Novel Insights into the Biotechnological Production of Haematococcus pluvialis-Derived Astaxanthin: Advances and Key Challenges to Allow Its Industrial Use as Novel Food Ingredient
Abstract
1. Introduction
2. Astaxanthin
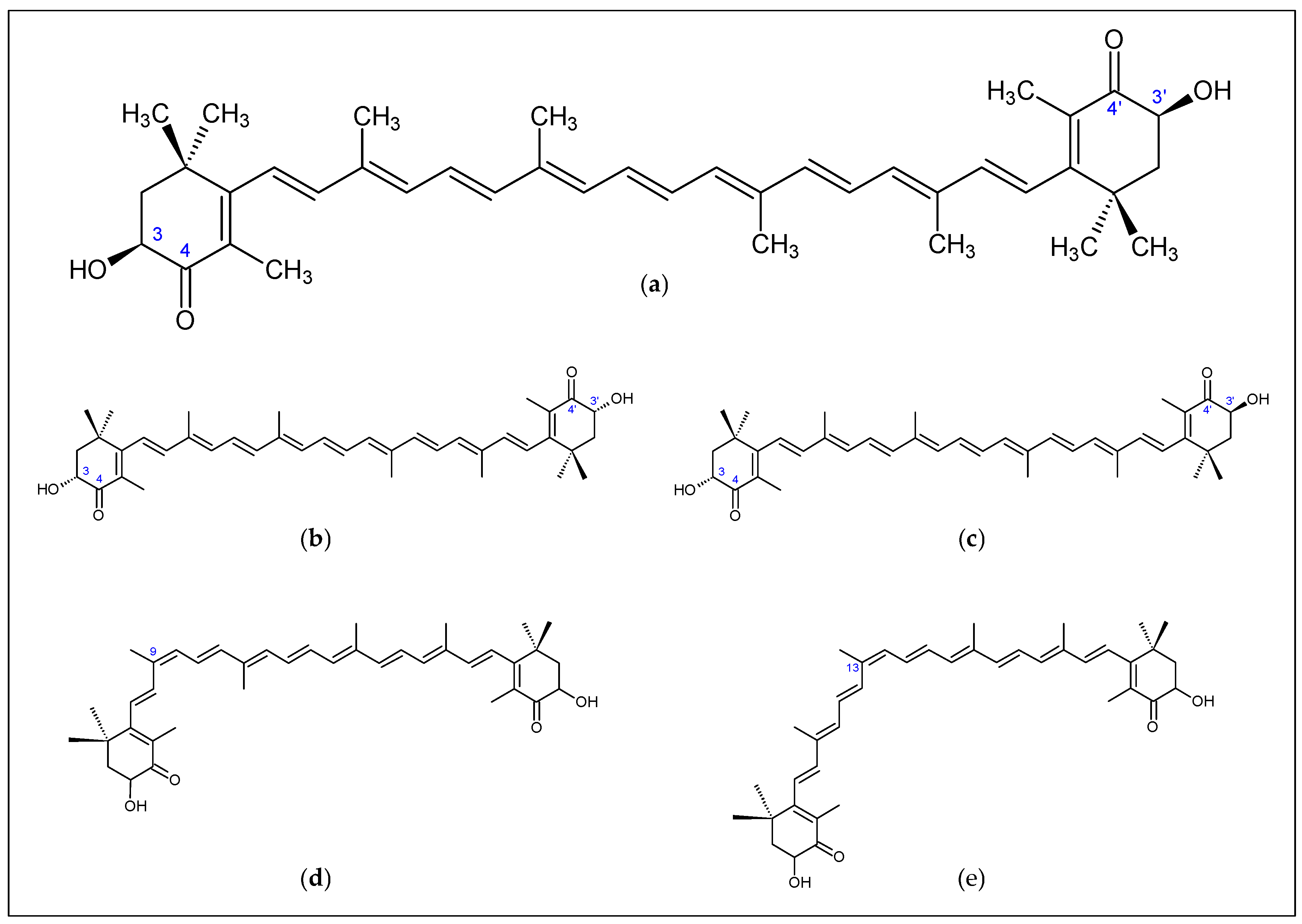
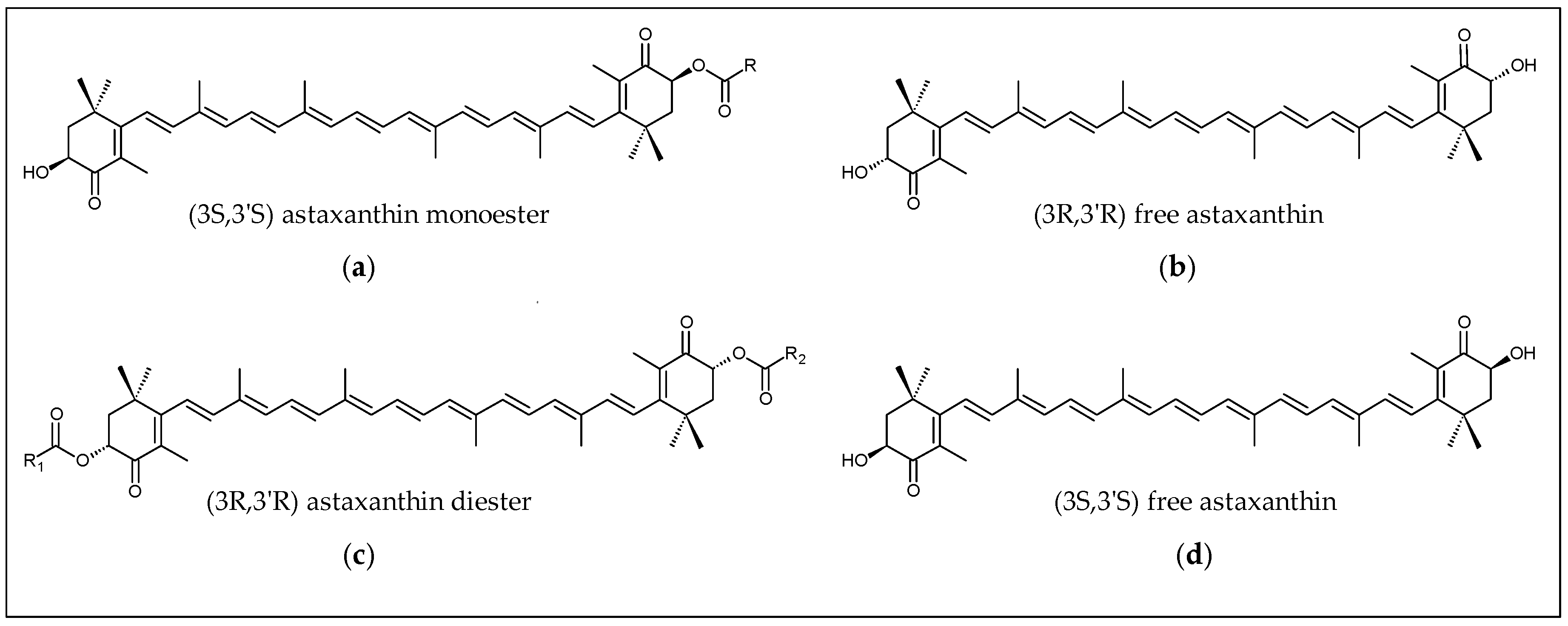
2.1. Biochemistry
2.2. Sources of Astaxanthin
2.3. Bioavailability and Metabolism of Carotenoids and Astaxanthin in Human
2.4. Toxicological Issues of Astaxanthin
2.5. Biological Activities of Astaxanthin
2.5.1. Antioxidant Activity
2.5.2. Anti-Inflammatory Activity
2.5.3. Roles of Astaxanthin in the Prevention of Cardiovascular Disease
2.5.4. Roles of Astaxanthin in the Prevention of Diabetes
2.5.5. Protective Effect of Astaxanthin on Liver
2.5.6. Immuno-Modulating Effects
2.5.7. Anti-Cancer Activities
2.5.8. Effects on the Nervous System and Cerebral and Visual Functions
3. Haematococcus pluvialis: Biological, Physiological, and Biochemical Aspects
3.1. Taxonomy and Distribution
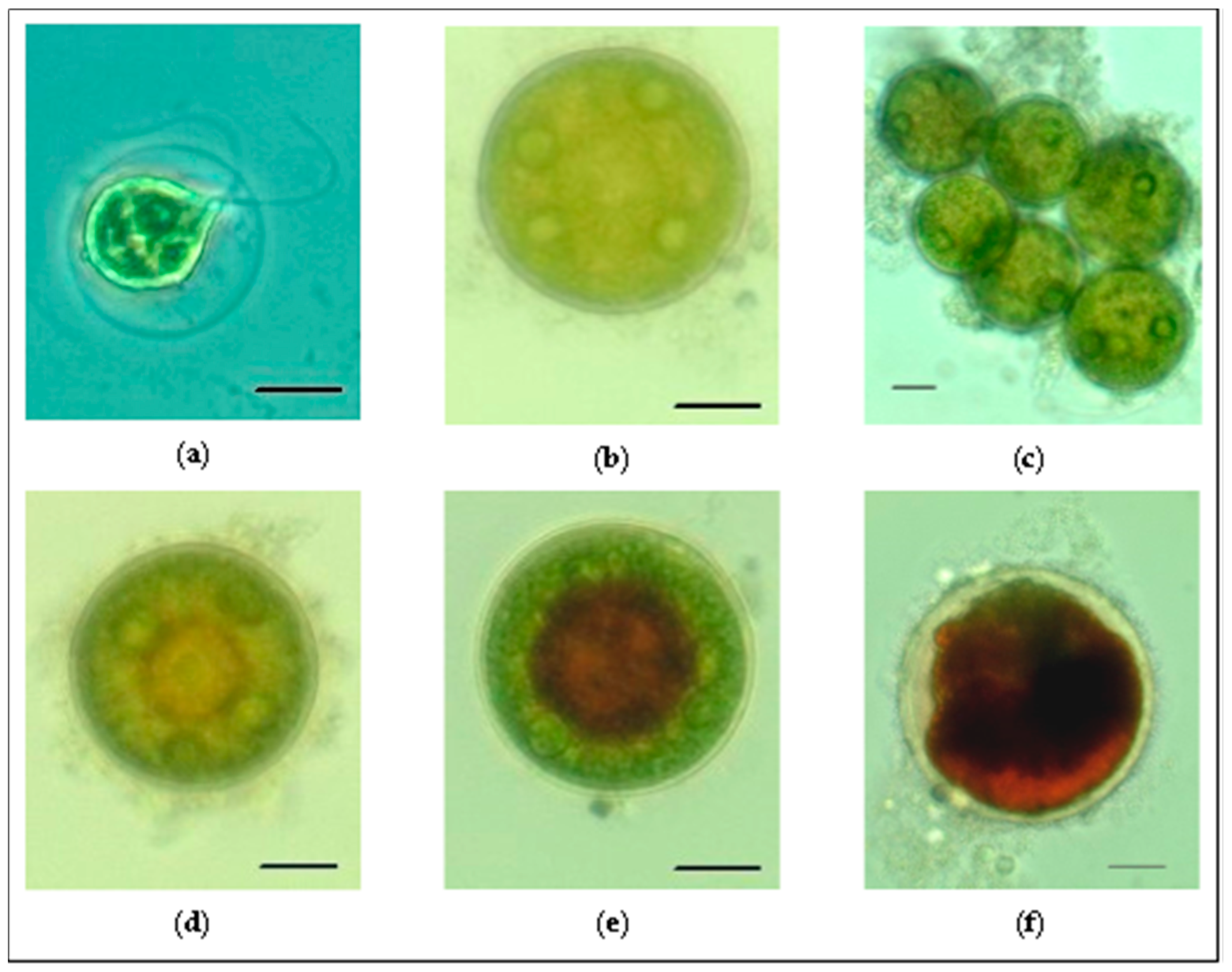
3.2. Morphology and Life Cycle
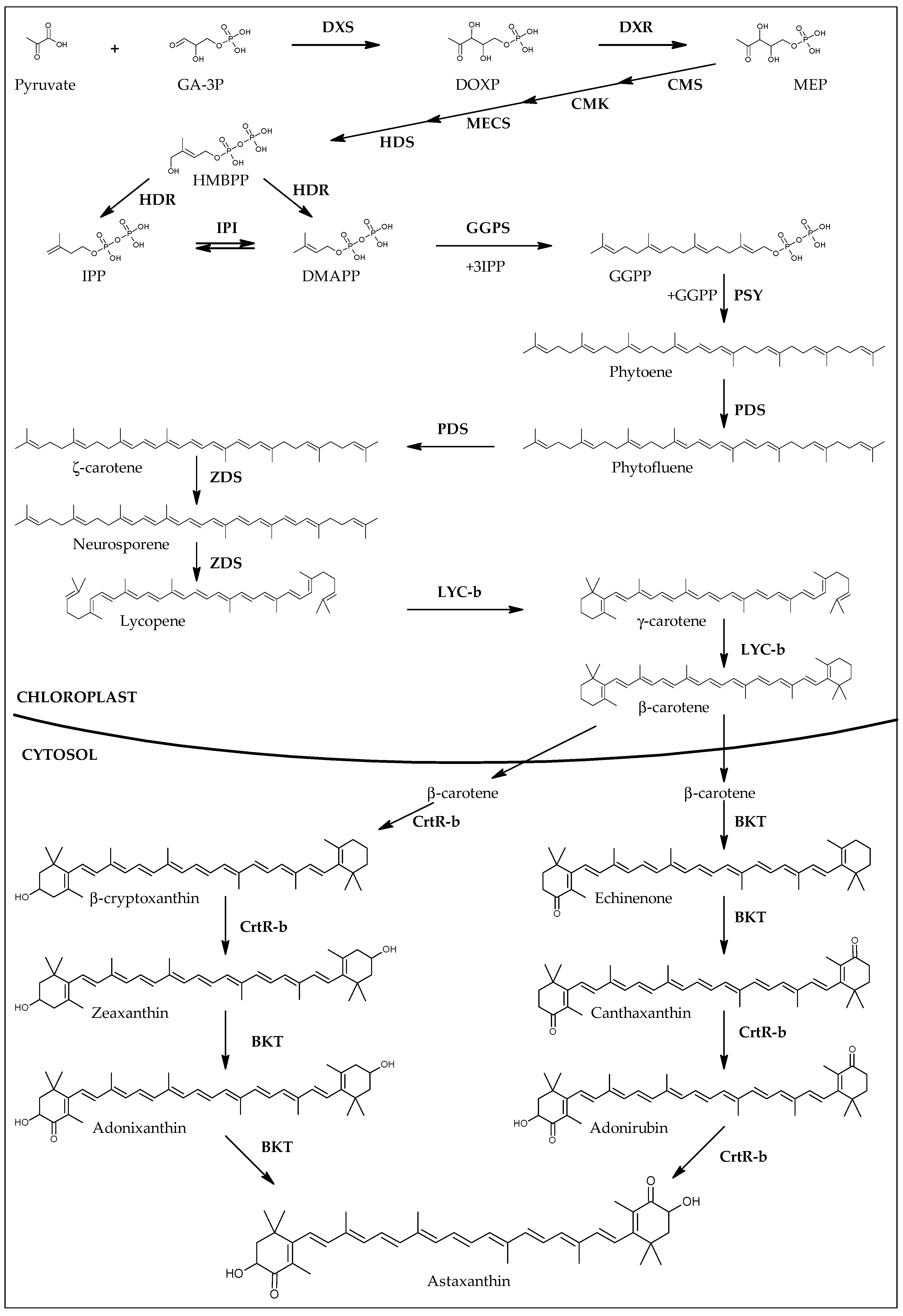
3.3. Carotenogenesis in H. pluvialis
3.4. Physiological Role of Astaxanthin Accumulation
3.5. Biochemical Composition of H. pluvialis Biomass
3.6. Cultivation of H. pluvialis for Astaxanthin Production
3.6.1. Cultivation Process
| Photobioreactor Type | Culture Volume (L) | Culture Conditions | Green Stage Biomass Productivity (g.L−1.d−1) | Red Stage Biomass Productivity (g.L−1.d−1) | Astaxanthin Content (% Dry Weight) | Astaxanthin Productivity (mg.L−1.day−1) | Ref. |
|---|---|---|---|---|---|---|---|
| airlift column | 30 | autrotrophy, batch, indoor | 0.03 | 0.01 | 2.7 | 0.44 (b) | [172] |
| bioreactor | 2.5 | mixotrophy, fed-batch, indoor | - | 0.137 | 2–2.3 | 3.2 (a) | [200] |
| tubular PBR/open pond | 25 000 | autrotrophy, batch, outdoor | 0.036–0.052 | - | 2.8–3 | - | [185] |
| Fermentor/vessel | 2.5 | heterotrophy/autrotrophy, fed-batch/batch, indoor | 0.275 | 0.05 | 2 | 4.4 (b) | [177] |
| flasks/Tubular PBR | 50 | autrotrophy, batch, indoor/outdoor | - | 0.05 | 3.6 | 7.2 (c, d) | [144] |
| bubbling column | 1.8 | autrotrophy, continuous, chemostat, indoor | - | 0.6 | 0.8 | 5.6 (a) | [178] |
| airlift tubular PBR | 55 | autrotrophy, batch, outdoor | - | 0.41 | 1.1 | 4.4 (a) | [171] |
| bubbling column | 55 | autrotrophy, batch, outdoor | - | 0.06 | 0.2 | 0.12 (a) | [171] |
| bubbling column | 1.8 | autrotrophy, continuous, indoor | 0.58 | - | - | - | [187] |
| airlift Tubular PBR | 220 | autrotrophy, continuous, outdoor | 0.68 | - | - | - | [187] |
| bubbling column | 1 | autrotrophy, fed-batch/batch, indoor | 0.36 | 0.14 | 3.6 | 12 (b) | [179] |
| Airlift column | 1 | autrotrophy, fed-batch/batch, indoor | 0.4 | - | - | 18 (b) | [179] |
| bubbling column | 1.8 | autrotrophy, continuous, chemostat, indoor | - | 1.9 | 1.1 | 21 (a) | [193] |
| bubbling column | 1.8 | autrotrophy, continuous, chemostat, indoor | - | 0.7 | 1 | 8 (a) | [186] |
| airlift tubular PBR | 50 | autrotrophy, continuous, chemostat, outdoor | - | 0.6–0.7 | 1.34 | 8 (a) | [186] |
| bubbling column | 0.5 | autrotrophy, batch, indoor | 0.5 | 0.21 | 4 | 11.5 (TC) (b) | [174] |
| flat panel/tubular PBRs | 2 000 | autrotrophy, batch, outdoor | 0.37 | 0.21 | 3.8 | 10.1 (TC) (b,c) | [174] |
| open circular pond | 3 | autrotrophy, batch, indoor | - | 0.15 | 2.8 | 4.3 (a) | [183] |
| flat type PBR | 1 | autrotrophy, fed-batch, indoor | 0.33 | - | 4.8 | 14 (b) | [182] |
| bag type PBR | 6 | autrotrophy, batch, indoor | - | 0.047 | - | 1.4 (a) | [181] |
| bubbling column | 0,6 | autrotrophy, batch, indoor/outdoor | - | 0.58 | 2.7 | 17.1 (c, d) | [180] |
| immobilized biofilm | 160 cm2 | autrotrophy, batch, indoor | - | 6.8 g.m−2.d−1 | 2.2 | 164.5 mg.m−2.d−1 (d) | [195] |
| microreactor | 0.1 10−3 | autrotrophy, batch, indoor | - | - | - | 45.25 (b) | [194] |
| bubbling column | 1 | heterotrophy/autrotrophy, batch, indoor | - | 0.3 | 2.37 | 10.5 (c) | [201] |
| bubbling column | 0.05 | mixotrophy, batch, indoor | 0.22–0.35 | - | - | 10.2 (b) | [202] |
| bottle/airlift PBR | 6.5 | autotrophy, batch, indoor | - | 0.16 | 4.94 | 7.8 (d) | [184] |
3.6.2. Impact of Physicochemical Factors on Growth and Productivity
Irradiance, Temperature, and pH of the Media
Nutrients Availability
Stress-Inducing Agents
Microbial Contamination
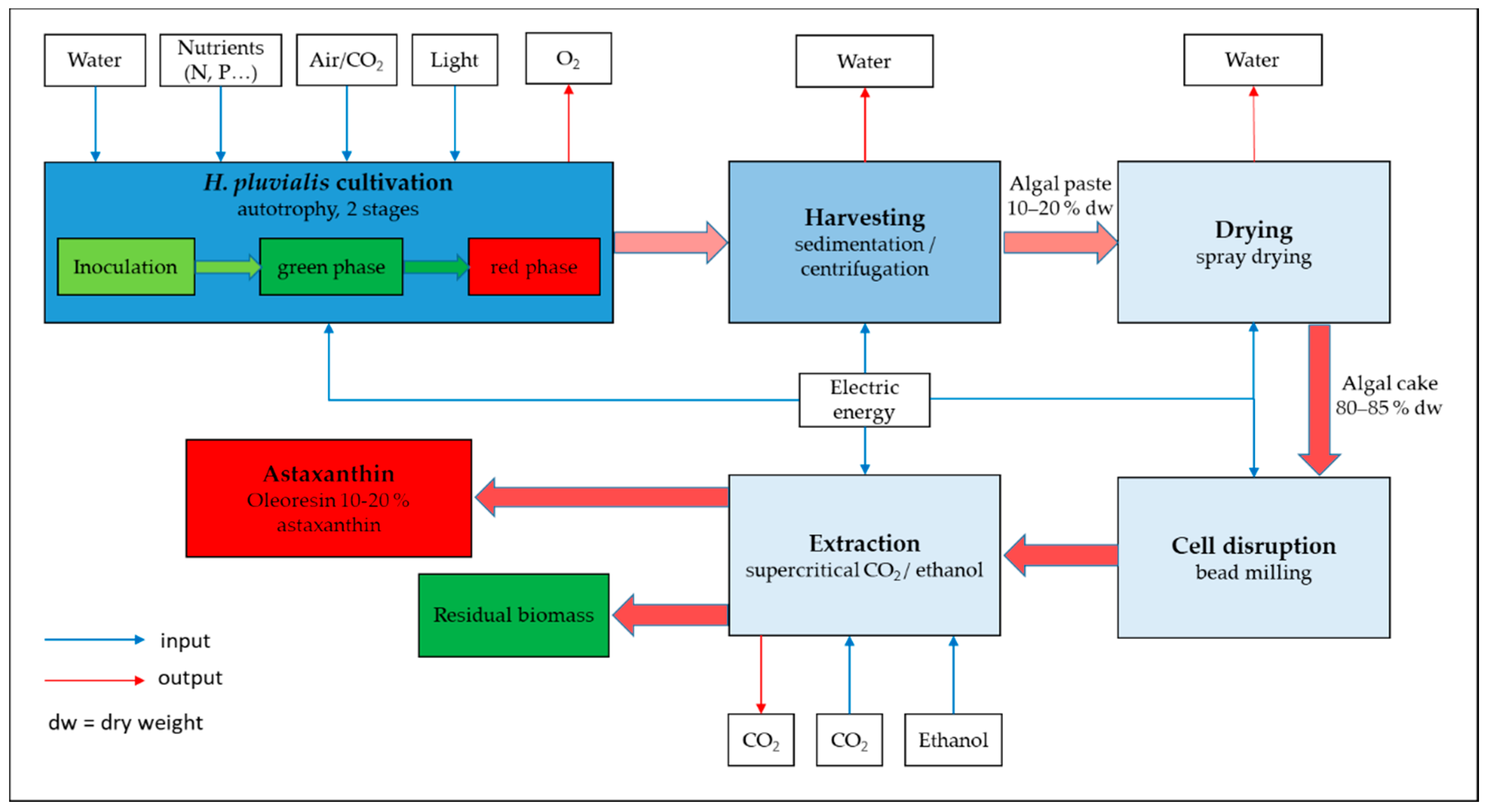
3.7. Downstream Processing
3.7.1. Harvesting of H. pluvialis Biomass
3.7.2. Drying of H. pluvialis Biomass
3.7.3. Pretreatment of H. pluvialis Biomass
3.7.4. Recovery of Astaxanthin from H. pluvialis
3.8. Economic Potential of H. pluvialis-Derived Astaxanthin
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Panis, G.; Carreon, J.R. Commercial astaxanthin production derived by green alga Haematococcus pluvialis: A microalgae process model and a techno-economic assessment all through production line. Algal Res. 2016, 18, 175–190. [Google Scholar] [CrossRef]
- Ahuja, K.; Rawat, A. Astaxanthin Market Size by Source (Synthetic, Natural), by Application (Dietary Supplement, Personal Care, Pharmaceuticals, Food & Beverages, Animal Feed {Aquaculture, Livestock, Pets}) Industry Outlook Report, Regional Analysis, Application Potential, Price Trends, Competitive Market Share & Forecast, 2019–2026. Available online: https://www.gminsights.com/industry-analysis/astaxanthin-market (accessed on 20 August 2020).
- Hu, J.; Nagarajan, D.; Zhang, Q.; Chang, J.-S.; Lee, D.-J. Heterotrophic cultivation of microalgae for pigment production: A review. Biotechnol. Adv. 2018, 36, 54–67. [Google Scholar] [CrossRef] [PubMed]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed). Scientific Opinion on the safety and efficacy of astaxanthin-dimethyldisuccinate (Carophyll® Stay-Pink 10%-CWS) for salmonids, crustaceans and other fish. EFSA J. 2019, 17, 42. [Google Scholar] [CrossRef]
- Han, D.; Li, Y.; Hu, Q. Biology and Commercial Aspects of Haematococcus pluvialis. In Handbook of Microalgal Culture: Applied Phycology and Biotechnology, 2nd ed.; Richmond, A., Hu, Q., Eds.; Wiley Blackwell: West Sussex, UK, 2013; pp. 388–405. [Google Scholar]
- Peng, J.; Xiang, W.; Tang, Q.; Sun, N.; Chen, F.; Yuan, J. Comparative analysis of astaxanthin and its esters in the mutant E1 of Haematococcus pluvialis and other green algae by HPLC with a C30 column. Sci. China C Life Sci. 2008, 51, 1108–1115. [Google Scholar] [CrossRef] [PubMed]
- EFSA NDA Panel (EFSA Panel on Nutrition, Novel Foods and Food Allergens). Scientific Opinion on the safety of astaxanthin for its use as a novel food in food supplements. EFSA J. 2020, 18, 9. [Google Scholar] [CrossRef]
- Hu, I.-C. Chapter 14—Production of potential coproducts from microalgae. In Biofuels from Algae. Biomass, Biofuels, Biochemicals, 2nd ed.; Pandey, A., Chang, J.S., Soccol, C.R., Lee, D.J., Chisti, Y., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 345–358. [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed). Opinion of the Scientific Panel on Additives and Products or Substances used in Animal Feed on the request from the European Commission on the safety of use of colouring agents in animal nutrition PART I. General Principles and Astaxanthin. EFSA J. 2005, 291, 1–40. [Google Scholar]
- Davinelli, S.; Nielsen, M.; Scapagnini, G. Astaxanthin in Skin Health, Repair, and Disease: A Comprehensive Review. Nutrients 2018, 10, 522. [Google Scholar] [CrossRef]
- Higuera-Ciapara, I.; Félix-Valenzuela, L.; Goycoolea, F.M. Astaxanthin: A Review of its Chemistry and Applications. Crit. Rev. Food Sci. Nutr. 2006, 46, 185–196. [Google Scholar] [CrossRef] [PubMed]
- Miki, W. Biological functions and activities of animal carotenoids. Pure Appl. Chem. 1991, 63, 141–146. [Google Scholar] [CrossRef]
- Vershinin, A. Biological functions of carotenoids—Diversity and evolution. BioFactors 1999, 10, 99–104. [Google Scholar] [CrossRef]
- Hussein, G.; Sankawa, U.; Goto, H.; Matsumoto, K.; Watanabe, H. Astaxanthin, a Carotenoid with Potential in Human Health and Nutrition. J. Nat. Prod. 2006, 69, 443–449. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.-P.; Chen, F. Purification of trans-astaxanthin from a high-yielding astaxanthin ester-producing strain of the microalga Haematococcus pluvialis. Food Chem. 2000, 68, 443–448. [Google Scholar] [CrossRef]
- Yuan, J.-P.; Chen, F. Kinetics for the reversible isomerization reaction of trans-astaxanthin. Food Chem. 2001, 73, 131–137. [Google Scholar] [CrossRef]
- Coral-Hinostroza, G.N.; Ytrestøyl, T.; Ruyter, B.; Bjerkeng, B. Plasma appearance of unesterified astaxanthin geometrical E/Z and optical R/S isomers in men given single doses of a mixture of optical 3 and 3′R/S isomers of astaxanthin fatty acyl diesters. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2004, 139, 99–110. [Google Scholar] [CrossRef]
- Schmidt, I.; Schewe, H.; Gassel, S.; Jin, C.; Buckingham, J.; Hümbelin, M.; Sandmann, G.; Schrader, J. Biotechnological production of astaxanthin with Phaffia rhodozyma/Xanthophyllomyces dendrorhous. Appl. Microbiol. Biotechnol. 2011, 89, 555–571. [Google Scholar] [CrossRef]
- Renstrøm, B.; Liaaen-Jensen, S. Fatty acid composition of some esterified carotenols. Comp. Biochem. Physiol. Part B Comp. Biochem. 1981, 69, 625–627. [Google Scholar] [CrossRef]
- Zhekisheva, M.; Zarka, A.; Khozin-Goldberg, I.; Cohen, Z.; Boussiba, S. Inhibition of astaxanthin synthesis under high irradiance does not abolish triacylglycerol accumulation in the green alga Haematococcus pluvialis (Chlorophyceae). J. Phycol. 2005, 41, 819–826. [Google Scholar] [CrossRef]
- Miao, F.; Lu, D.; Li, Y.; Zeng, M. Characterization of astaxanthin esters in Haematococcus pluvialis by liquid chromatography–atmospheric pressure chemical ionization mass spectrometry. Anal. Biochem. 2006, 352, 176–181. [Google Scholar] [CrossRef]
- Becker, E.W. Microalgae for Human and Animal Nutrition. In Handbook of Microalgal Culture: Applied Phycology and Biotechnology, 2nd ed.; Richmond, A., Hu, Q., Eds.; Wiley Blackwell: West Sussex, UK, 2013; pp. 461–503. [Google Scholar]
- Cunningham, F.X.; Gantt, E. A study in scarlet: Enzymes of ketocarotenoid biosynthesis in the flowers of Adonis aestivalis: Adonis β-ring oxygenases. Plant J. 2005, 41, 478–492. [Google Scholar] [CrossRef]
- Yuan, J.-P.; Peng, J.; Yin, K.; Wang, J.-H. Potential health-promoting effects of astaxanthin: A high-value carotenoid mostly from microalgae. Mol. Nutr. Food Res. 2011, 55, 150–165. [Google Scholar] [CrossRef]
- Ambati, R.; Phang, S.-M.; Ravi, S.; Aswathanarayana, R. Astaxanthin: Sources, Extraction, Stability, Biological Activities and Its Commercial Applications—A Review. Mar. Drugs 2014, 12, 128–152. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Chen, X.; Too, H.-P. Microbial astaxanthin biosynthesis: Recent achievements, challenges, and commercialization outlook. Appl. Microbiol. Biotechnol. 2020, 104, 5725–5737. [Google Scholar] [CrossRef] [PubMed]
- Foss, P.; Renstrøm, B.; Liaaen-Jensen, S. Natural occurrence of enantiomeric and Meso astaxanthin 7∗-crustaceans including zooplankton. Comp. Biochem. Physiol. Part B Comp. Biochem. 1987, 86, 313–314. [Google Scholar] [CrossRef]
- Kotake-Nara, E.; Nagao, A. Absorption and Metabolism of Xanthophylls. Mar. Drugs 2011, 9, 1024–1037. [Google Scholar] [CrossRef] [PubMed]
- Bohn, T. Bioavailability of Non-Provitamin A Carotenoids. Curr. Nutr. Food Sci. 2008, 4, 240–258. [Google Scholar] [CrossRef]
- Breithaupt, D.E.; Bamedi, A.; Wirt, U. Carotenol fatty acid esters: Easy substrates for digestive enzymes? Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2002, 132, 721–728. [Google Scholar] [CrossRef]
- Kistler, A.; Liechti, H.; Pichard, L.; Wolz, E.; Oesterhelt, G.; Hayes, A.; Maurel, P. Metabolism and CYP-inducer properties of astaxanthin in man and primary human hepatocytes. Arch. Toxicol. 2002, 75, 665–675. [Google Scholar] [CrossRef]
- Østerlie, M.; Bjerkeng, B.; Liaaen-Jensen, S. Plasma appearance and distribution of astaxanthin E/Z and R/S isomers in plasma lipoproteins of men after single dose administration of astaxanthin. J. Nutr. Biochem. 2000, 11, 482–490. [Google Scholar] [CrossRef]
- Okada, Y.; Ishikura, M.; Maoka, T. Bioavailability of Astaxanthin in Haematococcus Algal Extract: The Effects of Timing of Diet and Smoking Habits. Biosci. Biotechnol. Biochem. 2009, 73, 1928–1932. [Google Scholar] [CrossRef]
- Zhao, T.; Yan, X.; Sun, L.; Yang, T.; Hu, X.; He, Z.; Liu, F.; Liu, X. Research progress on extraction, biological activities and delivery systems of natural astaxanthin. Trends Food Sci. Technol. 2019, 91, 354–361. [Google Scholar] [CrossRef]
- Martínez-Álvarez, Ó.; Calvo, M.M.; Gómez-Estaca, J. Recent Advances in Astaxanthin Micro/Nanoencapsulation to Improve Its Stability and Functionality as a Food Ingredient. Mar. Drugs 2020, 18, 406. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Watanabe, M.; Takimoto, T.; Akiba, Y. Uptake and distribution of astaxanthin in several tissues and plasma lipoproteins in male broiler chickens fed a yeast (Phaffia rhodozyma) with a high concentration of astaxanthin. Br. Poult. Sci. 2004, 45, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Showalter, L.A.; Weinman, S.A.; Østerlie, M.; Lockwood, S.F. Plasma appearance and tissue accumulation of non-esterified, free astaxanthin in C57BL/6 mice after oral dosing of a disodium disuccinate diester of astaxanthin (HeptaxTM). Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2004, 137, 227–236. [Google Scholar] [CrossRef] [PubMed]
- Petri, D.; Lundebye, A.-K. Tissue distribution of astaxanthin in rats following exposure to graded levels in the feed. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2007, 145, 202–209. [Google Scholar] [CrossRef]
- Spiller, G.A.; Dewell, A. Safety of an Astaxanthin-Rich Haematococcus pluvialis Algal Extract: A Randomized Clinical Trial. J. Med. Food 2003, 6, 51–56. [Google Scholar] [CrossRef]
- Satoh, A.; Tsuji, S.; Okada, Y.; Murakami, N.; Urami, M.; Nakagawa, K.; Ishikura, M.; Katagiri, M.; Koga, Y.; Shirasawa, T. Preliminary Clinical Evaluation of Toxicity and Efficacy of A New Astaxanthin-rich Haematococcus pluvialis Extract. J. Clin. Biochem. Nutr. 2009, 44, 280–284. [Google Scholar] [CrossRef]
- Karppi; Rissanen; Nyyssönen; Kaikkonen; Olsson; Voutilainen. Salonen Effects of Astaxanthin Supplementation on Lipid Peroxidation. Int. J. Vitam. Nutr. Res. 2007, 77, 3–11. [Google Scholar] [CrossRef]
- Parisi, V.; Tedeschi, M.; Gallinaro, G.; Varano, M.; Saviano, S.; Piermarocchi, S. Carotenoids and Antioxidants in Age-Related Maculopathy Italian Study. Ophthalmology 2008, 115, 324–333.e2. [Google Scholar] [CrossRef]
- Kupcinskas, L.; Lafolie, P.; Lignell, Å.; Kiudelis, G.; Jonaitis, L.; Adamonis, K.; Andersen, L.P.; Wadström, T. Efficacy of the natural antioxidant astaxanthin in the treatment of functional dyspepsia in patients with or without Helicobacter pylori infection: A prospective, randomized, double blind, and placebo-controlled study. Phytomedicine 2008, 15, 391–399. [Google Scholar] [CrossRef]
- Choi, H.D.; Kim, J.H.; Chang, M.J.; Kyu-Youn, Y.; Shin, W.G. Effects of Astaxanthin on Oxidative Stress in Overweight and Obese Adults. Phytother. Res. 2011, 25, 1813–1818. [Google Scholar] [CrossRef]
- McNulty, H.P.; Byun, J.; Lockwood, S.F.; Jacob, R.F.; Mason, R.P. Differential effects of carotenoids on lipid peroxidation due to membrane interactions: X-ray diffraction analysis. Biochim. Biophys. Acta BBA Biomembr. 2007, 1768, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Naguib, Y.M.A. Antioxidant Activities of Astaxanthin and Related Carotenoids. J. Agric. Food Chem. 2000, 48, 1150–1154. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, E.; Mariutti, L.R.B.; Mercadante, A.Z. Scavenging Capacity of Marine Carotenoids against Reactive Oxygen and Nitrogen Species in a Membrane-Mimicking System. Mar. Drugs 2012, 10, 1784–1798. [Google Scholar] [CrossRef] [PubMed]
- Goto, S.; Kogure, K.; Abe, K.; Kimata, Y.; Kitahama, K.; Yamashita, E.; Terada, H. Efficient radical trapping at the surface and inside the phospholipid membrane is responsible for highly potent antiperoxidative activity of the carotenoid astaxanthin. Biochim. Biophys. Acta 2001, 1512, 251–258. [Google Scholar] [CrossRef]
- Nakajima, Y.; Inokuchi, Y.; Shimazawa, M.; Otsubo, K.; Ishibashi, T.; Hara, H. Astaxanthin, a dietary carotenoid, protects retinal cells against oxidative stress in-vitro and in mice in-vivo. J. Pharm. Pharmacol. 2008, 60, 1365–1374. [Google Scholar] [CrossRef]
- Yang, Y.; Seo, J.M.; Nguyen, A.; Pham, T.X.; Park, H.J.; Park, Y.; Kim, B.; Bruno, R.S.; Lee, J. Astaxanthin-Rich Extract from the Green Alga Haematococcus pluvialis Lowers Plasma Lipid Concentrations and Enhances Antioxidant Defense in Apolipoprotein E Knockout Mice. J. Nutr. 2011, 141, 1611–1617. [Google Scholar] [CrossRef]
- Ye, Q.; Huang, B.; Zhang, X.; Zhu, Y.; Chen, X. Astaxanthin protects against MPP+-induced oxidative stress in PC12 cells via the HO-1/NOX2 axis. BMC Neurosci. 2012, 13, 156. [Google Scholar] [CrossRef]
- Santos, S.D.; Cahú, T.B.; Firmino, G.O.; de Castro, C.C.M.M.B.; Carvalho, L.B., Jr.; Bezerra, R.S.; Filho, J.L.L. Shrimp Waste Extract and Astaxanthin: Rat Alveolar Macrophage, Oxidative Stress and Inflammation. J. Food Sci. 2012, 77, H141–H146. [Google Scholar] [CrossRef]
- Turkez, H.; Geyikoglu, F.; Yousef, M.I.; Togar, B.; Gürbüz, H.; Celik, K.; Akbaba, G.B.; Polat, Z. Hepatoprotective potential of astaxanthin against 2,3,7,8-tetrachlorodibenzo- p -dioxin in cultured rat hepatocytes. Toxicol. Ind. Health 2014, 30, 101–112. [Google Scholar] [CrossRef]
- Wolf, A.M.; Asoh, S.; Hiranuma, H.; Ohsawa, I.; Iio, K.; Satou, A.; Ishikura, M.; Ohta, S. Astaxanthin protects mitochondrial redox state and functional integrity against oxidative stress. J. Nutr. Biochem. 2010, 21, 381–389. [Google Scholar] [CrossRef]
- Campoio, T.R.; Oliveira, F.A.; Otton, R. Oxidative stress in human lymphocytes treated with fatty acid mixture: Role of carotenoid astaxanthin. Toxicol. In Vitro 2011, 25, 1448–1456. [Google Scholar] [CrossRef] [PubMed]
- Camera, E.; Mastrofrancesco, A.; Fabbri, C.; Daubrawa, F.; Picardo, M.; Sies, H.; Stahl, W. Astaxanthin, canthaxanthin and β-carotene differently affect UVA-induced oxidative damage and expression of oxidative stress-responsive enzymes. Exp. Dermatol. 2009, 18, 222–231. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, D.N.; Jena, G.B. Astaxanthin intervention ameliorates cyclophosphamide-induced oxidative stress, DNA damage and early hepatocarcinogenesis in rat: Role of Nrf2, p53, p38 and phase-II enzymes. Mutat. Res. Toxicol. Environ. Mutagen. 2010, 696, 69–80. [Google Scholar] [CrossRef] [PubMed]
- Barros, M.P.; Marin, D.P.; Bolin, A.P.; de Cássia Santos Macedo, R.; Campoio, T.R.; Fineto, C.; Guerra, B.A.; Polotow, T.G.; Vardaris, C.; Mattei, R.; et al. Combined astaxanthin and fish oil supplementation improves glutathione-based redox balance in rat plasma and neutrophils. Chem. Biol. Interact. 2012, 197, 58–67. [Google Scholar] [CrossRef]
- Speranza, L.; Pesce, M.; Patruno, A.; Franceschelli, S.; de Lutiis, M.A.; Grilli, A.; Felaco, M. Astaxanthin Treatment Reduced Oxidative Induced Pro-Inflammatory Cytokines Secretion in U937: SHP-1 as a Novel Biological Target. Mar. Drugs 2012, 10, 890–899. [Google Scholar] [CrossRef]
- Kishimoto, Y.; Tani, M.; Uto-Kondo, H.; Iizuka, M.; Saita, E.; Sone, H.; Kurata, H.; Kondo, K. Astaxanthin suppresses scavenger receptor expression and matrix metalloproteinase activity in macrophages. Eur. J. Nutr. 2010, 49, 119–126. [Google Scholar] [CrossRef]
- Lee, S.-J.; Bai, S.-K.; Lee, K.-S.; Namkoong, S.; Na, H.-J.; Ha, K.-S.; Han, J.-A.; Yim, S.-V.; Chang, K.; Kwon, Y.-G.; et al. Astaxanthin Inhibits Nitric Oxide Production and Inflammatory Gene Expression by Suppressing IκB Kinase-dependent NF-κB Activation. Mol. Cells 2003, 16, 97–105. [Google Scholar]
- Ohgami, K.; Shiratori, K.; Kotake, S.; Nishida, T.; Mizuki, N.; Yazawa, K.; Ohno, S. Effects of Astaxanthin on Lipopolysaccharide-Induced Inflammation in Vitro and in Vivo. Investig. Ophthalmol. Vis. Sci. 2003, 44, 2694. [Google Scholar] [CrossRef]
- Choi, S.-K.; Park, Y.-S.; Choi, D.-K.; Chang, H.-I. Effects of Astaxanthin on the Production of NO and the Expression of COX-2 and iNOS in LPS-Stimulated BV2 Microglial Cells. J. Microbiol. Biotechnol. 2008, 18, 1990–1996. [Google Scholar] [CrossRef]
- Terazawa, S.; Nakajima, H.; Shingo, M.; Niwano, T.; Imokawa, G. Astaxanthin attenuates the UVB-induced secretion of prostaglandin E2 and interleukin-8 in human keratinocytes by interrupting MSK1 phosphorylation in a ROS depletion-independent manner: Astaxanthin inhibits PGE2 and IL-8 secretion. Exp. Dermatol. 2012, 21, 11–17. [Google Scholar] [CrossRef]
- Wang, X.; Willén, R.; Wadström, T. Astaxanthin-Rich Algal Meal and Vitamin C Inhibit Helicobacter pylori Infection in BALB/cA Mice. Antimicrob. Agents Chemother. 2000, 44, 2452–2457. [Google Scholar] [CrossRef] [PubMed]
- Yasui, Y.; Hosokawa, M.; Mikami, N.; Miyashita, K.; Tanaka, T. Dietary astaxanthin inhibits colitis and colitis-associated colon carcinogenesis in mice via modulation of the inflammatory cytokines. Chem. Biol. Interact. 2011, 193, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Nagendraprabhu, P.; Sudhandiran, G. Astaxanthin inhibits tumor invasion by decreasing extracellular matrix production and induces apoptosis in experimental rat colon carcinogenesis by modulating the expressions of ERK-2, NFkB and COX-2. Investig. New Drugs 2011, 29, 207–224. [Google Scholar] [CrossRef]
- Iwamoto, T.; Hosoda, K.; Hirano, R.; Kurata, H.; Matsumoto, A.; Miki, W.; Kamiyama, M.; Itakura, H.; Yamamoto, S.; Kondo, K. Inhibition of Low-Density Lipoprotein Oxidation by Astaxanthin. J. Atheroscler. Thromb. 2000, 7, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Hussein, G.; Nakamura, M.; Zhao, Q.; Iguchi, T.; Goto, H.; Sankawa, U.; Watanabe, H. Antihypertensive and Neuroprotective Effects of Astaxanthin in Experimental Animals. Biol. Pharm. Bull. 2005, 28, 47–52. [Google Scholar] [CrossRef]
- Sasaki, Y.; Kobara, N.; Higashino, S.; Giddings, J.C.; Yamamoto, J. Astaxanthin inhibits thrombosis in cerebral vessels of stroke-prone spontaneously hypertensive rats. Nutr. Res. 2011, 31, 784–789. [Google Scholar] [CrossRef] [PubMed]
- Ikeuchi, M.; Koyama, T.; Takahashi, J.; Yazawa, K. Effects of Astaxanthin in Obese Mice Fed a High-Fat Diet. Biosci. Biotechnol. Biochem. 2007, 71, 893–899. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Hellsten, A.; Jacobsson, L.; Blomqvist, H.; Olsson, A.; Yuan, X. Alpha-tocopherol and astaxanthin decrease macrophage infiltration, apoptosis and vulnerability in atheroma of hyperlipidaemic rabbits. J. Mol. Cell. Cardiol. 2004, 37, 969–978. [Google Scholar] [CrossRef]
- Hussein, G.; Goto, H.; Oda, S.; Sankawa, U.; Matsumoto, K.; Watanabe, H. Antihypertensive Potential and Mechanism of Action of Astaxanthin: III. Antioxidant and Histopathological Effects in Spontaneously Hypertensive Rats. Biol. Pharm. Bull. 2006, 29, 684–688. [Google Scholar] [CrossRef]
- Monroy-Ruiz, J.; Sevilla, M.-Á.; Carrón, R.; Montero, M.-J. Astaxanthin-enriched-diet reduces blood pressure and improves cardiovascular parameters in spontaneously hypertensive rats. Pharmacol. Res. 2011, 63, 44–50. [Google Scholar] [CrossRef]
- Ishiki, M.; Nishida, Y.; Ishibashi, H.; Wada, T.; Fujisaka, S.; Takikawa, A.; Urakaze, M.; Sasaoka, T.; Usui, I.; Tobe, K. Impact of Divergent Effects of Astaxanthin on Insulin Signaling in L6 Cells. Endocrinology 2013, 154, 2600–2612. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Kim, Y.A.; Yokozawa, T. Protection against Oxidative Stress, Inflammation, and Apoptosis of High-Glucose-Exposed Proximal Tubular Epithelial Cells by Astaxanthin. J. Agric. Food Chem. 2009, 57, 8793–8797. [Google Scholar] [CrossRef] [PubMed]
- Marin, D.P.; Bolin, A.P.; Macedo, R.d.C.S.; Sampaio, S.C.; Otton, R. ROS production in neutrophils from alloxan-induced diabetic rats treated in vivo with astaxanthin. Int. Immunopharmacol. 2011, 11, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chen, Z.; Lu, W. Hypoglycemic effect of astaxanthin from shrimp waste in alloxan-induced diabetic mice. Med. Chem. Res. 2012, 21, 2363–2367. [Google Scholar] [CrossRef]
- Uchiyama, K.; Naito, Y.; Hasegawa, G.; Nakamura, N.; Takahashi, J.; Yoshikawa, T. Astaxanthin protects β-cells against glucose toxicity in diabetic db/db mice. Redox Rep. 2002, 7, 290–293. [Google Scholar] [CrossRef]
- Bhuvaneswari, S.; Yogalakshmi, B.; Sreeja, S.; Anuradha, C.V. Astaxanthin reduces hepatic endoplasmic reticulum stress and nuclear factor-κB-mediated inflammation in high fructose and high fat diet-fed mice. Cell Stress Chaperones 2014, 19, 183–191. [Google Scholar] [CrossRef]
- Arunkumar, E.; Bhuvaneswari, S.; Anuradha, C.V. An intervention study in obese mice with astaxanthin, a marine carotenoid—Effects on insulin signaling and pro-inflammatory cytokines. Food Funct. 2012, 3, 120–126. [Google Scholar] [CrossRef]
- Nakano, M.; Onodera, A.; Saito, E.; Tanabe, M.; Yajima, K.; Takahashi, J.; Chuyen, N.V. Effect of Astaxanthin in Combination with α-Tocopherol or Ascorbic Acid against Oxidative Damage in Diabetic ODS Rats. J. Nutr. Sci. Vitaminol. 2008, 54, 329–334. [Google Scholar] [CrossRef]
- Naito, Y.; Uchiyama, K.; Aoi, W.; Hasegawa, G.; Nakamura, N.; Yoshida, N.; Maoka, T.; Takahashi, J.; Yoshikawa, T. Prevention of diabetic nephropathy by treatment with astaxanthin in diabetic db/db mice. BioFactors 2004, 20, 49–59. [Google Scholar] [CrossRef]
- Yang, Y.; Bae, M.; Kim, B.; Park, Y.-K.; Koo, S.I.; Lee, J.-Y. Astaxanthin prevents and reverses the activation of mouse primary hepatic stellate cells. J. Nutr. Biochem. 2016, 29, 21–26. [Google Scholar] [CrossRef]
- Jia, Y.; Kim, J.-Y.; Jun, H.-J.; Kim, S.-J.; Lee, J.-H.; Hoang, M.H.; Hwang, K.-Y.; Um, S.-J.; Chang, H.I.; Lee, S.-J. The natural carotenoid astaxanthin, a PPAR-α agonist and PPAR-γ antagonist, reduces hepatic lipid accumulation by rewiring the transcriptome in lipid-loaded hepatocytes. Mol. Nutr. Food Res. 2012, 56, 878–888. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Kim, B.; Park, Y.-K.; Koo, S.I.; Lee, J.-Y. Astaxanthin prevents TGFβ1-induced pro-fibrogenic gene expression by inhibiting Smad3 activation in hepatic stellate cells. Biochim. Biophys. Acta Gen. Subj. 2015, 1850, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.O.; Kim, S.J.; Kim, H. Effect of astaxanthin on the hepatotoxicity, lipid peroxidation and antioxidative enzymes in the liver of CCl4-treated rats. Methods Find Exp. Clin. Pharmacol. 2001, 23, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Bhuvaneswari, S.; Arunkumar, E.; Viswanathan, P.; Anuradha, C.V. Astaxanthin restricts weight gain, promotes insulin sensitivity and curtails fatty liver disease in mice fed a obesity-promoting diet. Process Biochem. 2010, 45, 1406–1414. [Google Scholar] [CrossRef]
- Ni, Y.; Nagashimada, M.; Zhuge, F.; Zhan, L.; Nagata, N.; Tsutsui, A.; Nakanuma, Y.; Kaneko, S.; Ota, T. Astaxanthin prevents and reverses diet-induced insulin resistance and steatohepatitis in mice: A comparison with vitamin E. Sci. Rep. 2015, 5, 17192. [Google Scholar] [CrossRef]
- Lin, K.-H.; Lin, K.-C.; Lu, W.-J.; Thomas, P.-A.; Jayakumar, T.; Sheu, J.-R. Astaxanthin, a Carotenoid, Stimulates Immune Responses by Enhancing IFN-γ and IL-2 Secretion in Primary Cultured Lymphocytes in Vitro and ex Vivo. Int. J. Mol. Sci. 2015, 17, 44. [Google Scholar] [CrossRef]
- Park, J.S.; Mathison, B.D.; Hayek, M.G.; Massimino, S.; Reinhart, G.A.; Chew, B.P. Astaxanthin stimulates cell-mediated and humoral immune responses in cats. Vet. Immunol. Immunopathol. 2011, 144, 455–461. [Google Scholar] [CrossRef]
- Bennedsen, M.; Wang, X.; Willén, R.; Wadström, T.; Andersen, L.P. Treatment of H. pylori infected mice with antioxidant astaxanthin reduces gastric inflammation, bacterial load and modulates cytokine release by splenocytes. Immunol. Lett. 2000, 70, 185–189. [Google Scholar] [CrossRef]
- Chew, B.P.; Mathison, B.D.; Hayek, M.G.; Massimino, S.; Reinhart, G.A.; Park, J.S. Dietary astaxanthin enhances immune response in dogs. Vet. Immunol. Immunopathol. 2011, 140, 199–206. [Google Scholar] [CrossRef]
- McCall, B.; McPartland, C.; Moore, R.; Frank-Kamenetskii, A.; Booth, B. Effects of Astaxanthin on the Proliferation and Migration of Breast Cancer Cells in Vitro. Antioxidants 2018, 7, 135. [Google Scholar] [CrossRef]
- Wójcik, M.; Bobowiec, R.; Martelli, F. Effect of carotenoids on in vitro proliferation and differentiation of oval cells during neoplastic and non-neoplastic liver injuries in rats. J. Physiol. Pharmacol. 2008, 59, 203–213. [Google Scholar] [PubMed]
- Shao, Y.; Ni, Y.; Yang, J.; Lin, X.; Li, J.; Zhang, L. Astaxanthin Inhibits Proliferation and Induces Apoptosis and Cell Cycle Arrest of Mice H22 Hepatoma Cells. Med. Sci. Monit. 2016, 22, 2152–2160. [Google Scholar] [CrossRef] [PubMed]
- Palozza, P.; Torelli, C.; Boninsegna, A.; Simone, R.; Catalano, A.; Mele, M.C.; Picci, N. Growth-inhibitory effects of the astaxanthin-rich alga Haematococcus pluvialis in human colon cancer cells. Cancer Lett. 2009, 283, 108–117. [Google Scholar] [CrossRef]
- Jyonouchi, H.; Sun, S.; Iijima, K.; Gross, M.D. Antitumor activity of astaxanthin and its mode of action. Nutr. Cancer 2000, 36, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Prabhu, P.N.; Ashokkumar, P.; Sudhandiran, G. Antioxidative and antiproliferative effects of astaxanthin during the initiation stages of 1,2-dimethyl hydrazine-induced experimental colon carcinogenesis. Fundam. Clin. Pharmacol. 2009, 23, 225–234. [Google Scholar] [CrossRef]
- Tanaka, T.; Morishita, Y.; Suzui, M.; Kojima, T.; Okumura, A.; Mori, H. Chemoprevention of mouse urinary bladder carcinogenesis by the naturally occurring carotenoid astaxanthin. Carcinogenesis 1994, 15, 15–19. [Google Scholar] [CrossRef]
- Nakao, R.; Nelson, O.L.; Park, J.S.; Mathison, B.D.; Thompson, P.A.; Chew, B.P. Effect of Dietary Astaxanthin at Different Stages of Mammary Tumor Initiation in BALB/c Mice. Anticancer Res. 2010, 30, 2171–2175. [Google Scholar]
- Chew, B.P.; Wong, M.W.; Park, J.S.; Wong, T.S. Dietary beta-carotene and astaxanthin but not canthaxanthin stimulate splenocyte function in mice. Anticancer Res. 1999, 19, 5223–5227. [Google Scholar]
- Tanaka, T.; Kawamori, T.; Ohnishi, M.; Makita, H.; Mori, H.; Satoh, K.; Hara, A. Suppression of azoxymethane-induced rat colon carcinogenesis by dietary administration of naturally occurring xanthophylls astaxanthin and canthaxanthin during the postinitiation phase. Carcinogenesis 1995, 16, 2957–2963. [Google Scholar] [CrossRef]
- Kowshik, J.; Baba, A.B.; Giri, H.; Deepak Reddy, G.; Dixit, M.; Nagini, S. Astaxanthin Inhibits JAK/STAT-3 Signaling to Abrogate Cell Proliferation, Invasion and Angiogenesis in a Hamster Model of Oral Cancer. PLoS ONE 2014, 9, e109114. [Google Scholar] [CrossRef]
- Lu, Y.-P.; Liu, S.-Y.; Sun, H.; Wu, X.-M.; Li, J.-J.; Zhu, L. Neuroprotective effect of astaxanthin on H2O2-induced neurotoxicity in vitro and on focal cerebral ischemia in vivo. Brain Res. 2010, 1360, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Barros, M.; Poppe, S.; Bondan, E. Neuroprotective Properties of the Marine Carotenoid Astaxanthin and Omega-3 Fatty Acids, and Perspectives for the Natural Combination of Both in Krill Oil. Nutrients 2014, 6, 1293–1317. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.; Koh, H.-K.; Kim, D.-S. Down-regulation of IL-6 production by astaxanthin via ERK-, MSK-, and NF-κB-mediated signals in activated microglia. Int. Immunopharmacol. 2010, 10, 1560–1572. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Shibata, T.; Hisaka, S.; Osawa, T. Astaxanthin inhibits reactive oxygen species-mediated cellular toxicity in dopaminergic SH-SY5Y cells via mitochondria-targeted protective mechanism. Brain Res. 2009, 1254, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.-Q.; Sun, X.-B.; Xu, Y.-X.; Zhao, H.; Zhu, Q.-Y.; Zhu, C.-Q. Astaxanthin upregulates heme oxygenase-1 expression through ERK1/2 pathway and its protective effect against beta-amyloid-induced cytotoxicity in SH-SY5Y cells. Brain Res. 2010, 1360, 159–167. [Google Scholar] [CrossRef]
- Kim, J.-H.; Choi, W.; Lee, J.-H.; Jeon, S.-J.; Choi, Y.-H.; Kim, B.-W.; Chang, H.-I.; Nam, S.-W. Astaxanthin Inhibits H2O2-Mediated Apoptotic Cell Death in Mouse Neural Progenitor Cells via Modulation of P38 and MEK Signaling Pathways. J. Microbiol. Biotechnol. 2009, 19, 1355–1363. [Google Scholar] [CrossRef]
- Kim, J.-H.; Nam, S.-W.; Kim, B.-W.; Choi, W.; Lee, J.-H.; Kim, W.-J.; Choi, Y.-H. Astaxanthin Improves Stem Cell Potency via an Increase in the Proliferation of Neural Progenitor Cells. Int. J. Mol. Sci. 2010, 11, 5109–5119. [Google Scholar] [CrossRef]
- Li, Z.; Dong, X.; Liu, H.; Chen, X.; Shi, H.; Fan, Y.; Hou, D.; Zhang, X. Astaxanthin protects ARPE-19 cells from oxidative stress via upregulation of Nrf2-regulated phase II enzymes through activation of PI3K/Akt. Mol. Vis. 2013, 19, 1656–1666. [Google Scholar]
- Zhang, X.; Pan, L.; Wei, X.; Gao, H.; Liu, J. Impact of astaxanthin-enriched algal powder of Haematococcus pluvialis on memory improvement in BALB/c mice. Environ. Geochem. Health 2007, 29, 483–489. [Google Scholar] [CrossRef]
- Park, J.; Chyun, J.; Kim, Y.; Line, L.L.; Chew, B.P. Astaxanthin decreased oxidative stress and inflammation and enhanced immune response in humans. Nutr. Metab. 2010, 7, 18. [Google Scholar] [CrossRef]
- Kim, J.H.; Chang, M.J.; Choi, H.D.; Youn, Y.-K.; Kim, J.T.; Oh, J.M.; Shin, W.G. Protective effects of Haematococcus astaxanthin on oxidative stress in healthy smokers. J. Med. Food 2011, 14, 1469–1475. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, H.; Yanai, H.; Ito, K.; Tomono, Y.; Koikeda, T.; Tsukahara, H.; Tada, N. Administration of natural astaxanthin increases serum HDL-cholesterol and adiponectin in subjects with mild hyperlipidemia. Atherosclerosis 2010, 209, 520–523. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, K.; Kiko, T.; Miyazawa, T.; Carpentero Burdeos, G.; Kimura, F.; Satoh, A.; Miyazawa, T. Antioxidant effect of astaxanthin on phospholipid peroxidation in human erythrocytes. Br. J. Nutr. 2011, 105, 1563–1571. [Google Scholar] [CrossRef] [PubMed]
- Miyawaki, H.; Takahashi, J.; Tsukahara, H.; Takehara, I. Effects of Astaxanthin on Human Blood Rheology. J. Clin. Biochem. Nutr. 2008, 43, 69–74. [Google Scholar] [CrossRef]
- Katagiri, M.; Satoh, A.; Tsuji, S.; Shirasawa, T. Effects of astaxanthin-rich Haematococcus pluvialis extract on cognitive function: A randomised, double-blind, placebo-controlled study. J. Clin. Biochem. Nutr. 2012, 51, 102–107. [Google Scholar] [CrossRef]
- Nagaki, Y.; Hayasaka, S.; Yamada, T.; Hayasaka, Y.; Sanada, M.; Uonomi, T. Effects of astaxanthiq on accommodation, critical flicker fusion, and pattern visual evoked potential in visual display terminal workers. J. Trad. Med. 2002, 19, 170–173. [Google Scholar]
- McNulty, H.; Jacob, R.F.; Mason, R.P. Biologic Activity of Carotenoids Related to Distinct Membrane Physicochemical Interactions. Am. J. Cardiol. 2008, 101, S20–S29. [Google Scholar] [CrossRef] [PubMed]
- Gruszecki, W.I.; Strzałka, K. Carotenoids as modulators of lipid membrane physical properties. Biochim. Biophys. Acta Mol. Basis Dis. 2005, 1740, 108–115. [Google Scholar] [CrossRef]
- Pashkow, F.J.; Watumull, D.G.; Campbell, C.L. Astaxanthin: A Novel Potential Treatment for Oxidative Stress and Inflammation in Cardiovascular Disease. Am. J. Cardiol. 2008, 101, S58–S68. [Google Scholar] [CrossRef] [PubMed]
- Kishimoto, Y.; Yoshida, H.; Kondo, K. Potential Anti-Atherosclerotic Properties of Astaxanthin. Mar. Drugs 2016, 14, 35. [Google Scholar] [CrossRef]
- Ni, Y.; Zhuge, F.; Nagashimada, M.; Ota, T. Novel Action of Carotenoids on Non-Alcoholic Fatty Liver Disease: Macrophage Polarization and Liver Homeostasis. Nutrients 2016, 8, 391. [Google Scholar] [CrossRef] [PubMed]
- Faraone, I.; Sinisgalli, C.; Ostuni, A.; Armentano, M.F.; Carmosino, M.; Milella, L.; Russo, D.; Labanca, F.; Khan, H. Astaxanthin anticancer effects are mediated through multiple molecular mechanisms: A systematic review. Pharmacol. Res. 2020, 155, 104689. [Google Scholar] [CrossRef] [PubMed]
- Grimmig, B.; Kim, S.-H.; Nash, K.; Bickford, P.C.; Douglas Shytle, R. Neuroprotective mechanisms of astaxanthin: A potential therapeutic role in preserving cognitive function in age and neurodegeneration. GeroScience 2017, 39, 19–32. [Google Scholar] [CrossRef] [PubMed]
- Guiry, M.D.; Guiry, G.M. Haematococcus pluvialis Flotow 1844. Available online: http://www.algaebase.org (accessed on 18 August 2020).
- Hazen, T.E. The Life History of Sphaerella lacustris (Haematococcus pluvialis). Mem. Torrey Bot. Club 1899, 6, 211–246. [Google Scholar]
- Von Flotow, J. Observations about Haematococcus pluvialis. Negotiations of the Imperial Leopoldine-Carolinian German Academy of Natural Scientists 1844, 12, 413–606. Available online: https://img.algaebase.org/pdf/25E4D8D713c7824DEEYo81EE1F1A/44486.pdf (accessed on 8 October 2020).
- Pocock, M.A. Haematococcus in Southern Africa. Trans. R. Soc. S. Afr. 1960, 36, 5–55. [Google Scholar] [CrossRef]
- Pringsheim, E.G. Nutritional requirements of Haematococcus pluvialis and related species. J. Phycol. 1966, 2, 1–7. [Google Scholar] [CrossRef]
- Shah, M.M.R.; Liang, Y.; Cheng, J.J.; Daroch, M. Astaxanthin-Producing Green Microalga Haematococcus pluvialis: From Single Cell to High Value Commercial Products. Front. Plant Sci. 2016, 7, 531. [Google Scholar] [CrossRef]
- Dragos, N.; Bercea, V.; Bica, A.; Drugă, B.; Nicoară, A.; Coman, C. Astaxanthin production from a new strain of haematococcus pluvialis grown in batch culture. Ann. Rom. Soc. Cell Biol. 2010, 15, 353–361. [Google Scholar]
- Klochkova, T.A.; Kwak, M.S.; Han, J.W.; Motomura, T.; Nagasato, C.; Kim, G.H. Cold-tolerant strain of Haematococcus pluvialis (Haematococcaceae, Chlorophyta) from Blomstrandhalvøya (Svalbard). ALGAE 2013, 28, 185–192. [Google Scholar] [CrossRef]
- Chekanov, K.; Lobakova, E.; Selyakh, I.; Semenova, L.; Sidorov, R.; Solovchenko, A. Accumulation of Astaxanthin by a New Haematococcus pluvialis Strain BM1 from the White Sea Coastal Rocks (Russia). Mar. Drugs 2014, 12, 4504–4520. [Google Scholar] [CrossRef] [PubMed]
- Gacheva, G.; Dimitrova, P.; Pilarski, P. New strain Haematococcus cf. pluvialis Rozhen-12—Growth, biochemical characteristics and future perspectives. Genet. Plant Physiol. 2015, 5, 29–38. [Google Scholar]
- Kim, J.H.; Affan, M.A.; Jang, J.; Kang, M.-H.; Ko, A.-R.; Jeon, S.-M.; Oh, C.; Heo, S.-J.; Lee, Y.-H.; Ju, S.-J.; et al. Morphological, Molecular, and Biochemical Characterization of Astaxanthin-Producing Green Microalga Haematococcus sp. KORDI03 (Haematococcaceae, Chlorophyta) Isolated from Korea. J. Microbiol. Biotechnol. 2015, 25, 238–246. [Google Scholar] [CrossRef] [PubMed]
- Hagen, C.; Siegmund, S.; Braune, W. Ultrastructural and chemical changes in the cell wall of Haematococcus pluvialis (Volvocales, Chlorophyta) during aplanospore formation. Euro. J. Phycol. 2002, 37, 217–226. [Google Scholar] [CrossRef]
- Wayama, M.; Ota, S.; Matsuura, H.; Nango, N.; Hirata, A.; Kawano, S. Three-Dimensional Ultrastructural Study of Oil and Astaxanthin Accumulation during Encystment in the Green Alga Haematococcus pluvialis. PLoS ONE 2013, 8, e53618. [Google Scholar] [CrossRef]
- Schoefs, B.; Rmiki, N.-E.; Rachadi, J.; Lemoine, Y. Astaxanthin accumulation in Haematococcus requires a cytochrome P450 hydroxylase and an active synthesis of fatty acids. FEBS Lett. 2001, 500, 125–128. [Google Scholar] [CrossRef]
- Collins, A.M.; Jones, H.D.T.; Han, D.; Hu, Q.; Beechem, T.E.; Timlin, J.A. Carotenoid Distribution in Living Cells of Haematococcus pluvialis (Chlorophyceae). PLoS ONE 2011, 6, e24302. [Google Scholar] [CrossRef]
- Harker, M.; Tsavalos, A.J.; Young, A.J. Factors responsible for astaxanthin formation in the Chlorophyte Haematococcus pluvialis. Bioresour. Technol. 1996, 55, 207–214. [Google Scholar] [CrossRef]
- Torzillo, G.; Goksan, T.; Faraloni, C.; Kopecky, J.; Masojídek, J. Interplay between photochemical activities and pigment composition in an outdoor culture of Haematococcus pluvialis during the shift from the green to red stage. J. Appl. Phycol. 2003, 15, 127–136. [Google Scholar] [CrossRef]
- Han, D.; Wang, J.; Sommerfeld, M.; Hu, Q. Susceptibility and protective mechanisms of motile and non motile cells of Haematococcus pluvialis (Chlorophyceae) to photooxidative stress. J. Phycol. 2012, 48, 693–705. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K. The 1-deoxy-D-xylulose-5-phosphate pathway of isoprenoid biosynthesis in plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1999, 50, 47–65. [Google Scholar] [CrossRef] [PubMed]
- Disch, A.; Schwender, J.; Müller, C.; Lichtenthaler, H.K.; Rohmer, M. Distribution of the mevalonate and glyceraldehyde phosphate/pyruvate pathways for isoprenoid biosynthesis in unicellular algae and the cyanobacterium Synechocystis PCC 6714. Biochem. J. 1998, 333, 381–388. [Google Scholar] [CrossRef] [PubMed]
- Lemoine, Y.; Schoefs, B. Secondary ketocarotenoid astaxanthin biosynthesis in algae: A multifunctional response to stress. Photosynth. Res. 2010, 106, 155–177. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.J.; Lin, S.; Xu, W.; Cheung, P.C.K. Occurrence and biosynthesis of carotenoids in phytoplankton. Biotechnol. Adv. 2017, 35, 597–618. [Google Scholar] [CrossRef]
- Carol, P.; Stevenson, D.; Bisanz, C.; Breitenbach, J.; Sandmann, G.; Mache, R.; Coupland, G.; Kuntz, M. Mutations in the Arabidopsis Gene IMMUTANS Cause a Variegated Phenotype by Inactivating a Chloroplast Terminal Oxidase Associated with Phytoene Desaturation. Plant Cell 1999, 11, 57. [Google Scholar] [CrossRef]
- Wu, D.; Wright, D.A.; Wetzel, C.; Voytas, D.F.; Rodermel, S. The IMMUTANS Variegation Locus of Arabidopsis Defines a Mitochondrial Alternative Oxidase Homolog That Functions during Early Chloroplast Biogenesis. Plant Cell 1999, 11, 43–55. [Google Scholar] [CrossRef]
- Grünewald, K.; Hirschberg, J.; Hagen, C. Ketocarotenoid Biosynthesis Outside of Plastids in the Unicellular Green Alga Haematococcus pluvialis. J. Biol. Chem. 2001, 276, 6023–6029. [Google Scholar] [CrossRef]
- Ye, L.; Zhu, X.; Wu, T.; Wang, W.; Zhao, D.; Bi, C.; Zhang, X. Optimizing the localization of astaxanthin enzymes for improved productivity. Biotechnol. Biofuels 2018, 11, 278. [Google Scholar] [CrossRef]
- Chen, G.; Wang, B.; Han, D.; Sommerfeld, M.; Lu, Y.; Chen, F.; Hu, Q. Molecular mechanisms of the coordination between astaxanthin and fatty acid biosynthesis in Haematococcus pluvialis (Chlorophyceae). Plant J. 2015, 81, 95–107. [Google Scholar] [CrossRef]
- Asada, K. Production and Scavenging of Reactive Oxygen Species in Chloroplasts and Their Functions: Figure 1. Plant Physiol. 2006, 141, 391–396. [Google Scholar] [CrossRef]
- Wang, S.-B.; Chen, F.; Sommerfeld, M.; Hu, Q. Proteomic analysis of molecular response to oxidative stress by the green alga Haematococcus pluvialis (Chlorophyceae). Planta 2004, 220, 17–29. [Google Scholar] [CrossRef] [PubMed]
- Hagen, C.; Braune, W.; Bjorn, L.O. Functional aspects of secondary carotenoids in Haematococcus lacustris (Volvocales) III. Action as a sunshade. J. Phycol. 1994, 30, 241–248. [Google Scholar] [CrossRef]
- Ota, S.; Morita, A.; Ohnuki, S.; Hirata, A.; Sekida, S.; Okuda, K.; Ohya, Y.; Kawano, S. Carotenoid dynamics and lipid droplet containing astaxanthin in response to light in the green alga Haematococcus pluvialis. Sci. Rep. 2018, 8, 5617. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Sommerfeld, M.; Chen, F.; Hu, Q. Consumption of oxygen by astaxanthin biosynthesis: A protective mechanism against oxidative stress in Haematococcus pluvialis (Chlorophyceae). J. Plant Physiol. 2008, 165, 1783–1797. [Google Scholar] [CrossRef] [PubMed]
- Hagen, C.; Braune, W.; Greulich, F. Functional aspects of secondary carotenoids in Haematococcus lacustris [Girod] Rostafinski (Volvocales) IV. Protection from photodynamic damage. J. Photochem. Photobiol. B 1993, 20, 153–160. [Google Scholar] [CrossRef]
- Kobayashi, M.; Kakizono, T.; Nishio, N.; Nagai, S.; Kurimura, Y.; Tsuji, Y. Antioxidant role of astaxanthin in the green alga Haematococcus pluvialis. Appl. Microbiol. Biotechnol. 1997, 48, 351–356. [Google Scholar] [CrossRef]
- Kobayashi, M.; Sakamoto, Y. Singlet oxygen quenching ability of astaxanthin esters from the green alga. Biotechnol. Lett. 1999, 21, 265–269. [Google Scholar] [CrossRef]
- Hu, C.; Cui, D.; Sun, X.; Shi, J.; Xu, N. Primary metabolism is associated with the astaxanthin biosynthesis in the green algae Haematococcus pluvialis under light stress. Algal Res. 2020, 46, 101768. [Google Scholar] [CrossRef]
- Boussiba, S.; Vonshak, A. Astaxanthin Accumulation in the Green Alga Haematococcus pluvialis. Plant Cell Physiol. 1991, 32, 1077–1082. [Google Scholar] [CrossRef]
- Wang, B.; Zhang, Z.; Hu, Q.; Sommerfeld, M.; Lu, Y.; Han, D. Cellular Capacities for High-Light Acclimation and Changing Lipid Profiles across Life Cycle Stages of the Green Alga Haematococcus pluvialis. PLoS ONE 2014, 9, e106679. [Google Scholar] [CrossRef][Green Version]
- Recht, L.; Zarka, A.; Boussiba, S. Patterns of carbohydrate and fatty acid changes under nitrogen starvation in the microalgae Haematococcus pluvialis and Nannochloropsis sp. Appl. Microbiol. Biotechnol. 2012, 94, 1495–1503. [Google Scholar] [CrossRef] [PubMed]
- Saha, S.K.; McHugh, E.; Hayes, J.; Moane, S.; Walsh, D.; Murray, P. Effect of various stress-regulatory factors on biomass and lipid production in microalga Haematococcus pluvialis. Bioresour. Technol. 2013, 128, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Damiani, M.C.; Popovich, C.A.; Constenla, D.; Leonardi, P.I. Lipid analysis in Haematococcus pluvialis to assess its potential use as a biodiesel feedstock. Bioresour. Technol. 2010, 101, 3801–3807. [Google Scholar] [CrossRef] [PubMed]
- Zhekisheva, M.; Boussiba, S.; Khozin-Goldberg, I.; Zarka, A.; Cohen, Z. Accumulation of oleic acid in Haematococcus pluvialis (Chlorophyceae) under nitrogen starvation or high light is correlated with that of astaxanthin esters. J. Phycol. 2002, 38, 325–331. [Google Scholar] [CrossRef]
- Liang, C.; Zhai, Y.; Xu, D.; Ye, N.; Zhang, X.; Wang, Y.; Zhang, W.; Yu, J. Correlation between lipid and carotenoid synthesis and photosynthetic capacity in Haematococcus pluvialis grown under high light and nitrogen deprivation stress. Grasas Aceites 2015, 66, e077. [Google Scholar] [CrossRef]
- López, M.C.G.-M.; Sánchez, E.D.R.; López, J.L.C.; Fernández, F.G.A.; Sevilla, J.M.F.; Rivas, J.; Guerrero, M.G.; Grima, E.M. Comparative analysis of the outdoor culture of Haematococcus pluvialis in tubular and bubble column photobioreactors. J. Biotechnol. 2006, 123, 329–342. [Google Scholar] [CrossRef]
- Harker, M.; Tsavalos, A.J.; Young, A.J. Autotrophic growth and carotenoid production of Haematococcus pluvialis in a 30 liter air-lift photobioreactor. J. Ferment. Bioeng. 1996, 82, 113–118. [Google Scholar] [CrossRef]
- Boussiba, S.; Bing, W.; Yuan, J.-P.; Zarka, A.; Chen, F. Changes in pigments profile in the green alga Haematococcus pluvialis exposed to environmental stresses. Biotechnol. Lett. 1999, 21, 601–604. [Google Scholar] [CrossRef]
- Aflalo, C.; Meshulam, Y.; Zarka, A.; Boussiba, S. On the relative efficiency of two- vs. one-stage production of astaxanthin by the green alga Haematococcus pluvialis. Biotechnol. Bioeng. 2007, 98, 300–305. [Google Scholar] [CrossRef]
- Grung, M.; D’Souza, F.M.L.; Borowitzka, M.; Liaaen-Jensen, S. Algal Carotenoids 51. Secondary carotenoids 2. Haematococcus pluvialis aplanospores as a source of (3S, 3′S)-astaxanthin esters. J. Appl. Phycol. 1992, 4, 165–171. [Google Scholar] [CrossRef]
- Fan, L.; Vonshak, A.; Gabbay, R.; Hirschberg, J.; Cohen, Z.; Boussiba, S. The Biosynthetic Pathway of Astaxanthin in a Green Alga Haematococcus pluvialis as Indicated by Inhibition with Diphenylamine. Plant Cell Physiol. 1995, 36, 1519–1524. [Google Scholar] [CrossRef][Green Version]
- Hata, N.; Ogbonna, J.C.; Hasegawa, Y.; Taroda, H.; Tanaka, H. Production of astaxanthin by Haematococcus pluvialis in a sequential heterotrophic-photoautotrophic culture. J. Appl. Phycol. 2001, 13, 395–402. [Google Scholar] [CrossRef]
- Del Río, E.D.; Acién, F.G.; García-Malea, M.C.; Rivas, J.; Molina-Grima, E.; Guerrero, M.G. Efficient one-step production of astaxanthin by the microalga Haematococcus pluvialis in continuous culture. Biotechnol. Bioeng. 2005, 91, 808–815. [Google Scholar] [CrossRef] [PubMed]
- Ranjbar, R.; Inoue, R.; Katsuda, T.; Yamaji, H.; Katoh, S. High efficiency production of astaxanthin in an airlift photobioreactor. J. Biosci. Bioeng. 2008, 106, 204–207. [Google Scholar] [CrossRef]
- Wang, J.; Han, D.; Sommerfeld, M.R.; Lu, C.; Hu, Q. Effect of initial biomass density on growth and astaxanthin production of Haematococcus pluvialis in an outdoor photobioreactor. J. Appl. Phycol. 2013, 25, 253–260. [Google Scholar] [CrossRef]
- Yoo, J.J.; Choi, S.P.; Kim, B.W.; Sim, S.J. Optimal design of scalable photo-bioreactor for phototropic culturing of Haematococcus pluvialis. Bioprocess Biosyst. Eng. 2012, 35, 309–315. [Google Scholar] [CrossRef]
- Kang, C.D.; Han, S.J.; Choi, S.P.; Sim, S.J. Fed-batch culture of astaxanthin-rich Haematococcus pluvialis by exponential nutrient feeding and stepwise light supplementation. Bioprocess Biosyst. Eng. 2010, 33, 133–139. [Google Scholar] [CrossRef]
- Zhang, B.Y.; Geng, Y.H.; Li, Z.K.; Hu, H.J.; Li, Y.G. Production of astaxanthin from Haematococcus in open pond by two-stage growth one-step process. Aquaculture 2009, 295, 275–281. [Google Scholar] [CrossRef]
- Aslanbay Guler, B.; Deniz, I.; Demirel, Z.; Imamoglu, E. Computational fluid dynamics simulation in scaling-up of airlift photobioreactor for astaxanthin production. J. Biosci. Bioeng. 2020, 129, 86–92. [Google Scholar] [CrossRef]
- Olaizola, M. Commercial production of astaxanthin from Haematococcus pluvialis using 25,000-liter outdoor photobioreactors. J. Appl. Phycol. 2000, 12, 499–506. [Google Scholar] [CrossRef]
- García-Malea, M.C.; Acién, F.G.; Del Río, E.; Fernández, J.M.; Cerón, M.C.; Guerrero, M.G.; Molina-Grima, E. Production of astaxanthin by Haematococcus pluvialis: Taking the one-step system outdoors. Biotechnol. Bioeng. 2009, 102, 651–657. [Google Scholar] [CrossRef] [PubMed]
- García-Malea, M.C.; Acién, F.G.; Fernández, J.M.; Cerón, M.C.; Molina, E. Continuous production of green cells of Haematococcus pluvialis: Modeling of the irradiance effect. Enzyme Microb. Technol. 2006, 38, 981–989. [Google Scholar] [CrossRef]
- Nichols, H.W.; Bold, H.C. Trichosarcina polymorpha Gen. et Sp. Nov. J. Phycol. 1965, 1, 34–38. [Google Scholar] [CrossRef]
- Rippka, R.; Deruelles, J.; Waterbury, J.B.; Herdman, M.; Stanier, R.Y. Generic Assignments, Strain Histories and Properties of Pure Cultures of Cyanobacteria. J. Gen. Microbiol. 1979, 111, 1–61. [Google Scholar] [CrossRef]
- Zhao, Y.; Yue, C.; Geng, S.; Ning, D.; Ma, T.; Yu, X. Role of media composition in biomass and astaxanthin production of Haematococcus pluvialis under two-stage cultivation. Bioprocess Biosyst. Eng. 2019, 42, 593–602. [Google Scholar] [CrossRef] [PubMed]
- Fábregas, J.; Domínguez, A.; Regueiro, M.; Maseda, A.; Otero, A. Optimization of culture medium for the continuous cultivation of the microalga Haematococcus pluvialis. Appl. Microbiol. Biotechnol. 2000, 53, 530–535. [Google Scholar] [CrossRef]
- Hagen, C.; Grünewald, K.; Schmidt, S.; Müller, J. Accumulation of secondary carotenoids in flagellates of Haematococcus pluvialis (Chlorophyta) is accompanied by an increase in per unit chlorophyll productivity of photosynthesis. Eur. J. Phycol. 2000, 35, 75–82. [Google Scholar] [CrossRef]
- Del Río, E.; Acién, F.G.; García-Malea, M.C.; Rivas, J.; Molina-Grima, E.; Guerrero, M.G. Efficiency assessment of the one-step production of astaxanthin by the microalga Haematococcus pluvialis. Biotechnol. Bioeng. 2008, 100, 397–402. [Google Scholar] [CrossRef]
- Kwak, H.S.; Kim, J.Y.H.; Sim, S.J. A Microreactor System for Cultivation of Haematococcus pluvialis and Astaxanthin Production. J. Nanosci. Nanotechnol. 2015, 15, 1618–1623. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, J.; Wang, J.; Liu, T. Attached cultivation of Haematococcus pluvialis for astaxanthin production. Bioresour. Technol. 2014, 158, 329–335. [Google Scholar] [CrossRef]
- Kiperstok, A.C.; Sebestyén, P.; Podola, B.; Melkonian, M. Biofilm cultivation of Haematococcus pluvialis enables a highly productive one-phase process for astaxanthin production using high light intensities. Algal Res. 2017, 21, 213–222. [Google Scholar] [CrossRef]
- Kobayashi, M.; Kakizono, T.; Nagai, S. Astaxanthin production by a green alga, Haematococcus pluvialis accompanied with morphological changes in acetate media. J. Ferment. Bioeng. 1991, 71, 335–339. [Google Scholar] [CrossRef]
- Kobayashi, M.; Kakizono, T.; Yamaguchi, K.; Nishio, N.; Nagai, S. Growth and astaxanthin formation of Haematococcus pluvialis in heterotrophic and mixotrophic conditions. J. Ferment. Bioeng. 1992, 74, 17–20. [Google Scholar] [CrossRef]
- Chen, F.; Chen, H.; Gong, X. Mixotrophic and heterotrophic growth of Haematococcus lacustris and rheological behaviour of the cell suspensions. Bioresour. Technol. 1997, 62, 19–24. [Google Scholar] [CrossRef]
- Zhang, X.W.; Gong, X.-D.; Chen, F. Kinetic models for astaxanthin production by high cell density mixotrophic culture of the microalga Haematococcus pluvialis. J. Ind. Microbiol. Biotechnol. 1999, 23, 691–696. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Wang, B.; Hu, Q.; Sommerfeld, M.; Li, Y.; Han, D. A new paradigm for producing astaxanthin from the unicellular green alga Haematococcus pluvialis. Biotechnol. Bioeng. 2016, 113, 2088–2099. [Google Scholar] [CrossRef]
- Pan-utai, W.; Parakulsuksatid, P.; Phomkaivon, N. Effect of inducing agents on growth and astaxanthin production in Haematococcus pluvialis: Organic and inorganic. Biocatal. Agric. Biotechnol. 2017, 12, 152–158. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, C.; Liu, J.; Yang, N. A strategy for stimulating astaxanthin and lipid production in Haematococcus pluvialis by exogenous glycerol application under low light. Algal Res. 2020, 46, 101779. [Google Scholar] [CrossRef]
- Onorato, C.; Rösch, C. Comparative life cycle assessment of astaxanthin production with Haematococcus pluvialis in different photobioreactor technologies. Algal Res. 2020, 50, 102005. [Google Scholar] [CrossRef]
- Giannelli, L.; Yamada, H.; Katsuda, T.; Yamaji, H. Effects of temperature on the astaxanthin productivity and light harvesting characteristics of the green alga Haematococcus pluvialis. J. Biosci. Bioeng. 2015, 119, 345–350. [Google Scholar] [CrossRef]
- Fan, L.; Vonshak, A.; Boussiba, S. Effect of temperature and irradiance on growth of Haematococcus pluvialis (Chlorophyceae). J. Phycol. 1994, 30, 829–833. [Google Scholar] [CrossRef]
- Cifuentes, A.S.; González, M.A.; Vargas, S.; Hoeneisen, M.; González, N. Optimization of biomass, total carotenoids and astaxanthin production in Haematococcus pluvialis Flotow strain Steptoe (Nevada, USA) under laboratory conditions. Biol. Res. 2003, 36. [Google Scholar] [CrossRef] [PubMed]
- Harker, M.; Tsavalos, A.J.; Young, A.J. Use of response surface methodology to optimise carotenogenesis in the microalga, Haematococcus pluvialis. J. Appl. Phycol. 1995, 7, 399–406. [Google Scholar] [CrossRef]
- Choi, Y.E.; Yun, Y.-S.; Park, J.M. Evaluation of Factors Promoting Astaxanthin Production by a Unicellular Green Alga, Haematococcus pluvialis, with Fractional Factorial Design. Biotechnol. Prog. 2002, 18, 1170–1175. [Google Scholar] [CrossRef]
- Ma, R.; Thomas-Hall, S.R.; Chua, E.T.; Eltanahy, E.; Netzel, M.E.; Netzel, G.; Lu, Y.; Schenk, P.M. Blue light enhances astaxanthin biosynthesis metabolism and extraction efficiency in Haematococcus pluvialis by inducing haematocyst germination. Algal Res. 2018, 35, 215–222. [Google Scholar] [CrossRef]
- Tjahjono, A.E.; Hayama, Y.; Kakizono, T.; Terada, Y.; Nishio, N.; Nagai, S. Hyper-accumulation of astaxanthin in a green alga Haematococcus pluvialis at elevated temperatures. Biotechnol. Lett. 1994, 16, 133–138. [Google Scholar] [CrossRef]
- Borowitzka, M.A.; Huisman, J.M.; Osborn, A. Culture of the astaxanthin-producing green alga Haematococcus pluvialis. J. Appl. Phycol. 1991, 3, 295–304. [Google Scholar] [CrossRef]
- Wan, M.; Zhang, J.; Hou, D.; Fan, J.; Li, Y.; Huang, J.; Wang, J. The effect of temperature on cell growth and astaxanthin accumulation of Haematococcus pluvialis during a light–dark cyclic cultivation. Bioresour. Technol. 2014, 167, 276–283. [Google Scholar] [CrossRef]
- Hwang, S.-W.; Choi, H.I.; Sim, S.J. Acidic cultivation of Haematococcus pluvialis for improved astaxanthin production in the presence of a lethal fungus. Bioresour. Technol. 2019, 278, 138–144. [Google Scholar] [CrossRef]
- Dos Santos, A.C.; Lombardi, A.T. Growth, photosynthesis and biochemical composition of Haematococcus pluvialis at various pH. J. Algal Biomass Util. 2017, 8, 1–15. [Google Scholar]
- Sarada, R.; Tripathi, U.; Ravishankar, G.A. Influence of stress on astaxanthin production in Haematococcus pluvialis grown under different culture conditions. Process Biochem. 2002, 37, 623–627. [Google Scholar] [CrossRef]
- Sarada, R.; Bhattacharya, S.; Ravishankar, G.A. Optimization of culture conditions for growth of the green alga Haematococcus pluvialis. World J. Microbiol. Biotechnol. 2002, 18, 517–521. [Google Scholar] [CrossRef]
- Christian, D.; Zhang, J.; Sawdon, A.J.; Peng, C.-A. Enhanced astaxanthin accumulation in Haematococcus pluvialis using high carbon dioxide concentration and light illumination. Bioresour. Technol. 2018, 256, 548–551. [Google Scholar] [CrossRef] [PubMed]
- Orosa, M. Analysis and enhancement of astaxanthin accumulation in Haematococcus pluvialis. Bioresour. Technol. 2005, 96, 373–378. [Google Scholar] [CrossRef] [PubMed]
- Chekanov, K.; Schastnaya, E.; Solovchenko, A.; Lobakova, E. Effects of CO2 enrichment on primary photochemistry, growth and astaxanthin accumulation in the chlorophyte Haematococcus pluvialis. J. Photochem. Photobiol. B 2017, 171, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.Y.; Hong, M.E.; Jin, E.S.; Woo, H.M.; Sim, S.J. Improvement in modular scalability of polymeric thin-film photobioreactor for autotrophic culturing of Haematococcus pluvialis using industrial flue gas. Bioresour. Technol. 2018, 249, 519–526. [Google Scholar] [CrossRef]
- Kobayashi, M.; Kakizono, T.; Nagai, S. Enhanced Carotenoid Biosynthesis by Oxidative Stress in Acetate-Induced Cyst Cells of a Green Unicellular Alga, Haematococcus pluvialis. Appl. Environ. Microbiol. 1993, 59, 867–873. [Google Scholar] [CrossRef]
- Kobayashi, M.; Kurimura, Y.; Tsuji, Y. Light-independent, astaxanthin production by the green microalga Haematococcus pluvialis under salt stress. Biotechnol. Lett. 1997, 19, 507–509. [Google Scholar] [CrossRef]
- Scibilia, L.; Girolomoni, L.; Berteotti, S.; Alboresi, A.; Ballottari, M. Photosynthetic response to nitrogen starvation and high light in Haematococcus pluvialis. Algal Res. 2015, 12, 170–181. [Google Scholar] [CrossRef]
- Gutman, J.; Zarka, A.; Boussiba, S. The host-range of Paraphysoderma sedebokerensis, a chytrid that infects Haematococcus pluvialis. Eur. J. Phycol. 2009, 44, 509–514. [Google Scholar] [CrossRef]
- Gutman, J.; Zarka, A.; Boussiba, S. Evidence for the involvement of surface carbohydrates in the recognition of Haematococcus pluvialis by the parasitic blastoclad Paraphysoderma sedebokerensis. Fungal Biol. 2011, 115, 803–811. [Google Scholar] [CrossRef]
- Hoffman, Y.; Aflalo, C.; Zarka, A.; Gutman, J.; James, T.Y.; Boussiba, S. Isolation and characterization of a novel chytrid species (phylum Blastocladiomycota), parasitic on the green alga Haematococcus. Mycol. Res. 2008, 112, 70–81. [Google Scholar] [CrossRef] [PubMed]
- Strittmatter, M.; Guerra, T.; Silva, J.; Gachon, C.M.M. A new flagellated dispersion stage in Paraphysoderma sedebokerense, a pathogen of Haematococcus pluvialis. J. Appl. Phycol. 2016, 28, 1553–1558. [Google Scholar] [CrossRef] [PubMed]
- Barros, A.I.; Gonçalves, A.L.; Simões, M.; Pires, J.C.M. Harvesting techniques applied to microalgae: A review. Renew. Sustain. Energy Rev. 2015, 41, 1489–1500. [Google Scholar] [CrossRef]
- Lorenz, R.T.; Cysewski, G.R. Commercial potential for Haematococcus microalgae as a natural source of astaxanthin. Trends Biotechnol. 2000, 18, 160–167. [Google Scholar] [CrossRef]
- Olaizola, M. Commercial development of microalgal biotechnology: From the test tube to the marketplace. Biomol. Eng. 2003, 20, 459–466. [Google Scholar] [CrossRef]
- Pérez-López, P.; González-García, S.; Jeffryes, C.; Agathos, S.N.; McHugh, E.; Walsh, D.; Murray, P.; Moane, S.; Feijoo, G.; Moreira, M.T. Life cycle assessment of the production of the red antioxidant carotenoid astaxanthin by microalgae: From lab to pilot scale. J. Clean. Prod. 2014, 64, 332–344. [Google Scholar] [CrossRef]
- Li, J.; Zhu, D.; Niu, J.; Shen, S.; Wang, G. An economic assessment of astaxanthin production by large scale cultivation of Haematococcus pluvialis. Biotechnol. Adv. 2011, 29, 568–574. [Google Scholar] [CrossRef]
- Landels, A.; Beacham, T.A.; Evans, C.T.; Carnovale, G.; Raikova, S.; Cole, I.S.; Goddard, P.; Chuck, C.; Allen, M.J. Improving electrocoagulation floatation for harvesting microalgae. Algal Res. 2019, 39, 101446. [Google Scholar] [CrossRef]
- Khoo, K.S.; Lee, S.Y.; Ooi, C.W.; Fu, X.; Miao, X.; Ling, T.C.; Show, P.L. Recent advances in biorefinery of astaxanthin from Haematococcus pluvialis. Bioresour. Technol. 2019, 288, 121606. [Google Scholar] [CrossRef]
- Milledge, J.J. Energy Balance and Techno-Economic Assessment of Algal Biofuel Production Systems. Ph.D. Thesis, University of Southampton, Southampton, UK, 2013. [Google Scholar]
- Mata, T.M.; Martins, A.A.; Caetano, N.S. Microalgae for biodiesel production and other applications: A review. Renew. Sustain. Energy Rev. 2010, 14, 217–232. [Google Scholar] [CrossRef]
- Brennan, L.; Owende, P. Biofuels from microalgae—A review of technologies for production, processing, and extractions of biofuels and co-products. Renew. Sustain. Energy Rev. 2010, 14, 557–577. [Google Scholar] [CrossRef]
- Molina Grima, E.; Belarbi, E.-H.; Acién Fernández, F.G.; Robles Medina, A.; Chisti, Y. Recovery of microalgal biomass and metabolites: Process options and economics. Biotechnol. Adv. 2003, 20, 491–515. [Google Scholar] [CrossRef]
- Raposo, M.F.J.; Morais, A.M.M.B.; Morais, R.M.S.C. Effects of spray-drying and storage on astaxanthin content of Haematococcus pluvialis biomass. World J. Microbiol. Biotechnol. 2012, 28, 1253–1257. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, F.; Li, Y.; Fanning, K.; Netzel, M.; Schenk, P.M. Effect of drying, storage temperature and air exposure on astaxanthin stability from Haematococcus pluvialis. Food Res. Int. 2015, 74, 231–236. [Google Scholar] [CrossRef] [PubMed]
- Mercer, P.; Armenta, R.E. Developments in oil extraction from microalgae. Eur. J. Lipid Sci. Technol. 2011, 113, 539–547. [Google Scholar] [CrossRef]
- Show, K.-Y.; Lee, D.-J.; Tay, J.-H.; Lee, T.-M.; Chang, J.-S. Microalgal drying and cell disruption—Recent advances. Bioresour. Technol. 2015, 184, 258–266. [Google Scholar] [CrossRef] [PubMed]
- Greenwell, H.C.; Laurens, L.M.L.; Shields, R.J.; Lovitt, R.W.; Flynn, K.J. Placing microalgae on the biofuels priority list: A review of the technological challenges. J. R. Soc. Interface 2010, 7, 703–726. [Google Scholar] [CrossRef]
- Jaime, L.; Rodríguez-Meizoso, I.; Cifuentes, A.; Santoyo, S.; Suarez, S.; Ibáñez, E.; Señorans, F.J. Pressurized liquids as an alternative process to antioxidant carotenoids’ extraction from Haematococcus pluvialis microalgae. LWT Food Sci. Technol. 2010, 43, 105–112. [Google Scholar] [CrossRef]
- Nobre, B.; Marcelo, F.; Passos, R.; Beirão, L.; Palavra, A.; Gouveia, L.; Mendes, R. Supercritical carbon dioxide extraction of astaxanthin and other carotenoids from the microalga Haematococcus pluvialis. Eur. Food Res. Technol. 2006, 223, 787–790. [Google Scholar] [CrossRef]
- Kim, D.-Y.; Vijayan, D.; Praveenkumar, R.; Han, J.-I.; Lee, K.; Park, J.-Y.; Chang, W.-S.; Lee, J.-S.; Oh, Y.-K. Cell-wall disruption and lipid/astaxanthin extraction from microalgae: Chlorella and Haematococcus. Bioresour. Technol. 2016, 199, 300–310. [Google Scholar] [CrossRef] [PubMed]
- Razon, L.F.; Tan, R.R. Net energy analysis of the production of biodiesel and biogas from the microalgae: Haematococcus pluvialis and Nannochloropsis. Appl. Energy 2011, 88, 3507–3514. [Google Scholar] [CrossRef]
- Mendes-Pinto, M.M.; Raposo, M.F.J.; Bowen, J.; Young, A.J.; Morais, R. Evaluation of different cell disruption processes on encysted cells of Haematococcus pluvialis: Effects on astaxanthin recovery and implications for bio-availability. J. Appl. Phycol. 2001, 13, 19–24. [Google Scholar] [CrossRef]
- Bubrick, P. Production of astaxanthin from Haematococcus. Bioresour. Technol. 1991, 38, 237–239. [Google Scholar] [CrossRef]
- Sarada, R.; Vidhyavathi, R.; Usha, D.; Ravishankar, G.A. An Efficient Method for Extraction of Astaxanthin from Green Alga Haematococcus pluvialis. J. Agric. Food Chem. 2006, 54, 7585–7588. [Google Scholar] [CrossRef]
- Plechkova, N.V.; Seddon, K.R. Applications of ionic liquids in the chemical industry. Chem. Soc. Rev. 2008, 37, 123–150. [Google Scholar] [CrossRef]
- Kim, Y.-H.; Choi, Y.-K.; Park, J.; Lee, S.; Yang, Y.-H.; Kim, H.J.; Park, T.-J.; Hwan Kim, Y.; Lee, S.H. Ionic liquid-mediated extraction of lipids from algal biomass. Bioresour. Technol. 2012, 109, 312–315. [Google Scholar] [CrossRef]
- Choi, S.-A.; Oh, Y.-K.; Lee, J.; Sim, S.J.; Hong, M.E.; Park, J.-Y.; Kim, M.-S.; Kim, S.W.; Lee, J.-S. High-efficiency cell disruption and astaxanthin recovery from Haematococcus pluvialis cyst cells using room-temperature imidazolium-based ionic liquid/water mixtures. Bioresour. Technol. 2019, 274, 120–126. [Google Scholar] [CrossRef]
- Boonnoun, P.; Kurita, Y. Wet Extraction of Lipids and Astaxanthin from Haematococcus pluvialis by Liquefied Dimethyl Ether. J. Nutr. Food Sci. 2014, 4, 305. [Google Scholar] [CrossRef]
- Zou, T.-B.; Jia, Q.; Li, H.-W.; Wang, C.-X.; Wu, H.-F. Response Surface Methodology for Ultrasound-Assisted Extraction of Astaxanthin from Haematococcus pluvialis. Mar. Drugs 2013, 11, 1644–1655. [Google Scholar] [CrossRef]
- Dong, S.; Huang, Y.; Zhang, R.; Wang, S.; Liu, Y. Four Different Methods Comparison for Extraction of Astaxanthin from Green Alga Haematococcus pluvialis. Sci. World J. 2014, 2014, 694305. [Google Scholar] [CrossRef] [PubMed]
- Machmudah, S.; Shotipruk, A.; Goto, M.; Sasaki, M.; Hirose, T. Extraction of Astaxanthin from Haematococcus pluvialis Using Supercritical CO2 and Ethanol as Entrainer. Ind. Eng. Chem. Res. 2006, 45, 3652–3657. [Google Scholar] [CrossRef]
- Pan, J.-L.; Wang, H.-M.; Chen, C.-Y.; Chang, J.-S. Extraction of astaxanthin from Haematococcus pluvialis by supercritical carbon dioxide fluid with ethanol modifier: Supercritical CO2 fluid extraction of astaxanthin from microalgae. Eng. Life Sci. 2012, 12, 638–647. [Google Scholar] [CrossRef]
- Wang, L.; Yang, B.; Yan, B.; Yao, X. Supercritical fluid extraction of astaxanthin from Haematococcus pluvialis and its antioxidant potential in sunflower oil. Innov. Food Sci. Emerg. Technol. 2012, 13, 120–127. [Google Scholar] [CrossRef]
- Reyes, F.A.; Mendiola, J.A.; Ibañez, E.; del Valle, J.M. Astaxanthin extraction from Haematococcus pluvialis using CO2-expanded ethanol. J. Supercrit. Fluids 2014, 92, 75–83. [Google Scholar] [CrossRef]
- Cheng, X.; Qi, Z.; Burdyny, T.; Kong, T.; Sinton, D. Low pressure supercritical CO2 extraction of astaxanthin from Haematococcus pluvialis demonstrated on a microfluidic chip. Bioresour. Technol. 2018, 250, 481–485. [Google Scholar] [CrossRef]
- Molino, A.; Mehariya, S.; Iovine, A.; Larocca, V.; Di Sanzo, G.; Martino, M.; Casella, P.; Chianese, S.; Musmarra, D. Extraction of Astaxanthin and Lutein from Microalga Haematococcus pluvialis in the Red Phase Using CO2 Supercritical Fluid Extraction Technology with Ethanol as Co-Solvent. Mar. Drugs 2018, 16, 432. [Google Scholar] [CrossRef]
- Valderrama, J.O.; Perrut, M.; Majewski, W. Extraction of Astaxantine and Phycocyanine from Microalgae with Supercritical Carbon Dioxide. J. Chem. Eng. Data 2003, 48, 827–830. [Google Scholar] [CrossRef]
- Kang, C.D.; Sim, S.J. Direct extraction of astaxanthin from Haematococcus culture using vegetable oils. Biotechnol. Lett. 2008, 30, 441–444. [Google Scholar] [CrossRef]
- Salatti-Dorado, J.A.; García-Gómez, D.; Rodriguez-Ruiz, V.; Gueguen, V.; Pavon-Djavid, G.; Rubio, S. Multifunctional green supramolecular solvents for cost-effective production of highly stable astaxanthin-rich formulations from Haematococcus pluvialis. Food Chem. 2019, 279, 294–302. [Google Scholar] [CrossRef]
- Dietary Supplements Market Size, Share & Trends Analysis Report by Ingredient (Vitamins, Minerals), by Form, by Application, by End User, by Distribution Channel, by Region, and Segment Forecasts, 2020–2027. Available online: https://www.grandviewresearch.com/industry-analysis/dietary-supplements-market (accessed on 27 September 2020).
- Functional Foods Market Size, Share & Trends Analysis Report By Ingredient (Carotenoids, Prebiotics & Probiotics, Fatty Acids, Dietary Fibers), by Product, by Application, and Segment Forecasts, 2019–2025. Available online: https://www.grandviewresearch.com/industry-analysis/functional-food-market (accessed on 27 September 2020).

| Biological Activities | Experimental Models | References |
|---|---|---|
| antioxidant activity | liposomes | [45,46,47,48] |
| rat, human cells | [49,50,51,52,53,54,55,56] | |
| mouse, rat | [49,50,57,58] | |
| anti-inflammatory activity | mouse, human cells | [59,60,61,62,63,64] |
| mouse, rat | [61,62,65,66,67] | |
| preventive effects on cardiovascular disease | mouse, human cells | [60,68] |
| mouse, rat, rabbit | [50,69,70,71,72,73,74] | |
| prevention effects on diabetes | rat, pig cells | [75,76] |
| mouse, rat | [77,78,79,80,81,82,83] | |
| protective effects on liver | rat, human cells | [53,84,85,86] |
| mouse, rat | [71,87,88,89] | |
| immuno-modulating effects | mouse cells | [90] |
| mouse, dog, cat | [90,91,92,93] | |
| Anti-cancer activity | mouse, rat, human cells | [94,95,96,97] |
| mouse, rat, hamster | [57,66,67,96,98,99,100,101,102,103,104] | |
| effects on nervous system, cerebral and visual functions | mouse, rat, human cells | [49,51,105,106,107,108,109,110,111,112] |
| mouse, rat | [49,69,105,113] |
| Study Design | Population | Duration | Dosage mg/day | Outcomes | Ref. |
|---|---|---|---|---|---|
| open labelled | 24 healthy volunteers | 14 days | 1.8, 3.6, 14.4, 21.6 | ↑ LDL oxidation lag time | [68] |
| randomized double-blinded, placebo-controlled | 40 healthy non-smoking male volunteers | 3 months | 8 | ↓ 12- and 15-hydroxy fatty acids | [41] |
| randomized double-blinded, placebo-controlled | 42 healthy young female subjects | 8 weeks | 2, 8 | ↓ 8-OHdG and CRP, ↑ NK cell cytotoxic activity, ↑ total T and B cells, ↑ IFN-γ and IL-6 | [114] |
| randomized double-blinded | 23 overweight and obese healthy adults | 3 weeks | 5, 20 | ↓ MDA and ISP, ↑ SOD and TAC plasma levels | [44] |
| randomized trial | 39 heavy smoker subjects | 3 weeks | 5, 20, 40 | ↓ MDA and ISP, ↑ SOD and TAC plasma levels | [115] |
| randomized double-blinded, placebo-controlled | 61 healthy subjects with mild hyperlipidemia | 12 weeks | 6, 12, 18 | ↓ triglyceride, ↑ HDL-cholesterol and adiponectin | [116] |
| randomized, double-blinded, placebo-controlled | 30 middle-aged and senior healthy subjects | 12 weeks | 6, 12 | ↓ erythrocyte PLOOH levels | [117] |
| single-blinded, placebo-controlled | 20 healthy adult male subjects | 10 days | 6 | ↓ whole blood transit time | [118] |
| randomized, placebo- controlled | 12 biopsy-confirmed NASH patients | 24 weeks | 12 | improvement of steatohepatitis, ↓ total NAS score | [89] |
| open-labelled | 10 subjects with age-related forgetfulness | 12 weeks | 12 | ↑ performances in CogHealth and P300 cognitive tests | [40] |
| randomized, double-blinded, placebo-controlled | 96 subjects with age-related forgetfulness | 12 weeks | 6, 12 | ↑ scores in the CogHealth and GMLT cognitive tests | [119] |
| randomized, controlled | 27 patients with non-advanced AMD | 12 months | 4 | ↑ multifocal electroretinogram RAD for retinal eccentricity of 0° to 5° | [42] |
| randomized, double-blinded, placebo-controlled | 26 VDT workers | 4 weeks | 5 | improvement of accommodation amplitude | [120] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jannel, S.; Caro, Y.; Bermudes, M.; Petit, T. Novel Insights into the Biotechnological Production of Haematococcus pluvialis-Derived Astaxanthin: Advances and Key Challenges to Allow Its Industrial Use as Novel Food Ingredient. J. Mar. Sci. Eng. 2020, 8, 789. https://doi.org/10.3390/jmse8100789
Jannel S, Caro Y, Bermudes M, Petit T. Novel Insights into the Biotechnological Production of Haematococcus pluvialis-Derived Astaxanthin: Advances and Key Challenges to Allow Its Industrial Use as Novel Food Ingredient. Journal of Marine Science and Engineering. 2020; 8(10):789. https://doi.org/10.3390/jmse8100789
Chicago/Turabian StyleJannel, Samuel, Yanis Caro, Marc Bermudes, and Thomas Petit. 2020. "Novel Insights into the Biotechnological Production of Haematococcus pluvialis-Derived Astaxanthin: Advances and Key Challenges to Allow Its Industrial Use as Novel Food Ingredient" Journal of Marine Science and Engineering 8, no. 10: 789. https://doi.org/10.3390/jmse8100789
APA StyleJannel, S., Caro, Y., Bermudes, M., & Petit, T. (2020). Novel Insights into the Biotechnological Production of Haematococcus pluvialis-Derived Astaxanthin: Advances and Key Challenges to Allow Its Industrial Use as Novel Food Ingredient. Journal of Marine Science and Engineering, 8(10), 789. https://doi.org/10.3390/jmse8100789