Abstract
The Philippine archipelago is geographically positioned in the tropics with rich areas of marine biodiversity. Its marine sediments harbor actinomycetes that exhibit antibacterial activity. Screening of actinomycetes isolated from marine sediments collected near the coast of Islas de Gigantes, Iloilo showed one isolate that exhibited high activity against the multidrug-resistant Staphylococcus aureus (MRSA) strain carrying the Staphylococcal Cassette Chromosome mec (SCCmec) type 1 gene, a biomarker for drug resistance. The isolate was identified as Streptomyces sp. strain DSD011 based on its 16s rRNA and protein-coding genes (atpD, recA, rpoB, and trpB) sequences, and was found to be a new species of salt-tolerant marine Streptomyces. Further, the strain harbors both non-ribosomal peptide synthetase (NRPS) and type II polyketide synthase (PKS) in its genome. The targeted chromatographic isolation and chemical investigations by Liquid Chromatography Mass Spectrometry-Time of Flight (LCMS-TOF), tandem mass spectrometry (MS/MS), and Global Natural Product Social molecular networking (GNPS) of the antibiotics produced by the strain afforded the two polycyclic aromatic polyketide angucycline glycosides, fridamycin A (1) and fridamycin D (2), which are products of type II PKS biosynthesis. Compounds 1 and 2 displayed antibacterial activity against MRSA with minimum inhibitory concentration (MIC) of 500 μg/mL and 62.5 μg/mL, respectively. These results suggest that the underexplored marine sediments near the coast of Islas de Gigantes, Iloilo offer access to undiscovered Streptomyces species that are invaluable sources of antibiotic leads.
1. Introduction
The seafloor covers about 70% of the Earth’s surface and is carpeted by marine sediments, which are mixtures of complex organic and inorganic particles that accumulated due to accretion and erosion of the continents, oceanic biological activities, volcanic eruptions, and chemical processes within the ocean [1,2]. Given their vast coverage, marine sediments harbor remarkable diverse microbial communities accounting for 12–45% of the total microbial biomass or ~0.6–2% of the whole biosphere [3,4,5,6]. Remarkably, members of the soil-dwelling Actinobacteria (the order of actinomycetes in particular) are widely distributed in marine sediments found in intertidal zones, near shores [7,8,9,10], and deep-sea [10,11,12,13]. Actinomycetes, known for its antibiotic-producing ability [14], spend the majority of their life cycle as dormant spores [15] that are continually washed in large numbers from surrounding terrestrial niches through river runoffs into the marine environment where they can remain dormant [8,16]. These marine actinomycetes can survive in a very demanding, nutrient deficient, competitive, and hostile environment that is inundated with high salinity and high pressure [17,18,19]. As a result, some metabolic changes occur in these bacteria that trigger the production of secondary metabolites that are distinct from their terrestrial counterparts [19,20]. The diversity of marine sediment-derived actinomycetes is indisputable [7,21,22], with decreasing genetic diversity patterns in proportion with depth [13]. This enormous diversity and metabolic changes yield new or novel core structures with potential pharmaceutical applications [23,24,25,26,27,28] as antibacterial [24,27,29,30,31] and antitumor [32,33] agents. In particular, Salinosporamide A (Marizomib), a novel rare bicyclic beta-lactone gamma-lactam isolated from an obligate marine actinobacterium, Salinispora tropica [34], has been a significant representation of compound derived from marine actinobacteria leading to clinical trials in the treatment of cancer in humans [35,36].
Efforts in searching for bioactive compounds from marine sediment-derived actinomycetes gained the attention of many researchers in the last five years [7,12,37,38,39,40,41,42,43,44]. Some examples of marine actinomycetes that produce bioactive compounds include the marine sediment-derived Streptomyces sp. RKND004 isolated from Prince Edward Island, Canada that produces the anticancer polycyclic polyether natural products terrosamycins A and B [31]; a marine sediment-derived Streptomyces sp. EG1 collected from the North Coast of the Mediterranean Sea of Egypt which produces tetracene antibiotic mersaquinone [27], and deep-sea sediment-derived Streptomyces sp. SCSIO 11791 that produces two new chlorinated bis-indole alkaloid antibiotics namely, dionemycin and 6-OMe-7′,7″-dichorochromopyrrolic acid [45]. Most recently, our group discovered the antibiotic anthracycline shunt metabolites bisanhydroaklavinone and 1-hydroxybisanhydroaklavinone from a Philippine marine sediment-derived Streptomyces griseorubens strain DSD069, which destroys the cell membrane integrity of multidrug-resistant Staphylococcus aureus [24]. These anthracyclines shunt metabolites are precursors of anticancer anthracyclines such as doxorubicin, daunorubicin, and cinerubins. They were accumulated in the growth medium during the fermentation of Streptomyces griseorubens strain DSD069, which is a rare event occurring in a non-genetically modified Streptomyces strain [24]. This recent finding shows that Philippine marine sediment-derived actinomycetes are a potentially rich source of new or novel secondary metabolites.
As part of our continuing program to explore the biological and chemical profiles of Philippine marine sediment-derived actinomycetes for antibiotics against multidrug-resistant S. aureus (MRSA), we screened the actinomycete strains isolated from tropical marine sediments collected from Islas de Gigantes, Iloilo (Figure 1a). The Islas de Gigantes is an island chain within the larger Western Visayas archipelago in the Visayan Sea and Jintotolo Channel. We report here the isolation of marine sediment-derived actinomycete strain from Islas de Gigantes and its bioactivity against multidrug-resistant S. aureus (MRSA), a causative pathogen of various community and hospital-acquired infections that carries the SCCmec type 1 gene as a drug-resistant biomarker. The actinomycete isolate was identified as a Streptomyces sp. strain DSD011 based on phylogenetic marker genes using small subunit rRNA (i.e., 16s rRNA) and protein-coding genes (atpD, recA, rpoB, and trpB) sequence analyses and was found to belong to a new species of salt-tolerant marine Streptomyces. Further, the Streptomyces sp. strain DSD011 was screened for the presence of type I and II polyketide synthase (PKS) and non-ribosomal peptide synthetase (NRPS) biosynthetic genes. Our results showed that the Streptomyces sp. strain DSD011 harbors both type II polyketide synthase (PKS) and NRPS in its genome. The targeted chromatographic isolation and chemical investigations by LCMS, MS/MS, and Global Natural Product Social molecular networking (GNPS) of the antibiotics present in the crude fermentation extract of Streptomyces sp. strain DSD011 led to the discovery of type II PKS that are polycyclic aromatic polyketide angucycline glycosides fridamycin A (1) and fridamycin D (2) with activities against MRSA strain harboring the SCCmec gene. These results suggest that the under-explored marine sediments near the coast of Islas de Gigantes, Iloilo offer access to undiscovered Streptomyces species, which are valuable sources of antibiotic leads.
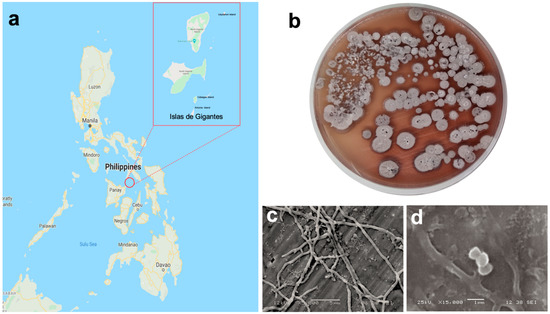
Figure 1.
(a) Map of the collection site (11°35′39″ N, 123°20′11″ E); (b) Streptomyces sp. strain DSD011 in MM1 marine media; scanning electron micrograph of strain DSD011 showing (c) branched hyphae; and (d) chain of smooth-surfaced spores, when grown in marine medium 1 (MM1) broth at 28 °C for 14 days.
2. Materials and Methods
2.1. Collection of Marine Sediments
Marine sediments were collected from Islas de Gigantes (11°35′39″ N, 123°20′11″ E) group of islands in March 2016. The locations sampled included the islands of Antonia, Bantigue, Cabugao, and Uaydahon. The marine sediment samples were collected by self-contained underwater breathing apparatus (SCUBA) diving from a depth of approximately 20 to 30 m and a distance of 300 to 500 m away from the shore of the nearest island. Samples of marine sediments were collected by digging a shallow trench of 0.3 m using a hand trowel technique. The habitats encountered at each sampling location were categorized as sand, silt, or reef. The sediments were collected in sterile 50 mL tubes and kept on ice until processed in the laboratory.
2.2. Culture-Dependent Isolation
The sediment samples were air-dried for 8 h in a biosafety hood prior to inoculation in the solid medium of ISP4 (International Streptomyces Project Medium 4) (Difco) with filtered seawater using the dry stamp method (DSM) and heat shock method (HSM) [19,24,46]. The inoculated plates were incubated at room temperature (25 to 28 °C) for 15 to 30 days.
2.3. Identification of Strain DSD011 by Phylogenetic Marker Genes Using Small Subunit rRNA (i.e., 16s rRNA) and Protein Coding Genes (atpD, recA, rpoB, and trpB) Sequence Analyses
The DNA of actinomycete strain was extracted using a DNeasy blood and tissue kit (Qiagen, Germany) according to the manufacturer’s instruction. For 16S rRNA gene amplification, universal primers 27F (5′-AGAGTTTGATCCTGGCTCAG-3′), 518F (5′-CCAGCAGCCGCGGTAATACG-3′), 800R (5′-TACCAGGGTATCTAATCC-3′), and 1492R (5′-TACGGCTACCTTGTTACGACTT-3′) were used in the PCR reaction. The reaction mix of 50 μL contained 5 μL Taq Polymerase (5 units/μL), 5 μL MgCl2 (50 mM), 5 μL Taq Buffer, 5 μL dNTPs (10 mM), 3 μL 27F (10 μM), 3 μL 1492R (10 μM), 19 μL nuclease-free H2O and 100 ng DNA template. The PCR conditions were as follows: initial denaturation at 98 °C for 3 min; 35 cycles at 98 °C for 10 s, 60 °C for 10 s and 72 °C for 60 s; a 10-min final extension at 72 °C using a thermocycler (Bio-Rad T100TM Thermal Cycler, Bio-Rad Laboratories, Inc., Singapore). The amplification products were cleaned up using a QIAquick PCR Purification Kit (Qiagen, Germany) according to the manufacturer’s protocol. The universal primers 27F, 518F, 800R, and 1492R were used to obtain the almost complete 16S rRNA sequences of the isolate (1401 bp). The species-level affiliation of the sequences was validated using sequences from the BLAST server from National Center for Biotechnology Information (NCBI) (U.S. National Library of Medicine, Rockville Pike, Bethesda, MD, USA) [47]. The phylogenetic tree was constructed using a multiple alignment with maximum likelihood method in Mega 7.0 software [48] and the resultant tree topologies were evaluated by bootstrap analysis based on 1000 replicates. The evolutionary distance was calculated using the Kimura two-parameter model for nucleotide sequences [49].
In addition to 16S rRNA, the multilocus sequence analysis (MLSA) was employed using four housekeeping genes, namely atpD (ATP synthase F1, beta subunit), recA (recombinase A), rpoB (RNA polymerase beta subunit), and trpB (tryptophane B, beta subunit) were amplified and sequenced. The primers shown in Supplemental Table S1 were used to amplify atpD, recA, rpoB, and trpB genes [50]. Each 50 µL PCR reaction contains 25 µL SYBR green supermix, 3 µL forward primer (20 µM), 3 µL reverse primer (20 µM), 14 µL nuclease-free H2O, and 5 µL DNA template. The PCR conditions were as follows: initial denaturation at 98 °C for 3 min; 35 cycles at 98 °C for 10 s, 60 °C for 10 s and 72 °C for 60 s, and a 5-min final extension at 72 °C using a thermocycler (Bio-Rad CFX96TM Real-Time System, Bio-Rad Laboratories, Inc., Singapore). The amplification products were cleaned up using a QIAquick PCR Purification Kit (Qiagen, Germany) according to the manufacturer’s protocol and sequenced. Gene sequences were combined to create a concatenated sequence for MLSA. Concatenated sequence alignment was done using MUSCLE and corrected using MEGA 7.0 Software (Pennsylvania State University, PA, USA) [48]. The resultant tree topologies were also evaluated by bootstrap analysis based on 1000 replicates. The evolutionary distance was calculated using the Kimura two-parameter model for nucleotide sequences [49].
2.4. PCR-Based Screening for Secondary Metabolite Biosynthetic Genes
Specific degenerate primers used in this study were designed to detect conserved regions of ketosynthase (KS) and adenylation (AD) domains necessary for polyketides and non-ribosomal peptides, respectively using the primers shown in the Supplemental Table S2 [51,52,53,54,55,56]. PCR amplification using the genomic DNA was carried out in a final volume of 20 μL containing 10 μL SYBR green, 1 μL 10 µM forward and 1 μL 10 µM reverse primers, 1 μL DMSO, and 140 ng DNA template. Three reaction mixes were prepared for every DNA sample. To amplify biosynthetic genes targeting type I polyketide synthase β-ketoacyl synthase (KS) domain fragments, two sets of primers were used: KSMAF and KSMBR, and KSF and KSR [51,52]. For the PCR protocol using PKS-I KSMAF and KSMBR primers, the reaction started with an initial denaturation at 95 °C for 5 min, followed by 40 cycles at 95 °C for 1 min, 60 °C for 1 min and 72 °C for 2 min, a 5 min final extension at 72 °C using a thermocycler (Bio-Rad CFX96TM Real-Time System, Bio-Rad Laboratories, Inc., Singapore). Gene amplification using PKS-I KSF and KSR primers was executed as follows: initial denaturation at 95 °C for 15 min, one cycle of 95 °C for 1 min, 65 °C for 1 min and 72 °C for 1 min, followed by 35 cycles of 95 °C for 1 min, 62 °C for 1 min and 72 °C for 1 min, with a 10-min final extension at 72 °C.
PKS-II ketoacyl synthase domain fragments were amplified using three sets of primers: KS1F and KS1R; KSα and KSβ; 540F and 1100R [53,54,55]. PCR protocol in detecting PKS-II type of polyketides were as follows: initial denaturation at 95 °C for 5 min; 40 cycles at 95 °C for 1 min, 58 °C for 1 min and 72 °C for 2 min; and a 10-min final extension at 72 °C. Meanwhile, for the amplification of AD gene fragments, NRPS A3F and A7R primers were used [56]. The PCR condition included an initial denaturation of 95 °C for 5 min, followed by 40 cycles of 95 °C for 30 s and 59 °C for 1.5 min, and 72 °C for 1 min, with a 10 min final extension at 72 °C. The amplified PCR products were viewed in 2% agarose gel using a Quantum CX5 Edge Gel Doc automated gel imaging and documentation systems (Vilber Lourmat, Collégien, France).
2.5. Phenotypic Characterization
2.5.1. Salt Tolerance
Pure culture of strain DSD011 was inoculated in Marine Medium 1 (MM1) [19,24] prepared with artificial seawater varying in NaCl concentrations (0%, 3%, 5%, 7%, 10%, 12%, 15%, and 20%) [57]. The plates were incubated at room temperature (25 to 28 °C) for 7 days and checked periodically for the growth of actinomycetes. Detection of diffusible pigments, aerial, and substrate mycelia were observed and determined the optimal salt concentration that the strain could tolerate.
2.5.2. Carbon Utilization Test
Cells of strain DSD011 were inoculated unto five marine media containing different carbon sources: glucose (0.05%), mannitol (0.05%), raffinose (0.05%), soluble starch (1.0%), and trehalose (0.05%) in w/v. Thereafter, the plates were incubated at room temperature (25 to 28 °C) for 7 days. Morphological characteristics were identified according to aerial mycelium, mycelium substratum, and diffusible pigments. The medium having prolific growth of the strain was determined as the best medium to utilize a particular carbon source for growth development [58].
2.5.3. Scanning Electron Microscopy
The strain DSD011 was grown on marine medium 1 (MM1) broth at 25–28 °C for 14 days and spore morphology was observed using a scanning electron microscope (JEOL JSM 5510LV, JEOL Ltd., Tokyo, Japan). The sample was fixed using a 2.5% glutaraldehyde solution at 4 °C for 2 h. Cells were precipitated using poly-L-lysine in aluminum foil, oven-dried for 10 min and washed with PBS. The sample was dehydrated by a series of gradient exchange using ethanol solutions (30%, 50%, 85%, 95%, and 100%), and stored again at 4 °C for 10 min before adding t-butyl alcohol. The sample was freeze-dried at −20 °C for 10 min prior to mounting of samples in metal swab to remove ice crystals and avoid damage of cells. Once the sample was mounted by sputter coating, the metal stub was viewed at an accelerating voltage from 10 kV to 25 kV and intact spore structures were then selected for examination up to 15,000× magnification.
2.6. Cultivation and Extraction of Biomass
The strain DSD011 was inoculated and allowed to grow in the marine medium 1 (MM1) agar and broth according to the published method and protocols [19,24]. The MM1 agar inoculated with the strain was incubated for 14 days at 25–28 °C. After 14 days of incubation, the biomass was harvested. The agar with the cells was extracted three times with ethyl acetate. The extract was concentrated in vacuo using a rotary evaporator, dried in vacuum, and stored in −20 °C until tested for antibacterial assay and purified by chromatographic separations.
2.7. Antibacterial Assay
2.7.1. Multidrug-Resistant Staphylococcus aureus (MRSA)
The target pathogen used in this study was S. aureus ATCC BAA- 44. This is an Iberian clone of MRSA containing SCCmec type 1 gene and resistant to various types of antibiotics such as ampicillin, amoxicillin/clavulanic acid, ciprofloxacin, cephalothin, doxycycline, gentamicin, erythromycin, imipenem, methicillin, penicillin, tetracycline, oxacillin, azithromycin, clindamycin, ceftriaxone, rifampin, amikacin, and tobramycin (Staphylococcus aureus subsp. aureus (ATCC® BAA-44™) [59]. These characteristics make S. aureus ATCC BAA-44 an ideal target in searching for an antibiotic that can combat multidrug-resistant pathogens.
It has to be emphasized that although tetracycline was listed as one of the antibiotics with resistance against S. aureus ATCC BAA-44, this antibiotic was used as the positive control in all the antibacterial assays covered in this work. According to the protocol described in this work, the S. aureus ATCC BAA-44 has an MIC value of 31.25–50 μg/mL for tetracycline [24].
2.7.2. Disk Diffusion Assay of the Crude Extract
The fermented crude extract was tested for preliminary antibacterial screening against multidrug-resistant S. aureus ATCC BAA-44 (MRSA) [59]. Briefly, the bacterial cells of MRSA were adjusted to 1 × 106 CFU/mL and were flooded accordingly into Mueller Hinton Agar (MHA) plates. Negative control (10 µL MeOH), positive control (0.5 mg tetracycline), and crude extracts (5 mg) were then dispensed in 6 mm circular disks and impregnated into agar plates with the pathogen. The plates were incubated for 18–24 h at 37 °C before measuring the zone of inhibition in mm. The assay was done in triplicates.
2.7.3. Antibacterial Activity by Microbroth Susceptibility Assay
Antibacterial activity was confirmed using microbroth susceptibility assay against MRSA using the protocol and method of Dalisay et al. [19] and Paderog et al. [24]. Accordingly, a volume of 5 μL of 5 mg/mL extract concentration was dispensed into a 96 well plate and 195 μL of Mueller Hinton Broth (MHB) seeded with test pathogen with an optical density of 1 × 106 CFU/mL. Tetracycline (5 mg/mL) was used as a positive control, dimethyl sulfoxide (DMSO) as a negative control, and MHB as blank control. Plates were incubated for 18–24 h at 37 °C. The optical density was measured at 600 nm using an absorbance microplate reader (BioTekTM ELx808TM, Biotek, Winooski, Vermont, USA). The threshold used for an acceptable antibacterial activity was 50% growth inhibition. The assay was done in triplicates.
2.7.4. Minimum Inhibitory Concentration
Serial dilution of the extracts was carried out by two-fold dilution starting from an initial test concentration of 1000 µg/mL to 1.9 µg/mL (compound 1) and 125 µg/mL to 0.122 µg/mL (compound 2). DMSO and tetracycline (125 µg/mL) were used as negative and positive controls, respectively. The bacterial suspension (195 µL) with an optical density of 1 × 106 CFU/mL was added into the wells. The negative control used in this assay was DMSO and the positive control was tetracycline (125 to 0.122 µg/mL). The plates were incubated for 18–24 h at 37 °C. The optical density was measured at 600 nm using an absorbance microplate reader (BioTekTM ELx808TM, Biotek, Winooski, VT, USA). The last concentration showing more than 90% growth inhibition was considered as the MIC90 (μg/mL) of the extract [24,60]. The assay was done in triplicates.
2.8. Targeted Chromatographic Isolation
2.8.1. Gel Filtration Chromatography
Approximately 450 mg of ethyl acetate crude extract was dissolved in 4.0 mL MeOH and centrifuged prior to loading to a 3.4 cm × 38 cm column Sephadex® LH-20. A total of 21 batches of gel filtration chromatography were performed giving a total of 7055.9 mg crude extract purified in Sephadex® LH-20. The column was eluted with MeOH and the fractions were collected at 2 min intervals. Each fraction was subjected to TLC analysis using 9.5:0.5 DCM/MeOH as the mobile phase. The eluted fractions were pooled according to their TLC profiles as observed under UV (254 and 365 nm). A total of 12 final gel filtration chromatography fractions (G1–G12) were obtained and dried in vacuo for succeeding antibacterial testing.
2.8.2. Flash Column Chromatography
To purify the active fractions (i.e., G6, 506.1 mg and G7, 143.5 mg) from the gel filtration chromatography, Isolera® One (Biotage, Uppsala, Sweden) normal-phase flash column chromatography system was used. The antibacterial fraction, G6 (106.2 mg representing only 20% total yield of Fraction G6), was dissolved in 500 µL MeOH and was directly applied on a 1 g samplet, dried under vacuum for 20 min and packed in a 10 g SNAP KP-SIL cartridge. The column volume of the cartridge was 15 mL and was eluted with dichloromethane/methanol (DCM/MeOH) gradient (0% to 100%) at a flow rate of 36 mL/min. Each fraction was collected, pooled according to their TLC profiles at 254 and 365 nm, and dried in vacuo for succeeding antibacterial testing. The same methods and conditions were applied for the separation of G7 (114.5 mg loaded in the column). Each individual fraction was collected, pooled under the absorbance range of 254 and 365 nm, and dried in vacuo for succeeding antibacterial screening.
2.8.3. High-Performance Liquid Chromatography (HPLC)
The antibacterial fractions from Section 2.8.2 (i.e., G6I5, 17 mg; G7I3-6, 38.1 mg) were purified by reversed-phase HPLC (Shimadzu Prominence) equipped with a photodiode array detector. For Fraction G6I5, the purification was performed using a reversed-phase C18 column (Phenomenex SynergiTM Hydro-RP, 250 mm × 10 mm, 10 μm, 80 Å). The mobile phase used was water/methanol (H2O/MeOH) in gradient at a flow rate of 5 mL/min from 0% to 100% MeOH. Compound 1 (4.0 mg, tR = 20 min) was obtained under these conditions. Similarly, Fraction G7I3-6 was purified using the same column as above but was eluted with water/acetonitrile (H2O/ACN) in gradient at a flow rate of 5 mL/min from 0% to 100% ACN to yield a semi-pure fraction, which was further purified using the same conditions in H2O/ACN in gradient at a flow rate of 3 mL/min from 20% water to 100% ACN to afford compound 2 (1.2 mg, tR = 14 min).
2.9. LCMS, MS/MS and Global Natural Product Social Molecular Networking (GNPS)
LCMS analyses were performed using a high-resolution mass spectrometer (Waters Xevo® G2-XS QTOF) equipped with StepWaveTM ion source technology, XS collision cell technology, QuanTOFTM technology, UPLC/TOFM® for high transmission mode, and UPLC/MSe for data acquisition. The stationary phase used was a Waters 100 Å, 1.8 µm, 1 × 150 mm column. Samples were reconstituted to 0.15 mg/mL and an optimized injection volume of 2 μL was adopted to avoid column overloading. The solvent system was water with 0.1% (v/v) formic acid (solvent A) and ACN with 0.1% (v/v) formic acid (solvent B). A flow rate of 0.22 mL/min was used with a column temperature of 50 °C. The interface used for MSe was electron spray ionization (ESI) with a collision energy of 6.0 eV for low energy and 10 to 50 eV for high energy ramp. The scan time was set to 0.250 s using polarity positive or negative mode with a mass range scan of m/z 50–1500. MS data were analyzed using Waters UNIFI Scientific Information System R and ChemspiderTM Database.
Mass spectra for the GNPS MASST Search tool were acquired on a ShimadzuTM LCMS-TQ 8045 instrument equipped with a Prominence HPLC system (Shimadzu Corporation, Japan) (SIL-20A HT autosampler, LC-20AD pump system, SDP-M20A UV Vis Detector) using Phenomenex SynergyTM column (particle size: 4 μm, diameter: 100.0 × 2.0 mm) in a gradient elution system from 100% water (solvent A) to 100 % ACN (solvent B) in 0.1% formic acid. The sample load was 5 μg of dried HPLC fractions. The instrument parameters for the elution of extract was performed in a solvent flow rate of 0.4 mL/min and with oven temperature at 40 °C, starting with 100% solvent A at 0.01 to 0.5 min, gradually increasing to 100% solvent B at 21 min to 26 min, then back to 100% solvent A at 28 min to 30 min. Data acquisition was stopped at 30 min. The interface used for MS was electron spray ionization (ESI). The block temperature was 400 °C, and DL temperature was 250 °C. The nebulizing gas flow rate was 3.0 L/min nitrogen while the drying gas flow rate was 10.0 L/min nitrogen. The event time was 0.1 s at Q1 scan positive mode and Q3 scan negative mode. The masses were analyzed in both positive and negative ion modes with range m/z 200–1500. MS/MS experiments were performed on the peaks of interest at 35 V collision energy.
A library search was performed using the online workflow at GNPS through MASST Search Tool (University of California, San Diego, San Diego, CA, USA) [61]. A single MS/MS spectrum was matched against all public spectral libraries that includes peptides, lipids, small molecules, and pharmacologically active natural products. The spectral match is required to have a parent mass tolerance of 2.0 Da, ion tolerance of 1.0 Da, score threshold of 0.59, and at least 4 matched peaks.
3. Results and Discussion
3.1. Isolation and Phenotypic Characterization of Actinomycete Strain DSD011
Actinomycete strain DSD011 was isolated from the marine sediments collected in the coast of Uaydahon Island (11°38′14.5″ N, 123°22′18.5″ E) in the Islas de Gigantes group of islands (Figure 1a). The strain was recovered in ISP4 marine media using the dry stamp method (DSM). The colony on MM1 agar at 14 days of incubation exhibited a tough, leathery, and filamentous colony with diffusible reddish-brown pigmentation (Figure 1b). The colony produced a red-brown substrate mycelium with branched hyphae (between 0.5–1.0 µm in diameter) (Figure 1c) grey aerial mycelium which differentiated into chains of smooth-surfaced spores (between 0.8 to 1 µm in diameter) (Figure 1d). These observed morphological features of strain DSD011 are diagnostic characteristics and typical morphological properties of the genus Streptomyces.
The strain DSD011 exhibited good growth in glucose and soluble starch and exhibited moderate growth in mannitol, trehalose, and raffinose as carbon sources (Table 1). Good growth of strain DSD011 occurred with NaCl concentrations of 0–5% (w/v) and moderate growth at 7% NaCl (Table 2). The morphological characteristics of strain DSD011 were affected by the culture conditions that led to the development of different substrate mycelium, aerial mycelium formation, and pigments. After 7 days of growth in a NaCl enriched medium, strain DSD011 produced white aerial mycelium and brownish substrate mycelium (Supplemental Table S3). A series of yellow to brown, light brown, and white substrate mycelium was observed in the carbon source utilization test. Sporulating aerial mycelium was white and gray in mannitol and glucose, respectively, while a yellowish-brown substrate mycelium was observed in starch. Interestingly, raffinose, soluble starch, and glucose did not produce aerial mycelia and diffusible pigment. Instead, white, light brown, and yellow substrate mycelia were observed, respectively (Supplemental Table S4).

Table 1.
Growth of Streptomyces sp. strain DSD011 in various carbon sources (% w/v).

Table 2.
Growth of Streptomyces sp. strain DSD011 in various NaCl concentrations (% w/v).
The fermentation crude extract of strain DSD011 showed activity against SCCmec type 1-harboring MRSA strain [59] with a 17 mm zone of inhibition at 5 mg/disk concentration (Supplemental Table S5). MRSA gives rise to a variety of infections acquired in community and hospital settings and affects vulnerable populations. Treatment of MRSA infections is difficult due to the limited range of effective antibiotics; hence it poses a significant health threat [62]. This indicates that new antibiotic leads are needed to overcome MRSA treatment challenges.
3.2. Genomic Characterization and Phylogenetic Analysis of Antibiotic Producing Actinomycete Strain DSD011
The molecular identification of strain DSD011 was performed with the sequence of the small subunit rRNA, i.e., 16S rRNA as a genetic marker. The phylogenetic analysis of the nearly complete 16S rRNA gene sequence (1401 bp) led to the identification of strain DSD011 (GenBank Accession No. MT820508), which is related to type strains assigned in the genus Streptomyces hence denoted as Streptomyces sp. strain DSD011. BLAST analysis revealed the highest 16S rRNA gene sequence similarity to Streptomyces cellulosae strain NBRC 13027 (99.93%, GenBank NR_112346.1) and Streptomyces pseudogriseolus strain NRRL B-3288 (99.79%, GenBank NR_043835.1). Both of these strains are terrestrial in origin. The related species of Streptomyces sp. strain DSD011 in the GenBank was used to create a phylogenetic tree (Figure 2) to visualize the relatedness of the species with the level of confidence calculated from 1000 replicates each for bootstrap analysis using the maximum-likelihood method [63]. The analysis revealed that the 16S rRNA gene sequence has a mean pairwise evolutionary distance of 0.037. It also revealed that the Streptomyces sp. strain DSD011 formed a distinct lineage among the most closely related species: Streptomyces cellulosae strain NBRC 13027 (99.93%, GenBank NR_112346.1) and Streptomyces cellulosae strain NRRL B-2889 (99.93%, GenBank NR_043815.1). However, it showed low bootstrap values indicating less significant separation of Streptomyces sp. strain DSD011 and related species.
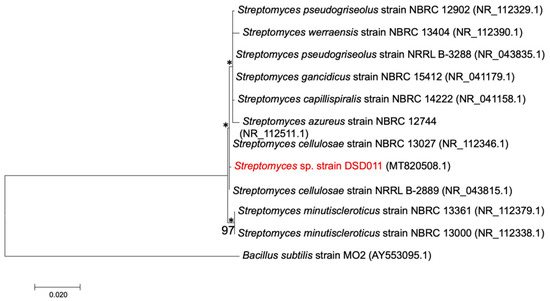
Figure 2.
Maximum-likelihood phylogenetic tree based in the nearly complete 16S rRNA gene sequences (1401 bp) of strain DSD011 showing the relationship with members of the genus Streptomyces with homology values of more than 99%. Bacillus subtilis strain MO2 (AY553095.1) was used as an outgroup. Asterisks denote branches that were also recovered using the neighbor-joining method. Bootstrap values above 50% (based on 1000 replications) are shown in the branch points. Bar, 0.020 substitutions per nucleotide position.
The close relationships between Streptomyces sp. strain DSD011 with Streptomyces cellulosae strain NBRC 13027 and Streptomyces cellulosae strain NRRL B-2889 were further confirmed by the multi-locus sequence analysis (MLSA). While most of the recent studies on MLSA analysis of Streptomyces species are based on five housekeeping genes—atpD (ATP synthase F1, beta subunit), gyrB (DNA gyrB subunit), recA (recombinase A), rpoB (RNA polymerase beta subunit), and trpB (tryptophane B, beta subunit) [64,65,66], our MLSA work covers four housekeeping genes: atpD, recA, rpoB, and trpB, which are also useful in studying the systematics of Streptomyces species [67]. We tested gyrB in strain DSD011, but a well-amplified product was not obtained; hence this gene was not included in the study. All the partial gene sequences of four housekeeping genes, atpD, recA, rpoB, and trpB that were obtained in this study were deposited in Genbank with accession numbers: MT844068, MT844069, MT844070, MT844071.
The concatenation of the four gene sequences (atpD, recA, rpoB, and trpB) yielded an alignment of 3567 nt. A concatenated MLSA tree (Figure 3) was constructed based on 1000 replicates each for bootstrap analysis using Kimura’s maximum-likelihood method [63]. The concatenated alignment of the four housekeeping genes has a mean pairwise evolutionary distance of 0.240 within the phylogenetic tree. MLSA provides an improved framework and a more stable topological structure supported by high bootstrap values ranging from 62–100%. The Streptomyces sp. strain DSD011 notably separated itself from its nearest lineage as indicated by the evolutionary distance value of 0.015 and 0.014 to its close neighboring species, Streptomyces sp. WAC 01438 (98.82% percent identity) and Streptomyces variabilis strain ARRS001 (99.57% percent identity), respectively. This result is consistent with the MLSA evolutionary genetic distances, which showed that nucleotide sequence distance with ≥0.007 value should retain the species status [64]. Our MLSA analysis showed that Streptomyces sp. strain DSD011 diverged from the related species on the database and showed good discriminatory ability to classify Streptomyces sp. strain DSD011 and related species into a more resolved separation on the phylogenetic tree, thus indicating that the strain DSD011 formed a new Streptomyces species.
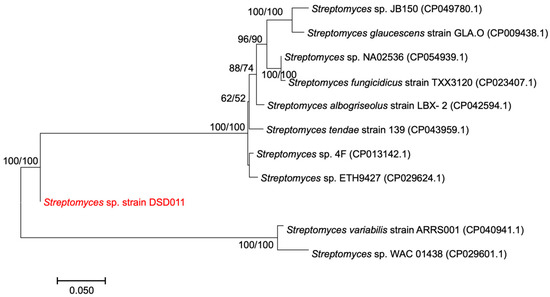
Figure 3.
Maximum-likelihood phylogenetic tree based on concatenated sequences of four housekeeping genes, atpD, recA, rpoB, and trpB, showing the phylogenetic relationship of strain DSD011 and its relative within the genus Streptomyces. Bootstrap values above 50% (based on 1000 replications) are shown in the branch points for maximum-likelihood (left) and maximum-parsimony (right) analyses. Bar, 0.050 substitutions per nucleotide position.
In this study, we have demonstrated that the 16S rRNA phylogeny along with multi-locus analysis (MLSA) of four housekeeping genes (atpD, recA, rpoB, and trpB) provided evidence to examine fine-scale interrelationships of Streptomyces sp. strain DSD011 and its reference strains match in the GenBank. Considered as a highly conserved gene with a minimal rate of nucleotide changes, 16S rRNA as a sole basis for bacterial phylogeny is weak and has a low differentiation power when dealing with closely-related species [68]. The MLSA used in this study are universal genes that serve as alternatives to 16S rRNA by using individually or concatenated analyzed sequences [64]. The phylogenetic analysis constructed based upon the 16S rRNA gene sequences and concatenated MLSA revealed differences between the phylogenetically coherent branches of Streptomyces sp. strain DSD011 and its reference matches. The Streptomyces sp. strain DSD011 was closely related to Streptomyces cellulosae strain NBRC 13027 (99.93% percent identity) based on the 16S rRNA phylogeny, as compared to that of the MLSA tree where Streptomyces variabilis strain ARRS001 (99.57% percent identity) was observed to be the closest relative. Moreover, individual housekeeping gene phylogenies demonstrated slight dissimilarities where the Streptomyces sp. strain DSD011 share close affinity with Streptomyces sp. 4F (98–99% percent identity) for genes associated with atpD, recA and rpoB, but was more affiliated only with Streptomyces variabilis strain ARRS001 (96.8% percent identity) for trpB gene. Thus, the incongruent results of two phylogenetic analyses indicate that Streptomyces sp. strain DSD011 may represent a new taxon.
To note, the closely related species of Streptomyces sp. strain DSD011 are terrestrial in origin. More interestingly, one related strain, Streptomyces sp. strain 4F, was isolated from the highly saline environment in the Great Salt Plains, Oklahoma [69]. It is possible that Streptomyces sp. strain DSD011 originated from terrestrial soil and its spores are washed in the marine environment and reside in marine sediments as dormant spores. It is noteworthy that Streptomyces sp. strain DSD011 grew well at 0 to 5% NaCl and showed moderate growth at 7% NaCl as shown in Table 2, suggesting that the strain developed growth adaptation in the marine environment and has evolved as a salt-tolerant Streptomyces.
3.3. Screening for Polyketide Synthase (PKS) and Non-Ribosomal Peptide Synthetase (NRPS) Secondary Metabolite Biosynthetic Genes
As major producers of natural products, Streptomyces are often surveyed for the presence, abundance, and novelty of PKS and NRPS genes to evaluate its potential as secondary metabolite producers. To determine the presence of type I and type II PKS and NRPS biosynthetic genes in Streptomyces sp. strain DSD011, we screened its genome by targeting the adenylation (AD), type I ketoacyl synthase (KS), and KSα and KSβ domains, respectively using PCR amplification approach [51,52,53,54,55,56]. The biosynthetic genes were considered present when a strong unambiguous amplicon could be observed in the agarose gel.
Our results revealed that Streptomyces sp. strain DSD011 harbors both NRPS and type II PKS in its genome. The presence of a distinct band at 700 base pair (bp) size indicated positive amplification of AD of NRPS. For type II PKS gene clusters, three sets of degenerate primers targeting different conserved regions of KSα and KSβ domain were used to screen for its presence: (1) KS1-F and KS1-R (with 613 bp PCR product) that target the KSα domain; (2) KSα and KSβ (with 800–900 bp PCR product) that target the KSα and KSβ domain complex; and (3) 540F and 1100R (with 554 bp PCR product) that target the 5’ of KSα domain. PCR screening showed that Streptomyces sp. strain DSD011 harbors all target regions for the type II PKS gene as indicated by the distinct bands with an amplicon size of 554–700 bp (Figure 4). Interestingly, Streptomyces sp. strain DSD011 harbors type II PKS genes targeting the KSα and KSβ domain complex at 600–700 bp, which is in contrast to the reported 800–900 expected length of target region [53]. Conversely, Streptomyces sp. strain DSD011 does not harbor type I PKS in this PCR screening as supported by the absence of distinct amplicon bands in 613 bp for the two target conserved sequences in the KS domain (Figure 4).
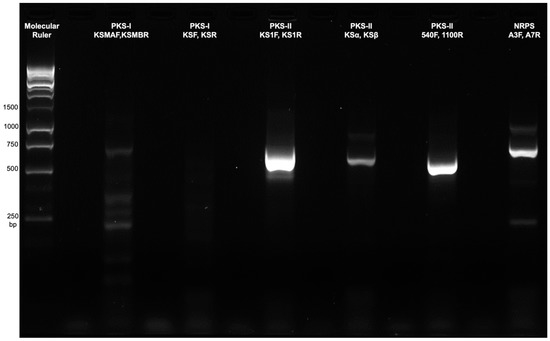
Figure 4.
Amplified PCR products of Streptomyces sp. strain DSD011 using degenerate primers to detect conserved regions of types I and II polyketide synthetase (PKS) and non-ribosomal peptide synthase (NRPS) domains.
Here, the PCR detected type II PKS gene of ketosynthase domains in Streptomyces sp. strain DSD011 indicates a high possibility of aromatic polyketides potentially produced by the strain. Notably, contrary to the expected length, KSα and KSβ gene fragment were amplified at 600–700 bp suggesting that type II PKS KSα domains were abundant in Streptomyces strain sp. DSD011 which can be associated with complex and diverse type II PKS compounds. The type II PKSs are mainly responsible for producing polycyclic aromatic polyketides compounds harboring at least one aromatic ring [70]. These compounds are an important type of natural product because of their potential pharmaceutical applications such as antibacterial, anticancer, and antiviral activities [71]. Although type II PKS and NRPS are the major enzymes for secondary metabolite biosynthesis found in Streptomyces sp. strain DSD011, their presence does not indicate expression or functionality but rather increases the likelihood of producing bioactive polyketides and non-ribosomal peptide compounds.
3.4. Isolation and Identification of Antibiotic Compounds 1 and 2
To identify the structures of the antibacterial compounds from Streptomyces sp. strain DSD011, the crude extract was fractionated by series of chromatography including gel filtration chromatography, flash chromatography, and semi-preparative reversed-phase HPLC, which afforded two pure compounds with UV activity at 254 nm. QTOF-MS and MS/MS data in combination with GNPS analysis revealed molecular ion peaks at m/z 487.1606 [M + H]+ (predicted molecular formula: C25H27O10, calc. mass = 487.1604, DBE = 13) and 597.1977 [M + H]+ (predicted molecular formula: C31H33O12, calc. mass: 597.1972, DBE = 16) that correspond to compounds 1, fridamycin A (Figure 5, Supplemental Table S6 and S7) and 2, fridamycin D (Figure 6, Supplemental Tables S8 and S9), respectively. Compounds 1 and 2 displayed antibacterial activity against multidrug-resistant S. aureus ATCC BAA-44 with MIC of 500 and 62.5 μg/mL, respectively (MIC of tetracycline = 31.25 μg/mL).
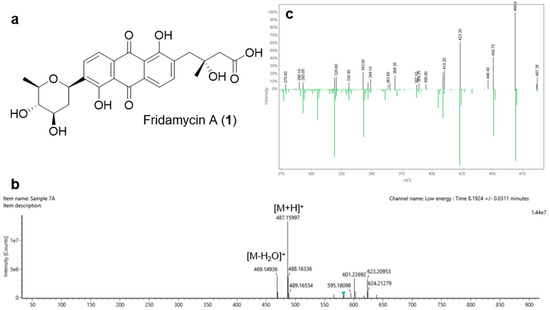
Figure 5.
(a) Chemical structure of the angucycline glycoside fridamycin A (1); (b) HRMS spectrum of 1; (c) Global Natural Product Social molecular networking (GNPS) mass mirror view of MS/MS from experimental data and GNPS Library Spectrum (bottom spectrum) that are associated to the structure of 1.
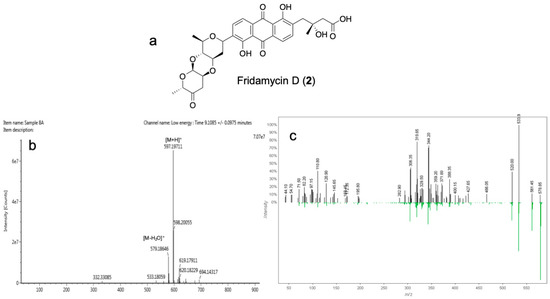
Figure 6.
(a) Chemical structure of the angucycline glycoside fridamycin D (2); (b) HRMS spectrum of 2; and (c) GNPS mass mirror view of MS/MS from experimental data and GNPS Library Spectrum (bottom spectrum) that are associated to the structure of 2.
In this work, we isolated two angucycline glycoside antibiotics, fridamycin A (1) and fridamycin D (2) that belongs to the angucycline class of natural products—the largest group of polycyclic aromatic polyketides produced by actinomycetes, mostly from Streptomyces species. These compounds are known to possess diverse pharmacological activities including cytotoxicity and antitumor antibacterial and antiviral properties [72,73]. Structurally, the angucycline compounds have a rich core structure that contains an angular benz[a]anthracene moiety that is glycosylated [72,73]. Fridamycin A (1) and fridamycin D (2) are non-classic congeners of glycosylated angucycline compounds with rearranged linear tetracyclic or tricyclic core units [72]. The biosynthetic origin of all angucyclines are polyketide derived, with type II polyketide synthases (PKS II) responsible for the initial decaketide backbone which is followed by ring closure reactions to form the benz[a]anthracene scaffold [73]. The isolation of 1 and 2 in this work is supported by the results on PKS screening, wherein Streptomyces sp. strain DSD011 showed unambiguous amplicon of type II PKS genes.
While older studies report that terrestrial Streptomyces produces angucycline glycosides, recent reports have shown that marine sediment-derived Streptomyces are also prolific producers of angucycline natural products [9,74,75,76,77]. Numerous angucycline compounds along with 1 and 2 that were isolated from marine sediment-derived Streptomyces were found to be cytotoxic against breast cancer cells (MCF-7, MDA-MB-231, and BT-474) [76,77], Jurkat cells [75], and hepatoma carcinoma cells (HepG-2, SMMC-7721, and plc-prf-5) [9,77]. Our work demonstrates for the first time that compounds 1 and 2 from marine sediment-derived Streptomyces sp. are antibacterial against multidrug-resistant S. aureus harboring the SCCmec gene, which is a biomarker of drug resistance. While the mode of action of angucycline glycosides as an anticancer is known for its DNA cleaving activity [73], there are no reports that describe the mechanism of their antibacterial activity. Hence, the promising antibacterial activities of compounds 1 and 2 against multidrug-resistant S. aureus warrant more research to investigate their antibacterial mechanism of action.
4. Conclusions
In conclusion, we have isolated and identified the Streptomyces sp. strain DSD011 from marine sediments of Islas de Gigantes, Iloilo, Philippines that possesses antibacterial property against multidrug-resistant S. aureus carrying the antibiotic drug resistance marker SCCmec type I gene. The Streptomyces sp. strain DSD011 is a new species of salt-tolerant marine Streptomyces and harbors type II PKS gene clusters responsible for the production of two polycyclic aromatic polyketide angucycline antibiotics, fridamycin A (1) and fridamycin D (2). This study demonstrates that marine sediments in the Philippine archipelago contain new or novel Streptomyces species that produce antibiotics. Further work that includes genomics for mining of novel biosynthetic genes and metabolomics for the detection of new chemical scaffolds will be needed for future studies.
Supplementary Materials
The following are available online at https://www.mdpi.com/2077-1312/8/10/734/s1. Supplemental Table S1. Primers for Multilocus Sequence Analysis [50]. Supplemental Table S2. Degenerate Primers for PCR Screening of Polyketide Synthase and Non-Ribosomal Synthetase. Supplemental Table S3. Growth Characteristics of Streptomyces sp. strain DSD011 in various NaCl concentrations. Supplemental Table S4. Growth Characteristics of Streptomyces sp. strain DSD011 in various carbon sources (% w/v) after 7 days of incubation. Supplemental Table S5. Antibacterial Activity of strain DSD011 crude fermented extract. Supplemental Table S6. Latest Library Spectrum Information on GNPS match for fridamycin A, 1 (m/z 487 [M + H]+). Supplemental Table S7. Waters UNIFI Scientific Information System Report for fridamycin A, 1 (m/z 487 [M + H]+). Supplemental Table S8. Latest Library Spectrum Information on GNPS match for fridamycin D, 2 (m/z 597 [M + H]+). Supplemental Table S9. Waters UNIFI Scientific Information System Report for fridamycin D, 2 (m/z 597 [M + H]+).
Author Contributions
Conceptualization, J.P.S. and D.S.D.; methodology, E.M.S., A.F.L.S., C.P.T., D.J.V.L.T., S.D.C.O., D.S.A., J.E.E.J., A.M.Q.R., I.G.M.V., L.D.D.A., C.F.S., C.A.V., J.P.S., and D.S.D.; software, E.M.S., A.F.L.S., C.P.T., and D.J.V.L.T.; validation, E.M.S., A.F.L.S., C.P.T., D.J.V.L.T., D.S.A., J.E.E.J., A.M.Q.R., I.G.M.V., L.D.D.A., C.F.S., C.A.V., J.P.S., and D.S.D.; formal analysis, E.M.S., A.F.L.S., C.P.T., D.J.V.L.T., S.D.C.O., D.S.A., J.E.E.J., A.M.Q.R., I.G.M.V., L.D.D.A., C.F.S., C.A.V., J.P.S., and D.S.D.; investigation, E.M.S., A.F.L.S., C.P.T., D.J.V.L.T., S.D.C.O., D.S.A., J.E.E.J., A.M.Q.R., I.G.M.V., L.D.D.A., C.F.S., C.A.V., J.P.S., and D.S.D.; resources, J.P.S. and D.S.D.; data curation, J.P.S., D.S.D., E.M.S., A.F.L.S., C.P.T., D.J.V.L.T., and D.S.A.; writing—original draft preparation, J.P.S., D.S.D., E.M.S., A.F.L.S., C.P.T., D.J.V.L.T., and D.S.A.; writing—review and editing, E.M.S., A.F.L.S., C.P.T., D.J.V.L.T., S.D.C.O., D.S.A., J.E.E.J., A.M.Q.R., I.G.M.V., L.D.D.A., C.F.S., C.A.V., J.P.S., and D.S.D.; visualization, E.M.S., A.F.L.S., C.P.T., D.J.V.L.T., J.P.S., and D.S.D.; supervision, J.P.S. and D.S.D.; project administration, J.P.S. and D.S.D.; funding acquisition, J.P.S. and D.S.D. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the Tuklas Lunas Development Center Program of the Department of Science and Technology—Philippine Council for Health Research and Development (DOST-PCHRD) and the University of San Agustin Professorial Chair Grant.
Acknowledgments
The authors acknowledge the invaluable support of Julius Tagomata and Cecilio Gonzales for collecting the marine sediment samples, and the local government of Islas de Gigantes, Carles, Iloilo, Philippines for the permission. In addition, Dalisay and Saludes wish to acknowledge the Balik Scientist Program of the Philippines Department of Science and Technology (DOST), through the Philippine Council for Health Research and Development (PCHRD), for the opportunity to serve the Filipino community through science, technology, and innovation. The Balik (Filipino word for Returning) Scientist Program (BSP) seeks highly trained Filipino scientists, technologists, experts, and professionals residing abroad to return to the Philippines and transfer their expertise to the local community for the acceleration of scientific, agro-industrial, and economic development of the country. Special thanks to Hannah Valencia, Abigael Quiachon, and Jasmine Velo for the technical support; Suzanne Ynion for administrative support; and Yzabel Layson for editing the manuscript. The authors thank Zhen Jie Low of Waters Pacific Pte Ltd., Singapore, Singapore for the QTOF-MS service; Ma. Wennie Pauline C. Caelian of SEAFDEC, Tigbauan, Iloilo for the SEM service; and Stephen Sabinay of WVSU Biotech Research and Learning Center for the use of gel doc imaging and documentation systems. The authors wish to thank the Research Information, Communication, and Utilization Division (RICUD) of DOST-PCHRD for supporting the publication of this manuscript.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Singh, S.; Thumar, J.; Gohel, S.; Kikani, B.; Shukla, R.; Sharma, A.; Dangar, K. Actinomycetes from marine habitats and their enzymatic potential. Mar. Enzym. Biocatal. Sources Biocatal. Charact. Bioprocesses Mar. Enzym. 2013, 191–214. [Google Scholar] [CrossRef]
- Tamelander, T.; Spilling, K.; Winder, M. Organic matter export to the seafloor in the Baltic Sea: Drivers of change and future projections. Ambio 2017, 46, 842–851. [Google Scholar] [CrossRef]
- Whitman, W.B.; Coleman, D.C.; Wiebe, W.J. Prokaryotes: The unseen majority. Proc. Natl. Acad. Sci. USA 1998, 95, 6578–6583. [Google Scholar] [CrossRef]
- Morono, Y.; Ito, M.; Hoshino, T.; Terada, T.; Hori, T.; Ikehara, M.; D’Hondt, S.; Inagaki, F. Aerobic microbial life persists in oxic marine sediment as old as 101.5 million years. Nat. Commun. 2020, 11, 3626. [Google Scholar] [CrossRef]
- Bar-On, Y.M.; Phillips, R.; Milo, R. The biomass distribution on Earth. Proc. Natl. Acad. Sci. USA 2018, 115, 6506–6511. [Google Scholar] [CrossRef]
- Kallmeyer, J.; Pockalny, R.; Adhikari, R.R.; Smith, D.C.; D’Hondt, S. Global distribution of microbial abundance and biomass in subseafloor sediment. Proc. Natl. Acad. Sci. USA 2012, 109, 16213–16216. [Google Scholar] [CrossRef] [PubMed]
- Jose, P.A.; Jha, B. Intertidal marine sediment harbours actinobacteria with promising bioactive and biosynthetic potential. Sci. Rep. 2017, 7, 10041. [Google Scholar] [CrossRef] [PubMed]
- Jensen, P.R.; Dwight, R.; Fenical, W. Distribution of actinomycetes in near-shore tropical marine sediments. Appl. Environ. Microbiol. 1991, 57, 1102–1108. [Google Scholar] [CrossRef] [PubMed]
- Peng, A.; Qu, X.; Liu, F.; Li, X.; Li, E.; Xie, W. Angucycline glycosides from an intertidal sediments strain streptomyces sp. and their cytotoxic activity against hepatoma carcinoma cells. Mar. Drugs 2018, 16, 470. [Google Scholar] [CrossRef] [PubMed]
- Prieto-Davó, A.; Villarreal-Gómez, L.J.; Forschner-Dancause, S.; Bull, A.T.; Stach, J.E.; Smith, D.C.; Rowley, D.C.; Jensen, P.R. Targeted search for actinomycetes from nearshore and deep-sea marine sediments. FEMS Microbiol. Ecol. 2013, 84, 510–518. [Google Scholar] [CrossRef]
- Wagner, M.; Abdel-Mageed, W.M.; Ebel, R.; Bull, A.T.; Goodfellow, M.; Fiedler, H.P.; Jaspars, M. Dermacozines H-J isolated from a deep-sea strain of Dermacoccus abyssi from Mariana Trench sediments. J. Nat. Prod. 2014, 77, 416–420. [Google Scholar] [CrossRef] [PubMed]
- Meena, B.; Anburajan, L.; Vinithkumar, N.V.; Kirubagaran, R.; Dharani, G. Biodiversity and antibacterial potential of cultivable halophilic actinobacteria from the deep sea sediments of active volcanic Barren Island. Microb. Pathog. 2019, 132, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Stach, J.E.; Maldonado, L.A.; Masson, D.G.; Ward, A.C.; Goodfellow, M.; Bull, A.T. Statistical approaches for estimating actinobacterial diversity in marine sediments. Appl. Environ. Microbiol. 2003, 69, 6189–6200. [Google Scholar] [CrossRef] [PubMed]
- Barka, E.A.; Vatsa, P.; Sanchez, L.; Gaveau-Vaillant, N.; Jacquard, C.; Meier-Kolthoff, J.P.; Klenk, H.P.; Clément, C.; Ouhdouch, Y.; van Wezel, G.P. Taxonomy, physiology, and natural products of Actinobacteria. Microbiol. Mol. Biol. Rev. 2016, 80, 1–43. [Google Scholar] [CrossRef] [PubMed]
- Mayfield, C.I.; Williams, S.T.; Ruddick, S.M.; Hatfield, H.L. Studies on the ecology of actinomycetes in soil IV. Observations on the form and growth of Streptomycetes in soil. Soil Biol. Biochem. 1972, 4, 79–91. [Google Scholar] [CrossRef]
- Cross, T. Aquatic actinomycetes: A critical survey of the occurrence, growth and role of actinomycetes in aquatic habitats. J. Appl. Bacteriol. 1981, 50, 397–423. [Google Scholar] [CrossRef] [PubMed]
- Soliev, A.B.; Hosokawa, K.; Enomoto, K. Bioactive pigments from marine bacteria: Applications and physiological roles. Evid. Based Complement. Alternat. Med. 2011, 2011, 670349. [Google Scholar] [CrossRef] [PubMed]
- Hassan, S.S.U.; Shaikh, A.L. Marine actinobacteria as a drug treasure house. Biomed. Pharmacother. 2017, 87, 46–57. [Google Scholar] [CrossRef]
- Dalisay, D.S.; Williams, D.E.; Wang, X.L.; Centko, R.; Chen, J.; Andersen, R.J. Marine sediment-derived Streptomyces bacteria from British Columbia, Canada are a promising microbiota resource for the discovery of antimicrobial natural products. PLoS ONE 2013, 8, e77078. [Google Scholar] [CrossRef]
- Valli, S.; Suvathi, S.S.; Aysha, O.S.; Nirmala, P.; Vinoth, K.P.; Reena, A. Antimicrobial potential of Actinomycetes species isolated from marine environment. Asian Pac. J. Trop. Biomed. 2012, 2, 469–473. [Google Scholar] [CrossRef]
- Manivasagan, P.; Kang, K.H.; Sivakumar, K.; Li-Chan, E.C.; Oh, H.M.; Kim, S.K. Marine actinobacteria: An important source of bioactive natural products. Environ. Toxicol. Pharmacol. 2014, 38, 172–188. [Google Scholar] [CrossRef] [PubMed]
- Claverías, F.P.; Undabarrena, A.; González, M.; Seeger, M.; Cámara, B. Culturable diversity and antimicrobial activity of Actinobacteria from marine sediments in Valparaíso bay, Chile. Front. Microbiol. 2015, 6, 737. [Google Scholar] [CrossRef] [PubMed]
- Velasco-Alzate, K.Y.; Bauermeister, A.; Tangerina, M.M.P.; Lotufo, T.M.C.; Ferreira, M.J.P.; Jimenez, P.C.; Padilla, G.; Lopes, N.P.; Costa-Lotufo, L.V. Marine bacteria from Rocas Atoll as a rich source of pharmacologically active compounds. Mar. Drugs 2019, 17, 671. [Google Scholar] [CrossRef] [PubMed]
- Paderog, M.J.V.; Suarez, A.F.L.; Sabido, E.M.; Low, Z.J.; Saludes, J.P.; Dalisay, D.S. Anthracycline shunt metabolites from Philippine marine sediment-derived Streptomyces destroy cell membrane integrity of multidrug-resistant Staphylococcus aureus. Front. Microbiol. 2020, 11, 743. [Google Scholar] [CrossRef]
- Stincone, P.; Brandelli, A. Marine bacteria as source of antimicrobial compounds. Crit. Rev. Biotechnol. 2020, 40, 306–319. [Google Scholar] [CrossRef]
- Ibrahimi, M.; Korichi, W.; Hafidi, M.; Lemee, L.; Ouhdouch, Y.; Loqman, S. Marine Actinobacteria: Screening for predation leads to the discovery of potential new drugs against multidrug-resistant bacteria. Antibiotics 2020, 9, 91. [Google Scholar] [CrossRef]
- Kim, M.C.; Cullum, R.; Hebishy, A.M.S.; Mohamed, H.A.; Faraag, A.H.I.; Salah, N.M.; Abdelfattah, M.S.; Fenical, W. Mersaquinone, a new tetracene derivative from the marine-derived Streptomyces sp. EG1 exhibiting activity against methicillin-resistant Staphylococcus aureus (MRSA). Antibiotics 2020, 9, 252. [Google Scholar] [CrossRef]
- Elmallah, M.I.Y.; Cogo, S.; Constantinescu, A.A.; Elifio-Esposito, S.; Abdelfattah, M.S.; Micheau, O. Marine Actinomycetes-derived secondary metabolites overcome trail-resistance via the intrinsic pathway through downregulation of survivin and XIAP. Cells 2020, 9, 1760. [Google Scholar] [CrossRef]
- Williams, D.E.; Dalisay, D.S.; Chen, J.; Polishchuck, E.A.; Patrick, B.O.; Narula, G.; Ko, M.; Av-Gay, Y.; Li, H.; Magarvey, N.; et al. Aminorifamycins and sporalactams produced in culture by a micromonospora sp. isolated from a northeastern-pacific marine sediment are potent antibiotics. Org. Lett. 2017, 19, 766–769. [Google Scholar] [CrossRef]
- Liang, L.; Haltli, B.A.; Kerr, R.G. Draft genome sequence of Streptomyces sp. strain Rknd-216, an antibiotic producer isolated from marine sediment in Prince Edward Island, Canada. Microbiol. Resour. Announc. 2019, 8, e00870–e00919. [Google Scholar] [CrossRef]
- Sproule, A.; Correa, H.; Decken, A.; Haltli, B.; Berrué, F.; Overy, D.P.; Kerr, R.G. Terrosamycins A and B, bioactive polyether ionophores from Streptomyces sp. RKND004 from Prince Edward Island sediment. Mar. Drugs 2019, 17, 347. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Wang, Y.; Yang, Y.; Chen, H. Shellmycin A-D, Novel Bioactive Tetrahydroanthra-γ-pyrone antibiotics from marine Streptomyces sp. Shell-016. Mar. Drugs 2020, 18, 58. [Google Scholar] [CrossRef]
- Zhou, W.; Fang, H.; Wu, Q.; Wang, X.; Liu, R.; Li, F.; Xiao, J.; Yuan, L.; Zhou, Z.; Ma, J.; et al. Ilamycin E, a natural product of marine actinomycete, inhibits triple-negative breast cancer partially through ER stress-CHOP-Bcl-2. Int. J. Biol. Sci. 2019, 15, 1723–1732. [Google Scholar] [CrossRef]
- Feling, R.H.; Buchanan, G.O.; Mincer, T.J.; Kauffman, C.A.; Jensen, P.R.; Fenical, W. Salinosporamide A: A highly cytotoxic proteasome inhibitor from a novel microbial source, a marine bacterium of the new genus Salinospora. Angew. Chem. Int. Ed. 2003, 4, 355–357. [Google Scholar] [CrossRef] [PubMed]
- Raninga, P.V.; Lee, A.; Sinha, D.; Dong, L.F.; Datta, K.K.; Lu, X.; Kalita-de Croft, P.; Dutt, M.; Hill, M.; Pouliot, N.; et al. Marizomib suppresses triple-negative breast cancer via proteasome and oxidative phosphorylation inhibition. Theranostics 2020, 10, 5259–5275. [Google Scholar] [CrossRef]
- Harrison, S.J.; Mainwaring, P.; Price, T.; Millward, M.J.; Padrik, P.; Underhill, C.R.; Cannell, P.K.; Reich, S.D.; Trikha, M.; Spencer, A. Phase I clinical trial of Marizomib (npi-0052) in patients with advanced malignancies including multiple myeloma: Study NPI-0052-102 Final Results. Clin. Cancer Res. 2016, 22, 4559–4566. [Google Scholar] [CrossRef] [PubMed]
- Kashfi, R.; Kelsey, C.; Gang, D.J.; Call, D.R.; Gang, D.R. Metabolomic diversity and identification of antibacterial activities of bacteria isolated from marine sediments in Hawai’i and Puerto Rico. Front. Mol. Biosci. 2020, 7, 23. [Google Scholar] [CrossRef] [PubMed]
- Gozari, M.; Zaheri, A.; Jahromi, S.T.; Karimzadeh, R. Screening and characterization of marine actinomycetes from the northern Oman Sea sediments for cytotoxic and antimicrobial activity. Int. Microbiol. 2019, 22, 521–530. [Google Scholar] [CrossRef]
- Parera-Valadez, Y.; Yam-Puc, A.; López-Aguiar, L.K.; Borges-Argáez, R.; Figueroa-Saldivar, M.A.; Cáceres-Farfán, M.; Márquez-Velázquez, N.A.; Prieto-Davó, A. Ecological strategies behind the selection of cultivable Actinomycete strains from the Yucatan Peninsula for the discovery of secondary metabolites with antibiotic activity. Microb. Ecol. 2019, 77, 839–851. [Google Scholar] [CrossRef] [PubMed]
- Quintero, M.; Velásquez, A.; Jutinico, L.M.; Jiménez-Vergara, E.; Blandón, L.M.; Martinez, K.; Lee, H.S.; Gómez-León, J. Bioprospecting from marine coastal sediments of Colombian Caribbean: Screening and study of antimicrobial activity. J. Appl. Microbiol. 2018, 125, 753–765. [Google Scholar] [CrossRef] [PubMed]
- Yang, N.; Song, F. Bioprospecting of novel and bioactive compounds from marine actinomycetes isolated from South China Sea sediments. Curr. Microbiol. 2018, 75, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Prieto-Davó, A.; Dias, T.; Gomes, S.E.; Rodrigues, S.; Parera-Valadez, Y.; Borralho, P.M.; Pereira, F.; Rodrigues, C.M.; Santos-Sanches, I.; Gaudêncio, S.P. The Madeira Archipelago as a significant source of marine-derived Actinomycete diversity with anticancer and antimicrobial potential. Front. Microbiol. 2016, 7, 1594. [Google Scholar] [CrossRef] [PubMed]
- Tangerina, M.M.; Correa, H.; Haltli, B.; Vilegas, W.; Kerr, R.G. Bioprospecting from cultivable bacterial communities of marine sediment and invertebrates from the underexplored Ubatuba region of Brazil. Arch. Microbiol. 2017, 199, 155–169. [Google Scholar] [CrossRef]
- Undabarrena, A.; Beltrametti, F.; Claverías, F.P.; González, M.; Moore, E.R.; Seeger, M.; Cámara, B. Exploring the diversity and antimicrobial potential of marine actinobacteria from the Comau Fjord in Northern Patagonia, Chile. Front. Microbiol. 2016, 7, 1135. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Yang, J.; Yu, J.; Li, J.; Yuan, J.; Wong, N.K.; Ju, J. Chlorinated bis-indole alkaloids from deep-sea derived Streptomyces sp. SCSIO 11791 with antibacterial and cytotoxic activities. J. Antibiot. 2020, 73, 542–547. [Google Scholar] [CrossRef]
- Mincer, T.J.; Jensen, P.R.; Kauffman, C.A.; Fenical, W. Widespread and persistent populations of a major new marine actinomycete taxon in ocean sediments. Appl. Environ. Microbiol. 2002, 68, 5005–5011. [Google Scholar] [CrossRef]
- Johnson, M.; Zaretskaya, I.; Raytselis, Y.; Merezhuk, Y.; McGinnis, S.; Madden, T.L. NCBI BLAST: A better web interface. Nucleic. Acids. Res. 2008, 36, W5–W9. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef]
- Kimura, M. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J. Mol. Evol. 1980, 16, 111–120. [Google Scholar] [CrossRef]
- Guo, Y.; Zheng, W.; Rong, X.; Huang, Y. A multilocus phylogeny of the Streptomyces griseus 16S rRNA gene clade: Use of multilocus sequence analysis for Streptomycete systematics. Int. J. Syst. Evol. Microbiol. 2008, 58, 149–159. [Google Scholar] [CrossRef]
- Ginolhac, A.; Jarrin, C.; Robe, P.; Perrière, G.; Vogel, T.M.; Simonet, P.; Nalin, R. Type I polyketide synthases may have evolved through horizontal gene transfer. J. Mol. Evol. 2005, 60, 716–725. [Google Scholar] [CrossRef] [PubMed]
- Izumikawa, M.; Murata, M.; Tachibana, K.; Ebizuka, Y.; Fujii, I. Cloning of modular type I polyketide synthase genes from salinomycin producing strain of Streptomyces albus. Bioorg. Med. Chem. 2003, 11, 3401–3405. [Google Scholar] [CrossRef]
- Al-Amoudi, S.; Essack, M.; Simões, M.F.; Bougouffa, S.; Soloviev, I.; Archer, J.A.; Lafi, F.F.; Bajic, V.B. Bioprospecting Red Sea coastal ecosystems for culturable microorganisms and their antimicrobial potential. Mar. Drugs 2016, 14, 165. [Google Scholar] [CrossRef] [PubMed]
- Zothanpuia; Passari, A.K.; Gupta, V.K.; Singh, B.P. Detection of antibiotic-resistant bacteria endowed with antimicrobial activity from a freshwater lake and their phylogenetic affiliation. PeerJ 2016, 4, e2103. [Google Scholar] [CrossRef] [PubMed]
- Wawrik, B.; Kerkhof, L.; Zylstra, G.J.; Kukor, J.J. Identification of unique type II polyketide synthase genes in soil. Appl. Environ. Microbiol. 2005, 71, 2232–2238. [Google Scholar] [CrossRef]
- Ayuso-Sacido, A.; Genilloud, O. New PCR primers for the screening of NRPS and PKS-I systems in actinomycetes: Detection and distribution of these biosynthetic gene sequences in major taxonomic groups. Microb. Ecol. 2005, 49, 10–24. [Google Scholar] [CrossRef]
- Mathew, B.T.; Torky, Y.; Amin, A.; Mourad, A.I.; Ayyash, M.M.; El-Keblawy, A.; Hilal-Alnaqbi, A.; AbuQamar, S.F.; El-Tarabily, K.A. Halotolerant marine rhizosphere-competent Actinobacteria promote Salicornia bigelovii growth and seed production using seawater irrigation. Front. Microbiol. 2020, 11, 552. [Google Scholar] [CrossRef]
- Kavitha, A.; Savithri, H.S. Biological significance of marine actinobacteria of east coast of Andhra Pradesh, India. Front. Microbiol. 2017, 8, 1201. [Google Scholar] [CrossRef]
- Staphylococcus Aureus Subsp. Aureus (ATCC® BAA-44™). Available online: https://atcc.org/Products/All/BAA-44.aspx#documentation (accessed on 15 August 2020).
- Andrews, J.M. Determination of minimum inhibitory concentrations. J. Antimicrob. Chemother. 2001, 48, 5–16. [Google Scholar] [CrossRef]
- Wang, M.; Carver, J.J.; Phelan, V.V.; Sanchez, L.M.; Garg, N.; Peng, Y. Sharing and community curation of mass spectrometry data with Global Natural Products Social Molecular Networking. Nat. Biotechnol. 2016, 34, 828–837. [Google Scholar] [CrossRef]
- Kemung, H.M.; Tan, L.T.; Khan, T.M.; Chan, K.G.; Pusparajah, P.; Goh, B.H.; Lee, L.H. Streptomyces as a prominent resource of future anti-MRSA drugs. Front. Microbiol. 2018, 9, 2221. [Google Scholar] [CrossRef] [PubMed]
- Saitou, N.; Nei, M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar] [PubMed]
- Rong, X.; Huang, Y. Taxonomic evaluation of the Streptomyces hygroscopicus clade using multilocus sequence analysis and DNA-DNA hybridization, validating the MLSA scheme for systematics of the whole genus. Syst. Appl. Microbiol. 2012, 35, 7–18. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Han, L.; Yu, M.; Cao, P.; Li, D.; Guo, X.; Liu, Y.; Wang, X.; Xiang, W. Characterization of Streptomyces sporangiiformans sp. nov., a novel soil Actinomycete with antibacterial activity against Ralstonia solanacearum. Microorganisms 2019, 7, 360. [Google Scholar] [CrossRef]
- Huang, X.; Kong, F.; Zhou, S.; Huang, D.; Zheng, J.; Zhu, W. Streptomyces tirandamycinicus sp. nov., a novel marine sponge-derived Actinobacterium with antibacterial potential against Streptococcus agalactiae. Front. Microbiol. 2019, 10, 482. [Google Scholar] [CrossRef]
- Labeda, D.P. Multilocus sequence analysis of phytopathogenic species of the genus Streptomyces. Int. J. Syst. Evol. Microbiol. 2011, 61, 2525–2531. [Google Scholar] [CrossRef]
- Johnson, J.S.; Spakowicz, D.J.; Hong, B.Y.; Petersen, L.M.; Demkowicz, P.; Chen, L.; Leopold, S.R.; Hanson, B.M.; Agresta, H.O.; Gerstein, M.; et al. Evaluation of 16S rRNA gene sequencing for species and strain-level microbiome analysis. Nat. Commun. 2019, 10, 5029. [Google Scholar] [CrossRef]
- Cornell, C.R.; Marasini, D.; Fakhr, M.K. Draft genome sequences of megaplasmid-bearing Streptomyces sp. strains BF-3 and 4F, isolated from the Great Salt Plains of Oklahoma. Genome Announc. 2018, 14, e00208–e00218. [Google Scholar] [CrossRef]
- Hertweck, C.; Luzhetskyy, A.; Rebets, Y.; Bechthold, A. Type II polyketide synthases: Gaining a deeper insight into enzymatic teamwork. Nat. Prod. Rep. 2007, 24, 162–190. [Google Scholar] [CrossRef]
- Katz, L.; Baltz, R.H. Natural product discovery: Past, present, and future. J. Ind. Microbiol. Biotechnol. 2016, 43, 155–176. [Google Scholar] [CrossRef]
- Rohr, J.; Thiericke, R. Angucycline group antibiotics. Nat. Prod. Rep. 1992, 9, 103–137. [Google Scholar] [CrossRef] [PubMed]
- Kharel, M.K.; Pahari, P.; Shepherd, M.D.; Tibrewal, N.; Nybo, S.E.; Shaaban, K.A.; Rohr, J. Angucyclines: Biosynthesis, mode-of-action, new natural products, and synthesis. Nat. Prod. Rep. 2012, 29, 264–325. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Qin, L.; Wang, Q.; Ding, W.; Chen, Z.; Ma, Z. Angucycline antibiotics and its derivatives from marine-derived actinomycete Streptomyces sp. A6H. Nat. Prod. Res. 2016, 30, 2551–2558. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Duan, Y.; Cui, Z.; Wang, Z.; Li, Z.; Zhang, Y.; Ju, J.; Huang, H. Cytotoxic rearranged angucycline glycosides from deep sea-derived Streptomyces lusitanus SCSIO LR32. J. Antibiot. 2017, 70, 819–822. [Google Scholar] [CrossRef] [PubMed]
- Qu, X.Y.; Ren, J.W.; Peng, A.H.; Lin, S.Q.; Lu, D.D.; Du, Q.Q.; Liu, L.; Li, X.; Li, E.W.; Xie, W.D. Cytotoxic, anti-migration, and anti-invasion activities on breast cancer cells of angucycline glycosides isolated from a marine-derived Streptomyces sp. Mar. Drugs 2019, 17, 277. [Google Scholar] [CrossRef]
- Huang, H.; Yang, T.; Ren, X.; Liu, J.; Song, Y.; Sun, A.; Ma, J.; Wang, B.; Zhang, Y.; Huang, C.; et al. Cytotoxic angucycline class glycosides from the deep sea actinomycete Streptomyces lusitanus SCSIO LR32. J. Nat. Prod. 2012, 75, 202–208. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).