First Application of a New Rapid Method of Age Determination in European Anchovy (Engraulis encrasicolus) by Fourier Transform Near-Infrared Spectroscopy
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection and Estimate of Age
2.2. Acquisition of Spectral Data
2.3. Choice of the Model
3. Results
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- ICES. Workshop 3 on Age Estimation of European Anchovy (Engraulis encrasicolus) (WKARA3; Outputs from 2021 Meeting); ICES Scientific Reports; ICES: Copenhagen, Denmark, 2023; p. 59. [Google Scholar]
- ICES. Report of Otolith Exchange Analysis for Anchovy. Coordinated by Begoña Villamor, Andres Uriarte and Gualtiero Basilone; ICES: Copenhagen, Denmark, 2018; p. 95. [Google Scholar]
- FAO. The State of Mediterranean and Black Sea Fisheries 2023; FAO: Rome, Italy, 2023; ISBN 978-92-5-138411-4. [Google Scholar]
- Cury, P. Small Pelagics in Upwelling Systems: Patterns of Interaction and Structural Changes in “Wasp-Waist” Ecosystems. ICES J. Mar. Sci. 2000, 57, 603–618. [Google Scholar] [CrossRef]
- Basilone, G.; Ferreri, R.; Barra, M.; Bonanno, A.; Pulizzi, M.; Gargano, A.; Fontana, I.; Giacalone, G.; Rumolo, P.; Mazzola, S.; et al. Spawning Ecology of the European Anchovy (Engraulis encrasicolus) in the Strait of Sicily: Linking Variations of Zooplankton Prey, Fish Density, Growth, and Reproduction in an Upwelling System. Prog. Oceanogr. 2020, 184, 102330. [Google Scholar] [CrossRef]
- Fanelli, E.; Da Ros, Z.; Menicucci, S.; Malavolti, S.; Biagiotti, I.; Canduci, G.; De Felice, A.; Leonori, I. The Pelagic Food Web of the Western Adriatic Sea: A Focus on the Role of Small Pelagics. Sci. Rep. 2023, 13, 14554. [Google Scholar] [CrossRef] [PubMed]
- European Union. Regulation (EU) Regulation (EU) 2017/1004 of the European Parliament and of the Council of 17 May 2017 on the Establishment of a Union Framework for the Collection, Management and Use of Data in the Fisheries Sector and Support for Scientific Advice Regarding the Common Fisheries Policy and Repealing Council Regulation (EC) No 199/2008 (Recast). 2017. Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=CELEX:32017R1004 (accessed on 11 May 2025).
- Lleonart, J.; Maynou, F. Fish Stock Assessments in the Mediterranean: State of the Art. Sci. Mar. 2003, 67, 37–49. [Google Scholar] [CrossRef]
- Leonori, I.; Tičina, V.; Giannoulaki, M.; Hattab, T.; Iglesias, M.; Bonanno, A.; Costantini, I.; Canduci, G.; Machias, A.; Ventero, A.; et al. History of Hydroacoustic Surveys of Small Pelagic Fish Species in the European Mediterranean Sea. Medit. Mar. Sci. 2021, 22, 751. [Google Scholar] [CrossRef]
- ICES. Report of the Workshop on Age Estimation of European Anchovy (Engraulis encrasicolus). WKARA2 2016 Report 28 November–2 December 2016. Pasaia, Spain; ICES: Copenhagen, Denmark, 2017; p. 223. [Google Scholar]
- Basilone, G.; Mangano, S.; Pulizzi, M.; Fontana, I.; Giacalone, G.; Ferreri, R.; Gargano, A.; Aronica, S.; Barra, M.; Genovese, S.; et al. European Anchovy (Engraulis encrasicolus) Age Structure and Growth Rate in Two Contrasted Areas of the Mediterranean Sea: The Paradox of Faster Growth in Oligotrophic Seas. Medit. Mar. Sci. 2017, 18, 504. [Google Scholar] [CrossRef]
- Williams, P. Near Infrared Technology: Getting the Best Out of Light, 1st ed.; African Sun Media: Stellenbosch, South Africa, 2019; ISBN 978-1-928480-31-0. [Google Scholar]
- Williams, P.; Norris, K.H.; American Association of Cereal Chemists (Eds.) Near-Infrared Technology in the Agricultural and Food Industries; American Association of Cereal Chemists: St. Paul, MN, USA, 1987; ISBN 978-0-913250-49-5. [Google Scholar]
- Siesler, H.W.; Ozaki, Y.; Kawata, S.; Heise, H.M. (Eds.) Near-Infrared Spectroscopy: Principles, Instruments, Applications, 1st ed.; Wiley: Hoboken, NJ, USA, 2001; ISBN 978-3-527-30149-2. [Google Scholar]
- Helser, T.E.; Benson, I.; Erickson, J.; Healy, J.; Kastelle, C.; Short, J.A. A Transformative Approach to Ageing Fish Otoliths Using Fourier Transform near Infrared Spectroscopy: A Case Study of Eastern Bering Sea Walleye Pollock (Gadus chalcogrammus). Can. J. Fish. Aquat. Sci. 2019, 76, 780–789. [Google Scholar] [CrossRef]
- Vance, C.K.; Tolleson, D.R.; Kinoshita, K.; Rodriguez, J.; Foley, W.J. Near Infrared Spectroscopy in Wildlife and Biodiversity. J. Near Infrared Spectrosc. 2016, 24, 1–25. [Google Scholar] [CrossRef]
- Helser, T.E.; Benson, I.M.; Barnett, B.K. (Eds.) Proceedings of the Research Workshop on the Rapid Estimation of Fish Age Using Fourier Transform Near Infrared Spectroscopy (FT-NIRS); AFSC Processed Report 2019-06; NOAA: Seattle, WA, USA, 2019; 195p. [Google Scholar] [CrossRef]
- Passerotti, M.S.; Helser, T.E.; Benson, I.M.; Barnett, B.K.; Ballenger, J.C.; Bubley, W.J.; Reichert, M.J.M.; Quattro, J.M. Age Estimation of Red Snapper (Lutjanus campechanus) Using FT-NIR Spectroscopy: Feasibility of Application to Production Ageing for Management. ICES J. Mar. Sci. 2020, 77, 2144–2156. [Google Scholar] [CrossRef]
- Wedding, B.B.; Forrest, A.J.; Wright, C.; Grauf, S.; Exley, P.; Poole, S.E. A Novel Method for the Age Estimation of Saddletail Snapper (Lutjanus malabaricus) Using Fourier Transform-near Infrared (FT-NIR) Spectroscopy. Mar. Freshwater Res. 2014, 65, 894. [Google Scholar] [CrossRef]
- Rigby, C.L.; Wedding, B.B.; Grauf, S.; Simpfendorfer, C.A. The Utility of near Infrared Spectroscopy for Age Estimation of Deepwater Sharks. Deep. Sea Res. Part I Oceanogr. Res. Pap. 2014, 94, 184–194. [Google Scholar] [CrossRef]
- Robins, J.B.; Wedding, B.B.; Wright, C.; Grauf, S.; Sellin, M.; Fowler, A.; Saunders, T.; Newman, S. Revolutionising Fish Ageing: Using Near Infrared Spectroscopy to Age Fish; Department of Agriculture and Fisheries: Brisbane, Australia, 2015. [Google Scholar]
- Rigby, C.L.; Wedding, B.B.; Grauf, S.; Simpfendorfer, C.A. Novel Method for Shark Age Estimation Using near Infrared Spectroscopy. Mar. Freshwater Res. 2016, 67, 537. [Google Scholar] [CrossRef]
- Wright, C.; Wedding, B.B.; Grauf, S.; Whybird, O.J. Age Estimation of Barramundi (Lates calcarifer) over Multiple Seasons from the Southern Gulf of Carpentaria Using FT-NIR Spectroscopy. Mar. Freshwater Res. 2021, 72, 1268–1279. [Google Scholar] [CrossRef]
- Healy, J.; Helser, T.E.; Benson, I.M.; Tornabene, L. Aging Pacific Cod (Gadus macrocephalus) from Otoliths Using Fourier-transformed Near-infrared Spectroscopy. Ecosphere 2021, 12, e03697. [Google Scholar] [CrossRef]
- Arrington, M.B.; Helser, T.E.; Benson, I.M.; Essington, T.E.; Matta, M.E.; Punt, A.E. Rapid Age Estimation of Longnose Skate (Raja Rhina) Vertebrae Using Nearinfrared Spectroscopy. Mar. Freshwater Res. 2021, 73, 71–80. [Google Scholar] [CrossRef]
- Basilone, G.; Lo Cicero, G.; Fortuna, M.; Luviner, A.; Ferreri, R.; Aronica, S.; Genovese, S.; Giacalone, G.; Fontana, I.; Bonanno, A. Fourier-Transform near-Infrared Spectroscopy First Application to Age Determination in European Fish Species: The Case of the Atlantic Horse Mackerel from the Central Mediterranean Sea. Front. Mar. Sci. 2024, 11, 1429150. [Google Scholar] [CrossRef]
- Benson, I.M.; Barnett, B.K.; Helser, T.E. Classification of Fish Species from Different Ecosystems Using the near Infrared Diffuse Reflectance Spectra of Otoliths. J. Near Infrared Spectrosc. 2020, 28, 224–235. [Google Scholar] [CrossRef]
- Basilone, G.; Ferreri, R.; Bonanno, A.; Genovese, S.; Barra, M.; Aronica, S. Age and Growth of European Sardine (Sardina Pilchardus) in the Central Mediterranean Sea: Implication for Stock Assessment. Fishes 2023, 8, 202. [Google Scholar] [CrossRef]
- Pinardi, N.; Zavatarelli, M.; Adani, M.; Coppini, G.; Fratianni, C.; Oddo, P.; Simoncelli, S.; Tonani, M.; Lyubartsev, V.; Dobricic, S.; et al. Mediterranean Sea Large-Scale Low-Frequency Ocean Variability and Water Mass Formation Rates from 1987 to 2007: A Retrospective Analysis. Prog. Oceanogr. 2015, 132, 318–332. [Google Scholar] [CrossRef]
- Conzen, J.P. Multivariate Calibration. A Practical Guide for Developing Methods in the Quantitative Analytical Chemistry, 3rd English ed.; Bruker Optik GmbH: Ettlingen, Germany, 2014; ISBN 3-929431-13-3. [Google Scholar]
- Chen, J.; Wang, X.Z. A New Approach to Near-Infrared Spectral Data Analysis Using Independent Component Analysis. J. Chem. Inf. Comput. Sci. 2001, 41, 992–1001. [Google Scholar] [CrossRef]
- Couture, J.J.; Singh, A.; Rubert-Nason, K.F.; Serbin, S.P.; Lindroth, R.L.; Townsend, P.A. Spectroscopic Determination of Ecologically Relevant Plant Secondary Metabolites. Methods Ecol. Evol. 2016, 7, 1402–1412. [Google Scholar] [CrossRef]
- Ogle, D.H. Introductory Fisheries Analyses with R, 1st ed.; Chapman and Hall/CRC: Boca Raton, FL, USA, 2016; ISBN 978-1-315-37198-6. [Google Scholar]
- Beamish, R.J.; Fournier, D.A. A Method for Comparing the Precision of a Set of Age Determinations. Can. J. Fish. Aquat. Sci. 1981, 38, 982–983. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023. [Google Scholar]
- Aldanondo, N.; Cotano, U.; Etxebeste, E. Growth of Young-of-the-Year European Anchovy (Engraulis encrasicolus L.) in the Bay of Biscay. Sci. Mar. 2010, 75, 227–235. [Google Scholar] [CrossRef]
- Brunel, T.; Dickey-Collas, M. Effects of Temperature and Population Density on von Bertalanffy Growth Parameters in Atlantic Herring: A Macro-Ecological Analysis. Mar. Ecol. Prog. Ser. 2010, 405, 15–28. [Google Scholar] [CrossRef]
- Bacha, M.; Amara, R. Inter-Cohort Differences in Growth, Condition and Feeding of Juvenile Anchovy (Engraulis encrasicolus) in the Gulf of Béjaia (Algerian Coast, SW Mediterranean): Implications for Recruitment Success. Fish. Res. 2012, 129–130, 73–81. [Google Scholar] [CrossRef]
- Radtke, R.; Shafer, D. Environmental Sensitivity of Fish Otolith Microchemistry. Mar. Freshwater Res. 1992, 43, 935. [Google Scholar] [CrossRef]
- Tabouret, H.; Lord, C.; Bareille, G.; Pécheyran, C.; Monti, D.; Keith, P. Otolith Microchemistry in Sicydium Punctatum: Indices of Environmental Condition Changes after Recruitment. Aquat. Living Resour. 2011, 24, 369–378. [Google Scholar] [CrossRef]
- ICES. Working Group on Biological Parameters (WGBIOP); ICES Scientific Reports; ICES: Copenhagen, Denmark, 2024; p. 372. [Google Scholar]
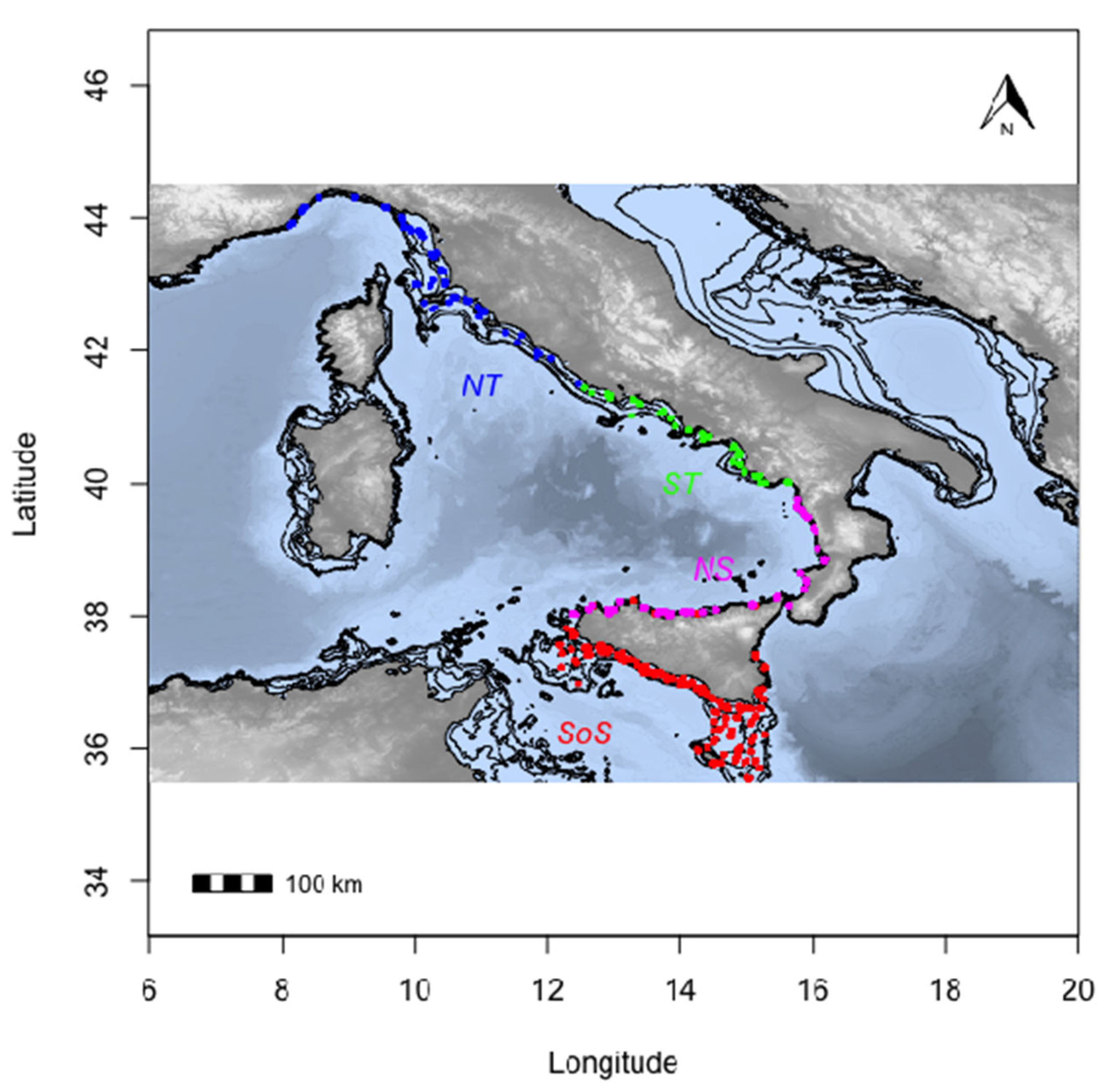
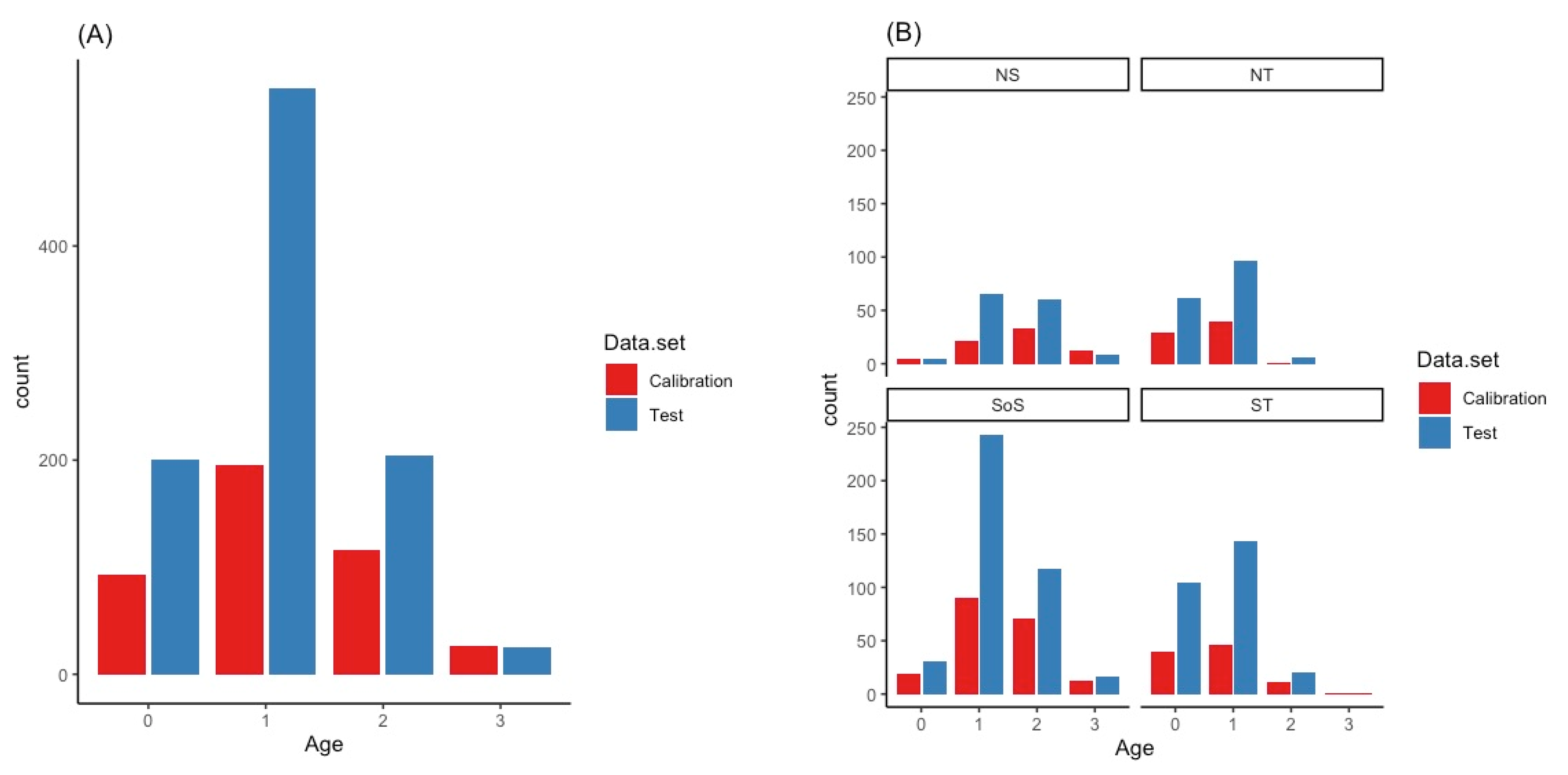


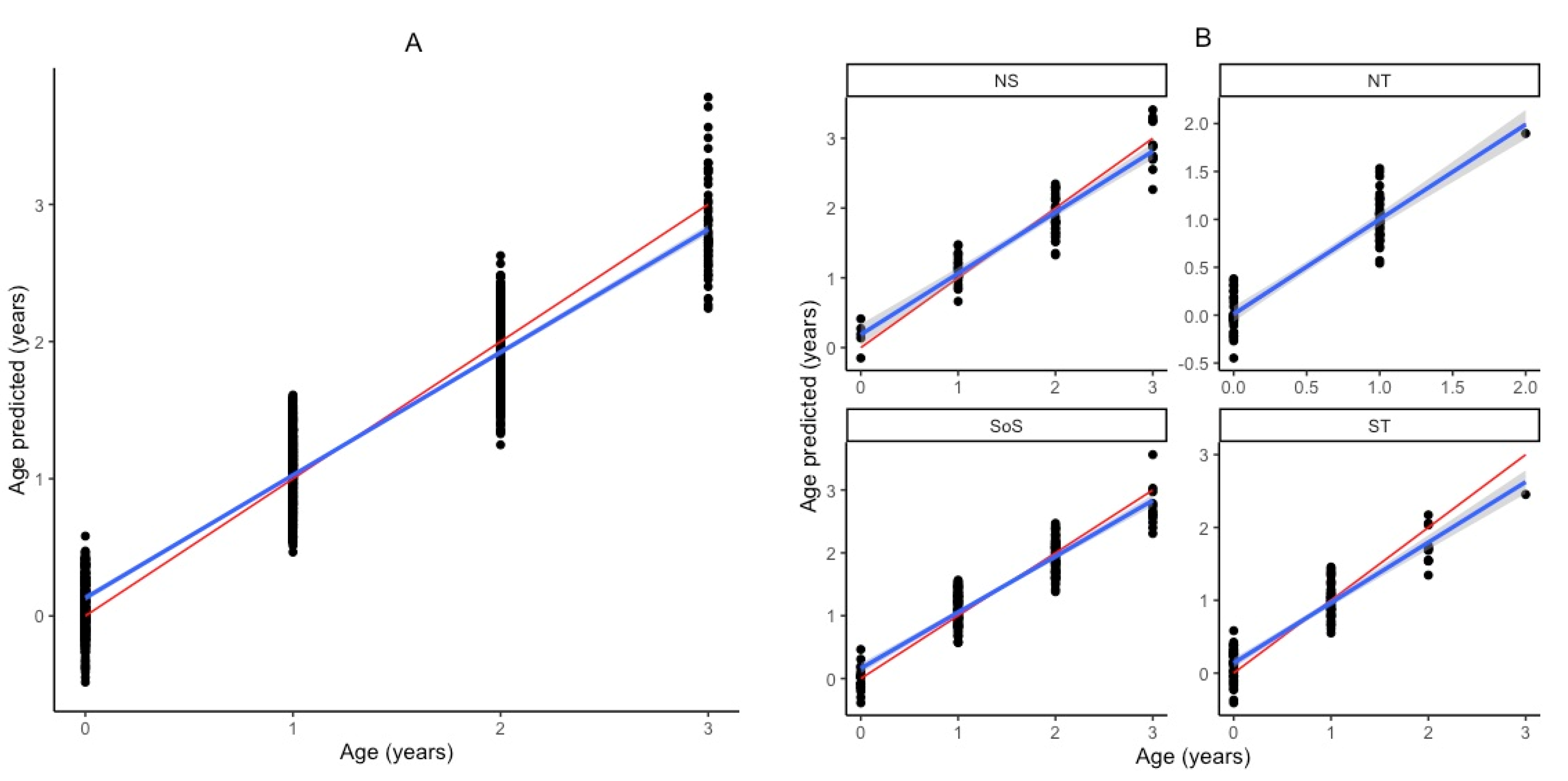
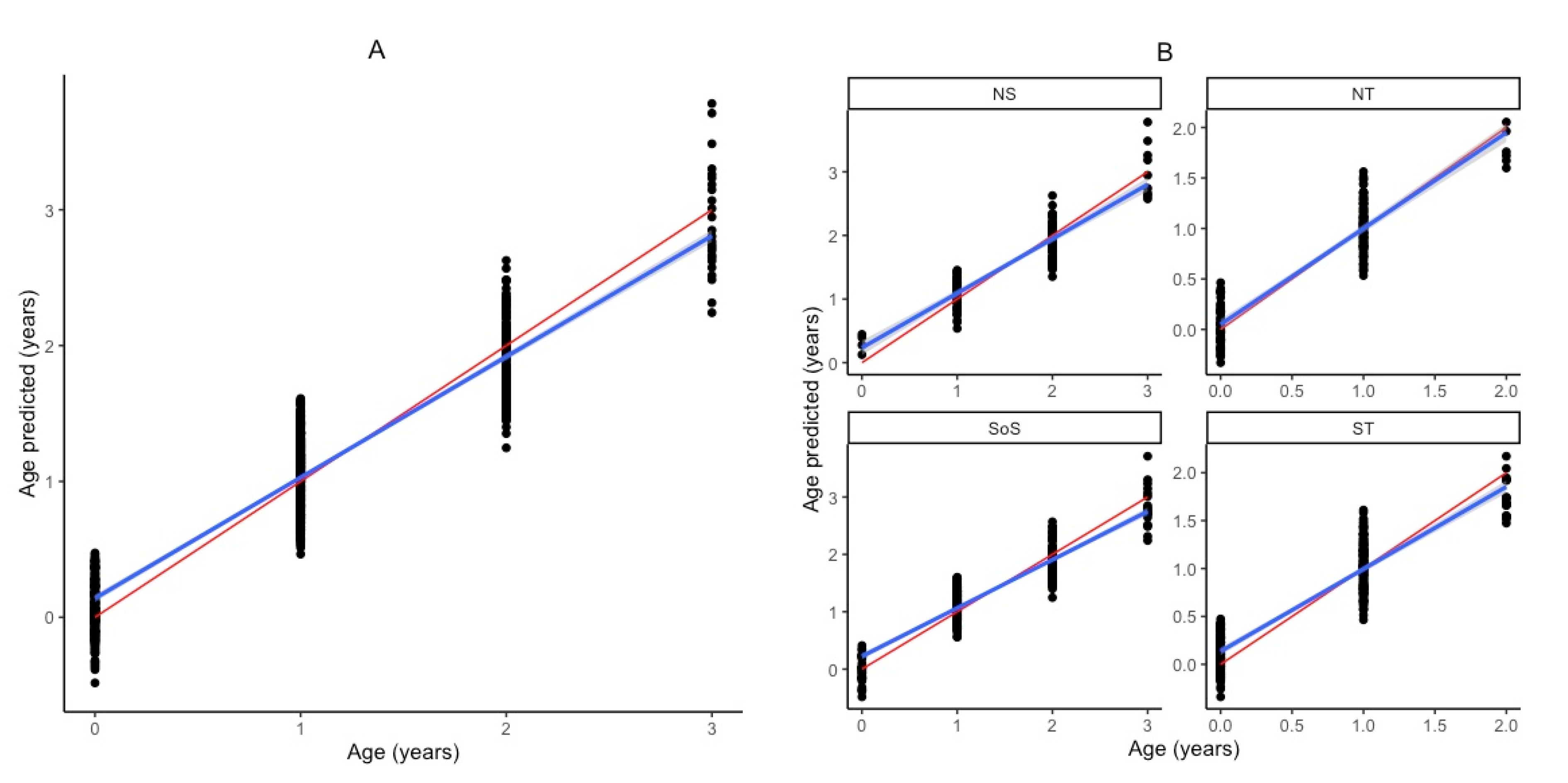
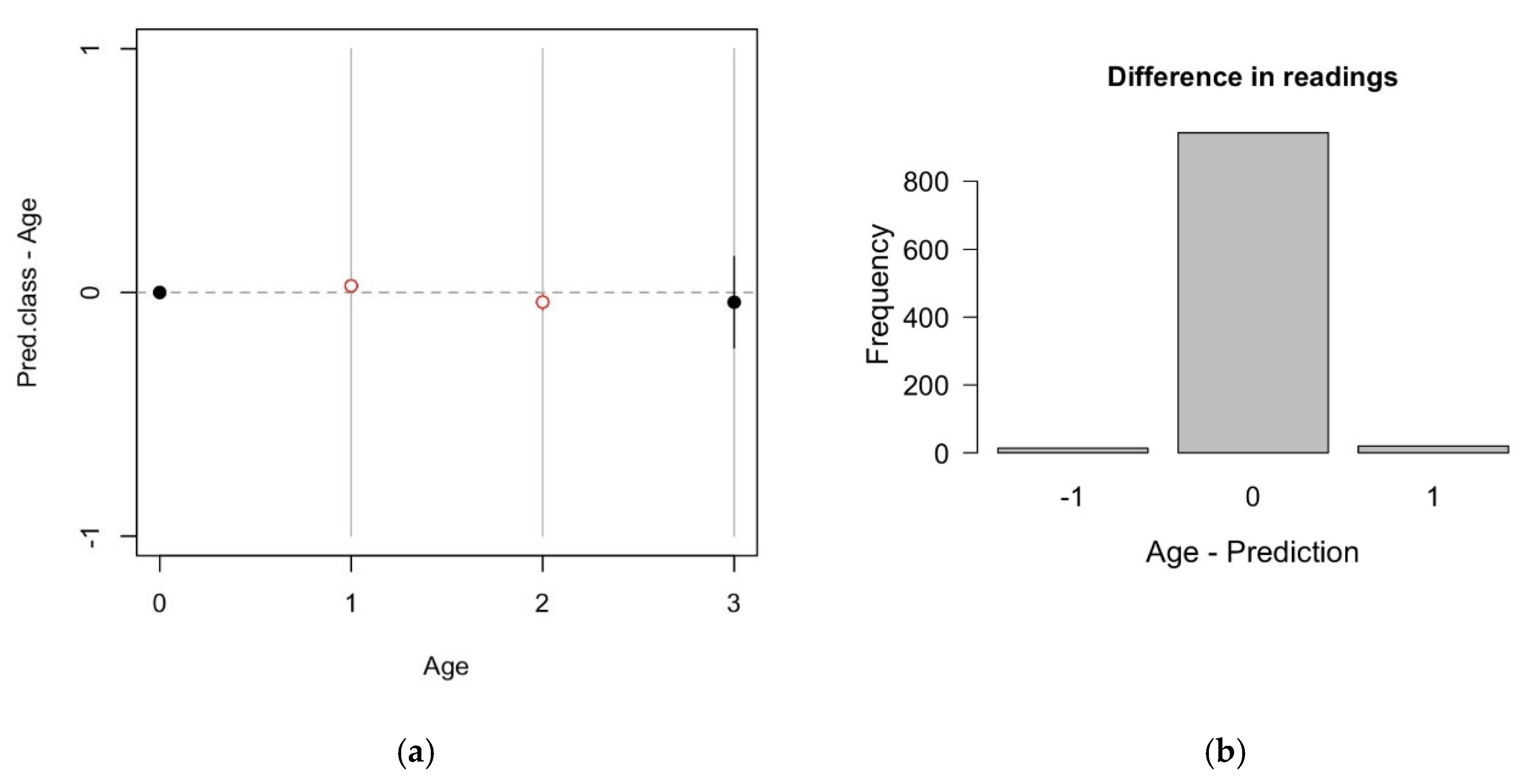
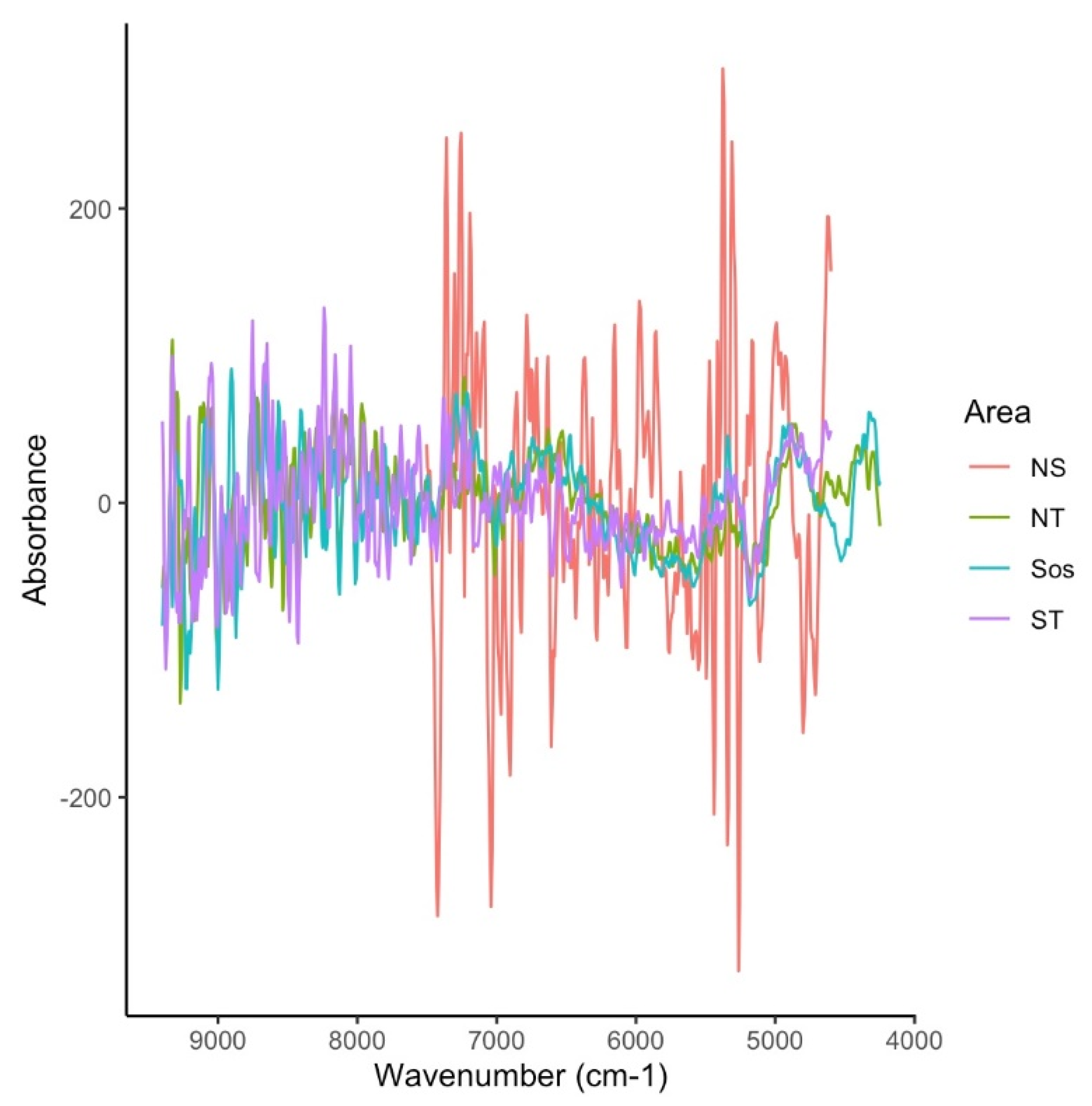
| Study | Species | Area | Age Range (Years) | Structure | n | R2 | RMSECV | RMSE% | Bias | Rank | Wavenumber Range |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Wedding et al. [19] | Lutjanus malabaricus | AU | 1–23 | Otolith | 169 | 0.94 | 1.35 | 5.87 | −0.005 | 4 | 7400–4000 |
| Rigby et al. [20] | Squalus megalops | AU | 5–25 | Vertebrae | 97 | 0.89 | 1.85 | 7.4 | −0.004 | 4 | 9300–8200 |
| 7800–6800 | |||||||||||
| 4600–4000 | |||||||||||
| 5–25 | Dorsal fin spine | 97 | 0.82 | 2.41 | 9.64 | −0.008 | 3 | 9300–8200 | |||
| 7800–6800 | |||||||||||
| 4600–4000 | |||||||||||
| Squalus montalbani | AU | 5–25 | Fin clip | 97 | 0.76 | 2.67 | 10.7 | −0.058 | 7 | 9300–8200 | |
| 7800–6800 | |||||||||||
| 4600–4000 | |||||||||||
| 3–31 | Dorsal fin spine | 95 | 0.73 | 2.96 | 9.54 | 0.052 | 4 | 9300–8200 | |||
| 7800–6800 | |||||||||||
| 4600–4000 | |||||||||||
| Robins et al. [21] | Lates calcarifer | AU | 2–12 | Otolith | 298 | 0.86 | 0.75 | 6.25 | 0.3 | 3 | 4832–4327 |
| Pagrus auratus | AU | 3–25 | Otolith | 306 | 0.88 | 1.53 | 6.12 | −0.060 | 2 | 6160–4580 | |
| Rigby et al. [22] | Sphyrna mokarran | AU | 0–10 | Vertebrae | 76 | 0.89 | 0.87 | 8.52 | 0.012 | 5 | 9200–4000 |
| Carcharhinus sorrah | AU | 0–10 | Vertebrae | 99 | 0.84 | 0.88 | 8.97 | −0.007 | 5 | 9200–4000 | |
| Wright et al. [23] | Lates calcarifer | AU | 1–10 | Otolith | 397 | 0.86 | 7.11 | - | −0.005 | - | 7255–4140 |
| Helser et al. [15] | Gadus chalcogrammus | US | 1–16 | Otolith | 202 | 0.95 | 0.78 | 4.87 | 0.002 | - | 6821–5269 |
| - | 5022–4171 | ||||||||||
| Passerotti et al. [18] | Lutjanus campechanus | US | 0–38 | Otolith | 510 | 0.94 | 1.58 | 4.16 | 0.001 | 9 | 7600–4100 |
| Healy et al. [24] | Gadus macrocephalus | US | 1–11 | Otolith | 498 | 0.84 | 0.588 | - | −0.070 | 10 | 7464–3952 |
| Arrington et al. [25] | Raja rhina | US | 0–14 | Vertebrae | 633 | 0.86 | 1.38 | 9.87 | - | - | 12,000–4000 |
| Basilone et al. [26] | Trachurus trachurus | EU | 0–14 | Otolith | 1084 | 0.95 | 0.41 | 3.42 | 0.002 | 10 | 7504–4248 |
| Sampling Date | Area | n | Fish Size Range (cm) | Age Range |
|---|---|---|---|---|
| 2023 (July) | SoS | 288 | 11–16 | 1–3 |
| NS | 110 | 9–16 | 0–2 | |
| ST | 168 | 8–15 | 0–2 | |
| 2022 (August–September) | SoS | 228 | 6–15 | 0–2 |
| NS | 42 | 12–15 | 1–2 | |
| NT | 232 | 5–13 | 0–2 | |
| ST | 203 | 7–15 | 0–3 | |
| 2021 (July–August) | SoS | 2 | 15–16 | 2–3 |
| NS | 15 | 15–16 | 2–3 | |
| 2020 (August) | SoS | 2 | 15–16 | 2–3 |
| NS | 3 | 15–16 | 2–3 | |
| 2019 (August) | SoS | 14 | 15–16 | 2–3 |
| 2018 (August) | SoS | 14 | 15–16 | 2–3 |
| NS | 7 | 15–16 | 2–3 | |
| 2017 (July–August) | SoS | 7 | 15–16 | 2–3 |
| NS | 24 | 15–17 | 2–3 | |
| 2015 (July–August) | SoS | 7 | 15–16 | 2–3 |
| NS | 2 | 15–16 | 2–3 | |
| 2014 (June–July) | SoS | 5 | 15–16 | 2–3 |
| NS | 3 | 15–16 | 2–3 | |
| 2013 (May–June) | SoS | 13 | 15–16 | 2–3 |
| NS | 3 | 14–15 | 3 | |
| 2012 (June–July) | SoS | 20 | 13–16 | 2–3 |
| Calibration Model | n | Max | Rank | R2 | RMSECV | RMSE% | Bias | RPD | Slope | Offset | Wavenumber Range | Pre-Processing |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AR | 436 | 3 | 9 | 89.7 | 0.271 | 9 | −0.001 | 3.12 | 0.906 | 0.111 | 9400–4248 | First Derivative |
| SoS | 195 | 3 | 6 | 84.22 | 0.303 | 10.1 | 0.001 | 2.52 | 0.855 | 0.204 | 9400–4248 | None |
| NT | 69 | 3 | 6 | 88.83 | 0.174 | 5.8 | −0.001 | 2.99 | 0.892 | 0.065 | 9400–4248 | None |
| ST | 100 | 3 | 6 | 83.64 | 0.282 | 9.4 | 0.005 | 2.47 | 0.855 | 0.098 | 9400–4600 | None |
| NS | 72 | 3 | 8 | 88.19 | 0.285 | 9.5 | 0.011 | 2.91 | 0.891 | 0.18 | 7504–4600 | None |
| Test Set vs. Calibration Test | n | Max. Age | R2 | RMSEP | RMSE% | Bias | RPD | Slope | Offset |
|---|---|---|---|---|---|---|---|---|---|
| ST vs. (AR) | 271 | 3 | 0.91 | 0.246 | 5.92 | −0.0411 | 2.5 | 0.857 | 0.137 |
| ST vs. (ST) | 271 | 3 | 0.91 | 0.256 | 8.53 | −0.0359 | 2.39 | 0.906 | 0.099 |
| AR vs. (AR) | 977 | 3 | 0.93 | 0.263 | 8.76 | −0.0236 | 2.74 | 0.888 | 0.141 |
| NS vs. (AR) | 138 | 3 | 0.9 | 0.285 | 9.5 | −0.0128 | 2.32 | 0.856 | 0.234 |
| NS vs. (NS) | 138 | 3 | 0.86 | 0.337 | 11.23 | −0.0243 | 1.96 | 0.812 | 0.313 |
| NT vs. (AR) | 163 | 3 | 0.91 | 0.225 | 7.55 | −0.0161 | 2.43 | 0.95 | 0.049 |
| NT vs. (NT) | 163 | 3 | 0.9 | 0.235 | 7.83 | 0.0259 | 2.33 | 0.802 | 0.106 |
| SoS vs. (AR) | 405 | 3 | 0.9 | 0.281 | 9.36 | −0.0187 | 2.36 | 0.84 | 0.225 |
| SoS vs. (SoS) | 405 | 3 | 0.88 | 0.314 | 10.46 | −0.002 | 2.11 | 0.777 | 0.29 |
| Parameters | Estimate | Std. Error | t Value | Pr(>|t|) | |
|---|---|---|---|---|---|
| Obs. | Linf | 165 | 1.93506 | 85.30 | <2 × 10−16 *** |
| K | 0.76 | 0.03686 | 20.75 | <2 × 10−16 *** | |
| t0 | −0.98 | 0.03711 | −26.43 | <2 × 10−16 *** | |
| Pred. | Linf | 164 | 1.93409 | 84.69 | <2 × 10−16 *** |
| K | 0.78 | 0.03889 | 20.14 | <2 × 10−16 *** | |
| t0 | −0.97 | 0.03807 | −25.51 | <2 × 10−16 *** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Basilone, G.; Fortuna, M.; Lo Cicero, G.; Genovese, S.; Giacalone, G.; Fontana, I.; Bonanno, A.; Aronica, S.; Ferreri, R. First Application of a New Rapid Method of Age Determination in European Anchovy (Engraulis encrasicolus) by Fourier Transform Near-Infrared Spectroscopy. J. Mar. Sci. Eng. 2025, 13, 961. https://doi.org/10.3390/jmse13050961
Basilone G, Fortuna M, Lo Cicero G, Genovese S, Giacalone G, Fontana I, Bonanno A, Aronica S, Ferreri R. First Application of a New Rapid Method of Age Determination in European Anchovy (Engraulis encrasicolus) by Fourier Transform Near-Infrared Spectroscopy. Journal of Marine Science and Engineering. 2025; 13(5):961. https://doi.org/10.3390/jmse13050961
Chicago/Turabian StyleBasilone, Gualtiero, Miryam Fortuna, Gabriella Lo Cicero, Simona Genovese, Giovanni Giacalone, Ignazio Fontana, Angelo Bonanno, Salvatore Aronica, and Rosalia Ferreri. 2025. "First Application of a New Rapid Method of Age Determination in European Anchovy (Engraulis encrasicolus) by Fourier Transform Near-Infrared Spectroscopy" Journal of Marine Science and Engineering 13, no. 5: 961. https://doi.org/10.3390/jmse13050961
APA StyleBasilone, G., Fortuna, M., Lo Cicero, G., Genovese, S., Giacalone, G., Fontana, I., Bonanno, A., Aronica, S., & Ferreri, R. (2025). First Application of a New Rapid Method of Age Determination in European Anchovy (Engraulis encrasicolus) by Fourier Transform Near-Infrared Spectroscopy. Journal of Marine Science and Engineering, 13(5), 961. https://doi.org/10.3390/jmse13050961







