Abstract
Methanol is a promising low-carbon fuel that can effectively reduce environmental pollution from ships compared to traditional fuels. The timing of methanol injection is a major factor affecting the performance of internal combustion engines, and either too late or too early injection can severely impact the combustion efficiency of an engine. This paper focused on a 4135Aca marine diesel engine produced by the Shanghai Diesel Engine Factory in China. Using CONVERGE/3.0 software for numerical simulation, the study analyzed the impact of methanol injection timing on the combustion and emission characteristics of marine diesel engines. It was found that the determination of methanol injection timing should comprehensively consider the effects of the combustion start point, mixture quality, flame front propagation speed, and evaporation heat absorption. Appropriate methanol injection timing can improve the combustion duration, cylinder pressure, and heat release rate, enhancing the power performance of marine diesel engines. This study shows that methanol injection at −30 °CA can effectively control the in-cylinder combustion process, improve combustion efficiency, and significantly reduce the emissions of pollutants such as soot (by 60.5%), HC (by 3.6%), CO (by 95.3%), etc. However, it can lead to an increase in NOx (by 3.7%) generation under high-temperature conditions. This research can provide a certain reference for the engineering application of methanol direct injection engines for ships.
1. Introduction
With the development of international globalization trade, the environmental harm caused by ship exhaust emissions has become increasingly serious. In order to reduce costs, shipping companies are exploring the adoption of more energy-efficient technologies and alternative fuels. These efforts aim to improve operational efficiency and promote the sustainable development of the industry [1,2]. Currently, there is a growing number of carbon reduction regulations in the shipping sector, including legislative actions by the European Union and the International Maritime Organization (IMO). Shipping companies are required to take more environmentally friendly measures to reduce greenhouse gas emissions from ships [3,4]. The MARPOL Convention requires all ships to calculate their existing ship energy efficiency index (EEXI) and report the annual operational carbon intensity indicator (CII) rating from 1 January 2023. Carbon reduction in shipping is urgent.
On 3 July 2023, the 80th session of the IMO Marine Environment Protection Committee (MEPC 80) was held in London, UK. The meeting adopted the revised “2023 Ship Greenhouse Gas Emission Reduction Strategy”, proposing a new goal of “achieving net-zero emissions around 2050” and setting an effective timetable for mid-term emission reduction measures. Green shipping is the future development trend of the global shipping industry, and methanol is a key transitional fuel for achieving the carbon reduction goals of the shipping industry.
Methanol, as a renewable and low-carbon alternative fuel, has broad application prospects in the maritime industry. By studying the combustion characteristics of methanol fuel in marine engines, technical support can be provided for the widespread application of methanol fuel, thus accelerating its commercialization process. Methanol not only has clear advantages over traditional fuels but also has relatively mature storage and transportation methods and lower technical requirements compared to other alternative fuels such as hydrogen and ammonia. Researching its application prospects in marine power systems will help to provide more options for energy diversification and the greening of shipping [5].
Research on low-carbon fuels and net zero emission internal combustion engines is increasingly attracting attention, which imposes higher standards and requirements on ship power plants. Clarkson Research statistics show that more than 40% of new orders signed since the beginning of 2023 are powered by alternative fuels, with the share of methanol alternative fuels rapidly increasing. In the first half of 2024, the number of new alternative fuel ship orders accounted for about one-third of the total orders. In June 2024, the world’s first methanol dual-fuel operating container ship was delivered in Zhoushan, China. In recent years, some researchers have conducted studies on the application of engine control strategies in methanol/diesel dual-fuel modes. Studies by Varol [6] and Wu [7] and others have shown that adding methanol, ethanol, n-butanol, and other alcohols to traditional fossil fuels can significantly reduce carbon monoxide and hydrocarbon compound emissions, with methanol being the most effective, but it also increases fuel consumption and CO2 emissions. Other studies have shown that methanol can effectively alleviate the knocking problem in internal combustion engines due to high octane numbers. Zhang et al. [8] and others found that alcohol fuels can effectively reduce soot emissions caused by insufficient oxygen content in fuel during engine combustion. Various tests have shown that inlet temperature has a significant impact on the combustion efficiency of alcohols. By adjusting the air–fuel ratio, the engine’s peak pressure and heat release rate can be effectively controlled, thereby optimizing its performance and efficiency. To further investigate the effects of methanol combustion in marine engines, several studies have been conducted using computational fluid dynamics (CFD) modeling and experimental analysis.
Karvounis and Theotokatos [9] developed a validated CFD model for a large marine four-stroke dual-fuel engine with a 90% methanol energy fraction. It was found that injecting methanol at 80 °CA BTDC and pilot diesel at 12 °CA BTDC achieved combustion efficiency up to 99% and thermal efficiencies of 46%, 45%, and 43% at high, medium, and low loads, respectively, while complying with IMO Tier III NOx limits. This indicates that the combination of advanced methanol injection timing with pilot diesel contributes significantly to optimal combustion performance.
Karvounis et al. [10] extended this research by analyzing port and direct methanol injection in a 10.5 MW marine dual-fuel engine. It was found that direct injection enabled up to 95% methanol energy fraction while maintaining knock-free combustion and reducing NOx emissions by 85%, whereas premixed combustion exhibited trade-offs in efficiency. These findings highlight the advantages of direct injection strategies in maximizing methanol utilization in marine applications.
Yu et al. [11] developed a 3D CFD model with CONVERGE/3.0 code to optimize fuel injector configurations and injection strategies for a diesel/methanol dual direct injection marine engine with a 96.5% methanol energy ratio (MER). The authors show that advancing methanol injection timing to 25 °CA BTDC increased indicated thermal efficiency (ITE) from 45.5% to 48.3%, while raising injection pressure to 56 MPa improved indicated thermal efficiency by 15.9% compared to 26.4 MPa, achieving efficient and stable combustion with lower emissions. This underscores the critical role of injection timing and pressure in enhancing engine performance.
In addition to injection location, different injection strategies have been examined to further optimize combustion performance.
Dierickx et al. [12] conducted an experimental study on a dual-fuel marine engine to evaluate methanol–water blends for knock suppression, NOx reduction, and efficiency improvements. It was found that methanol/water weight by weight shares of 50%/50% (MeOH-50) increased brake thermal efficiency by up to 4.9% compared to diesel-only operation, while pure methanol achieved the highest methanol energy fraction of 76%. NOx emissions decreased with higher water content, allowing MeOH-50 and MeOH-64 to meet IMO Tier III limits, though they had lower greenhouse gas reduction potential due to reduced diesel substitution. This indicates that blending water with methanol can improve efficiency and emissions performance while still meeting regulatory standards. In another study, Dierickx et al. [13] compared single-point injection (SPI) and multiple-point injection strategies for dual-fuel methanol–diesel engines in terms of the methanol energy fraction (MEF), brake thermal efficiency (BTE), and NOx emissions. It was found that SPI achieved the highest MEF of 84% and higher BTE at high MEFs, while MPI resulted in lower NOx emissions. This comparison highlights the trade-offs between different injection strategies in optimizing performance metrics.
Sun et al. [14] further refined injection strategies by optimizing a fuel injection strategy for a marine methanol/diesel direct dual-fuel stratification engine with a 95% methanol substitution rate. It was found that a methanol two-stage injection strategy reduced equivalent indicated specific fuel consumption by 3.95% while lowering NOx emissions, though it led to higher HC, CO, and soot emissions due to decreased combustion completeness. This demonstrates the complexity of optimizing injection strategies to balance efficiency and emissions across different pollutants.
Yu and Wen [15] conducted a numerical study on a diesel/methanol dual direct injection marine engine to optimize pre-injection strategies for efficiency and emissions. It was found that pre-injecting methanol at 35 °CA BTDC with a 15% pre-injection ratio increased indicated thermal efficiency by 19.9% compared to pure-diesel mode and reduced CO2 emissions by 19.4%, while improper timing led to combustion instability and increased soot, CO, and HC emissions. These results emphasize the importance of precise timing in pre-injection strategies to achieve desired performance outcomes.
Wu et al. [16] injected methanol at the intake port with heated inlet air in a diesel engine, and the Taguchi methodology was applied to optimize injection parameters. Results of the study showed that the optimal settings reduced smoke emissions by 41.5%, NOx by 61.7%, HC by 8.6%, and CO by 32.4% while improving brake thermal efficiency. Also, the authors indicate that confirmation tests showed that the predictions agreed with experimental results within a 95% confidence level. This further validates the effectiveness of the optimization methodology employed in their study.
Valera et al. [17] evaluated the potential of methanol as a substitute for diesel in a 16-cylinder locomotive engine using a novel co-axial injection concept, with a focus on three methanol injection strategies. The results indicated that the first injection strategy (ITM1) achieved higher in-cylinder pressure and comparable heat release rates while effectively reducing NOx emissions; optimal co-axial injector design parameters included diesel nozzle diameters of 0.2 mm and methanol nozzle diameters of 0.35 mm, with nine methanol nozzles, leading to significant reductions in the environmental impact of the engine. This suggests that innovative injector designs can enhance the combustion efficiency and environmental performance of methanol-fueled engines.
Güdden et al. [18] studied a 5L single-cylinder diesel engine converted to a port fuel injected (PFI) spark-ignited methanol combustion system, finding that methanol significantly improved engine performance, achieving brake thermal efficiencies over 44% and complying with IMO Tier III NOx emission limits without exhaust aftertreatment. While preignitions were not an issue, the application of an oxidation catalyst was recommended to address unburned methanol and formaldehyde emissions. These findings reinforce the potential of methanol as a cleaner alternative fuel in marine engines, highlighting the need for further research on emission control technologies.
Yusri et al. [19] and others found that alcohol fuels play an important role in improving the combustion efficiency of internal combustion engines. Compared with pure diesel, methanol–diesel blended fuels have higher combustion efficiency. In the operation of internal combustion engines, the addition of methanol can have a certain impact on emissions. After adding methanol to a diesel engine, the nitrogen oxide emissions have decreased and soot emissions have also been significantly reduced. Sayin et al. [20] and others adjusted the methanol–diesel blending ratio and found that injection pressure and fuel injection time are important factors affecting the working characteristics of diesel engines. The higher the methanol mass fraction, the easier it is to cause an increase in fuel consumption due to its lower energy density. Experimental results show that as the injection pressure increases, the emissions of HC and CO are reduced.
In recent years, CFD technology has developed rapidly, and numerical simulation methods can effectively reduce costs and shorten the research and development cycle [21,22,23]. In the current global carbon peak and carbon neutrality context, the use of green methanol fuel in ship engines is a practical and feasible solution [24,25], which can gradually meet the IMO shipping industry carbon emission targets. This article conducts CFD modeling on the 4135 Aca-type marine diesel engine to find a low-carbon path suitable for marine diesel engines. Using CONVERGE simulation to analyze the combustion and emission characteristics of an in-cylinder methanol engine at a rated speed of 1270 r/min, this study explores the feasibility of using methanol as a fuel for marine diesel engines, optimizes its combustion process, reduces carbon emissions, and improves efficiency. It also evaluates the impact of different alcohol injection times on combustion and environmental performance through CFD simulation analysis.
2. CFD Modeling and Validation
2.1. Test Bench and CFD Model
This study focuses on a marine 4-stroke diesel engine manufactured by Shanghai Diesel Engine Co., Ltd. (Shanghai, China), with the engine model being 4135Aca, a straight 4-cylinder engine. The dimensions of the 4135 Aca marine tested diesel engine are shown in Table 1.

Table 1.
Dimensions of a 4135 Aca marine tested diesel engine.
The environmental temperature measured in the experiment was 24.3 °C, the environmental atmospheric pressure was 101.01 kPa, and the relative humidity of the air was 63.5%. The 4135Aca marine diesel engine test bench is shown in Figure 1, using a Kistler 6613CG1 pressure sensor (Winterthur, Switzerland), a Kistler 2614CK1 angular encoder, and a Kistler KiBox 2893BK8 combustion analyzer (Winterthur, Switzerland) to monitor the in-cylinder combustion conditions. The test engine bench used a hydraulic dynamometer (Nantong, China) and monitoring system, employing a constant speed and constant torque mode to achieve the specified test conditions. An AVL SPC478 particulate matter sampling system (AVL List GmbH, Graz, Austria) was used to collect particulate matter emissions from marine engines under steady-state and transient conditions.

Figure 1.
4135Aca marine diesel engine test bench.
The particulate matter (PM) sampling filters were weighed in a particle weighing room that met the requirements of relevant domestic and international regulations for measuring pollutant emissions from marine diesel engines, with a constant temperature and humidity environment. The environmental weighing box model used for weighing was RXCH500-Ⅱ, and the ultra-microbalance model was Sartorius MSA2.7S-0CE-DF (Germany). The ultra-microbalance had a range of 0-2.1 mg and a resolution of 0.01 μg. A Horiba PG-350 analyzer (Japan) was used to measure emissions of carbon monoxide, carbon dioxide, nitrogen oxides, etc. The fuel consumption was measured using a ToCeil-CMFD015 circular fuel consumption meter (Shanghai, China), with a measurement accuracy of 0.12%. The main technical parameters of the test data acquisition and analysis instruments are listed in Table 2.

Table 2.
Technical parameters of data acquisition and analysis instruments.
The simulation software CONVERGE V3.0 used in this study was developed by Convergent Science [26]. This software has a rich set of physical models, covering all the elements required for the analysis of turbulence, spray, combustion, and emissions. Using SOLID WORKS 2023, a 3-dimensional model of the diesel engine combustion chamber was drawn based on the structural parameters of the combustion chamber, and the 3-dimensional simulation model of the engine is shown in Figure 2. Due to the symmetry of the cylinder, this article establishes a 1/4 cylinder model of the diesel engine, which can effectively improve the simulation efficiency while ensuring the reliability of the results. In this study, it is found that the results of the 1/4 cylinder model are in good agreement with the results of the whole machine.

Figure 2.
A 4135Aca marine diesel engine combustion chamber CFD model.
As shown in Figure 2, in this study, a combustion chamber model was established that includes the piston top surface, cylinder head, cylinder wall, and two virtual longitudinal sections of the combustion chamber. This model was used to simulate the spatial positions and gas dynamics inside the combustion chamber during the engine combustion process. During the simulation, the combustion chamber was kept sealed, and the simulation time range was set from 132 °CA before top dead center (BTDC) to 132 °CA after top dead center (ATDC), which sufficiently covered the dynamic changes in the gas inside the combustion chamber. With this combustion chamber model, it is possible to gain a deeper understanding of the movement and interaction of various components during the combustion process. At the same time, it can also accurately simulate the changes in parameters such as gas pressure and temperature inside the combustion chamber.
For model initialization, taking the verification condition as an example, the diesel engine intake was air, with consideration of 60% relative humidity. The initial composition inside the cylinder included nitrogen N2, oxygen O2, carbon dioxide CO2, and water vapor H2O, with the quantitative relationships between the components represented by mass fractions. The initial settings are shown in Table 3. The turbulence model used in this study was the RNG k-ε equation based on the RANS method; the combustion model employed the detailed chemical reaction kinetics (SAGE) model. The NOx emission model was based on the Zeldovich mechanism. The fuel breakup was modeled using the KH-RT model.

Table 3.
Boundary and initial conditions according to the CFD model.
2.2. CFD Model Validation
The density distribution of the grid has a certain impact on the accuracy of the simulation results. A larger grid may not be accurate enough, while a smaller grid can lead to excessively long computation times. CONVERGE does not require users to manually divide the grid; instead, it only needs the basic grid size of the model to be provided. Then, it uses its mature embedded encryption and adaptive encryption technology based on temperature or velocity (adaptive mesh refinement, AMR) to achieve a reasonable distribution of the model grid. The model used in this article was obtained by measuring the dimensions of the combustion chamber of the marine diesel engine used in this study, and the three-dimensional graphics of the combustion chamber for this simulation were drawn using SOLID WORK 2023 software and imported into CONVERGE V3.0 software. Through multiple runs and debugging of the model, the final basic grid size of the model was determined to be 4 mm, with the basic grid number of the simplified combustion chamber model being about 100,000 and the peak grid after embedded encryption or adaptive encryption being nearly 450,000.
As shown in Figure 3 and Table 4, the simulation results closely match the experimental data under the operating conditions of 1270 r/min and 240 NM torque. According to the CFD analysis results, the simulation model built in this article shows high accuracy and precision under the experimental conditions. The simulated cylinder pressure fits well with the experimental data, with an error within 5%, meeting the requirements for simulation accuracy in this study.
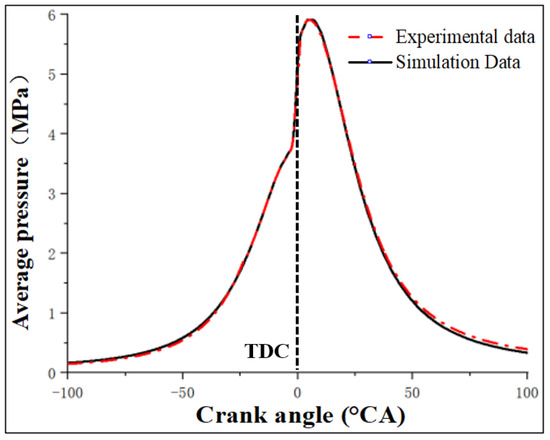
Figure 3.
Comparison of working condition simulation and test cylinder pressure value.

Table 4.
Comparison of parameters between test and simulation values.
After verifying the accuracy of combustion performance prediction, the validation of the emission model needs to be conducted. Figure 4 shows a comparison between simulated and experimental values of marine engine emissions under different operating conditions. As can be seen from the figure, based on the simulation results, the calculated NOx emissions under different operating conditions are basically consistent with the measured data. The relative error between the simulated and experimental values of engine NOx emissions under the 75% load base condition is less than 5%.
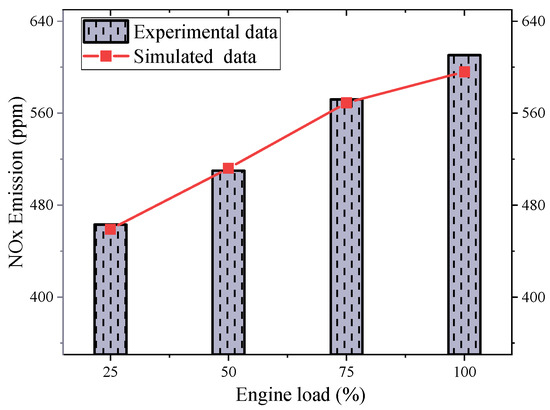
Figure 4.
Comparison of simulated and experimental emissions data under different conditions.
3. Simulation Results Analysis
This study used CONVERGE software to simulate the diesel engine’s alcohol injection process. The effects of different alcohol injection timings (−20 °CA BTDC, −30 °CA BTDC, −40 °CA BTDC, and −50 °CA BTDC) on combustion and emission performance were investigated in detail through simulation research.
3.1. Influence of Methanol Injection Timing on Engine Combustion Characteristics
From Figure 5, it can be clearly observed that the concentration distribution of combustible gases in the cylinder varies significantly at different methanol injection timings just before ignition. Particularly at −20 °CA methanol injection timing, it can be seen that the methanol has just been injected and has not yet distributed evenly, mainly concentrating at the left end of the cylinder, forming a locally over-rich mixture area. The over-rich mixture area is one of the main reasons for the decrease in combustion efficiency and the increase in emissions. When the mixture is too concentrated, the fuel-to-oxygen ratio is improper, which may lead to incomplete combustion during the combustion process. This incomplete combustion not only reduces the engine’s combustion efficiency but also results in unoxidized fuel residues remaining in the combustion chamber, leading to the production of more emissions.
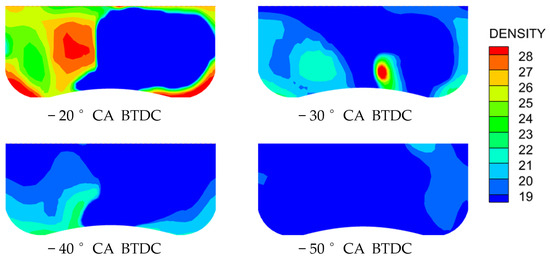
Figure 5.
Concentration fields under four methanol injection timings.
The unequal methanol distribution at −20 °CA injection timing emphasizes how crucial it is to maximize fuel–air mixing in order to improve combustion efficiency. Over-rich zones could be decreased by promoting greater atomization and improving mixture homogeneity through the use of a multi-stage injection method or a higher injection pressure. Furthermore, by analyzing in-cylinder turbulence effects using computational fluid dynamics simulations, spray penetration and evaporation characteristics may be further optimized, guaranteeing more thorough combustion and reduced emissions.
Figure 6 shows the cylinder average pressure at different methanol injection timings at a speed of 1270 r/min. As the methanol injection timing is delayed, it can be observed that the maximum combustion pressure of the engine first increases and then decreases until the end of the injection. When the methanol injection timing is set at −30 °CA, the maximum in-cylinder pressure reaches its highest value, at 11.6 MPa. As the methanol injection timing is advanced or delayed, the peak pressure within the cylinder decreases, indicating that the combustion efficiency is also affected, which in turn impacts the engine’s performance.
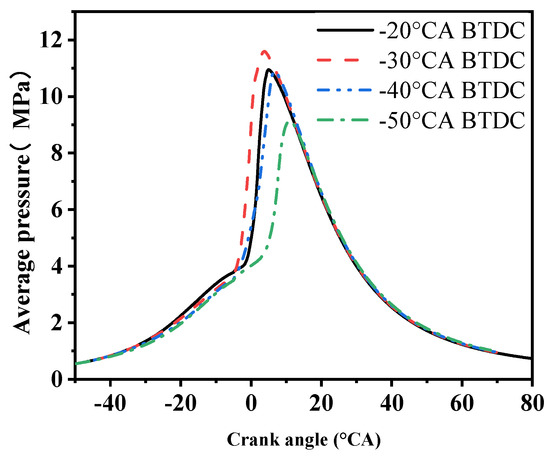
Figure 6.
Cylinder pressure under four methanol injection timings.
When methanol is injected at −50 °CA, the peak cylinder pressure is the lowest, at only 9.1 MPa. This is due to the methanol fuel being injected too early, corresponding to a lower cylinder temperature at the time of injection. Additionally, due to methanol’s high latent heat of vaporization, this results in poor atomization and a deterioration of the combustion process. If the methanol injection timing is too late, there is insufficient time for the methanol and air to mix evenly, leading to poor combustion. When the methanol injection timing is at −30 °CA, the methanol and air are well mixed. This allows for a more rapid and uniform combustion process, resulting in the highest cylinder pressure.
Figure 7 shows the cylinder temperature variation curves at different methanol injection timings. As the methanol injection timing is delayed, the overall cylinder temperature exhibits a trend of first increasing and then decreasing, similar to the cylinder pressure curves. The delay in injection timing leads to a decrease in the highest cylinder temperature. When the injection timing is at −30 °CA, the peak of the cylinder temperature curve reaches 2177 K, with a very small difference from the 2166 K at −40 °CA injection timing; at −50 °CA injection timing, the peak of the cylinder temperature curve is the lowest, at only 2063 K.
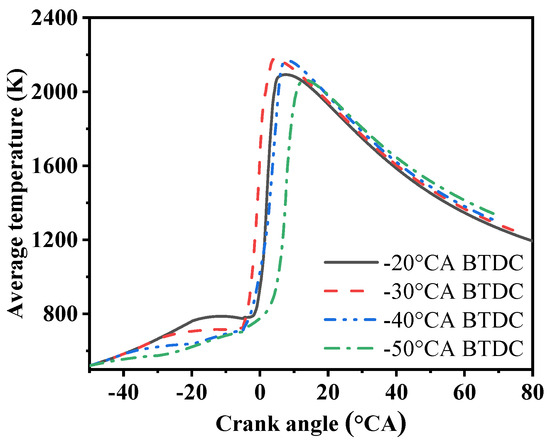
Figure 7.
Average temperature under four methanol injection timings.
When the methanol injection timing is relatively late (−20 °CA BTDC), because the methanol does not have sufficient time to be evenly mixed with the air in the cylinder, the combustion condition in the cylinder is poor and the combustion efficiency will decrease accordingly. This is, on the one hand, because the latent heat of vaporization of methanol is large and a longer mixing time and a higher temperature are required during atomization. On the other hand, it is because the combustible mixture gas is not evenly distributed, which further aggravates the downward trend of combustion efficiency. In order to improve this situation, measures need to be taken to ensure the uniform mixing of the fuel and optimize the methanol injection timing, thereby improving the combustion efficiency and performance.
Figure 8 shows the changes in the heat release rate (HRR) in the cylinder under four different injection timings. It can be observed that the peak values of the heat release rate at different methanol injection timings exhibit a clear trend of first decreasing and then increasing. This change shows different characteristics at different injection timings. At −20 °CA, the heat release rate reaches the highest peak, up to 1151 J/°CA, and the delay period is significantly shortened. However, at −30 °CA, although the heat release rate slightly decreases, it still remains at a high level, reaching 1062 J/°CA. Notably, at −30 °CA, the heat release rate curve (HRR) exhibits a double-peak phenomenon, which may be related to subtle changes in the injection timing. When further delayed to −40 °CA, the peak heat release rate significantly drops to only 692 J/°CA, and there is also a clear trend of decreased combustion quality in the cylinder.
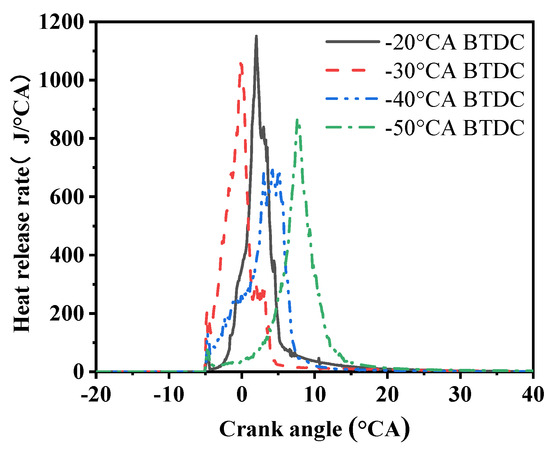
Figure 8.
HRR under four methanol injection timings.
The appearance of the double peaks may be related to early methanol injection timing. When methanol is injected too early, the temperature and pressure of the mixture are not sufficient to support the rapid development of the combustion reaction, resulting in a large distance between the ignition starting point and the top dead center (TDC) crank angle, forming the double-peak phenomenon. In this case, the fuel in the cylinder mixture may partially burn but is not completely consumed, leading to the first peak in the heat release rate curve. As the methanol injection timing gets closer to the ignition timing, the temperature and pressure in the cylinder begin to rise due to the sharp increase in the mixture temperature, which quickly reaches the minimum conditions for combustion. This process shortens the distance between the combustion initiation point and the TDC, making the combustion reaction more rapid and simultaneously shortening the delay period.
Both too early or too late methanol injection timing can seriously affect the combustion efficiency of the internal combustion engine. Appropriate methanol injection timing can effectively control the combustion period, in-cylinder pressure, and heat release rate, thereby improving the power performance of the internal combustion engine. This study indicates that at −30 °CA BTDC, methanol injection is the optimal timing. At this moment, the injected methanol can effectively control the combustion process and improve the combustion efficiency.
3.2. Impact of Methanol Injection Timing on Emission Characteristics
Figure 9 shows the soot and NOx emissions at four different methanol injection timings. The NOx emissions exhibit different trends at various methanol injection timings. Specifically, at an injection timing of −40 °CA, the NOx emissions reach the highest level, indicating that the engine produces the most nitrogen oxides at this timing. In contrast, at −30 °CA, the NOx emissions are lower and are almost the same as those at −40 °CA. However, when further delayed to −50 °CA, there is a significant reduction in NOx emissions.
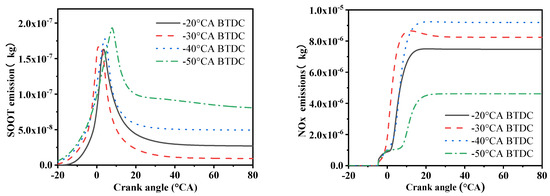
Figure 9.
NOx and soot emissions under four methanol injection timings.
The formation of NOx involves multiple pathways, including high temperatures, oxygen-rich conditions, and the duration of the reaction. Analyzing these pathways can help better understand the mechanism of NOx formation. The study found that when the intake air volume and methanol injection volume are the same, the oxygen concentration in the cylinder remains consistent. The duration of the reaction affects NOx production, with higher temperatures accelerating the reaction rate and promoting NOx formation. In chemical reactions, higher thermal energy aids in the production of NOx. This means that in high-temperature environments, especially during the combustion process, nitrogen and oxygen may react at a faster rate, resulting in more NOx production.
Referring to Figure 9, it can be observed that at −50 °CA, there is a phenomenon of oxygen deficiency in some parts of the cylinder. This means that at one or more points within the cylinder, the combustion rate may slow down, extending the combustion time, resulting in lower temperatures in the combustion chamber and thus producing less NOx. In comparison, at −30 °CA and −40 °CA crank angles, the combustion effect is better, but due to the higher cylinder temperatures, the amount of NOx generated is higher.
Figure 10 indicates that as the methanol injection timing is delayed, the soot emissions gradually decrease and then begin to increase again. When the methanol injection timing is delayed, the mixing time of methanol with air is relatively extended, which also delays the timing of methanol molecules appearing at the active centers in the cylinder. Therefore, at this point, the concentration gradient of methanol and air in the cylinder is smaller, and the soot emissions also decrease significantly. However, when the methanol injection timing is further delayed, the situation begins to change. At this point, the effect of methanol’s latent heat of vaporization on the cylinder temperature is no longer significant, and under high-temperature and low-oxygen conditions, the concentration changes in the mixture are not very obvious. Methanol injection at −30 °CA can effectively reduce the soot emissions produced by hydrocarbon fuels in high-temperature and oxygen-deficient environments. This is mainly because the temperature and pressure conditions in the combustion chamber at −30 °CA are favorable for the oxidation reaction of methanol, thereby reducing the generation of incomplete combustion products and reducing soot emissions.
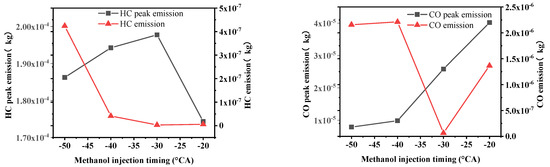
Figure 10.
Peak emissions of HC and CO under four methanol injection timings.
Figure 10 shows the HC and CO emissions at four different methanol injection timings. As seen in Figure 8, the HC emission peak is highest at −30 °CA, reaching 198 mg, and the lowest HC emission is at −20 °CA, which is only 174 mg. After combustion, the HC emissions are highest at −50 °CA, reaching 0.42 mg, and the lowest at −30 °CA, at 0.0038 mg.
Figure 10 shows that the peak CO emissions are the highest under the −20 °CA condition, reaching 40.5 mg, and the lowest under the −50 °CA condition, at only 7.89 mg. The CO emissions at the end of combustion in the cylinder are the highest under the −50 °CA condition, reaching 2.22 mg, and the lowest under the −30 °CA condition, at 0.0677 mg.
The highest CO emissions after combustion under the −50 °CA condition are mainly due to the incomplete combustion of methanol, where the mixture and temperature do not reach the ideal effect and CO cannot be completely converted to CO2. The lowest CO emissions under the −30 °CA condition are because the temperature and the quality of the mixture are more ideal compared to the other methanol injection timings.
Figure 11 reveals that an increase in CO emissions is observed when methanol is injected at −20 °CA. This phenomenon can be explained by the delayed methanol injection timing, which leads to an uneven concentration of the methanol–air mixture, causing an incomplete combustion process and resulting in the production of CO. The delay in methanol injection timing also affects the change in cylinder temperature. As the injection timing is delayed, the cylinder temperature gradually decreases. Since the formation of CO is closely related to the temperature during the combustion process, the decrease in cylinder temperature causes the CO emissions to continue to rise after complete combustion.
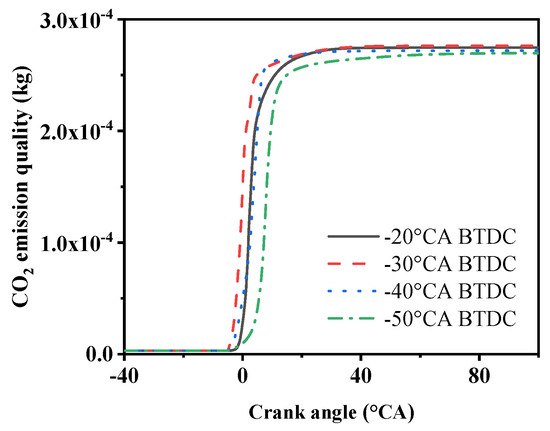
Figure 11.
CO2 emission under four methanol injection timings.
As shown in Figure 11, the graph depicts the mass of CO2 produced at four different methanol injection timings. It can be observed from the graph that changing the timing of methanol injection has a relatively small impact on the total CO2 emissions. The highest mass of CO2 is produced at −30 °CA, amounting to 276 mg, while the lowest mass of CO2 is produced at −50 °CA, at 269 mg. A comparison reveals that the production of CO2 follows a similar trend to that of CO, which is because CO and CO2 can react and convert into each other. When the combustion is complete, the final emission amounts are similar.
4. Conclusions
This study, based on CFD three-dimensional model simulation calculations, simulates the impact of different methanol injection timings on the performance of marine dual-fuel engines. The main conclusions are as follows:
Both too early or too late methanol injection timings may lead to uneven fuel distribution or over-concentration, which in turn affects combustion efficiency and emissions. After considering factors such as the mixture concentration gradient, cylinder pressure and temperature, and heat release rate, a comparison of three different injection timings found that −30 °CA BTDC injection timing is the most effective. At this timing, the mixture gas can be better mixed in the cylinder and distributed more uniformly, and the peak cylinder pressure and peak cylinder temperature at the moment of injection are the highest at the ignition timing, with the peak HRR only slightly lower than that at −20 °CA BTDC. Meanwhile, at this timing, the emissions of soot, HC, and CO are the lowest, indicating a more environmentally friendly emission level. Overall, −30 °CA BTDC injection timing has optimal combustion efficiency and emission levels. Choosing this timing for methanol injection can achieve complete fuel combustion while minimizing the emission of harmful gases, thus achieving a comprehensive optimization of engine performance and environmental friendliness. The use of CFD simulation can effectively predict the performance of marine methanol engines. Future work will primarily focus on optimizing the internal structure of the combustion chamber to better accommodate methanol fuel in marine engines, achieving a cleaner and more efficient combustion process.
Future studies may examine the effects of injection pressure, spray aiming, and turbulence intensity on fuel–air mixing and combustion efficiency in addition to improving injection time. Methanol consumption in marine engines may be further improved by advanced combustion techniques like reactivity-controlled compression ignition (RCCI) or partially premixed compression ignition (PPCI).
Author Contributions
Methodology, H.G. and H.D.; Software, C.Y.; Investigation, V.B. and F.B.; Resources, C.Y.; Data curation, H.G.; Writing—original draft, H.G.; Writing—review & editing, Veysi Bashan; Visualization, X.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Scientific and Innovative Action Plan of Shanghai (grant no. 23230750300).
Data Availability Statement
Data is contained within the article.
Conflicts of Interest
The authors declare that there are no conflicts of interest regarding the publication of this paper.
References
- Karl, O.; David, R.; Terese, L. Combustion of liquid ammonia and diesel in a compression ignition engine operated in high-pressure dual fuel mode. Fuel 2024, 360, 130269. [Google Scholar]
- Kumar, D.; Sonawane, U.; Chandra, K.; Agarwal, A.K. Experimental investigations of methanol fumigation via port fuel injection in preheated intake air in a single cylinder dual-fuel diesel engine. Fuel 2022, 324, 124340. [Google Scholar] [CrossRef]
- Duan, X.; Zhang, S.; Liu, Y.; Li, Y.; Liu, J.; Lai, M.-C.; Deng, B. Numerical investigation the effects of the twin-spark plugs coupled with EGR on the combustion process and emissions characteristics in a lean burn natural gas SI engine. Energy 2020, 206, 118181. [Google Scholar] [CrossRef]
- Panda, K.; Ramesh, A. HCCI combustion of methanol along with diesel through novel injection strategies and its potential over conventional dual fuel combustion. Fuel 2022, 324, 124766. [Google Scholar] [CrossRef]
- Garcia, A.; Monsalve-Serrano, J.; Villalta, D.; Guzmán-Mendoza, M. Methanol and OMEx as fuel candidates to fulfill the potential EURO VII emissions regulation under dual-mode dual-fuel combustion. Fuel 2021, 287, 119548. [Google Scholar] [CrossRef]
- Li, X.; Yan, P.; Li, H.-M.; Zheng, L.; Shen, G.; Hu, Y.-C.; Han, D. Numerical Study of the Effect of Direct-Injection Timing of Methanol and Excess Air Ratio on the Combustion Characteristics of a Marine Diesel-Methanol Dual-Fuel Engine. In Proceedings of the Energy and Propulsion Conference and Exhibition, Greenville, SC, USA, 7–9 November 2023; 1626p. [Google Scholar]
- Wu, H.; Nithyanandan, K.; Zhou, N.; Leeet, T.H.; Lee, C.-F.F.; Zhang, C. Impacts of acetone on the spray combustion of Acetone–Butanol–Ethanol (ABE)-Diesel blends under low ambient temperature. Fuel 2015, 142, 109–116. [Google Scholar] [CrossRef]
- Zhang, C.; Wu, H. Combustion characteristics and performance of a methanol fueled homogenous charge compression ignition (HCCI) engine. J. Energy Inst. 2016, 89, 346–353. [Google Scholar] [CrossRef]
- Karvounis, P.; Theotokatos, G. Parametric optimisation of diesel–methanol injection timings of a dual-fuel marine engine operating with high methanol fraction using CFD. Appl. Therm. Eng. 2025, 264, 125433. [Google Scholar] [CrossRef]
- Karvounis, P.; Theotokatos, G.; Patil, C.; Xiang, L.; Ding, Y. Parametric investigation of diesel–methanol dual fuel marine engines with port and direct injection. Fuel 2025, 381, 133441. [Google Scholar] [CrossRef]
- Yu, Y.; Wen, H.; Shen, J.; Li, J.; Xu, C.; Jing, H. Exploration and optimization of injection strategy for medium-speed diesel/methanol dual direct-injection marine engine under extremely high methanol energy ratio. Energy Sources Part Recovery Util. Environ. Eff. 2025, 47, 1289–1307. [Google Scholar] [CrossRef]
- Dierickx, J.; Dejaegere, Q.; Peeters, J.; Sileghem, L.; Verhelst, S. Performance and emissions of a high-speed marine dual-fuel engine operating with methanol-water blends as a fuel. Fuel 2023, 333, 126349. [Google Scholar] [CrossRef]
- Dierickx, J.; Verbiest, J.; Janvier, T.; Peeters, J.; Sileghem, L.; Verhelst, S. Retrofitting a high-speed marine engine to dual-fuel methanol-diesel operation: A comparison of multiple and single point methanol port injection. Fuel Commun. 2021, 7, 100010. [Google Scholar] [CrossRef]
- Sun, W.; Jiang, M.; Guo, L.; Zhang, H.; Jia, Z.; Qin, Z.; Zeng, W.; Lin, S.; Zhu, G.; Ji, S.; et al. Numerical study of injection strategies for marine methanol/diesel direct dual fuel stratification engine. J. Clean. Prod. 2023, 1, 138505. [Google Scholar] [CrossRef]
- Yu, Y.; Wen, H. Investigation on efficient and clean combustion pre-injection strategy of a diesel/methanol dual direct-injection marine engine under full load. Case Stud. Therm. Eng. 2024, 1, 104472. [Google Scholar] [CrossRef]
- Wu, H.-W.; Fan, C.M.; He, J.Y.; Hsu, T.-T. Optimal factors estimation for diesel/methanol engines changing methanol injection timing and inlet air temperature. Energy 2017, 141, 1819–1828. [Google Scholar] [CrossRef]
- Valera, H.; Kumar, D.; Agarwal, A.K. Evaluating the effect of variable methanol injection timings in a novel co-axial fuel injection system equipped locomotive engine. J. Clean. Prod. 2022, 1, 131452. [Google Scholar] [CrossRef]
- Güdden, A.; Pischinger, S.; Geiger, J.; Heuser, B.; Müther, M. An experimental study on methanol as a fuel in large bore high speed engine applications—Port fuel injected spark ignited combustion. Fuel 2021, 1, 121292. [Google Scholar] [CrossRef]
- Yusri, I.; Mamat, R.; Najafi, G.; Razman, A.; Awad, O.I.; Azmi, W.; Ishak, W.; Shaiful, A. Alcohol based automotive fuels from first four alcohol family in compression and spark ignition engine: A review on engine performance and exhaust emissions. Renew. Sustain. Energy Rev. 2017, 77, 169–181. [Google Scholar] [CrossRef]
- Sayin, C.; Ozsezen, N.A.; Canakci, M. The influence of operating parameters on the performance and emissions of a DI diesel engine using methanol-blended-diesel fuel. Fuel 2009, 89, 1407–1414. [Google Scholar] [CrossRef]
- Wyer, K.E.; Kelleghan, D.B.; Blanes-Vidal, V.; Schauberger, G.; Curran, T.P. Ammonia emissions from agriculture and their contribution to fine particulate matter: A review of implications for human health. J. Environ. Manag. 2022, 323, 116285. [Google Scholar] [CrossRef]
- Gawale, R.G.; Srinivasulu, N.G. Experimental investigation of ethanol/diesel and ethanol/biodiesel on dual fuel mode HCCI engine for different engine load conditions. Fuel 2020, 263, 116725. [Google Scholar] [CrossRef]
- Ma, B.; Yao, A.; Yao, C.; Chen, C.; Qu, G.; Wang, W.; Ai, Y. Multiple combustion modes existing in the engine operating in diesel methanol dual fuel. Energy 2021, 234, 121285. [Google Scholar] [CrossRef]
- Gong, C.; Peng, L.; Chen, Y.; Liu, J.; Liu, F.; Han, Y. Computational study of intake temperature effects on mixture formation, combustion and unregulated emissions of a DISI methanol engine during cold start. Fuel 2018, 234, 1269–1277. [Google Scholar] [CrossRef]
- Li, Z.; Wang, Y.; Yin, Z.; Gao, Z.; Wang, Y.; Zhen, X. To achieve high methanol substitution ratio and clean combustion on a diesel/methanol dual fuel engine: A comparison of diesel methanol compound combustion (DMCC) and direct dual fuel stratification (DDFS) strategies. Fuel 2021, 304, 121466. [Google Scholar] [CrossRef]
- Richards, K.J.; Senecal, P.K.; Pomraning, E. CONVERGE 4.1*; Convergent Science: Madison, WI, USA, 2025. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).