Growth Aspects and Biochemical Composition of Synechococcus sp. MK568070 Cultured in Oil Refinery Wastewater
Abstract
1. Introduction
2. Materials and Methods
2.1. Wastewater Properties
2.2. Cyanobacteria Isolation and Culture Maintenance
2.3. NH4+ and Salinity Tolerance Testing
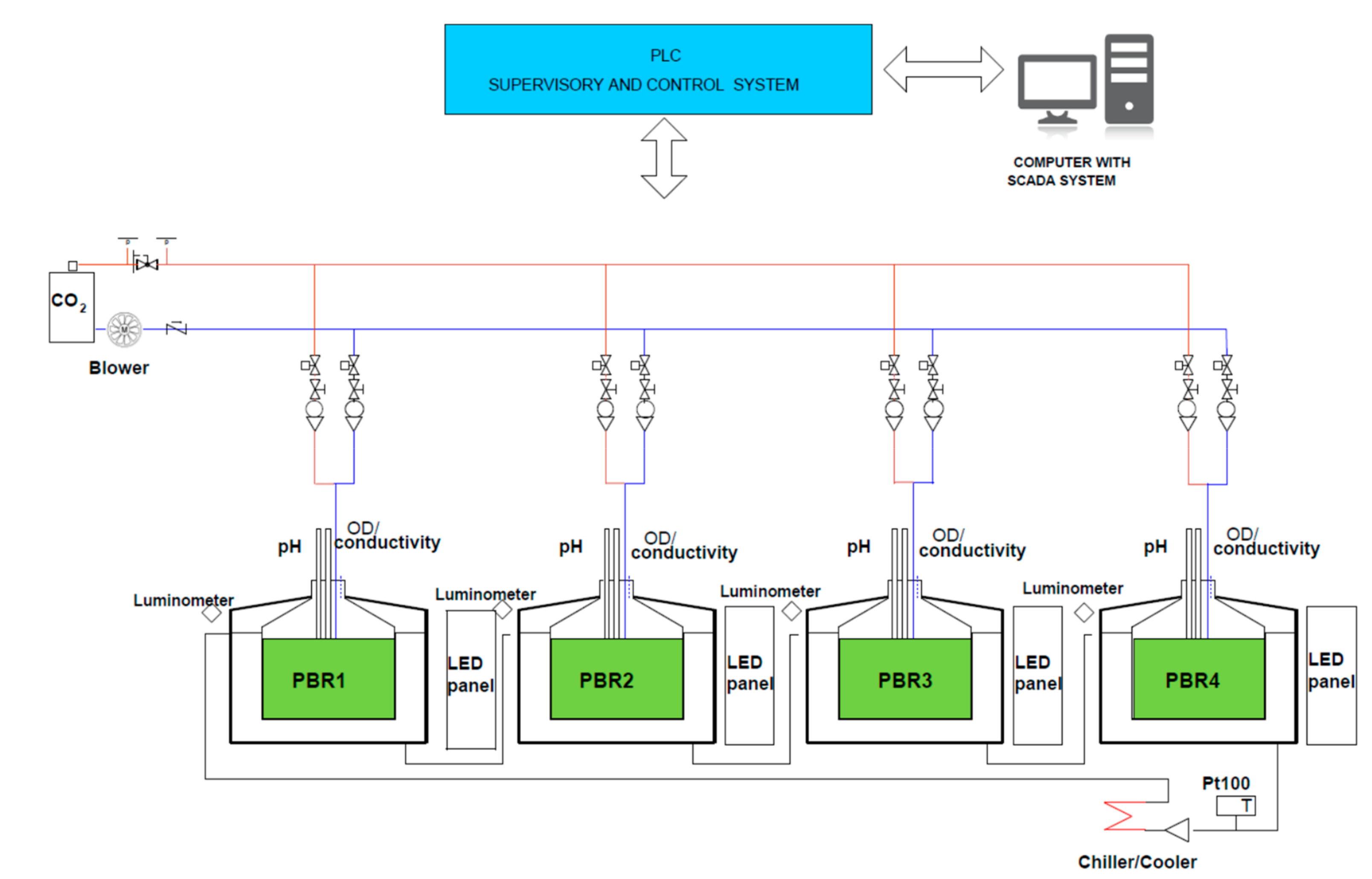
2.4. Experiments in Photobioreactors (PBRs)
2.4.1. Effect of N-Source on Growth of Synechococcus sp. MK568070
2.4.2. Biochemical Aspects of Synechococcus sp. MK568070 Growth on WW
2.5. Analytical Prcedures
2.5.1. BIOMASS Measurements
2.5.2. Nutrient-Determination
2.5.3. Lipid Extraction and Fatty Acid Analysis
2.5.4. Total Carbohydrate Determination
2.5.5. Protein Determination
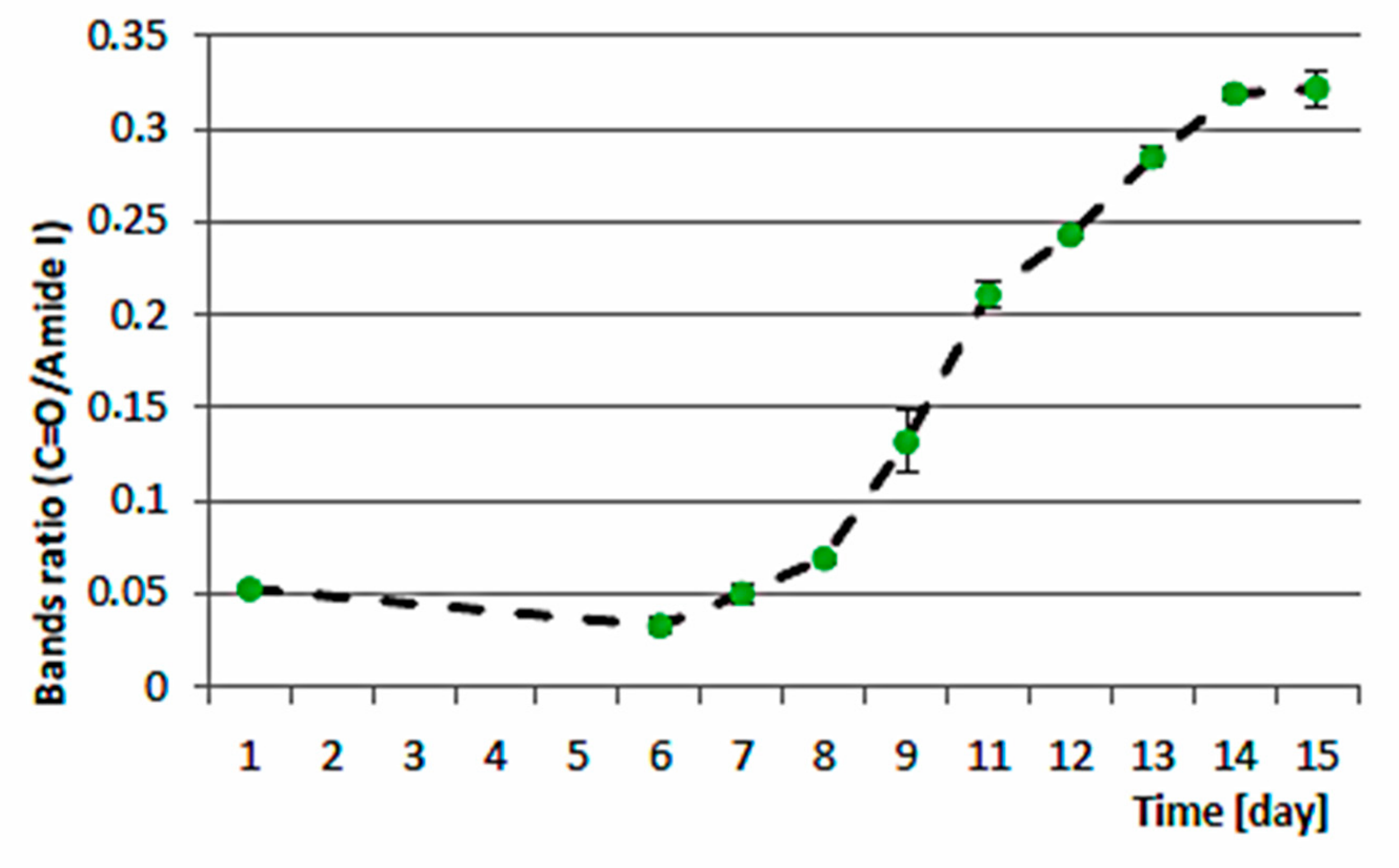
2.5.6. FTIR Analysis
3. Results
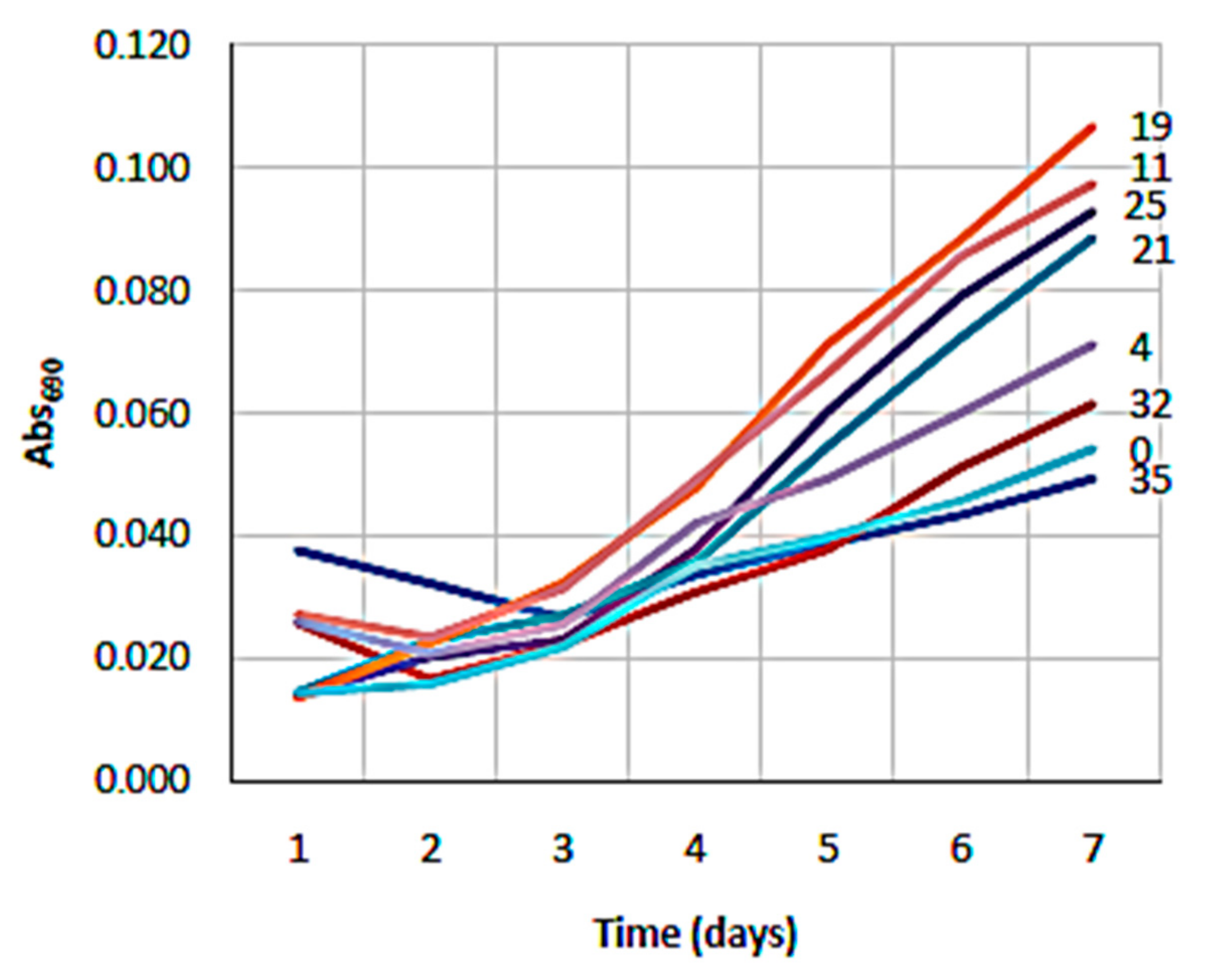
3.1. NH4 and Salinity Tolerance Testing
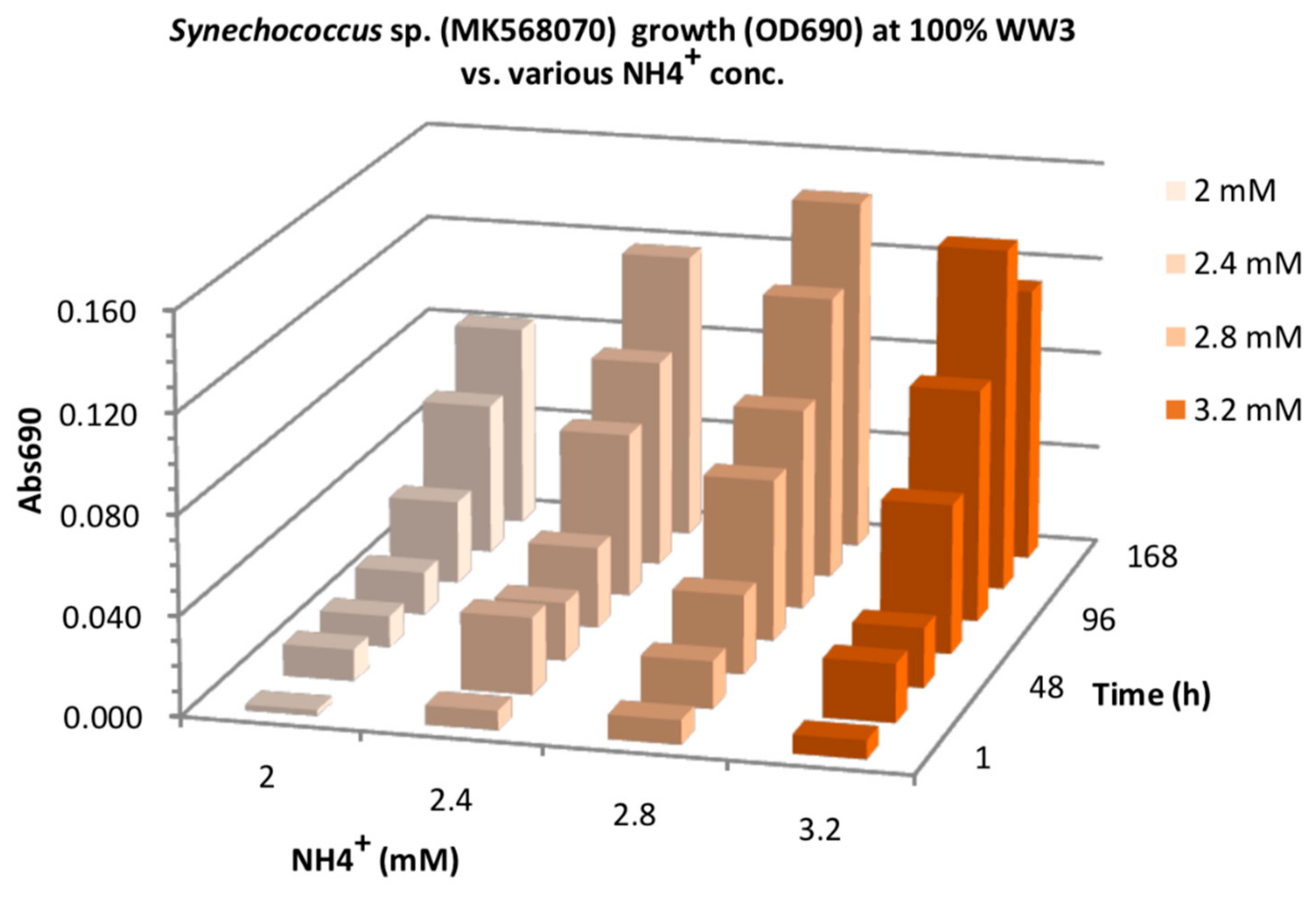
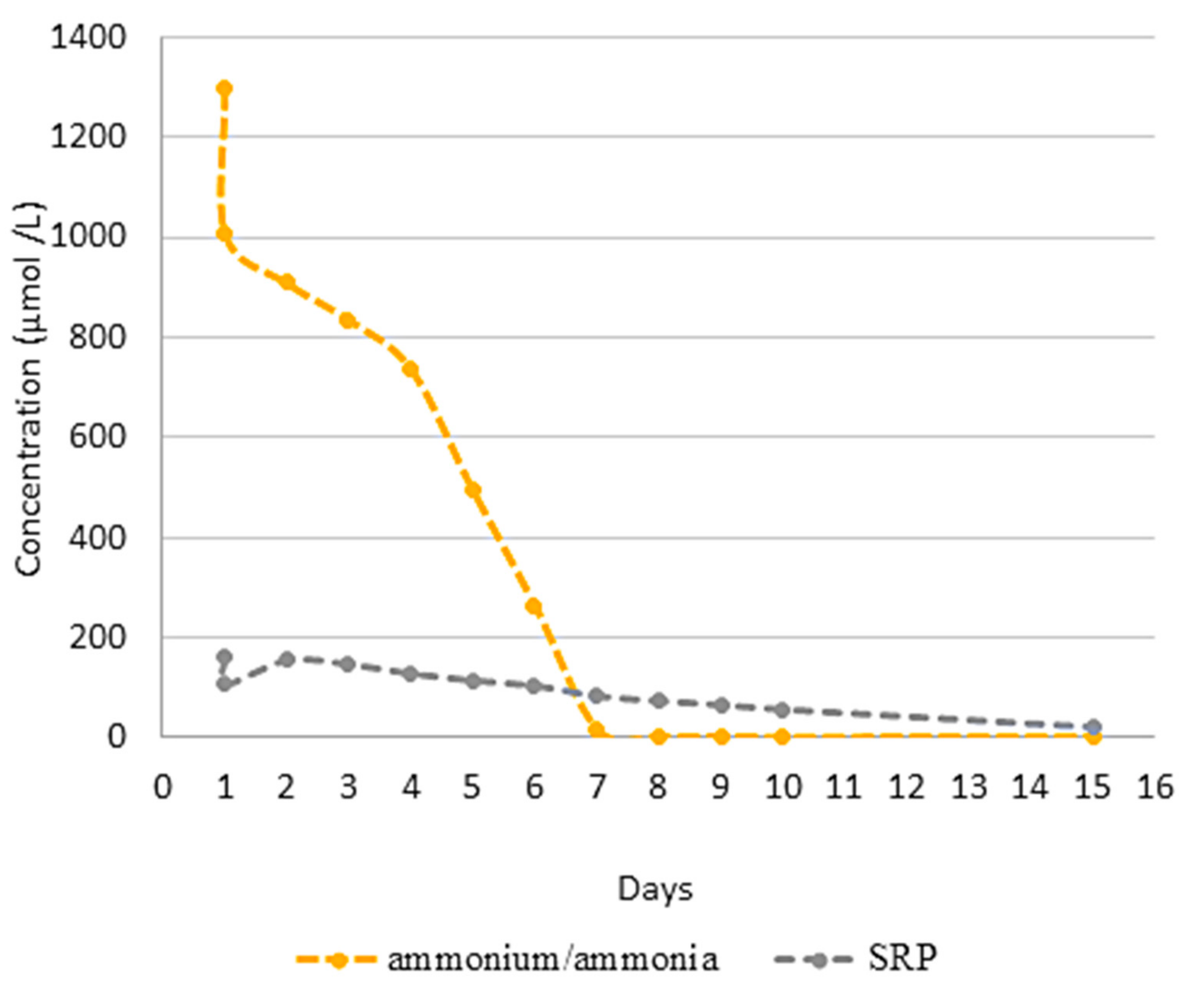
3.2. Effect of N-Source on Growth of Synechococcus sp. MK568070
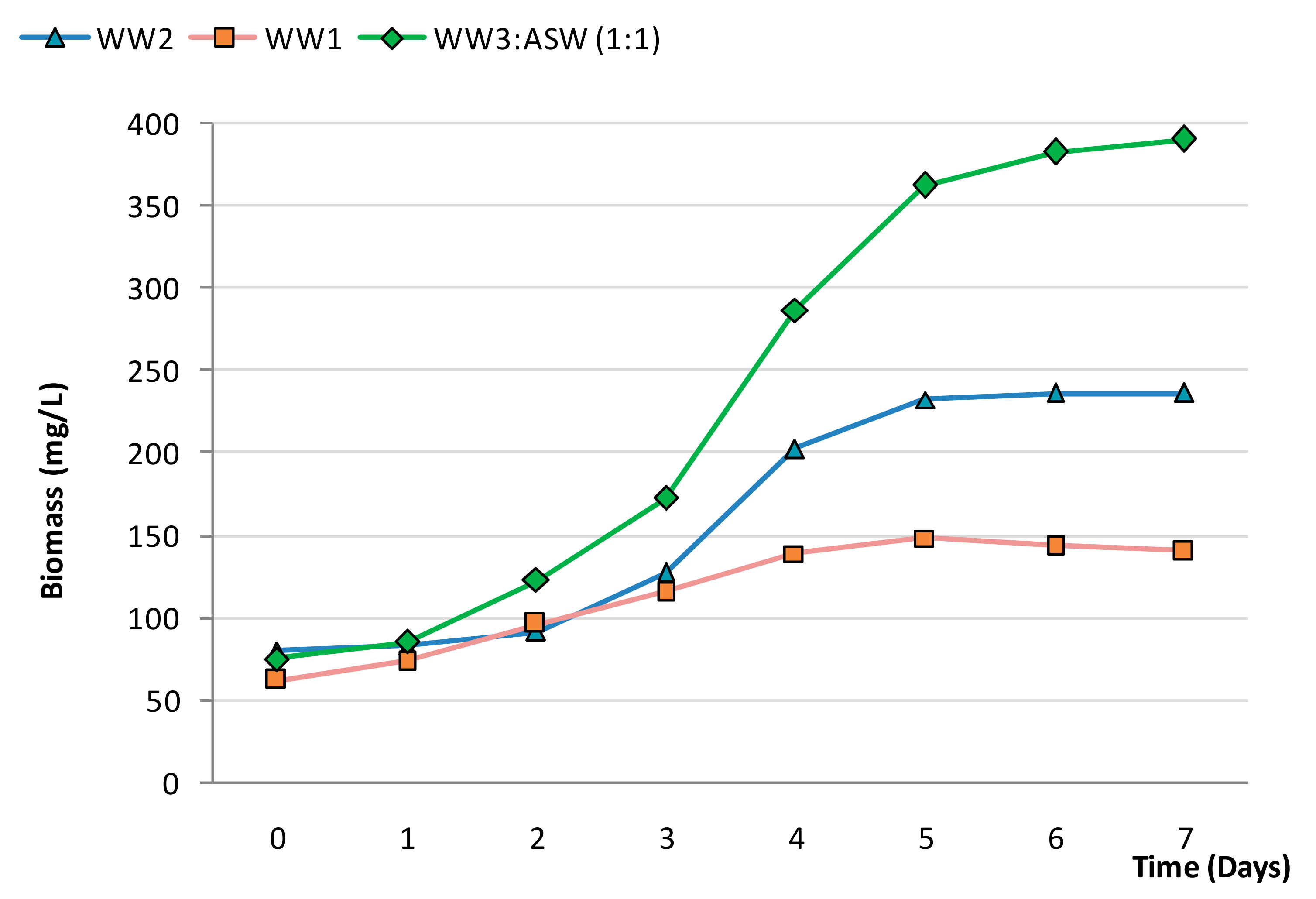
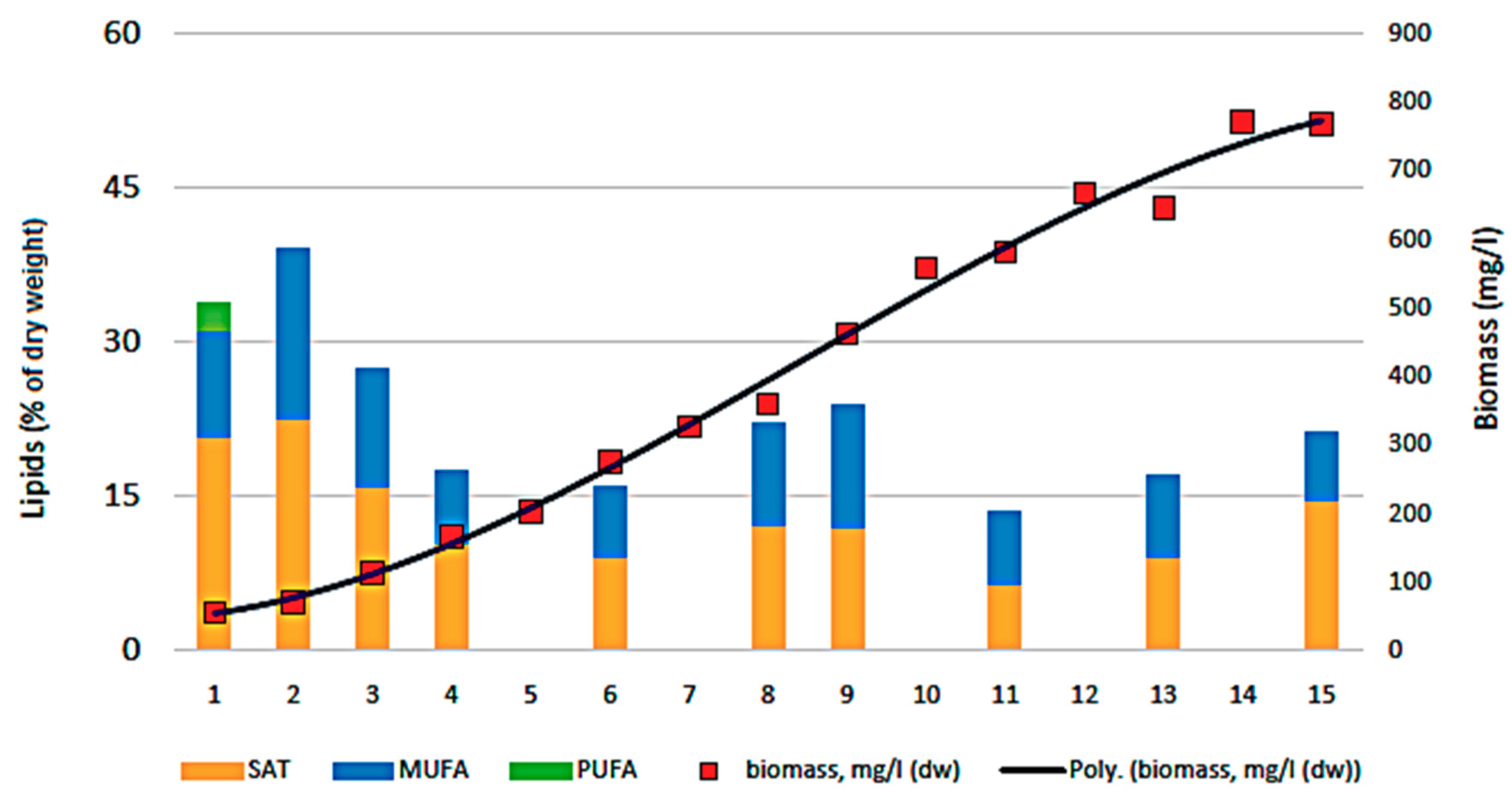
3.3. Biocheimcal Aspects of Synechococcus sp. MK568070 Growth on WW
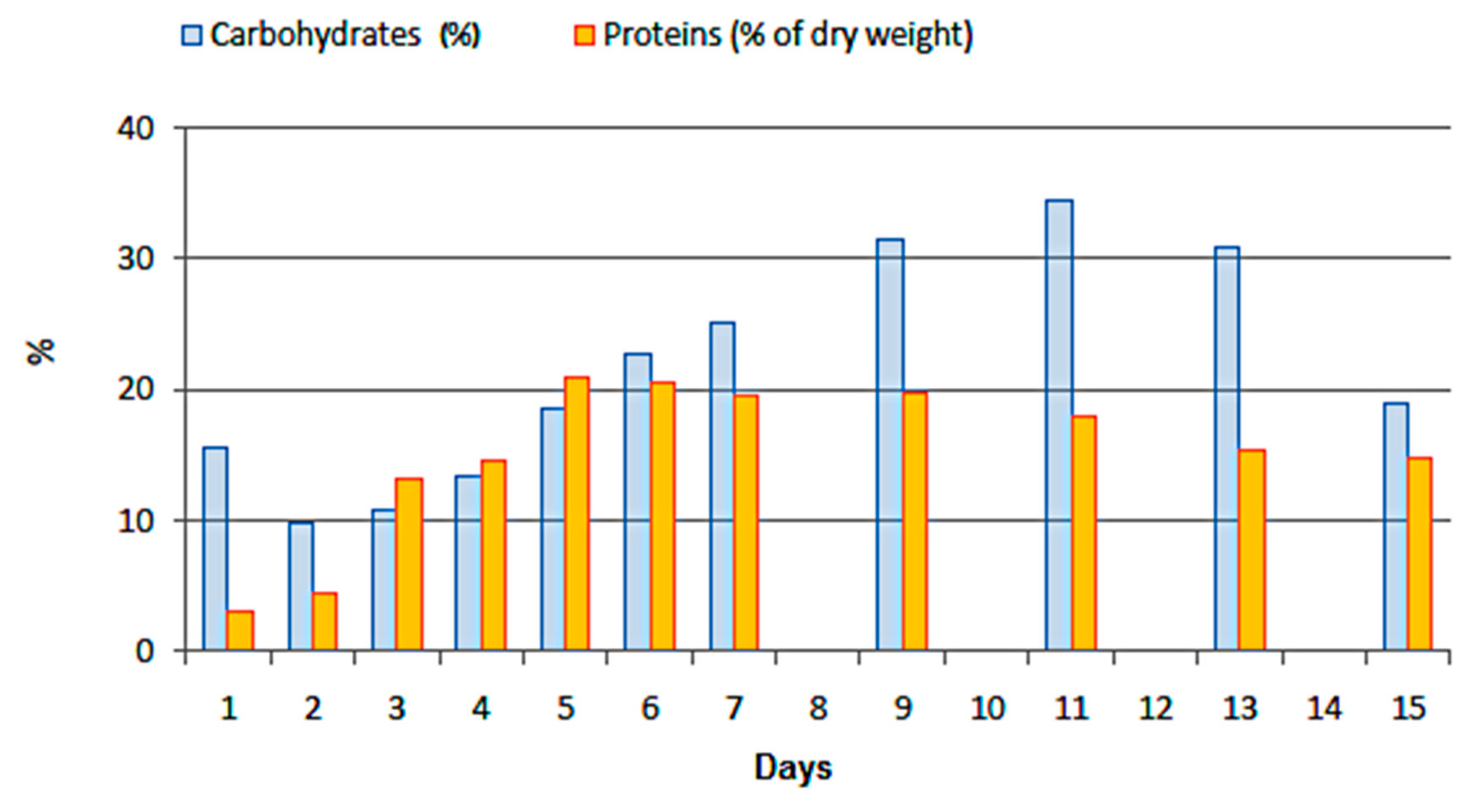
3.4. Carbohydrates and Proteins
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kuan, D.; Duff, S.; Posarac, D.; Bi, X. Growth optimization of Synechococcus elongatus PCC7942 in lab flasks and a 2-D photobioreactor. Can. J. Chem. Eng. 2015, 93, 640–647. [Google Scholar] [CrossRef]
- Santos-Merino, M.; Garcillán-Barcia, M.P.; De La Cruz, F. Engineering the fatty acid synthesis pathway in Synechococcus elongatus PCC 7942 improves omega-3 fatty acid production. Biotechnol. Biofuels 2018, 11, 239. [Google Scholar] [CrossRef]
- Safafar, H.; Hass, M.Z.; Møller, P.; Holdt, S.L.; Jacobsen, C. High EPA-Biomass from Nannochloropsissalina Cultivated in Flat-Panel Photo-Bioreactor on a Process Water-Enriched Growth Medium. Mar. Drugs 2016, 14, 144. [Google Scholar] [CrossRef]
- Eltgroth, M.L.; Watwood, R.L.; Wolfe, G.V. Production and cellular localization of neutral long-chain lipids in the haptophyte algae Isochrysisgalbana and Emilianiahuxley. J. Phycol. 2005, 41, 1000–1009. [Google Scholar] [CrossRef]
- Chen, X.; Li, Z.; He, N.; Zheng, Y.; Li, H.; Wang, H.; Wang, Y.; Lu, Y.; Li, Q.; Peng, Y. Nitrogen and phosphorus removal from anaerobically digested wastewater by microalgae cultured in a novel membrane photobioreactor. Biotechnol. Biofuels 2018, 11, 190. [Google Scholar] [CrossRef] [PubMed]
- Beacham, T.A.; Sweet, J.B.; Allen, M.J. Large scale cultivation of genetically modified microalgae: A new era for environmental risk assessment. Algal Res. 2017, 25, 90–100. [Google Scholar] [CrossRef]
- Collos, Y.; Harrison, P.J. Acclimation and toxicity of high ammonium concentrations to unicellular algae. Mar. Pollut. 2014, 80, 8–23. [Google Scholar] [CrossRef]
- Alcantara, C.; Dominguez, J.M.; Garcia, D.; Blanco, S.; Perez, R.; Garcia-Encina, P.A.; Munoz, R. Evaluation of wastewater treatment in a novel anoxic-aerobic algal-bacterial photobioreactor with biomass recycling through carbon and nitrogen mass balances. Bioresour. Technol. 2015, 191, 173–186. [Google Scholar] [CrossRef]
- Renuka, N.; Sood, A.; Prasanna, R.; Ahluwalia, A.S. Phycoremediation of wastewaters: A synergistic approach using microalgae for bioremediation and biomass generation. Int. J. Environ. Sci. Technol. 2015, 12, 1443–1460. [Google Scholar] [CrossRef]
- Silva-Benavides, A.M.; Torzillo, G. Nitrogen and phosphorus removal through laboratory batch cultures of microalgae Chlo- rella vulgaris and cyanobacterium Planktothrix isothrix grown as monoalgal and as co-cultures. J. Appl. Phycol. 2011, 24, 267–276. [Google Scholar] [CrossRef]
- El-Sheekh, M.M.; El-Shouny, W.A.; Osman, M.E.; El-Gammal, E.W. Growth and heavy metals removal efficiency of Nostoc muscorum and Anabaena subcylindrica in sewage and industrial wastewater effluents. Environ. Toxicol. Pharmacol. 2005, 19, 357–365. [Google Scholar] [CrossRef]
- Chinnasamy, S.; Bhatnagar, A.; Hunt, R.W.; Das, K. Microalgae cultivation in a wastewater dominated by carpet mill effluents for biofuel applications. Bioresour. Technol. 2010, 101, 3097–3105. [Google Scholar] [CrossRef]
- Schwarzenbach, R.P.; Escher, B.I.; Fenner, K.; Hofstetter, T.B.; Johnson, C.A.; Von Gunten, U.; Wehrli, B. The challenge of micropolutants in aquatic systems. Science 2016, 313, 1072–1077. [Google Scholar] [CrossRef]
- El-Sheekh, M.M.; Hamouda, R.A.; Nizam, A.A. Biodegradation of crude oil by Scenedesmus obliquus and Chlorella vulgaris growing under heterotrophic conditions. Int. Biodeterior. Biodegradation 2013, 82, 67–72. [Google Scholar] [CrossRef]
- Hu, Q.; Zeng, R.; Zhang, S.-X.; Yang, Z.-H.; Huang, H. Production of Microalgal Lipids as Biodiesel Feedstock with Fixation of CO2 by Chlorella vulgaris. Food Technol. Biotechnol. 2014, 52, 285–291. [Google Scholar]
- Islam, M.A.; Magnusson, M.; Brown, R.J.; Ayoko, G.A.; Nabi, M.N.; Heimann, K. Microalgal Species selection for Biodiesel Production Based on Fuel Properties Derived frmFattty Acid Profiles. Energies 2013, 6, 5676–5702. [Google Scholar] [CrossRef]
- Motwalli, O.; Essack, M.; Salhi, A.; Hanks, J.; Mijakovic, I.; Bajic, V.B. BioPS: System for screening and assessment of biofuel-production potential of cyanobacteria. PLoS ONE 2018, 13, e0202002. [Google Scholar] [CrossRef]
- Yoshino, T.; Kakunaka, N.; Liang, Y.; Ito, Y.; Maeda, Y.; Nomaguchi, T.; Matsunaga, T.; Tanaka, T. Production of ω3 fatty acids in marine cyanobacterium Synechococcus sp. strain NKBG 15041c via genetic engineering. Appl. Microbiol. Biotechnol. 2017, 101, 6899–6905. [Google Scholar] [CrossRef]
- Cardoso, S.M.; Carvalho, L.; Silva, P.; Rodrigues, M.; Pereira, O.; Pereira, L.; De Carvalho, L. Bioproducts from Seaweeds: A Review with Special Focus on the Iberian Peninsula. Org. Chem. 2014, 18, 896–917. [Google Scholar] [CrossRef]
- Tibocha-Bonilla, J.D.; Zuñiga, C.; Godoy-Silva, R.D.; Zengler, K. Advances in metabolic modelingof oleaginous microalgae. Biotechnol. Biofuels 2018, 11, 241. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.C.; Sinha, R.P.; Häder, D.-P. Role of Lipids and Fatty Acids in Stress Tolerance in Cyanobacteria. Acta Protozool. 2002, 41, 297–308. [Google Scholar]
- Murphy, J.; Riley, J. A modified single solution method for the determination of phosphate in natural waters. Anal. Chim. Acta 1962, 27, 31–36. [Google Scholar] [CrossRef]
- Iglewicz, B.; Hoaglin, D.C. The ASQC Basic References in Quality Control: Statistical Techniques. In How to Detect and Handle Outliers; Mykytka, E.F., Ed.; ASQC Quality Press: Milwaukee, WI, USA, 1993; Volume 16, pp. 1–87. [Google Scholar]
- Bowen, C.E.; Bidwell, J.P. Ionization of Ammonia in Seawater: Effects of Temperature, pH, and Salinity. J. Fish. Res. Board Can. 1978, 35, 12–16. [Google Scholar]
- Parsons, T.R.; Maita, Y.; Lalli, C.M. A Manual of Chemical and Biological Methods for Seawater, Analysis; Pergamon Press: Elmsford, NY, USA, 1984; pp. 149–153. [Google Scholar]
- Ivančić, I.; Degobbis, D. An optimal manual procedure for ammonia analysis in natural waters by the indophenol blue method. Water Res. 1984, 18, 1143–1147. [Google Scholar] [CrossRef]
- Bligh, E.G.; Dyer, W.J. A rapid method for total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef] [PubMed]
- Miller, L. Gas–liquid chromatography of cellular fatty acid as abacterial identification aid. HP Gas Chromatogr. Appl. Note 1985, 228, 37. [Google Scholar]
- Loewus, F.A. Improvement in Anthrone Method for Determination of Carbohydrates. Anal. Chem. 1952, 24, 219. [Google Scholar] [CrossRef]
- Safafar, H.; Nørregaard, P.U.; Ljubic, A.; Møller, P.; Holdt, S.L.; Jacobsen, C. Enhancement of Protein and Pigment Content in Two Chlorella Species Cultivated on Industrial Process Water. J. Mar. Sci. Eng. 2016, 4, 84. [Google Scholar] [CrossRef]
- Driver, T.; Bajhaiya, A.K.; Allwood, J.W.; Goodacre, R.; Pittman, J.K.; Dean, A.P. Metabolic responses of eukaryotic microalgae to environmental stress limit the ability of FT-IR spectroscopy for species identification. Algal Res. 2015, 11, 148–155. [Google Scholar] [CrossRef]
- Pistorius, A.M.; DeGrip, W.J.; Egorova-Zachernyuk, T.A.; Egorova-Zachernyuk, T.A. Monitoring of biomass composition from microbiological sources by means of FT-IR spectroscopy. Biotechnol. Bioeng. 2009, 103, 123–129. [Google Scholar] [CrossRef]
- Laurens, L.M.L.; Wolfrum, E.J. Feasibility of spectroscopic characterisation of algal lipids: Chemometric correlation of NIR and FTIR spectra with exogenous lipids in algal biomass. Bioenerg. Res. 2011, 4, 22–35. [Google Scholar] [CrossRef]
- Mayers, J.J.; Flynn, K.J.; Shields, R.J. Rapid determination of bulk microalgal biochemical composition by Fourier-Transform Infrared spectroscopy. Bioresour. Technol. 2013, 148, 215–220. [Google Scholar] [CrossRef]
- Giordano, M.; Kansiz, M.; Heraud, P.; Beardall, J.; Wood, B.; McNaughton, D. Fourier transform infrared spectroscopy as a novel tool to investigate changes in intracellular macromolecular pools in the marine microalgae Chaetocerosmuellerii (Bacillariophyceae). J. Phycol. 2001, 37, 271–279. [Google Scholar] [CrossRef]
- Hyenstrand, P.; Burkert, U.; Pettersson, A.; Blomqvist, P. Competition between the green alga Scenedesmusand the cyanobacterium Synechococcusunder different modes of inorganic nitrogen supply. Hydrobiologia 2000, 435, 91–98. [Google Scholar] [CrossRef]
- Wen, X.; Du, K.; Wang, Z.; Peng, X.; Luo, L.; Tao, H.; Xu, Y.; Zhang, D.; Geng, Y.; Li, Y. Effective cultivation of microalgae for biofuel production: A pilot scale evaluation of a novel oleaginous microalga Graesiella sp. WBG-1. Biotechnol. Biofuels 2016, 9, 123. [Google Scholar] [CrossRef] [PubMed]
- Sarmah, P.; Rout, J. Efficient biodegradation of low-density polyethylene by cyanobacteriaisolated from submerged polyethylene surface in domesticsewage water. Environ. Sci. Pollut. Res. 2018, 25, 33508–33520. [Google Scholar] [CrossRef] [PubMed]
- ZeaObando, C.; Linossier, I.; Dufosse, L.; Zubia, M.; Tunin-Ley, A.; Turquet, J.; Grondin, I.; Tremblay, R.; Fay, F.; Rehel, K. Fatty Acid Profiling of Tropical Microalgae and Cyanobacteria Strains Isolated From Southwest Indian Ocean Islands. J. Mar. Biol. Aquacult. 2017, 3, 1–14. [Google Scholar]
- Patel, V.K.; Sundaram, S.; Patel, A.K.; Kalra, A. Characterization of Seven Species of Cyanobacteria for High-Quality Biomass Production. Arab J. Sci. Eng. 2018, 43, 109–121. [Google Scholar] [CrossRef]
- De Farias Silva, C.; Sforza, E.; Bertucco, A. Enhancing Carbohydrate Productivity in Photosynthetic Microorganism Production: A Comparison between Cyanobacteria and Microalgae and the Effect of Cultivation Systems. In Advances in Feedstock Conversion Technologies for Alternative Fuels and Bioproducts; Hosseini, M., Ed.; Chapter 3; Woodhead Publishing: Cambridge, UK, 2019. [Google Scholar]
- Dillschneider, R.; Steinweg, C.; Rosello-Sastre, R.; Posten, C. Biofuels from microalgae: Photoconversion efficiency during lipid accumulation. Bioresour. Technol. 2013, 142, 647–654. [Google Scholar] [CrossRef] [PubMed]
- Concas, A.; Lutzu, G.A.; Pisu, M.; Cao, G. Experimental analysis and novel modeling of semi-batch photobioreactors operated with Chlorella vulgaris and fed with 100% (v/v) CO2. Chem. Eng. J. 2012, 213, 203–213. [Google Scholar] [CrossRef]
- Chi, X.; Yang, Q.; Zhao, F.; Qin, S.; Yang, Y.; Shen, J.; Lin, H. Comparative analysis of fatty acid desaturases in cyanobacterial genomes. Comp. Funct. Genom. 2008, 2008, 284508. [Google Scholar] [CrossRef]
| Wastewater Type | NO2− (µM) | NO3− (µM) | NH4+/NH3 (mM) | DIN (mM) | SRP (mM) | DOP (µM) | DIN/SRP |
|---|---|---|---|---|---|---|---|
| WW1 | 2.2 | 5.1 | 0.8 | 0.8 | 0.1 | 1.86 | 8 |
| WW2 | 23.7 | 204.0 | 0.6 | 0.8 | 0.16 | 18.15 | 6 |
| WW3 | 0.1 | 4.5 | 2.5 | 2.5 | 0.001 | 2.09 | 1897 |
| Days of Experiment | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 6 | 8 | 9 | 11 | 13 | 15 | |
| C11:0 | 0.53 | 0.39 | 0.21 | |||||||
| C12:0 | 0.21 | 0.34 | 0.29 | 0.22 | ||||||
| C13:0 | ||||||||||
| C14:1 | 0.33 | 0.81 | 0.71 | 0.54 | 0.48 | 0.58 | 0.59 | 0.59 | ||
| C14:0 | 11.08 | 19.92 | 21.83 | 21.33 | 19.78 | 19.66 | 18.22 | 16.78 | 19.73 | 13.26 |
| C15:1 | ||||||||||
| C15:0i/a | 0.29 | 0.26 | 0.15 | |||||||
| C15:0 | 0.80 | 0.64 | 0.53 | 0.44 | 0.38 | 0.59 | 0.61 | 0.36 | 1.23 | |
| C16:1c | 19.69 | 31.90 | 32.12 | 31.82 | 29.88 | 29.48 | 30.65 | 29.37 | 31.91 | 21.27 |
| C16:1t | 0.70 | 0.68 | 0.60 | 0.64 | 0.25 | 0.28 | 0.65 | 1.75 | ||
| C16:0 | 40.16 | 31.44 | 28.60 | 30.92 | 29.56 | 29.75 | 25.19 | 24.59 | 27.41 | 43.04 |
| C17:0 i/a | 0.45 | 0.44 | 0.52 | 0.58 | 0.51 | |||||
| C17:1 | 0.27 | 0.25 | 0.26 | 0.23 | 0.18 | |||||
| C17:0 | 1.71 | 1.03 | 0.85 | 0.75 | 0.79 | 1.01 | 1.10 | 0.63 | 2.51 | |
| C18:2(n-6) | 8.10 | |||||||||
| C18:1c | 8.58 | 8.49 | 6.54 | 7.27 | 11.33 | 13.05 | 17.38 | 20.65 | 12.71 | 5.94 |
| C18:1t | 0.19 | 2.15 | ||||||||
| C18:0 | 6.91 | 3.64 | 3.73 | 3.61 | 3.36 | 3.27 | 3.59 | 3.57 | 2.94 | 8.77 |
| C20:1 | 0.61 | |||||||||
| C20:0 | 0.59 | 0.28 | ||||||||
| SAT | 61.25 | 55.00 | 56.75 | 57.77 | 53.89 | 54.68 | 49.75 | 47.78 | 52.16 | 68.82 |
| MUFA | 30.18 | 40.39 | 40.40 | 39.80 | 42.35 | 43.65 | 49.13 | 51.31 | 46.05 | 31.11 |
| PUFA | 8.10 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| DETRIT | 2.77 | 0.00 | 2.36 | 1.38 | 1.18 | 1.62 | 2.67 | 2.78 | 1.84 | 3.74 |
| C14 | 11.42 | 19.92 | 22.64 | 22.04 | 20.32 | 20.14 | 18.81 | 17.37 | 20.32 | 13.26 |
| C16 | 60.55 | 63.34 | 61.40 | 62.74 | 60.04 | 59.86 | 56.09 | 54.24 | 59.97 | 66.06 |
| C18 | 23.58 | 12.12 | 10.27 | 10.88 | 14.69 | 16.32 | 20.98 | 24.40 | 15.65 | 16.86 |
| UND | 0.89 | 0.73 | 0.72 | 0.69 | 0.79 | 0.80 | 0.99 | 1.07 | 0.88 | 0.45 |
| Wave Number (cm−1) | Functional Group |
|---|---|
| 3400–3200 | ν OH (water, alcohol), ν N-H (Amide A, proteins) |
| 2960 | νasCH3, aliphatic stretching, methyl group |
| 2930 | νas CH2, aliphatic stretching, methylene group |
| 2850 | ν CH2, v CH3, methylene and methyl group |
| 1745 | ν C=O, ester of lipids and fatty acids |
| 1655 | ν C=O, Protein (Amide I) |
| 1545 | δ N-H, ν C-N, Protein (Amide II) |
| 1420–470 | δas CH2, δas CH3, aliphatic stretching, methyl and methylene group |
| 1390 | δaCH2, CH2 CH3Proteins/Carboxylic groups |
| 1200–900 | ν C-O-C Polysaccharides |
| 1075 and 950 | ν Si-O, Silicate frustules |
| 980–940 | P-O-P Polyphosphate |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blažina, M.; Haberle, I.; Hrustić, E.; Budiša, A.; Petrić, I.; Konjević, L.; Šilović, T.; Djakovac, T.; Geček, S. Growth Aspects and Biochemical Composition of Synechococcus sp. MK568070 Cultured in Oil Refinery Wastewater. J. Mar. Sci. Eng. 2019, 7, 164. https://doi.org/10.3390/jmse7060164
Blažina M, Haberle I, Hrustić E, Budiša A, Petrić I, Konjević L, Šilović T, Djakovac T, Geček S. Growth Aspects and Biochemical Composition of Synechococcus sp. MK568070 Cultured in Oil Refinery Wastewater. Journal of Marine Science and Engineering. 2019; 7(6):164. https://doi.org/10.3390/jmse7060164
Chicago/Turabian StyleBlažina, Maria, Ines Haberle, Enis Hrustić, Andrea Budiša, Ines Petrić, Lucija Konjević, Tina Šilović, Tamara Djakovac, and Sunčana Geček. 2019. "Growth Aspects and Biochemical Composition of Synechococcus sp. MK568070 Cultured in Oil Refinery Wastewater" Journal of Marine Science and Engineering 7, no. 6: 164. https://doi.org/10.3390/jmse7060164
APA StyleBlažina, M., Haberle, I., Hrustić, E., Budiša, A., Petrić, I., Konjević, L., Šilović, T., Djakovac, T., & Geček, S. (2019). Growth Aspects and Biochemical Composition of Synechococcus sp. MK568070 Cultured in Oil Refinery Wastewater. Journal of Marine Science and Engineering, 7(6), 164. https://doi.org/10.3390/jmse7060164







