Effects of Low pH and Low Salinity Induced by Meltwater Inflow on the Behavior and Physical Condition of the Antarctic Limpet, Nacella concinna
Abstract
1. Introduction
2. Material and Methods
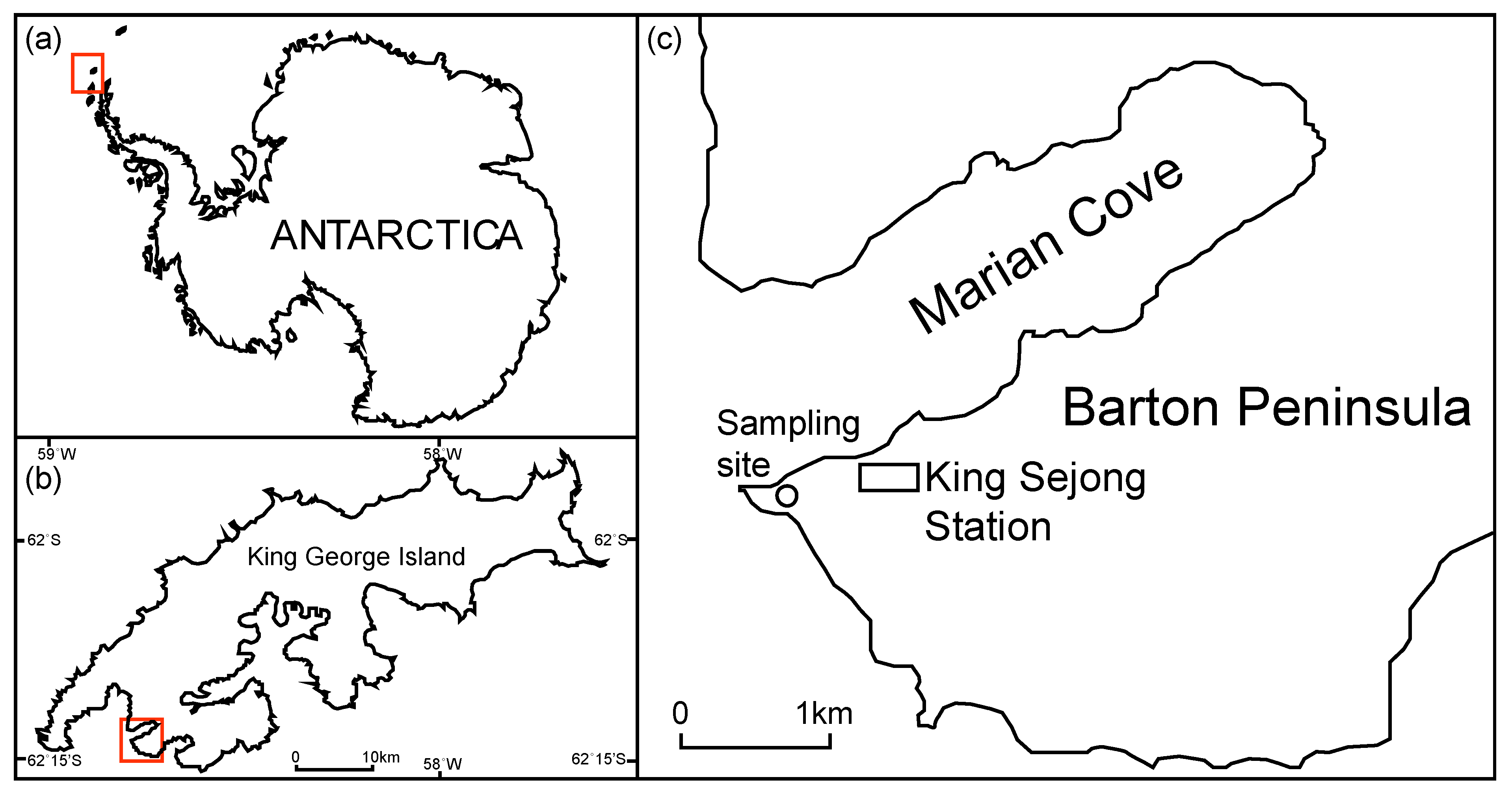
2.1. Sampling of Limpets and Preparation for the Experiments
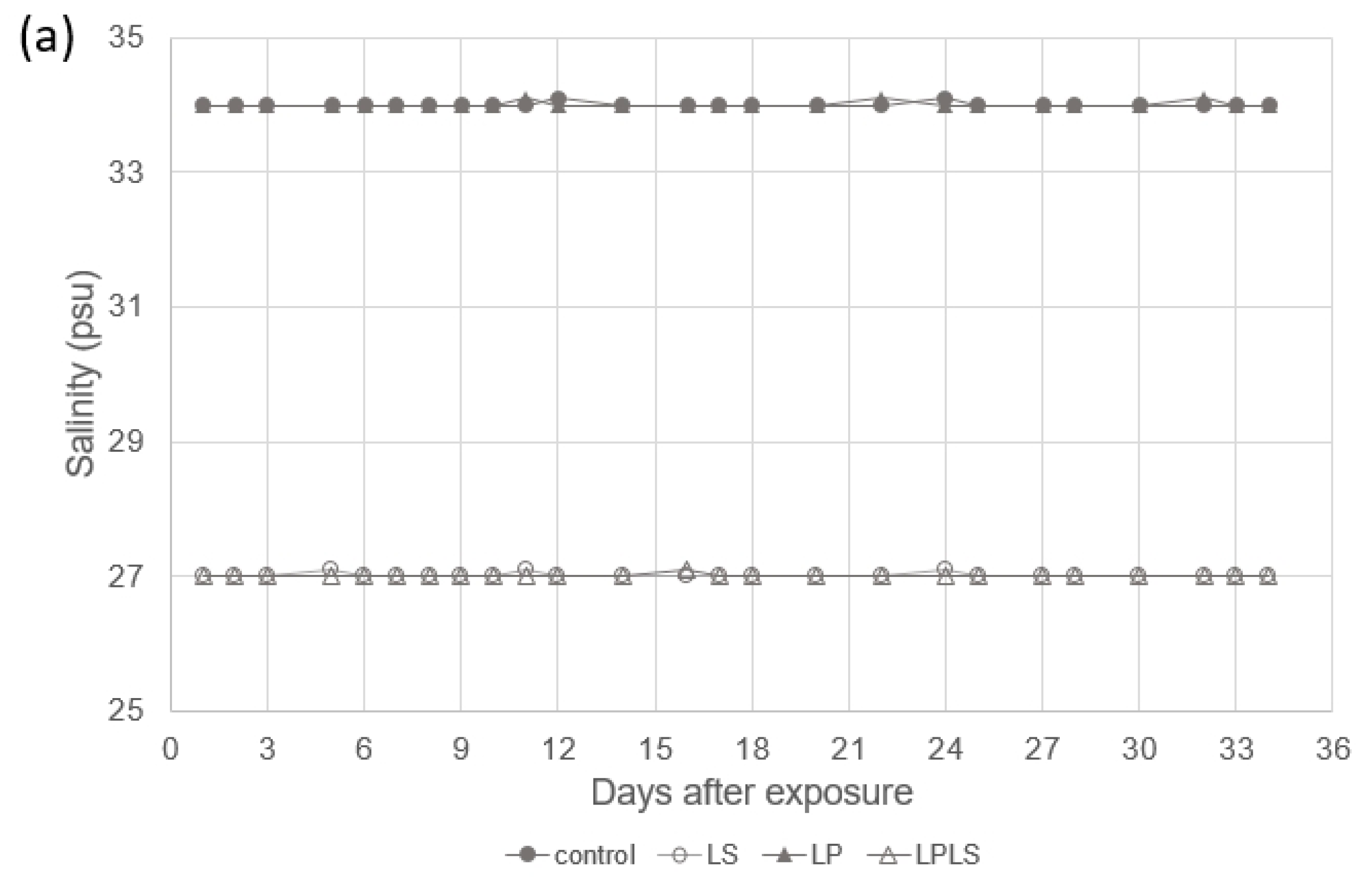
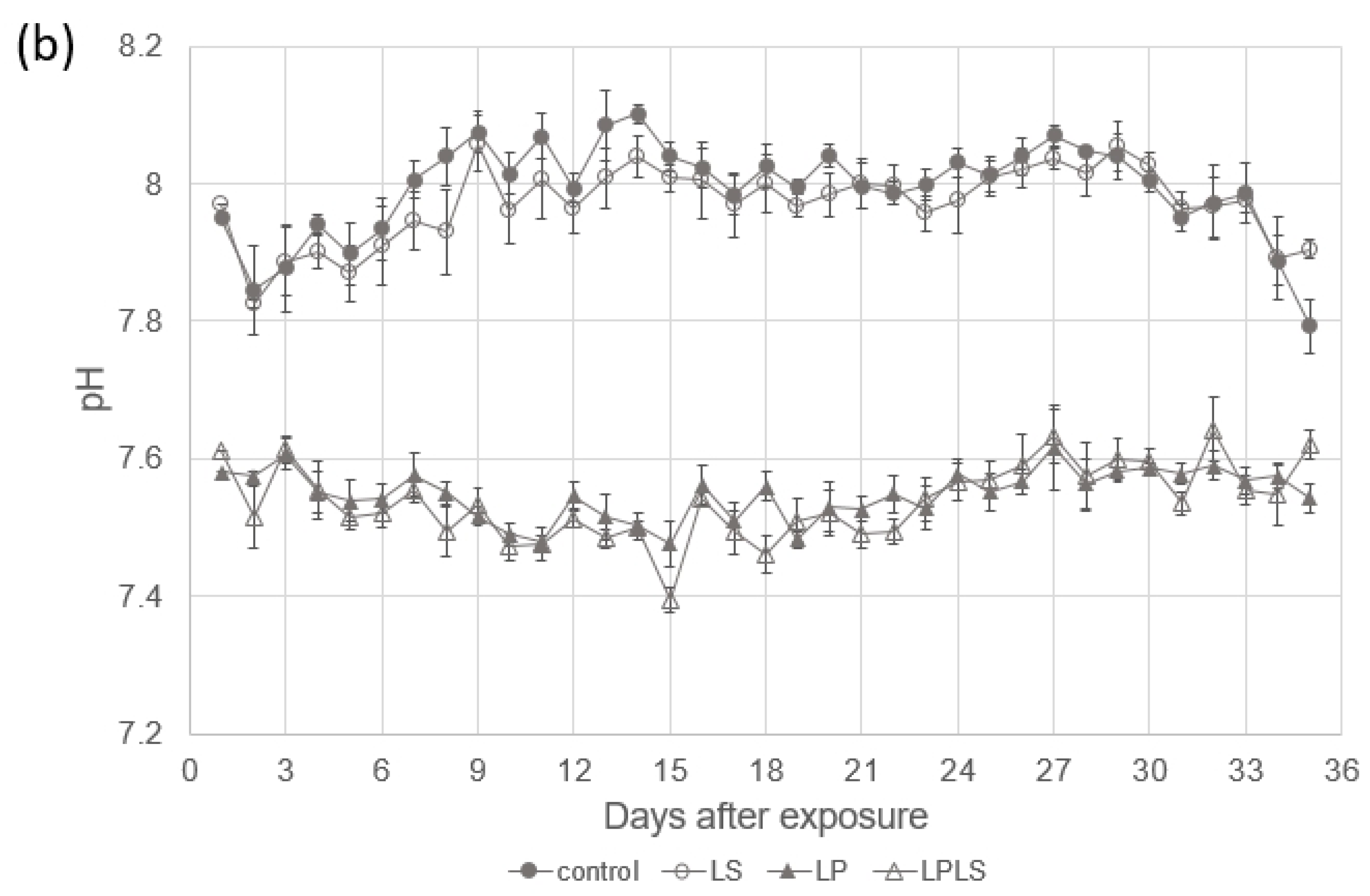
2.2. Experimental Setup
2.3. Total Alkalinity (TA)/Dissolved Inorganic Carbon (DIC) Analysis
2.4. Effects of Low pH and Low Salinity on the Behavioral Response and Physical Condition of the Limpets
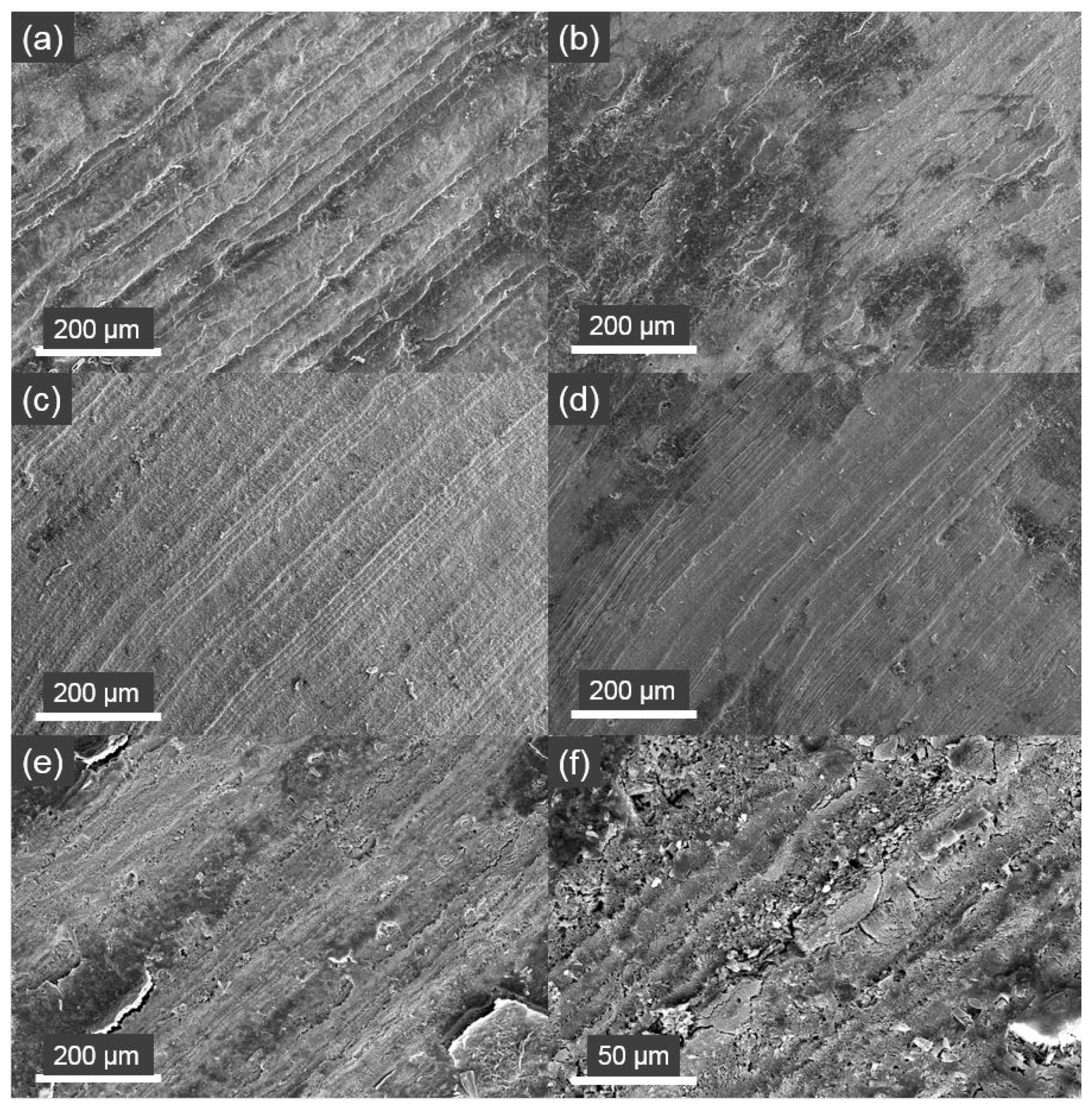
2.5. SEM Image Analysis on Shell Dissolution
2.6. Statistical Analyses
3. Results
3.1. Experimental Treatment
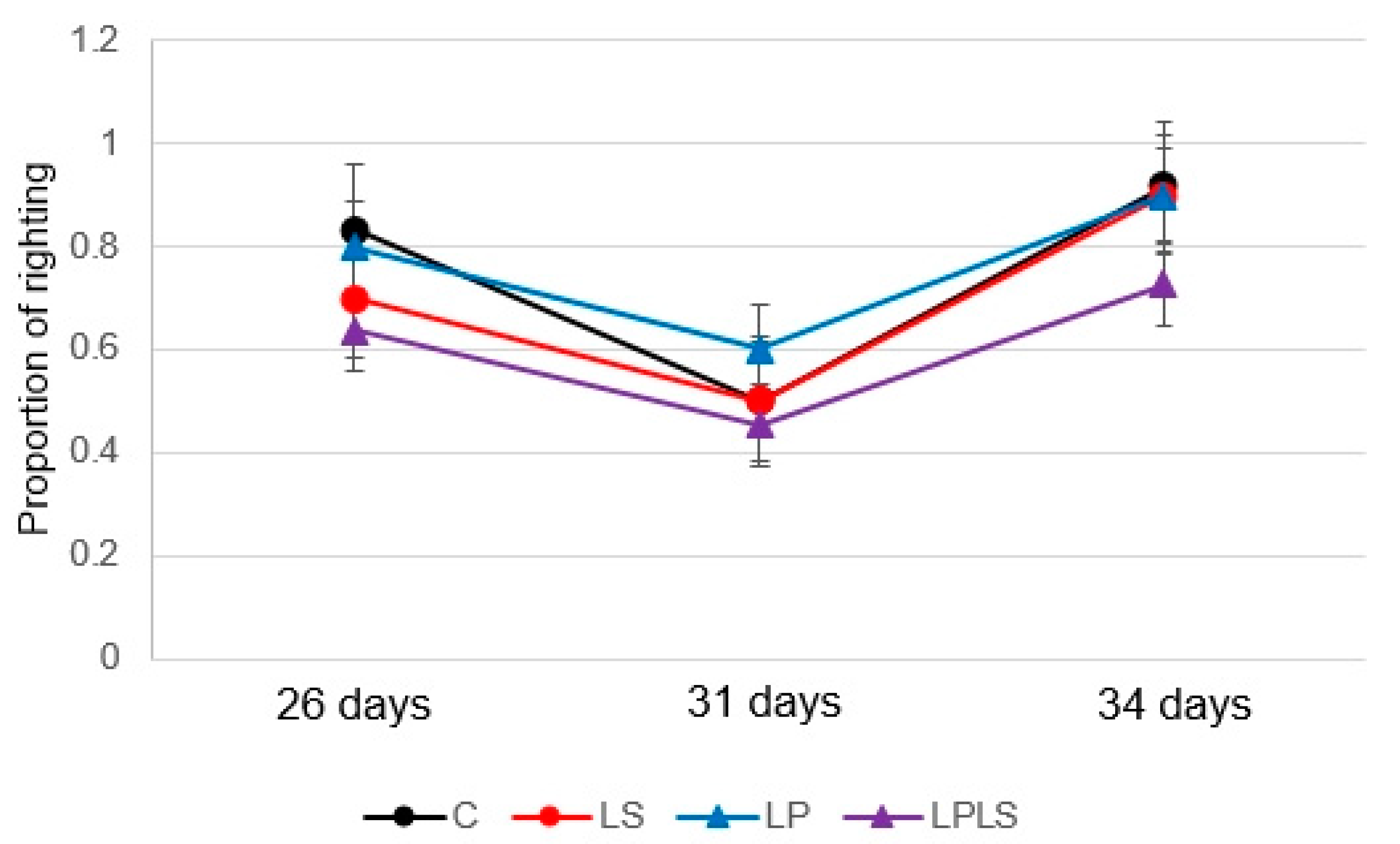
3.2. Behavioral Response—Righting
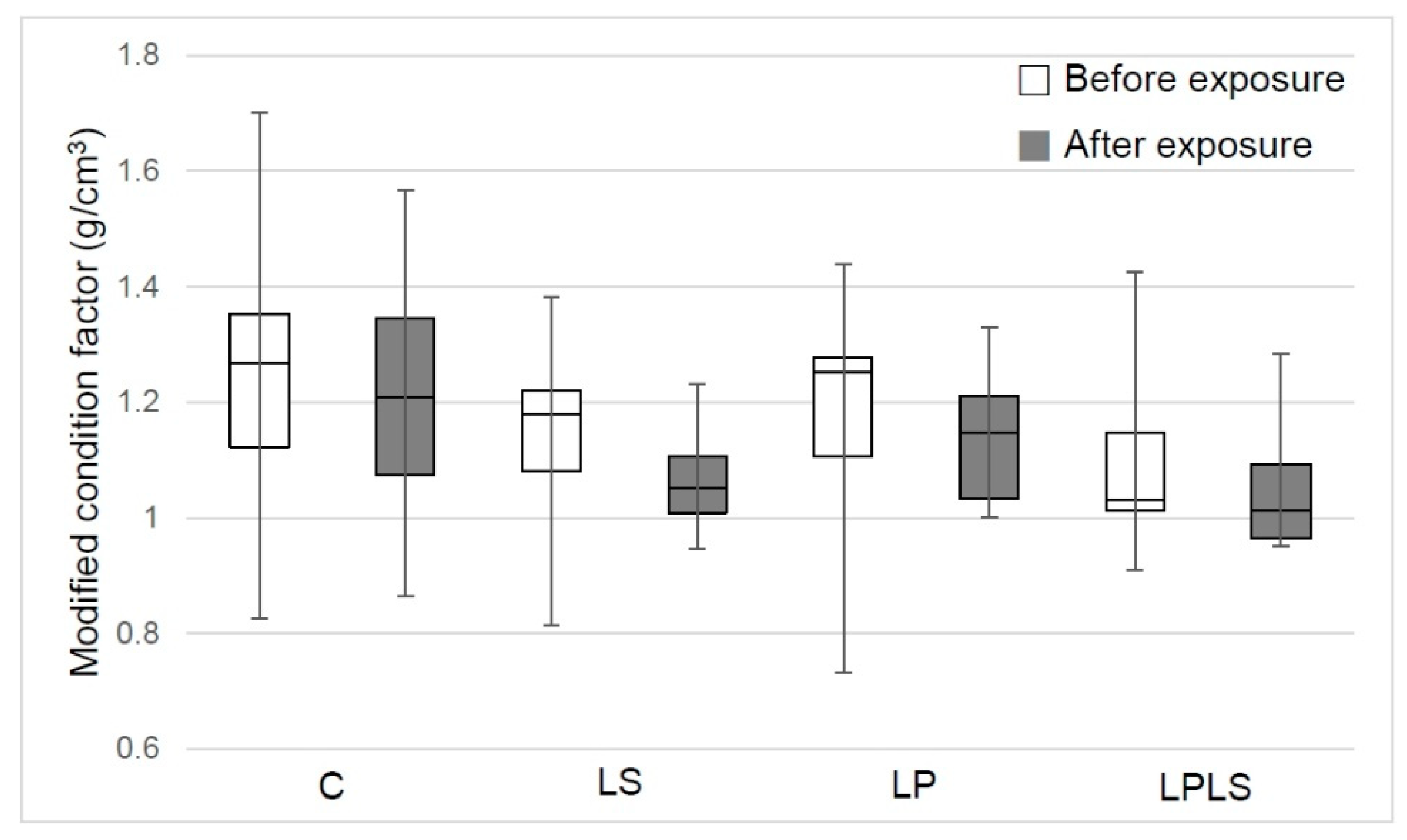
3.3. Mortality and Condition Factor
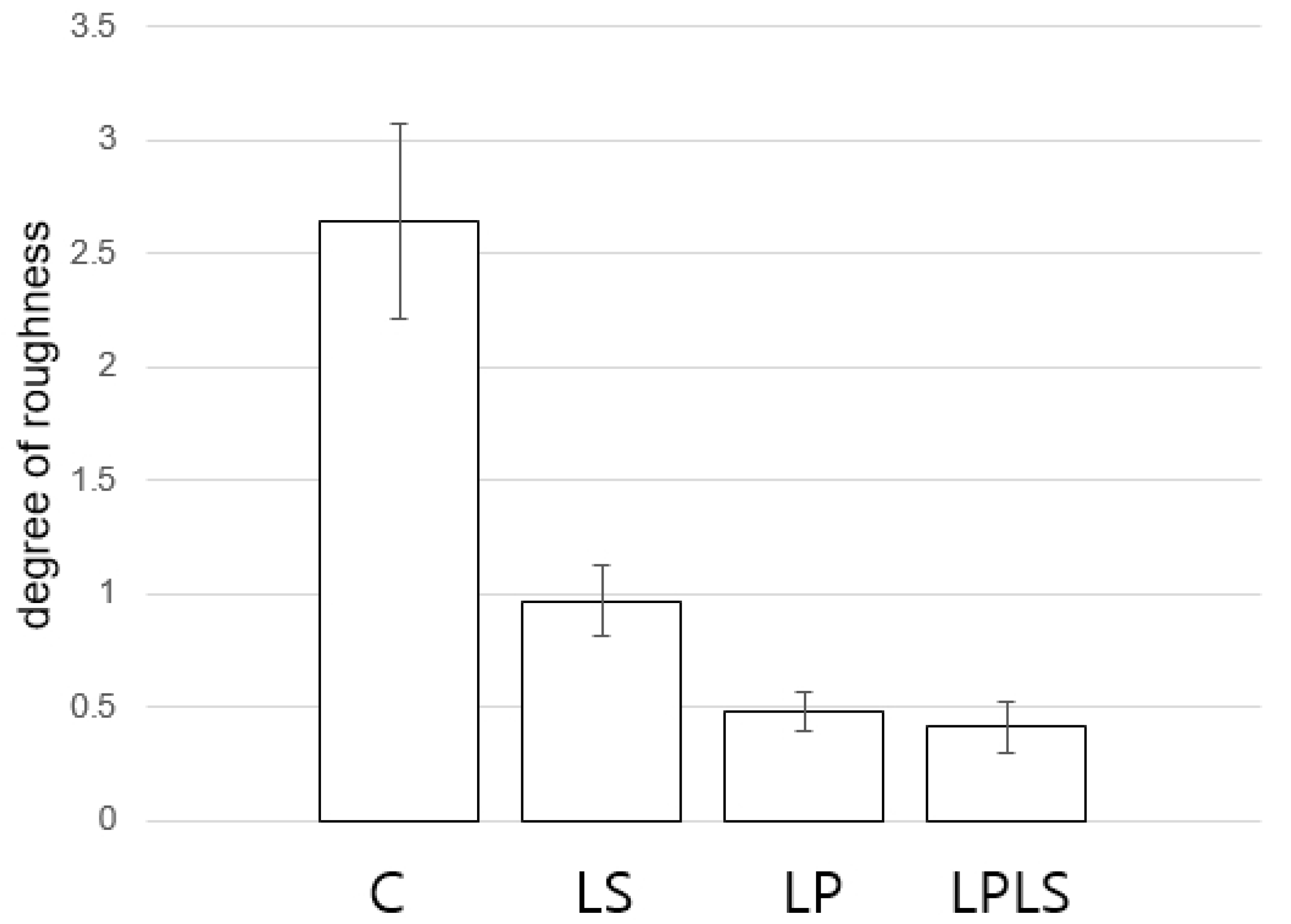
3.4. Shell Dissolution
4. Discussions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Caldeira, K.; Wickett, M.E. Oceanography: Anthropogenic carbon and ocean pH. Nature 2003, 425, 365. [Google Scholar] [CrossRef] [PubMed]
- Pachauri, R.K.; Allen, M.R.; Barros, V.R.; Broome, J.; Cramer, W.; Christ, R.; Church, J.A.; Clarke, L.; Dahe, Q.; Dasgupta, P. Climate Change 2014: Synthesis Report. Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; IPCC: Geneva, Switzerland, 2014. [Google Scholar]
- Orr, J.C.; Fabry, V.J.; Aumont, O.; Bopp, L.; Doney, S.C.; Feely, R.A.; Gnanadesikan, A.; Gruber, N.; Ishida, A.; Joos, F. Anthropogenic ocean acidification over the twenty-first century and its impact on calcifying organisms. Nature 2005, 437, 681–686. [Google Scholar] [CrossRef] [PubMed]
- McNeil, B.I.; Matear, R.J. Southern Ocean acidification: A tipping point at 450-ppm atmospheric CO2. Proc. Natl. Acad. Sci. USA 2008, 105, 18860–18864. [Google Scholar] [CrossRef] [PubMed]
- Hauri, C.; Friedrich, T.; Timmermann, A. Abrupt onset and prolongation of aragonite undersaturation events in the Southern Ocean. Nat. Clim. Chang. 2016, 6, 172. [Google Scholar] [CrossRef]
- Yamamoto-Kawai, M.; McLaughlin, F.A.; Carmack, E.C.; Nishino, S.; Shimada, K. Aragonite undersaturation in the Arctic Ocean: Effects of ocean acidification and sea ice melt. Science 2009, 326, 1098–1100. [Google Scholar] [CrossRef]
- Manno, C.; Morata, N.; Primicerio, R. Limacina retroversa’s response to combined effects of ocean acidification and sea water freshening. Estuar. Coast. Shelf Sci. 2012, 113, 163–171. [Google Scholar] [CrossRef]
- Mattsdotter Björk, M.; Fransson, A.; Chierici, M.; Torstensson, A. Ocean acidification state in western Antarctic surface waters: Controls and interannual variability. Biogeosciences 2014, 11, 57–73. [Google Scholar] [CrossRef]
- Cook, A.J.; Vaughan, D.G.; Luckman, A.J.; Murray, T. A new Antarctic Peninsula glacier basin inventory and observed area changes since the 1940s. Antarct. Sci. 2014, 26, 614–624. [Google Scholar] [CrossRef]
- Rignot, E.; Mouginot, J.; Scheuchl, B.; van den Broeke, M.; van Wessem, M.J.; Morlighem, M. Four decades of Antarctic Ice Sheet mass balance from 1979–2017. Proc. Natl. Acad. Sci. USA 2019, 116, 1095–1103. [Google Scholar] [CrossRef]
- Cook, A.; Holland, P.; Meredith, M.; Murray, T.; Luckman, A.; Vaughan, D.J.S. Ocean forcing of glacier retreat in the western Antarctic Peninsula. Science 2016, 353, 283–286. [Google Scholar] [CrossRef]
- Spence, P.; Holmes, R.M.; Hogg, A.M.; Griffies, S.M.; Stewart, K.D.; England, M.H. Localized rapid warming of West Antarctic subsurface waters by remote winds. Nat. Clim. Chang. 2017, 7, 595. [Google Scholar] [CrossRef]
- Ha, S.-Y.; Ahn, I.; Moon, H.-W.; Choi, B.; Shin, K.-H. Tight trophic association between benthic diatom blooms and shallow-water megabenthic communities in a rapidly deglaciated Antarctic fjord. Estuar. Coast. Shelf Sci. 2019, 218, 258–267. [Google Scholar] [CrossRef]
- Moon, H.-W.; Hussin, W.M.R.W.; Kim, H.-C.; Ahn, I. The impacts of climate change on Antarctic nearshore mega-epifaunal benthic assemblages in a glacial fjord on King George Island: Responses and implications. Ecol. Indic. 2015, 57, 280–292. [Google Scholar] [CrossRef]
- Lim, H.S.; Park, Y.; Lee, J.-Y.; Yoon, H.I. Geochemical characteristics of meltwater and pondwater on Barton and Weaver Peninsulas of King George Island, West Antarctica. Geochem. J. 2014, 48, 409–422. [Google Scholar] [CrossRef]
- Yoo, K.-C.; Lee, M.K.; Yoon, H.I.; Lee, Y.I.; Kang, C.Y. Hydrography of Marian Cove, King George Island, West Antarctica: Implications for ice-proximal sedimentation during summer. Antarct. Sci. 2015, 27, 185–196. [Google Scholar] [CrossRef]
- Sahade, R.; Lagger, C.; Torre, L.; Momo, F.; Monien, P.; Schloss, I.; Barnes, D.K.; Servetto, N.; Tarantelli, S.; Tatián, M.J.S.A. Climate change and glacier retreat drive shifts in an Antarctic benthic ecosystem. Sci. Adv. 2015, 1, e1500050. [Google Scholar] [CrossRef]
- Kim, D. Seasonality of marine algae and grazers of an Antarctic rocky intertidal, with emphasis on the role of the limpet Nacella concinna Strebel (Gastropoda: Patellidae). Ber. Zur Polar-Und Meeresforsch. (Rep. Polar Mar. Res.) 2001, 397, 43. [Google Scholar]
- Dierssen, H.M.; Smith, R.C.; Vernet, M. Glacial meltwater dynamics in coastal waters west of the Antarctic peninsula. Proc. Natl. Acad. Sci. USA 2002, 99, 1790–1795. [Google Scholar] [CrossRef]
- Smale, D.A. Ice disturbance intensity structures benthic communities in nearshore Antarctic waters. Mar. Ecol. Prog. Ser. 2007, 349, 89–102. [Google Scholar] [CrossRef]
- Kim, I.; Kim, G.; Choy, E.J. The significant inputs of trace elements and rare earth elements from melting glaciers in Antarctic coastal waters. Polar Res. 2015, 34, 24289. [Google Scholar] [CrossRef]
- Ahn, I.; Chung, K.H.; Choi, H.J. Influence of glacial runoff on baseline metal accumulation in the Antarctic limpet Nacella concinna from King George Island. Mar. Pollut. Bull. 2004, 1, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Straneo, F.; Curry, R.G.; Sutherland, D.A.; Hamilton, G.S.; Cenedese, C.; Våge, K.; Stearns, L.A. Impact of fjord dynamics and glacial runoff on the circulation near Helheim Glacier. Nat. Geosci. 2011, 4, 322–327. [Google Scholar] [CrossRef]
- Peck, L.S. Prospects for surviving climate change in Antarctic aquatic species. Front. Zool. 2005, 2, 9. [Google Scholar] [CrossRef] [PubMed]
- Roberts, D.; Hodgson, D.A.; McMinn, A.; Verleyen, E.; Terry, B.; Corbett, C.; Vyverman, W. Recent rapid salinity rise in three East Antarctic lakes. J. Paleolimnol. 2006, 36, 385–406. [Google Scholar] [CrossRef]
- Torre, L.; Tabares, P.C.C.; Momo, F.; Meyer, J.F.; Sahade, R. Climate change effects on Antarctic benthos: A spatially explicit model approach. Clim. Chang. 2017, 141, 733–746. [Google Scholar] [CrossRef]
- Park, S.; Ahn, I.; Sin, E.; Shim, J.; Kim, T. Ocean freshening and acidification differentially influence mortality and behavior of the Antarctic amphipod Gondogeneia antarctica. Mar. Environ. Res. 2019, 154, 104847. [Google Scholar] [CrossRef]
- Gunderson, A.R.; Armstrong, E.J.; Stillman, J.H. Multiple stressors in a changing world: The need for an improved perspective on physiological responses to the dynamic marine environment. Annu. Rev. Mar. Sci. 2016, 8, 357–378. [Google Scholar] [CrossRef]
- Ingels, J.; Vanreusel, A.; Brandt, A.; Catarino, A.I.; David, B.; De Ridder, C.; Dubois, P.; Gooday, A.J.; Martin, P.; Pasotti, F. Possible effects of global environmental changes on Antarctic benthos: A synthesis across five major taxa. Ecol. Evol. 2012, 2, 453–485. [Google Scholar] [CrossRef]
- Davenport, J. Tenacity of the Antarctic limpet Nacella concinna. J. Molluscan Stud. 1988, 54, 355–356. [Google Scholar] [CrossRef]
- Walker, A. Introduction to the ecology of the Antarctic limpet Patinigera polaris (Hombron and Jaquinot) at Signy island, South Orkney islands. Br. Antarct. Surv. Bull. 1972, 28, 49–71. [Google Scholar]
- Picken, G. The distribution, growth, and reproduction of the Antarctic limpet Nacella (Patinigera) concinna (Strebel, 1908). J. Exp. Mar. Biol. Ecol. 1980, 42, 71–85. [Google Scholar] [CrossRef]
- Bowden, D.A. Quantitative characterization of shallow marine benthic assemblages at Ryder Bay, Adelaide Island, Antarctica. Mar. Biol. 2005, 146, 1235–1249. [Google Scholar] [CrossRef]
- Cadée, G.C. Shell damage and shell repair in the Antarctic limpet Nacella concinna from King George Island. J. Sea Res. 1999, 41, 149–161. [Google Scholar] [CrossRef]
- Suda, C.N.; Vani, G.S.; de Oliveira, M.F.; Rodrigues, E.; Lavrado, H.P. The biology and ecology of the Antarctic limpet Nacella concinna. Polar Biol. 2015, 38, 1949–1969. [Google Scholar] [CrossRef]
- Thor, P.; Dupont, S. Transgenerational effects alleviate severe fecundity loss during ocean acidification in a ubiquitous planktonic copepod. Glob. Chang. Biol. 2015, 21, 2261–2271. [Google Scholar] [CrossRef] [PubMed]
- Davenport, J. Meltwater effects on intertidal Antarctic limpets, Nacella concinna. J. Mar. Biol. Assoc. UK 2001, 81, 643–649. [Google Scholar] [CrossRef]
- Guinotte, J.M.; Fabry, V.J. Ocean acidification and its potential effects on marine ecosystems. Ann. N. Y. Acad. Sci. 2008, 1134, 320–342. [Google Scholar] [CrossRef] [PubMed]
- McClintock, J.B.; Angus, R.A.; Mcdonald, M.R.; Amsler, C.D.; Catledge, S.A.; Vohra, Y.K. Rapid dissolution of shells of weakly calcified Antarctic benthic macroorganisms indicates high vulnerability to ocean acidification. Antarct. Sci. 2009, 21, 449–456. [Google Scholar] [CrossRef]
- Cummings, V.; Hewitt, J.; Van Rooyen, A.; Currie, K.; Beard, S.; Thrush, S.; Norkko, J.; Barr, N.; Heath, P.; Halliday, N.J. Ocean acidification at high latitudes: Potential effects on functioning of the Antarctic bivalve Laternula elliptica. PLoS ONE 2011, 6, e16069. [Google Scholar] [CrossRef]
- Seibel, B.A.; Maas, A.E.; Dierssen, H.M. Energetic plasticity underlies a variable response to ocean acidification in the pteropod, Limacina helicina antarctica. PLoS ONE 2012, 7, e30464. [Google Scholar] [CrossRef]
- Schram, J.B.; Schoenrock, K.M.; McClintock, J.B.; Amsler, C.D.; Angus, R.A. Multiple stressor effects of near-future elevated seawater temperature and decreased pH on righting and escape behaviors of two common Antarctic gastropods. J. Exp. Mar. Biol. Ecol. 2014, 457, 90–96. [Google Scholar] [CrossRef]
- Lee, S.H.; Joo, H.M.; Joo, H.; Kim, B.K.; Song, H.J.; Jeon, M.; Kang, S.-H. Large contribution of small phytoplankton at Marian Cove, King George Island, Antarctica, based on long-term monitoring from 1996 to 2008. Polar Biol. 2015, 38, 207–220. [Google Scholar] [CrossRef]
- Haugan, P.M.; Drange, H. Effects of CO2 on the ocean environment. Energy Convers. Manag. 1996, 37, 1019–1022. [Google Scholar] [CrossRef]
- Gradinger, R.; Schnack-Schiel, S. Potential effect of ice formation on Antarctic pelagic copepods: Salinity induced mortality of Calanus propinquus and Metridia gerlachei in comparison to sympagic acoel turbellarians. Polar Biol. 1998, 20, 139–142. [Google Scholar] [CrossRef]
- Pierrot, D.; Lewis, E.; Wallace, D. MS Excel Program Developed for CO2 System Calculations; ORNL/CDIAC-105a; Carbon Dioxide Information Analysis Center, Oak Ridge National Laboratory, US Department of Energy: Oak Ridge, TN, USA, 2006; Volume 10. [Google Scholar]
- Obermüller, B.E.; Morley, S.A.; Clark, M.S.; Barnes, D.K.; Peck, L.S. Antarctic intertidal limpet ecophysiology: A winter–summer comparison. J. Exp. Mar. Biol. Ecol. 2011, 403, 39–45. [Google Scholar] [CrossRef]
- Peck, L.S.; Webb, K.E.; Bailey, D.M. Extreme sensitivity of biological function to temperature in Antarctic marine species. Funct. Ecol. 2004, 18, 625–630. [Google Scholar] [CrossRef]
- Weihe, E.; Abele, D. Differences in the physiological response of inter-and subtidal Antarctic limpets Nacella concinna to aerial exposure. Aquat. Biol. 2008, 4, 155–166. [Google Scholar] [CrossRef]
- Bednaršek, N.; Tarling, G.A.; Bakker, D.C.; Fielding, S.; Cohen, A.; Kuzirian, A.; McCorkle, D.; Lézé, B.; Montagna, R. Description and quantification of pteropod shell dissolution: A sensitive bioindicator of ocean acidification. Glob. Chang. Biol. 2012, 18, 2378–2388. [Google Scholar] [CrossRef]
- Jeong, W.; Weon, J.I. Mar Behavior and Quantitative Evaluation of Urethane-Acrylate Coatings with a Different Gloss. Polymer 2013, 37, 455–461. [Google Scholar] [CrossRef]
- Tukey, J.W. Exploratory Data Analysis; Addison-Wesley: Boston, MA, USA, 1977; Volume 2. [Google Scholar]
- Nolan, C.P. Size, shape and shell morphology in the Antarctic limpet Nacella concinna at Signy Island, South Orkney Islands. J. Molluscan Stud. 1991, 57, 225–238. [Google Scholar] [CrossRef]
- Morley, S.; Clark, M.; Peck, L. Depth gradients in shell morphology correlate with thermal limits for activity and ice disturbance in Antarctic limpets. J. Exp. Mar. Biol. Ecol. 2010, 390, 1–5. [Google Scholar] [CrossRef]
- Harper, E.M.; Clark, M.S.; Hoffman, J.I.; Philipp, E.E.; Peck, L.S.; Morley, S.A. Iceberg scour and shell damage in the Antarctic bivalve Laternula elliptica. PLoS ONE 2012, 7, e46341. [Google Scholar] [CrossRef] [PubMed]
- Kapsenberg, L.; Kelley, A.L.; Shaw, E.C.; Martz, T.R.; Hofmann, G.E. Near-shore Antarctic pH variability has implications for the design of ocean acidification experiments. Sci. Rep. 2015, 5, 9638. [Google Scholar] [CrossRef]
- Dong, Y.W.; Zhang, S.J.F.E. Ecological relevance of energy metabolism: Transcriptional responses in energy sensing and expenditure to thermal and osmotic stresses in an intertidal limpet. Funct. Ecol. 2016, 30, 1539–1548. [Google Scholar] [CrossRef]
- Roleda, M.Y.; Boyd, P.W.; Hurd, C.L. Before ocean acidification: Calcifier chemistry lessons 1. J. Phycol. 2012, 48, 840–843. [Google Scholar] [CrossRef]
- Cyronak, T.; Schulz, K.G.; Jokiel, P.L. The Omega myth: What really drives lower calcification rates in an acidifying ocean. ICES J. Mar. Sci. 2016, 73, 558–562. [Google Scholar] [CrossRef]
- Cross, E.L.; Peck, L.S.; Harper, E.M. Ocean acidification does not impact shell growth or repair of the Antarctic brachiopod Liothyrella uva (Broderip, 1833). J. Exp. Mar. Biol. Ecol. 2015, 462, 29–35. [Google Scholar] [CrossRef]
- San Chan, V.B.; Li, C.; Lane, A.C.; Wang, Y.; Lu, X.; Shih, K.; Zhang, T.; Thiyagarajan, V. CO2-driven ocean acidification alters and weakens integrity of the calcareous tubes produced by the serpulid tubeworm, Hydroides elegans. PLoS ONE 2012, 7, e42718. [Google Scholar] [CrossRef]
- Shabica, S.V. The Natural History of the Antarctic Limpet Patinigera polaris (Hombron and Jacquinot). Ph.D. Thesis, University of Oregon, Eugene, OR, USA, 1976. [Google Scholar]
| Group | C | LS | LP | LPLS |
|---|---|---|---|---|
| pH(NBS) | 8.01 ± 0.01 | 7.99 ± 0.01 | 7.61 ± 0.02 | 7.59 ± 0.01 |
| Salinity (psu) | 34.0 ± 0.0 | 27.0 ± 0.0 | 34.0 ± 0.0 | 27.0 ± 0.0 |
| Water temp. (°C) | 1.0 ± 0.3 | 0.9 ± 0.5 | 1.0 ± 0.4 | 0.8 ± 0.6 |
| TA (μmol/kg) | 2498.1 ± 47.7 | 2052.5 ± 95.2 | 2569.5 ± 101.5 | 2135.7 ± 96.0 |
| DIC (μmol/kg) | 2401.5 ± 47.1 | 2003.9 ± 95.8 | 2606.3 ± 108.5 | 2187.9 ± 102.9 |
| pCO2 (μatm) | 594.2 ± 13.8 | 552.5 ± 30.4 | 1595.9 ± 118.5 | 1496.3 ± 97.8 |
| Ωcal | 2.09 ± 0.12 | 1.33 ± 0.05 | 0.87 ± 0.02 | 0.59 ± 0.02 |
| Ωarg | 1.32 ± 0.08 | 0.82 ± 0.03 | 0.55 ± 0.02 | 0.36 ± 0.01 |
| N | 7 | 4 | 5 | 4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sin, E.; Ahn, I.-Y.; Park, S.; Kim, T. Effects of Low pH and Low Salinity Induced by Meltwater Inflow on the Behavior and Physical Condition of the Antarctic Limpet, Nacella concinna. J. Mar. Sci. Eng. 2020, 8, 822. https://doi.org/10.3390/jmse8100822
Sin E, Ahn I-Y, Park S, Kim T. Effects of Low pH and Low Salinity Induced by Meltwater Inflow on the Behavior and Physical Condition of the Antarctic Limpet, Nacella concinna. Journal of Marine Science and Engineering. 2020; 8(10):822. https://doi.org/10.3390/jmse8100822
Chicago/Turabian StyleSin, Eunchong, In-Young Ahn, Seojeong Park, and Taewon Kim. 2020. "Effects of Low pH and Low Salinity Induced by Meltwater Inflow on the Behavior and Physical Condition of the Antarctic Limpet, Nacella concinna" Journal of Marine Science and Engineering 8, no. 10: 822. https://doi.org/10.3390/jmse8100822
APA StyleSin, E., Ahn, I.-Y., Park, S., & Kim, T. (2020). Effects of Low pH and Low Salinity Induced by Meltwater Inflow on the Behavior and Physical Condition of the Antarctic Limpet, Nacella concinna. Journal of Marine Science and Engineering, 8(10), 822. https://doi.org/10.3390/jmse8100822






