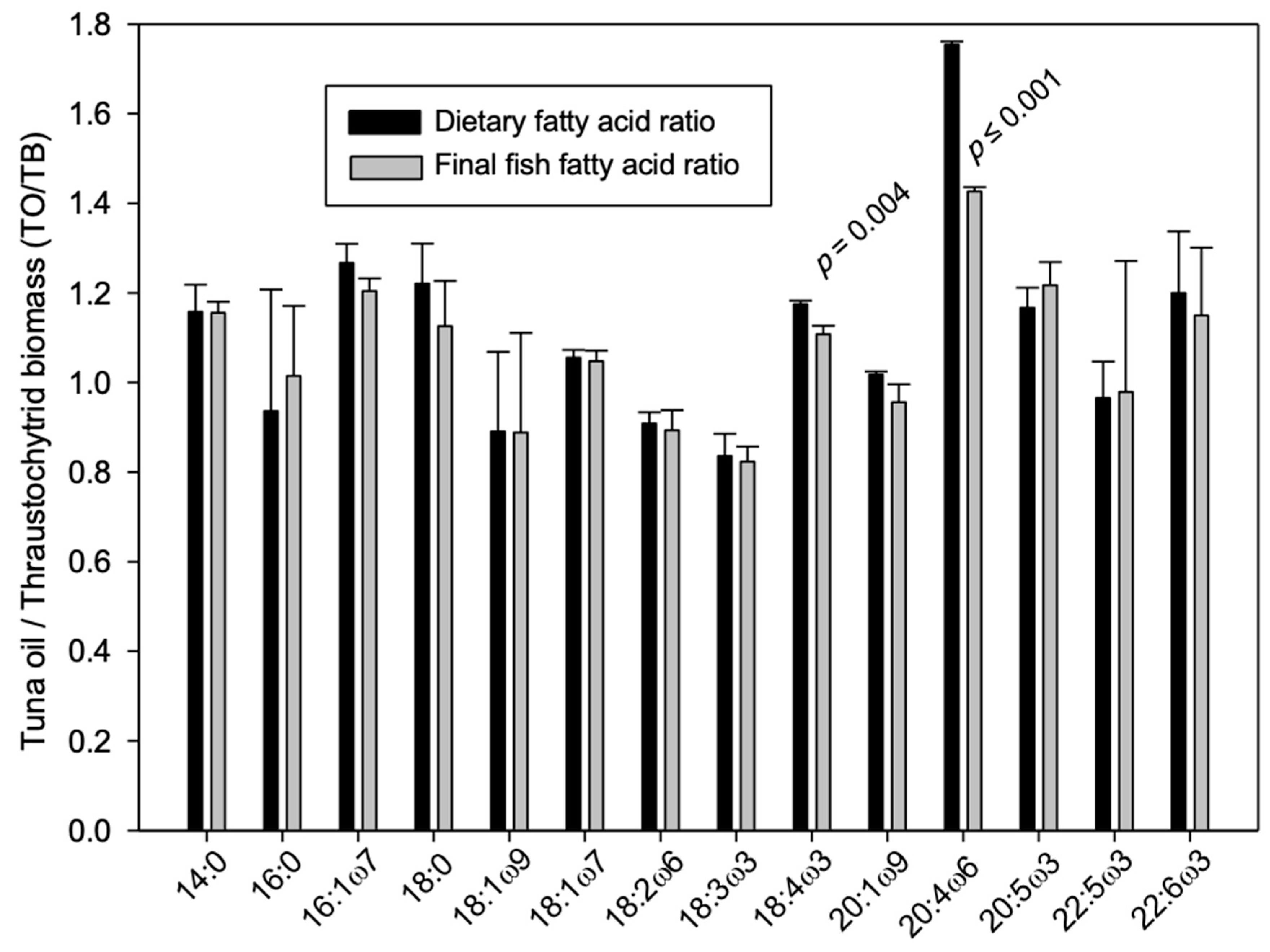
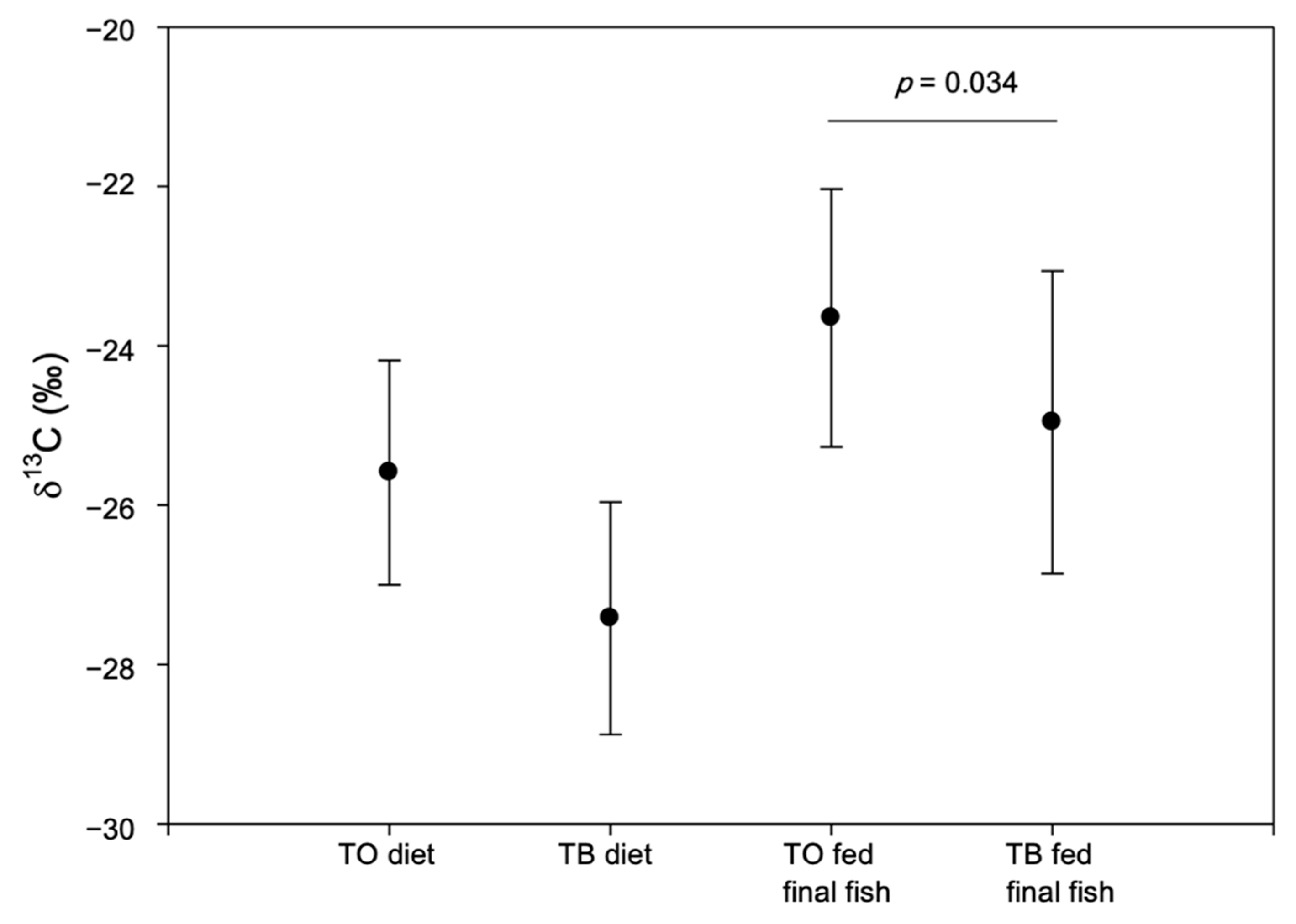
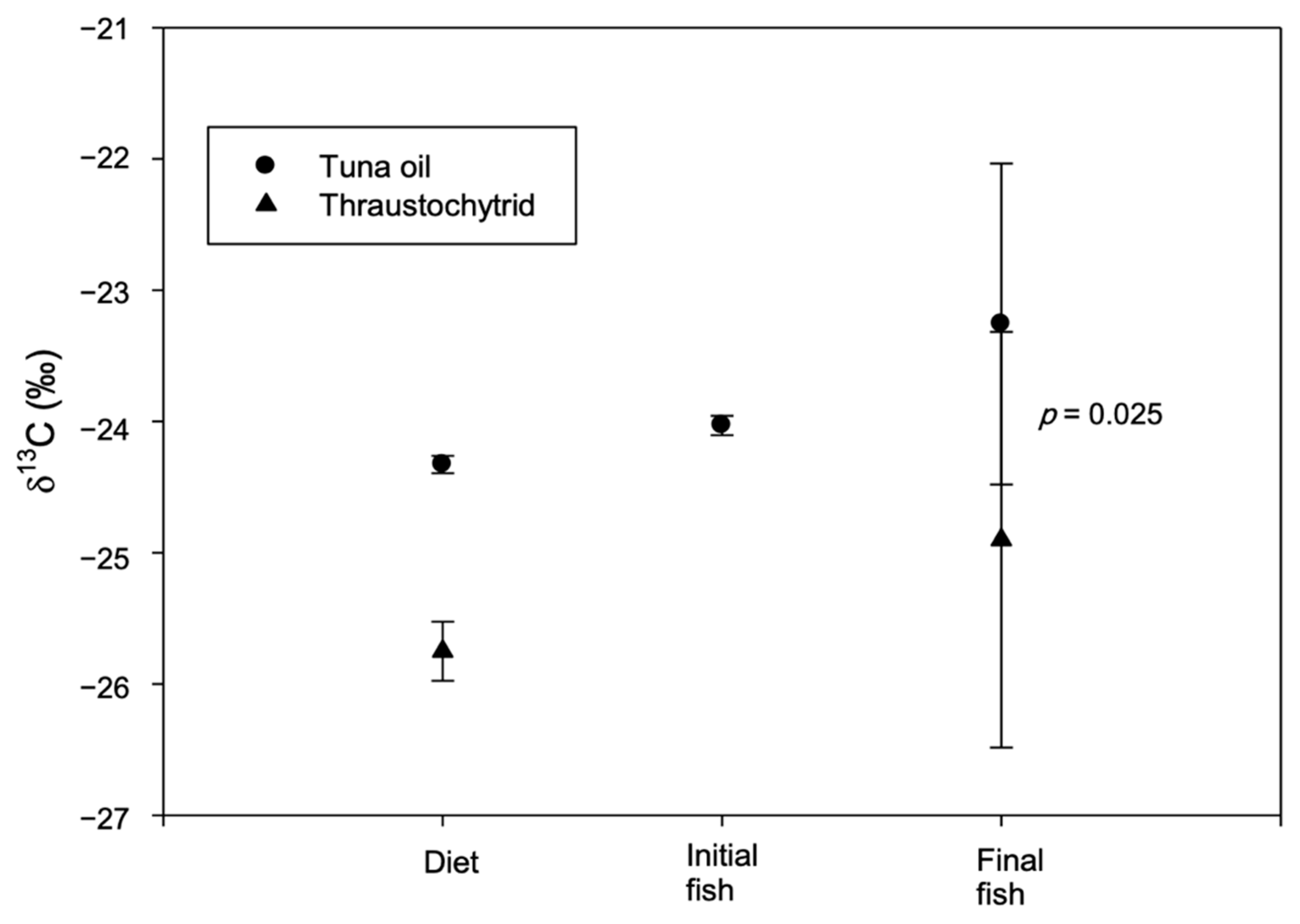
3. Results and Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Caamaño, E.; Loperena, L.; Hinzpeter, I.; Pradel, P.; Gordillo, F.; Corsini, G.; Tello, M.; Lavín, P.; González, A.R. Isolation and molecular characterization of Thraustochytrium strain isolated from Antarctic Peninsula and its biotechnological potential in the production of fatty acids. Braz. J. Microbiol. 2017, 48, 671–679. [Google Scholar] [CrossRef]
- Stefánsson, M.Ö.; Baldursson, S.; Magnússon, K.P.; Eyþórsdóttir, A.; Einarsson, H. Isolation, Characterization and biotechnological potentials of Thraustochytrids from Icelandic waters. Mar. Drugs 2019, 17, 449. [Google Scholar] [CrossRef]
- Lee Chang, K.J.; Dunstan, G.A.; Abell, G.; Clementson, L.; Blackburn, S.; Nichols, P.D.; Koutoulis, A. Biodiscovery of new Australian thraustochytrids for production of biodiesel and long-chain omega-3 oils. Appl. Microbiol. Biotechnol. 2012, 93, 2215–2231. [Google Scholar] [CrossRef]
- Marchan, L.F.; Chang, K.J.L.; Nichols, P.D.; Polglase, J.L.; Mitchell, W.J.; Gutierrez, T. Screening of new British thraustochytrids isolates for docosahexaenoic acid (DHA) production. J. Appl. Phycol. 2017, 29, 2831–2843. [Google Scholar] [CrossRef]
- Ou, M.-C.; Yeong, H.-Y.; Pang, K.-L.; Phang, S.-M. Fatty acid production of tropical thraustochytrids from Malaysian mangroves. Bot. Mar. 2016, 59, 321–338. [Google Scholar] [CrossRef]
- Raghukumar, S. Ecology of the marine portists, the Labyrinthulomycetes (thraustochytrids and labyrinthulids). Eur. J. Protistol. 2002, 38, 127–145. [Google Scholar] [CrossRef]
- Maas, P.A.Y.; Kleinschuster, S.J.; Dykstra, M.J.; Smolowitz, R.; Parent, J. Molecular characterization of QPX (Quahog Parasite Unknown), a pathogen of Mercenaria mercenaria. J. Shellfish Res. 1999, 18, 561–567. [Google Scholar]
- Raghukumar, S. Bactivory: A novel dual role for thraustochytrids in the sea. Mar. Biol. 1992, 113, 165–169. [Google Scholar] [CrossRef]
- Marchan, L.F.; Chang, K.J.L.; Nichols, P.D.; Mitchell, W.J.; Polglase, J.L.; Gutierrez, T. Taxonomy, ecology and biotechnological applications of thraustochytrids: A review. Biotechnol. Adv. 2018, 36, 26–46. [Google Scholar] [CrossRef]
- Cavalier-Smith, T.; Allsopp, M.T.E.P.; Chao, E.E. Thraustochytrids are chromists, not fungi: 18s rRNA signatures of Heterokonta. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 1994, 346, 387–397. [Google Scholar]
- Leander, C.A.; Porter, D.; Leander, B.S. Comparative morphology and molecular phylogeny of aplanochytrids (Labyrinthulomycota). Eur. J. Protistol. 2004, 40, 317–328. [Google Scholar] [CrossRef]
- Porter, D. Phylum Labyrinthulomycota. In Handbook of Protoctista; Margulis, L., Corliss, J.O., Melkonian, M., Chapman, D.J., Eds.; Jones and Bartlett: Boston, MA, USA, 1990; pp. 388–398. [Google Scholar]
- Leyland, B.; Leu, S.; Boussiba, S. Are thraustochytrids algae? Fungal Biol. 2017, 121, 835–840. [Google Scholar] [CrossRef] [PubMed]
- Barclay, W.; Meager, K.; Abril, J. Heterotrophic production of long chain omega-3 fatty acids utilizing algae and algae-like microorganisms. J. Appl. Phycol. 1994, 6, 123–129. [Google Scholar] [CrossRef]
- Barclay, W.; Weaver, C.; Metz, J.; Hansen, J. Development of a docosahexaenoic acid production technology using Schizochytrium: Historical perspective and update. In Single Cell Oils—Microbial and Algal Oils, 2nd ed.; Cohen, Z., Ratledge, C., Eds.; AOCS Press: Champaign, IL, USA, 2010; pp. 75–96. [Google Scholar]
- Lee Chang, K.; Nichols, C.; Blackburn, S.; Dunstan, G.; Koutoulis, A.; Nichols, P. Comparison of Thraustochytrids Aurantiochytrium sp., Schizochytrium sp., Thraustochytrium sp., and Ulkenia sp. for production of biodiesel, long-chain omega-3 oils, and exopolysaccharide. Mar. Biotechnol. 2014, 16, 396–411. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Barrow, C.J.; Puri, M. Omega-3 biotechnology: Thraustochytrids as a novel source of omega-3 oils. Biotechnol. Adv. 2012, 30, 1733–1745. [Google Scholar] [CrossRef] [PubMed]
- Allen, K.M.; Habte-Tsion, H.-M.; Thompson, K.R.; Filer, K.; Tidwell, J.H.; Kumar, V. Freshwater microalgae (Schizochytrium sp.) as a substitute to fish oil for shrimp feed. Sci. Rep. 2019, 9, 6178. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.R.; Nichols, P.D.; Carter, C.G. Replacement of fish oil with thraustochytrid Schizochytrium sp. L oil in Atlantic salmon parr (Salmo salar L) diets. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2007, 148, 382–392. [Google Scholar] [CrossRef]
- Sarker, P.K.; Kapuscinski, A.R.; Lanois, A.J.; Livesey, E.D.; Bernhard, K.P.; Coley, M.L. Towards sustainable aquafeeds: Complete substitution of fish oil with marine microalga Schizochytrium sp. improves growth and fatty acid deposition in juvenile Nile tilapia (Oreochromis niloticus). PLoS ONE 2016, 11, e0156684. [Google Scholar] [CrossRef]
- Sprague, M.; Walton, J.; Campbell, P.; Strachan, F.; Dick, J.R.; Bell, J.G. Replacement of fish oil with a DHA-rich algal meal derived from Schizochytrium sp. on the fatty acid and persistent organic pollutant levels in diets and flesh of Atlantic salmon (Salmo salar, L.) post-smolts. Food Chem. 2015, 185, 413–421. [Google Scholar] [CrossRef]
- Zhang, C. Determination of the digestibility of a whole-cell DHA-rich algal product and its effect on the lipid composition of rainbow trout and Atlantic salmon. Ph.D. Thesis, University of Saskatchewan, Saskatoon, SK, Cananda, 2013. [Google Scholar]
- Carter, C.; Bransden, M.; Lewis, T.; Nichols, P. Potential of thraustochytrids to partially replace fish oil in Atlantic salmon feeds. Mar. Biotechnol. 2003, 5, 480–492. [Google Scholar] [CrossRef]
- Lee Chang, K.J.; Paul, H.; Nichols, P.D.; Koutoulis, A.; Blackburn, S.I. Australian thraustochytrids: Potential production of dietary long-chain omega-3 oils using crude glycerol. J. Funct. Foods 2015, 19, 810–820. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Stanley, G.S. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [PubMed]
- Jones, D.B. Factors for Converting Percentages of Nitrogen in Foods and Feeds into Percentages of Proteins; US Department of Agriculture: Washington, DC, USA, 1931; Volume 183, pp. 1–21.
- Lee Chang, K.J.; Mansour, M.P.; Dunstan, G.A.; Blackburn, S.I.; Koutoulis, A.; Nichols, P.D. Odd-chain polyunsaturated fatty acids in thraustochytrids. Phytochemistry 2011, 72, 1460–1465. [Google Scholar] [CrossRef] [PubMed]
- Mizambwa, H.E. Effects of replacing fish oil with microalgae biomass (Schizochytrium spp) as a source of n-3 LC-PUFA to Atlantic salmon (Salmo salar) on growth performance, fillet quality and fatty acid composition. Master’s Thesis, Norwegian University of Life Sciences, Ås, Norway, 2017. [Google Scholar]
- Hixson, S.M.; Parrish, C.C.; Anderson, D.M. Changes in tissue lipid and fatty acid composition of farmed rainbow trout in response to dietary camelina oil as a replacement of fish oil. Lipids 2014, 49, 97–111. [Google Scholar] [CrossRef] [PubMed]
- Katan, T.; Caballero-Solares, A.; Taylor, R.G.; Rise, M.L.; Parrish, C.C. Effect of plant-based diets with varying ratios of ω6 to ω3 fatty acids on growth performance, tissue composition, fatty acid biosynthesis and lipid-related gene expression in Atlantic salmon (Salmo salar). Comp. Biochem. Physiol. Part D Genom. Proteom. 2019, 30, 290–304. [Google Scholar] [CrossRef]
- Bransden, M.P.; Carter, C.G.; Nichols, P.D. Replacement of fish oil with sunflower oil in feeds for Atlantic salmon (Salmo salar L.): Effect on growth performance, tissue fatty acid composition and disease resistance. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2003, 135, 611–625. [Google Scholar] [CrossRef]
- Cashion, T.; Le Manach, F.; Zeller, D.; Pauly, D. Most fish destined for fishmeal production are food-grade fish. Fish Fish. 2017, 18, 837–844. [Google Scholar] [CrossRef]
- Lee Chang, K.J.; Dumsday, G.; Nichols, P.D.; Dunstan, G.A.; Blackburn, S.I.; Koutoulis, A. High cell density cultivation of a novel Aurantiochytrium sp. strain TC 20 in a fed-batch system using glycerol to produce feedstock for biodiesel and omega-3 oils. Appl. Microbiol. Biotechnol. 2013, 97, 6907–6918. [Google Scholar] [CrossRef]
| Composition (%) | Tuna Oil Control | Thraustochytrid Biomass Test |
|---|---|---|
| Fishmeal | 79.0 | 76.0 |
| Wheat flour | 7.0 | 5.6 |
| Vitamin premix | 2.0 | 2.0 |
| Soy lecithin | 1.0 | 1.0 |
| CaHPO4 | 1.0 | 1.0 |
| Betaine | 1.00 | 1.00 |
| Mineral premix | 0.52 | 0.52 |
| Stay-C | 0.14 | 0.14 |
| Vitamin E (50%) | 0.03 | 0.03 |
| Astaxanthin | 0.01 | 0.01 |
| Choline chloride | 0.50 | 0.50 |
| Thraustochytrid biomass (TB) | 0.0 | 5.0 |
| % Oil in feed | Control | Thraustochytrid |
| Tuna Oil (TO) | 1.80 | 0.0 |
| Canola Oil (CO) (control) | 6.0 | 7.2 |
| Diet Chemical Composition | ||
| Dry matter content (%) | 94.1 | 96.7 |
| Protein (%) | 62.6 | 61.8 |
| Lipid (%) | 17.7 | 18.9 |
| Ash (%) | 12.4 | 12.9 |
| Energy (kJ/g) | 22.9 | 22.9 |
| Culture Performance | Tuna Oil Control | Thraustochytrid Biomass Test |
|---|---|---|
| Initial weight (g) | 0.8 ± 0.0 | 0.8 ± 0.0 |
| Final weight (g) | 15.5 ± 0.5 | 15.8 ± 0.3 |
| Body weight gain (fold increase) | 18.6± 0.5 | 18.9± 0.3 |
| SGR (%BW day−1) | 4.2 ± 0.0 | 4.2 ± 0.0 |
| Survival (%) | 99.7 ± 0.3 | 99.7 ± 0.3 |
| Feed offered (mg day/fish) | 203.1 ± 0.5 | 200.5 ± 1.0 |
| Final weight coefficient of variation (%) | 30.6 ± 2.4 | 28.6 ± 1.9 |
| Food conversion ratio (FCR) | 1.0 ± 0.0 | 0.9 ± 0.0 |
| Whole Fish Composition (on DM Basis, n = 3) | ||
| Dry matter content (%) | 25.9 ± 0.5 | 26.3 ± 0.3 |
| Protein (%) | 58.9 ± 0.6 | 57.8 ± 0.4 |
| Lipid (%) | 37.1 ± 0.5 | 37.8 ± 0.9 |
| Ash (%) | 6.2 ± 0.2 | 5.8 ± 0.4 |
| Energy (kJ/g) | 27.7 ± 0.1 | 27.2 ± 0.2 |
| % Fatty Acid | Tuna Oil Control | SD | Thraustochytrid Biomass Test | SD | Initial Fish | SD | Final Fish-Tuna Oil Control | SD | Final Fish-Thraustochytrid Biomass Test | SD |
|---|---|---|---|---|---|---|---|---|---|---|
| Saturated Fatty Acids | ||||||||||
| 14:0 | 4.3 b | 0.1 | 3.8 d | 0.0 | 5.7 a | 0.0 | 3.9 c | 0.0 | 3.4 e | 0.0 |
| 16:0 | 14.8 c | 0.3 | 15.8 b | 0.1 | 16.8 a | 0.1 | 14.9 c | 0.1 | 14.7 c | 0.1 |
| 18:0 | 3.8 d | 0.1 | 3.1 e | 0.0 | 4.8 a | 0.0 | 4.6 b | 0.1 | 4.1 c | 0.1 |
| 24:0 | 1.6 a | 0.0 | 1.5 a | 0.0 | 0.0 b | 0.0 | 0.2 b | 0.4 | 0.0 b | 0.0 |
| Subtotal | 24.6 | 0.3 | 24.1 | 0.1 | 27.4 | 0.1 | 23.6 | 0.4 | 22.1 | 0.1 |
| Monounsaturated Fatty Acids | ||||||||||
| 16:1ω7c | 4.6 b | 0.0 | 3.7 d | 0.0 | 7.5 a | 0.0 | 4.4 c | 0.0 | 3.6 d | 0.0 |
| 18:1ω9c | 31.6 d | 0.1 | 35.5 b | 0.1 | 14.7 e | 0.0 | 32.8 c | 0.2 | 36.9 a | 0.0 |
| 18:1ω7c | 2.5 d | 0.0 | 2.3 e | 0.0 | 3.0 a | 0.0 | 2.8 b | 0.0 | 2.7 c | 0.0 |
| 20:1ω9 | 1.6 c | 0.0 | 1.6 c | 0.0 | 1.2 d | 0.0 | 2.4 b | 0.0 | 2.5 a | 0.0 |
| Subtotal | 40.3 | 0.1 | 43.1 | 0.1 | 26.5 | 0.0 | 42.3 | 0.2 | 45.7 | 0.0 |
| Polyunsaturated Fatty Acids | ||||||||||
| 18:2ω6 | 7.5 b | 0.0 | 8.2 a | 0.0 | 5.3 e | 0.1 | 6.3 d | 0.0 | 7.1 c | 0.0 |
| 18:3ω3 | 3.9 b | 0.0 | 4.7 a | 0.0 | 1.0 e | 0.0 | 3.0 d | 0.0 | 3.7 c | 0.0 |
| 18:4ω3 | 0.9 b | 0.0 | 0.7 c | 0.0 | 1.1 a | 0.0 | 0.7 d | 0.0 | 0.6 e | 0.0 |
| 20:4ω6 | 0.6 b | 0.0 | 0.3 e | 0.0 | 1.6 a | 0.0 | 0.5 c | 0.0 | 0.4 d | 0.0 |
| 20:5ω3 | 7.8 b | 0.0 | 6.7 c | 0.0 | 8.7 a | 0.0 | 3.3 d | 0.0 | 2.7 e | 0.1 |
| 22:5ω3 | 0.1 c | 0.1 | 0.2 c | 0.1 | 2.4 a | 0.0 | 0.9 b | 0.3 | 0.9 b | 0.0 |
| 22:6ω3 | 11.0 d | 0.1 | 9.2 e | 0.1 | 21.8 a | 0.1 | 15.1 b | 0.1 | 13.2 c | 0.1 |
| Subtotal | 31.7 | 0.1 | 29.9 | 0.1 | 41.9 | 0.1 | 29.9 | 0.3 | 28.6 | 0.1 |
| FAME mg/g of Dry Weight | 708.0 | 26.3 | 713.7 | 9.7 | 708.0 | 13.0 | ||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee Chang, K.J.; Parrish, C.C.; Simon, C.J.; Revill, A.T.; Nichols, P.D. Feeding Whole Thraustochytrid Biomass to Cultured Atlantic Salmon (Salmo salar) Fingerlings: Culture Performance and Fatty Acid Incorporation. J. Mar. Sci. Eng. 2020, 8, 207. https://doi.org/10.3390/jmse8030207
Lee Chang KJ, Parrish CC, Simon CJ, Revill AT, Nichols PD. Feeding Whole Thraustochytrid Biomass to Cultured Atlantic Salmon (Salmo salar) Fingerlings: Culture Performance and Fatty Acid Incorporation. Journal of Marine Science and Engineering. 2020; 8(3):207. https://doi.org/10.3390/jmse8030207
Chicago/Turabian StyleLee Chang, Kim Jye, Christopher C. Parrish, Cedric J. Simon, Andrew T. Revill, and Peter D. Nichols. 2020. "Feeding Whole Thraustochytrid Biomass to Cultured Atlantic Salmon (Salmo salar) Fingerlings: Culture Performance and Fatty Acid Incorporation" Journal of Marine Science and Engineering 8, no. 3: 207. https://doi.org/10.3390/jmse8030207
APA StyleLee Chang, K. J., Parrish, C. C., Simon, C. J., Revill, A. T., & Nichols, P. D. (2020). Feeding Whole Thraustochytrid Biomass to Cultured Atlantic Salmon (Salmo salar) Fingerlings: Culture Performance and Fatty Acid Incorporation. Journal of Marine Science and Engineering, 8(3), 207. https://doi.org/10.3390/jmse8030207






