Diagenetic Barite Growths in the Mixing Zone of a Carbonate Coastal Aquifer
Abstract
1. Introduction
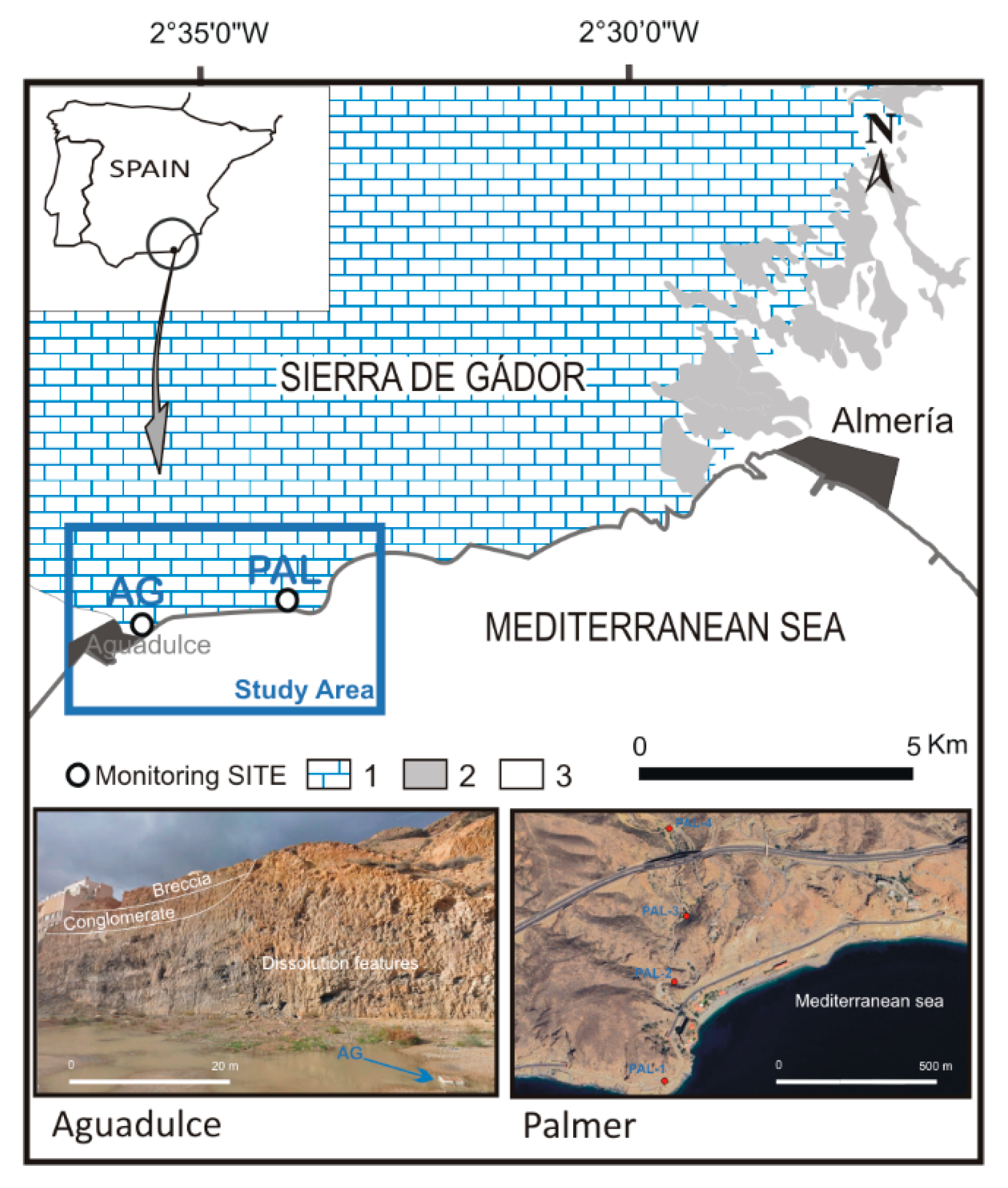
2. Geological Setting
3. Methods
4. Results
4.1. Aguadulce Site
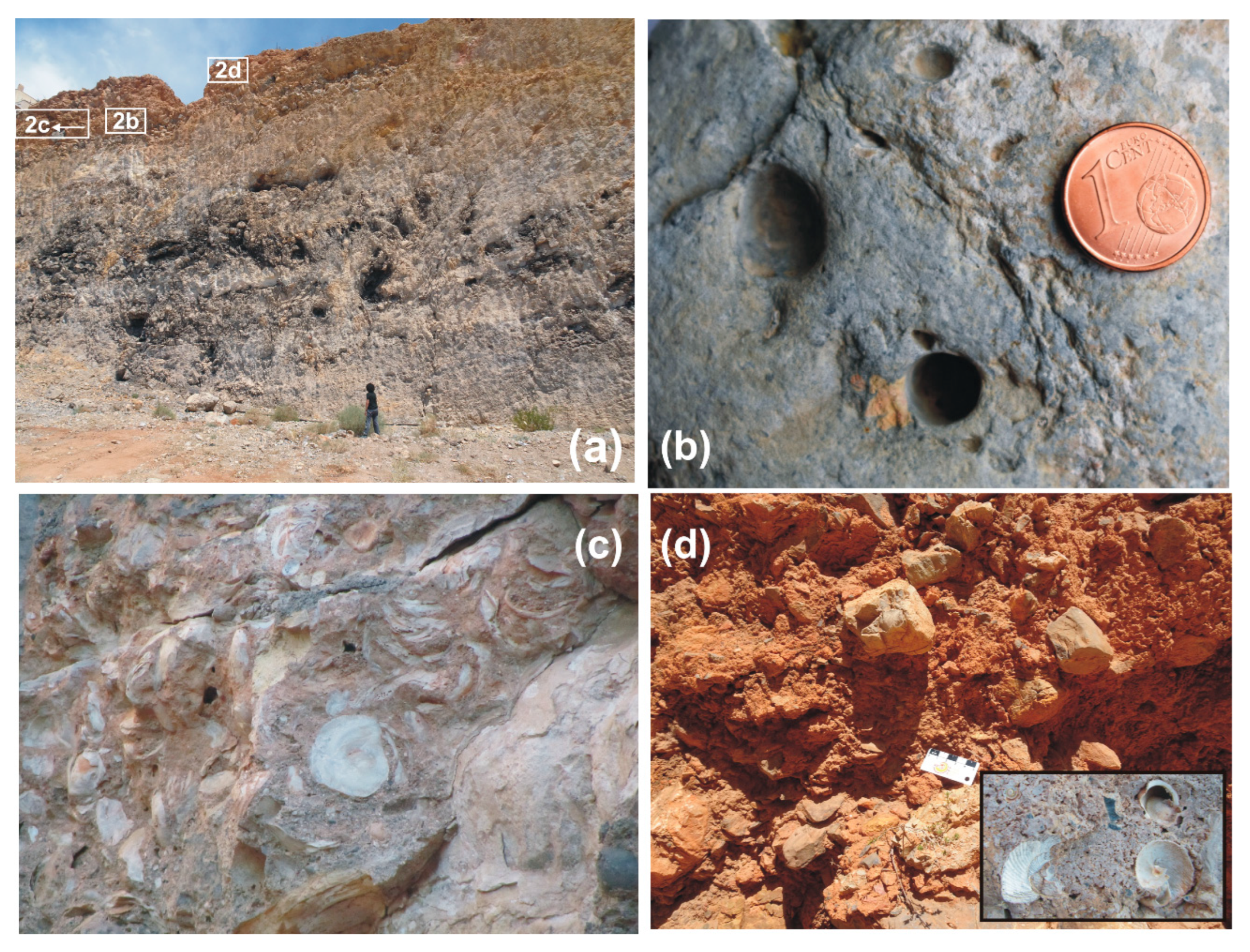
4.1.1. Sedimentary Record
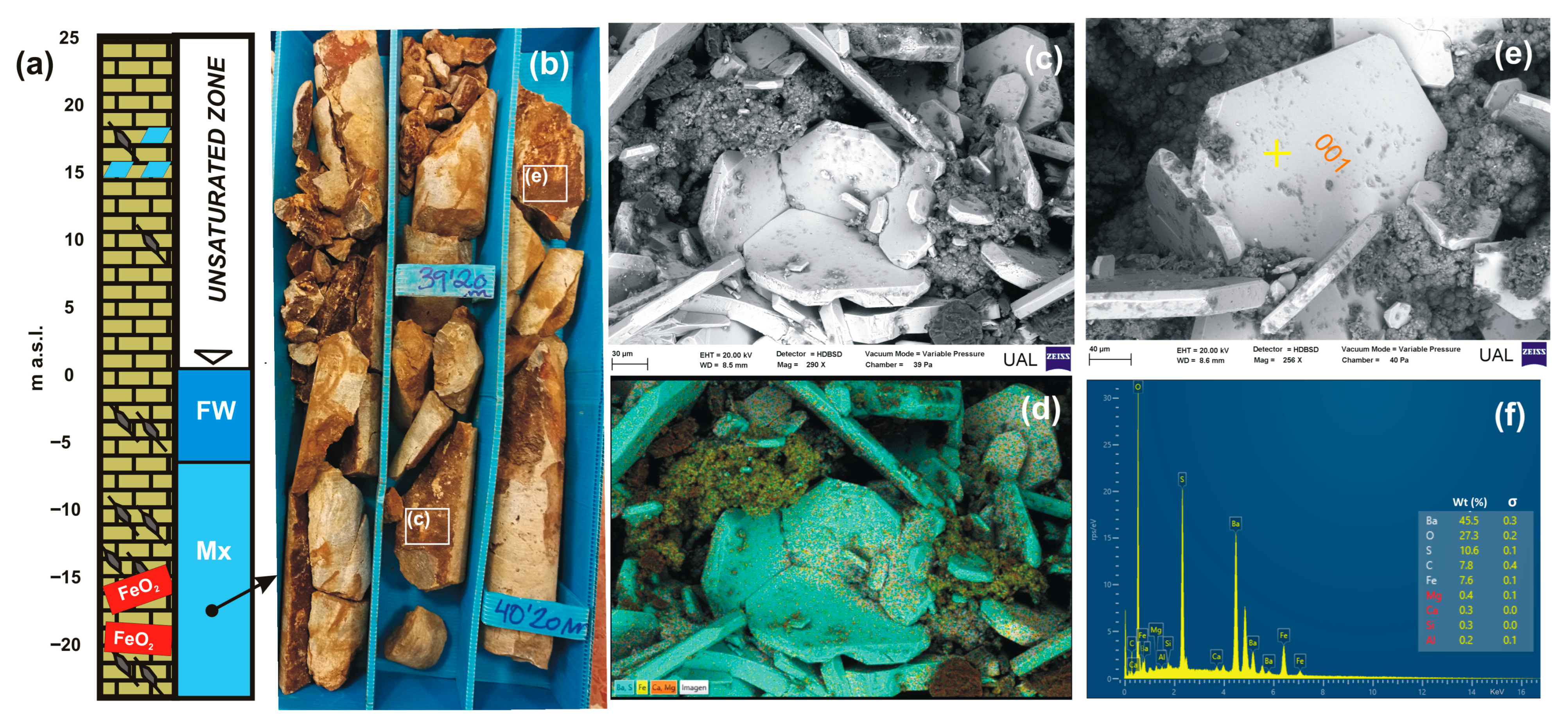
4.1.2. Diagenetic Features
4.1.3. Microscopy and Analysis of Precipitated Minerals
4.1.4. Hydrochemical Analysis
4.2. Palmer Site
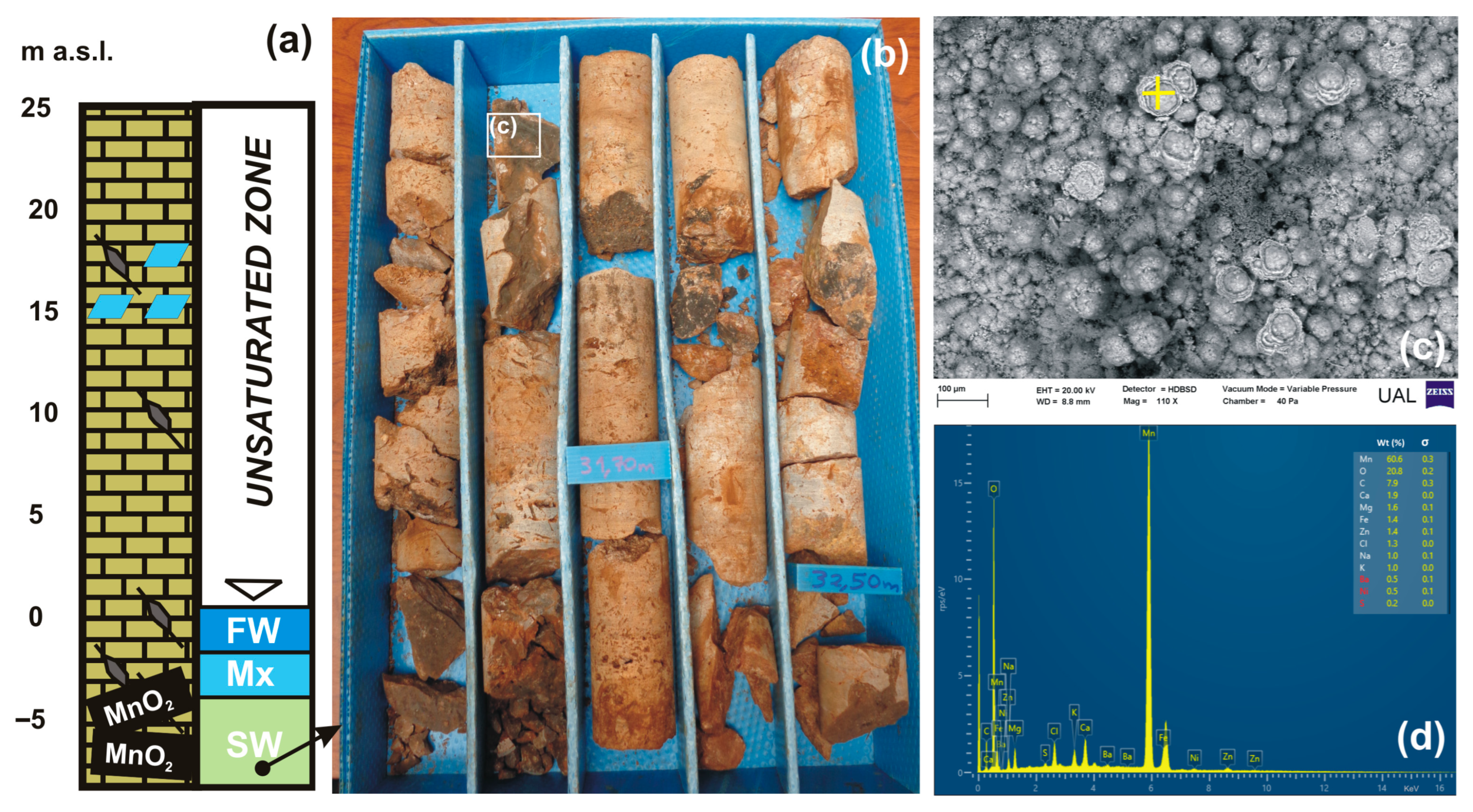
4.2.1. Diagenetic Features
4.2.2. Microscopy and Analysis of Precipitated Minerals
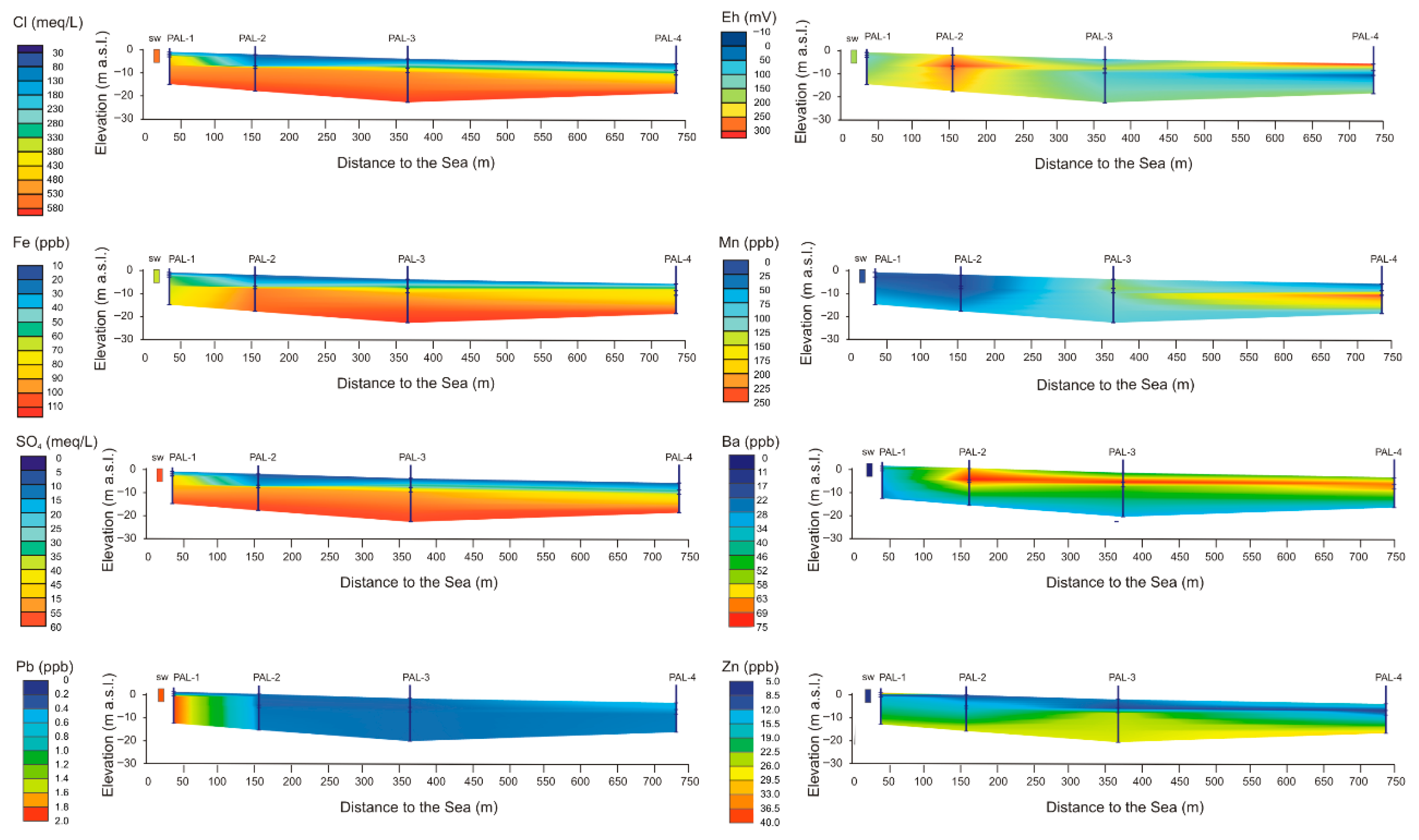
4.2.3. Hydrochemical Analysis
5. Discussion
5.1. Interpretation of the Sedimentary Record and Diagenetic Features
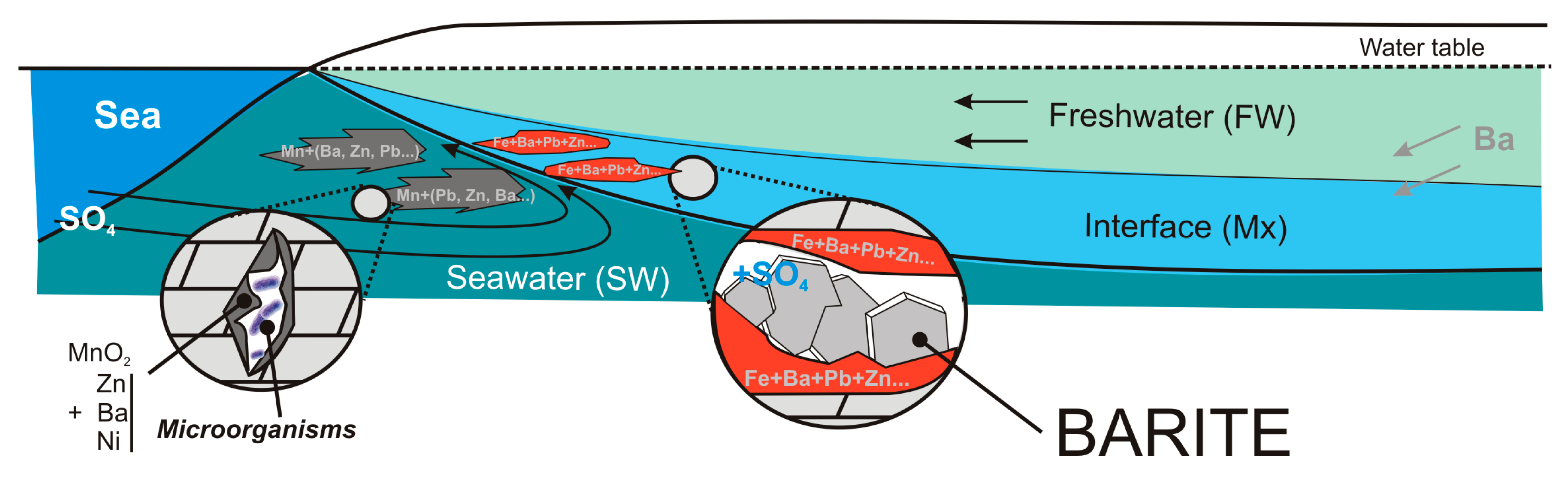
5.2. Diagenetic Barite Formation Model
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Appelo, C.A.J.; Postma, D. Geochemistry, Groundwater and Pollution; CRC Press: Boca Raton, FL, USA, 2005. [Google Scholar]
- McAllister, S.M.; Barnett, J.M.; Heiss, J.W.; Findlay, A.J.; MacDonald, D.J.; Dow, C.L.; Luther, G.W.; Michael, H.A.; Chan, C.S. Dynamic hydrologic and biogeochemical processes drive microbially enhanced iron and sulfur cycling within the intertidal mixing zone of a beach aquifer. Limnol. Oceanogr. 2015, 60, 329–345. [Google Scholar] [CrossRef]
- Heiss, J.W.; Post, V.E.; Laattoe, T.; Russoniello, C.J.; Michael, H.A. Physical controls on biogeochemical processes in intertidal zones of beach aquifers. Water Resour. Res. 2017, 53, 9225–9244. [Google Scholar] [CrossRef]
- Moore, W.S.; Joye, S.B. Saltwater intrusion and submarine groundwater discharge: Acceleration of biogeochemical reactions in changing coastal aquifers. Front. Earth Sci. 2021, 9, 600710. [Google Scholar] [CrossRef]
- Petrash, D.A.; Bialik, O.M.; Staudigel, P.T.; Konhauser, K.O.; Budd, D.A. Biogeochemical reappraisal of the freshwater–seawater mixing-zone diagenetic model. Sedimentology 2021, 68, 1797–1830. [Google Scholar] [CrossRef]
- Goyetche, T.; Luquot, L.; Carrera, J.; Martínez-Pérez, L.; Folch, A. Identification and quantification of chemical reactions in a coastal aquifer to assess submarine groundwater discharge composition. Sci. Total. Environ. 2022, 838, 155978. [Google Scholar] [CrossRef]
- Abarca, E.; Clement, T.P. A novel approach for characterizing the mixing zone of a saltwater wedge. Geophys. Res. Lett. 2009, 36, L06402. [Google Scholar] [CrossRef]
- Chang, S.W.; Clement, T.P. Experimental and numerical investigation of saltwater intrusion dynamics in flux-controlled groundwater systems. Water Resour. Res. 2012, 48, W09527. [Google Scholar] [CrossRef]
- Lu, C.; Chen, Y.; Zhang, C.; Luo, J. Steady-state freshwater–seawater mixing zone in stratified coastal aquifers. J. Hydrol. 2013, 505, 24–34. [Google Scholar] [CrossRef]
- Ataie-Ashtiani, B.; Volker, R.E.; Lockington, D.A. Tidal effects on sea water intrusion in unconfined aquifers. J. Hydrol. 1999, 216, 17–31. [Google Scholar] [CrossRef]
- Cartwright, N.; Li, L.; Nielsen, P. Response of the salt–freshwater interface in a coastal aquifer to a wave-induced groundwater pulse: Field observations and modelling. Adv. Water Resour. 2004, 27, 297–303. [Google Scholar] [CrossRef]
- Vallejos, A.; Sola, F.; Pulido-Bosch, A. Processes Influencing groundwater level and the freshwater-saltwater Interface in a coastal aquifer. Water Resour. Manag. 2015, 29, 679–697. [Google Scholar] [CrossRef]
- Smart, P.L.; Dawans, J.M.; Whitaker, F.F. Carbonate dissolution in a modern mixing zone, South Andros, Bahamas. Nature 1988, 335, 811–813. [Google Scholar] [CrossRef]
- Rezaei, M.; Sanz, E.; Raeisi, E.; Ayora, C.; Vázquez-Suñé, E.; Carrera, J. Reactive transport modeling of calcite dissolution in the fresh-salt water mixing zone. J. Hydrol. 2005, 311, 282–298. [Google Scholar] [CrossRef]
- Sanford, W.E.; Konikow, L.F. Simulation of Calcite Dissolution and Porosity Changes in Saltwater Mixing Zones in Coastal Aquifers. Water Resour. Res. 1989, 25, 655–667. [Google Scholar] [CrossRef]
- Hanshaw, B.B.; Back, W.; Deike, R.G. A geochemical hypothesis for dolomitization by ground water. Econ. Geol. 1971, 66, 710–724. [Google Scholar] [CrossRef]
- Badiozamani, K. The Dorag Dolomitization Model, Application to the Middle Ordovician of Wisconsin. J. Sediment. Res. 1973, 43, 965–984. [Google Scholar] [CrossRef]
- Land, L.S.; Salem, M.R.I.; Morrow, D.W. Paleo-hydrology of ancient dolomites: Geochemical evidence. AAPG Bull. 1975, 59, 1602–1625. [Google Scholar]
- Magaritz, M.; Goldenberg, L.; Kafri, U.; Arad, A. Dolomite formation in the seawater–freshwater interface. Nature 1980, 287, 622–624. [Google Scholar] [CrossRef]
- Machel, H.-G.; Mountjoy, E.W. Chemistry andenvironments of dolomitization—A reappraisal. Earth-Sci. Rev. 1986, 23, 175–222. [Google Scholar] [CrossRef]
- Melim, L.; Swart, P.K.; Eberli, G.P. Mixing-Zone Diagenesis in the Subsurface of Florida and the Bahamas. J. Sediment. Res. 2004, 74, 904–913. [Google Scholar] [CrossRef]
- Gaswirth, S.B.; Budd, D.A.; Farmer, G.L. The role and impact of freshwater–seawater mixing zones in the maturation of regional dolomite bodies within the proto Floridan Aquifer, USA. Sedimentology 2007, 54, 1065–1092. [Google Scholar] [CrossRef]
- Spiteri, C.; Slomp, C.P.; Charette, M.A.; Tuncay, K.; Meile, C. Flow and nutrient dynamics in a subterranean estuary (Waquoit Bay, MA, USA): Field data and reactive transport modeling. Geochim. Cosmochim. Acta 2008, 72, 3398–3412. [Google Scholar] [CrossRef]
- Ruiz-González, C.; Rodellas, V.; Garcia-Orellana, J. The microbial dimension of submarine groundwater discharge: Current challenges and future directions. FEMS Microbiol. Rev. 2021, 45, fuab010. [Google Scholar] [CrossRef]
- Seibert, S.L.; Massmann, G.; Meyer, R.; Post, V.E.A.; Greskowiak, J. Impact of mineral reactions and surface complexation on the transport of dissolved species in a subterranean estuary: Application of a comprehensive reactive transport modeling approach. Adv. Water Resour. 2024, 191, 104763. [Google Scholar] [CrossRef]
- Seibert, S.L.; Massmann, G.; Meyer, R.; Post, V.E.A.; Greskowiak, J. Reactive transport modeling to reveal the impacts of beach morphodynamics, storm floods and seasonal groundwater recharge on the biogeochemistry of sandy subterranean estuaries. Adv. Water Resour. 2025, 196, 104884. [Google Scholar] [CrossRef]
- Penny, E.; Lee, M.-K.; Morton, C. Groundwater and microbial processes of Alabama coastal plain aquifers. Water Resour. Res. 2003, 39, 1320. [Google Scholar] [CrossRef]
- Vallejos, A.; Sola, F.; Vargas-García, M.C.; Mancuso, M. Microbial-induced MnO2 precipitation in a carbonate coastal aquifer. Sci. Total. Environ. 2024, 915, 169968. [Google Scholar] [CrossRef]
- Majamäki, R.; Wasiljeff, J.; Purkamo, L.; Hultman, J.; Asmala, E.; Yli-Hemminki, P.; Jørgensen, K.S.; Koho, K.; Kuva, J.; Virtasalo, J.J. Microbially Enhanced Growth and Metal Capture by Ferromanganese Concretions in a Laboratory Experiment. Geobiology 2025, 23, e70010. [Google Scholar] [CrossRef]
- Chapelle, F. Geochemistry of Groundwater. In Treatise on Geochemistry; Elsevier: Amsterdam, The Netherlands, 2003; Volume 5, pp. 425–449. [Google Scholar]
- Stumm, W.; Morgan, J.J. Aquatic Chemistry, Chemical Equilibria and Rates in Natural Waters, 3rd ed.; John Wiley & Sons, Inc.: New York, NY, USA, 1996. [Google Scholar]
- Hansel, C.; Benner, S.; Neiss, J.; Dohnalkova, A.; Kukkadapu, R.; Fendorf, S. Secondary Mineralization Pathways Induced by Dissimilatory Iron Reduction of Ferrihydrite under Advective Flow. Geochim. Cosmochim. Acta 2003, 67, 2977–2992. [Google Scholar] [CrossRef]
- Wehrmann, L.M.; Riedinger, N.; Brunner, B.; Kamyshny, A., Jr.; Herbert, L.C.; Brüchert, V.; Jørgensen, B.B.; Ferdelman, T.G.; Formolo, M.J. Iron-controlled oxidative sulfur cycling recorded in the distribution and isotopic composition of sulfur species in glacially influenced fjord sediments of west Svalbard. Chem. Geol. 2017, 466, 678–695. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhang, C.; Cao, W.; Hofmann, H.; Wang, T.; Li, L. Oxidative precipitation of Fe (II) in porous media: Laboratory experiment and numerical simulation. ACS ES&T Water 2023, 3, 963–973. [Google Scholar] [CrossRef]
- Senn, A.-C.; Hug, S.J.; Kaegi, R.; Hering, J.G.; Voegelin, A. Arsenate co-precipitation with Fe(II) oxidation products and retention or release during precipitate aging. Water Res. 2018, 131, 334–345. [Google Scholar] [CrossRef]
- Fernex, F.; Février, G.; Bénaïm, J.; Arnoux, A. Copper, lead and zinc trapping in Mediterranean deep-sea sediments: Probable coprecipitation with Mn and Fe. Chem. Geol. 1992, 98, 293–306. [Google Scholar] [CrossRef]
- Tebo, B.M.; Bargar, J.R.; Clement, B.G.; Dick, G.J.; Murray, K.J.; Parker, D.; Verity, R.; Webb, S.M. Biogenic manganese oxides: Properties and mechanisms of formation. Annu. Rev. Earth Planet. Sci. 2004, 32, 287–328. [Google Scholar] [CrossRef]
- Sugiyama, M.; Hori, T. Geochemical behavior of barium in the vicinity of a MnO2/Mn2+ redox front formed in a eutrophic lake. Jpn. J. Limnol. 1994, 55, 27–37. [Google Scholar] [CrossRef]
- Ren, Y.; Guan, Y.; Sun, X.; Xu, L.; Xiao, Z.; Deng, Y.; He, W. Nano-mineralogy and growth environment of Fe-Mn polymetallic crusts and nodules from the South China Sea. Front. Mar. Sci. 2023, 10, 1141926. [Google Scholar] [CrossRef]
- Torres, M.E.; Brumsack, H.J.; Bohrmann, G.; Emeis, K.C. Barite fronts in continental margin sediments: A new look at barium remobilization in the zone of sulfate reduction and formation of heavy barites in diagenetic fronts. Chem. Geol. 1996, 127, 125–139. [Google Scholar] [CrossRef]
- Riedinger, N.; Kasten, S.; Gröger, J.; Franke, C.; Pfeifer, K. Active and buried authigenic barite fronts in sediments from the Eastern Cape Basin. Earth Planet. Sci. Lett. 2006, 24, 876–887. [Google Scholar] [CrossRef]
- Griffith, E.M.; Paytan, A. Barite in the ocean–occurrence, geochemistry and palaeoceanographic applications. Sedimentology 2012, 59, 1817–1835. [Google Scholar] [CrossRef]
- Zhou, X.; Chen, D.; Dong, S.; Zhang, Y.; Guo, Z.; Wei, H.; Yu, H. Diagenetic barite deposits in the Yurtus Formation in Tarim Basin, NW China: Implications for barium and sulfur cycling in the earliest Cambrian. Precambrian Res. 2015, 263, 79–87. [Google Scholar] [CrossRef]
- Jewell, P. Bedded barite in the geologic record. SEPM Spec. Public 2000, 66, 147–161. [Google Scholar]
- Laurent, D.; Lopez, M.; Combes, P.J.; Guerrot, C.; Spangenberg, J.E.; Gaucher, E.C. Synsedimentary to early diage-netic rejuvenation of barite-sulfides ore deposits: Example of the Triassic intrakarstic mineralization in the Lodève basin (France). Mar. Pet. Geol. 2020, 119, 104464. [Google Scholar] [CrossRef]
- Baena, J.; Voermans, F. Mapa Geológico de España, 1:50.000. Plan MAGNA. Hoja 1044 (Alhama de Almería); IGME: Madrid, Spain, 1983. [Google Scholar]
- Martin-Rojas, I.; Somma, R.; Delgado, F.; Estévez, A.; Iannace, A.; Zamparelli, V. The Triassic platform of the Gador-Turon unit (Alpujarride complex, Betic Cordillera, southeast Spain): Climate versus tectonic factors controlling platform architecture. Facies 2012, 58, 297–323. [Google Scholar] [CrossRef]
- Ros-Franch, S.; Sola, F.; Braga, J.C.; Márquez-Aliaga, A. Bivalve shell beds in the Triassic of the Sierra De Gádor (Al-pujárride complex, Betic Cordillera, SE Spain). J. Iber. Geol. 2025, 1–21. [Google Scholar] [CrossRef]
- Sælen, G.; Braga, J.C.; Sola, F. Oyster shells, bulk carbonate sediment, and meteoric calcite cement as recorders of oceanic and radiogenic 87Sr/86Sr in mixed heterozoan carbonates and terrigenous sediment. J. Sediment. Res. 2022, 92, 50–65. [Google Scholar] [CrossRef]
- Sola, F.; Braga, J.C.; Sælen, G. Contradictory coeval vertical facies changes in upper Miocene heterozoan carbonate–terrigenous deposits (Sierra de Gádor, Almería, SE Spain). J. Sediment. Res. 2022, 92, 257–274. [Google Scholar] [CrossRef]
- Sola, F.; Puga-Bernabéu, Á.; Aguirre, J.; Braga, J.C. Heterozoan carbonate deposition on a steep basement escarpment (Late Miocene, Almería, south-east Spain). Sedimentology 2017, 64, 1107–1131. [Google Scholar] [CrossRef]
- Sola, F.; Puga-Bernabéu, Á.; Aguirre, J.; Braga, J.C. Origin, evolution and sedimentary processes associated with a late Miocene submarine landslide, southeast Spain. Sediment. Geol. 2018, 364, 351–366. [Google Scholar] [CrossRef]
- Zazo, C.; Goy, J.L.; Dabrio, C.J.; Lario, J.; González-Delgado, J.A.; Bardají, T.; Hillaire-Marcel, C.; Cabero, A.; Ghaleb, B.; Borja, F.; et al. Retracing the Quaternary history of sea-level changes in the Spanish Mediterranean–Atlantic coasts: Geomorphological and sedimentological approach. Geomorphology 2013, 196, 36–49. [Google Scholar] [CrossRef]
- Díaz-Puga, M.A.; Vallejos, A.; Daniele, L.; Sola, F.; Rodríguez-Delgado, D.; Molina, L.; Pulido-Bosch, A. An oceanographic survey for the detection of a possible submarine groundwater discharge in the coastal zone of Campo de Dalias, SE Spain. In Advances in the Research of Aquatic Environment; Springer: Berlin/Heidelberg, Germany, 2011; Volume 1, pp. 417–424. [Google Scholar]
- Parkhurst, D.L.; Appelo, C.A.J. Description of input and examples for PHREEQC version 3—A computer program for speciation, batch-reaction, one-dimensional transport, and inverse geochemical calculations. In Modeling Techniques; U.S. Geological Survey Techniques and Methods: Denver, CO, USA, 2013; Chapter A43; 497p. [Google Scholar]
- Goy, J.L.; Zazo, C. Synthesis of the quaternary in the Almeria littoral neotectonic activity and its morphologic features, Western Betics, Spain. Tectonophysics 1986, 130, 259–270. [Google Scholar] [CrossRef]
- Zazo, C.; Goy, J.L.; Dabrio, C.J.; Bardají, T.; Hillaire-Marcel, C.; Ghaleb, B.; González-Delgado, J.A.; Soler, V. Pleistocene raised marine terraces of the Spanish Mediterranean and Atlantic coasts: Records of coastal uplift, sea-level highstands and climate changes. Mar. Geol. 2003, 194, 103–133. [Google Scholar] [CrossRef]
- Marín-Lechado, C.; Galindo-Zaldívar, J.; Rodríguez-Fernández, L.R.; Serrano, I.; Pedrera, A. Active faults, seismicity and stresses in an internal boundary of a tectonic arc (Campo de Dalías and Níjar, southeastern Betic Cordilleras, Spain). Tectonophysics 2005, 396, 81–96. [Google Scholar] [CrossRef]
- Pedrera, A.; Marín-Lechado, C.; Stich, D.; Ruiz-Constán, A.; Galindo-Zaldívar, J.; Rey-Moral, C.; de Lis Mancilla, F. Nucleation, linkage and active propagation of a segmented Quaternary normal-dextral fault: The Loma del Viento fault (Campo de Dalías, Eastern Betic Cordillera, SE Spain). Tectonophysics 2012, 522, 208–217. [Google Scholar] [CrossRef]
- Back, W.; Hanshaw, B.B.; Herman, J.S.; Van Driel, J.N. Differential dissolution of a Pleistocene reef in the ground-water mixing zone of coastal Yucatan, Mexico. Geology 1986, 14, 137–140. [Google Scholar] [CrossRef]
- Baceta, J.; Wright, V.; Pujalte, V. Palaeo-mixing zone karst features from Palaeocene carbonates of north Spain: Criteria for recognizing a potentially widespread but rarely documented diagenetic system. Sediment. Geol. 2001, 139, 205–216. [Google Scholar] [CrossRef]
- Calner, M.; Lehnert, O.; Nõlvak, J. Palaeokarst evidence for widespread regression and subaerial exposure in the middle Katian (Upper Ordovician) of Baltoscandia: Significance for global climate. Palaeogeogr. Palaeoclim. Palaeoecol. 2010, 296, 235–247. [Google Scholar] [CrossRef]
- Li, Z.; Goldstein, R.H.; Franseen, E.K. Meteoric calcite cementation: Diagenetic response to relative fall in sea-level and effect on porosity and permeability, Las Negras area, southeastern Spain. Sediment. Geol. 2017, 348, 1–18. [Google Scholar] [CrossRef]
- Charette, M.A.; Sholkovitz, E.R. Oxidative precipitation of groundwater-derived ferrous iron in the subterranean estuary of a coastal bay. Geophys. Res. Lett. 2002, 29, 85-1–85-4. [Google Scholar] [CrossRef]
- Cao, W.; Hofmann, H.; Yan, G.; Scheuermann, A. Porewater exchange and iron transformation in a coastal groundwater system: A field investigation, driving mechanisms analysis, and conceptual model. Front. Mar. Sci. 2024, 11, 1385517. [Google Scholar] [CrossRef]
- Charette, M.A.; Sholkovitz, E.R.; Hansel, C.M. Trace element cycling in a subterranean estuary: Part 1. Geochemistry of the permeable sediments. Geochim. Cosmochim. Acta 2005, 69, 2095–2109. [Google Scholar] [CrossRef]
- Charette, M.A.; Sholkovitz, E.R. Trace element cycling in a subterranean estuary: Part 2. Geochemistry of the pore water. Geochim. Cosmochim. Acta 2006, 70, 811–826. [Google Scholar] [CrossRef]
- Cao, W.; Yan, G.; Hofmann, H.; Scheuermann, A. State of the Art on Fe Precipitation in Porous Media: Hydrogeochemical Processes and Evolving Parameters. J. Mar. Sci. Eng. 2024, 12, 690. [Google Scholar] [CrossRef]
- Cao, W.; Hofmann, H.; Scheuermann, A. Iron Curtain Formation in Coastal Aquifers: Insights from Darcy-Scale Experiments and Reactive Transport Modelling. J. Mar. Sci. Eng. 2025, 13, 1909. [Google Scholar] [CrossRef]
- Testa, J.M.; Charette, M.A.; Sholkovitz, E.R.; Allen, M.C.; Rago, A.; Herbold, C.W. Dissolved iron cycling in the subterranean estuary of a coastal bay: Waquoit Bay, Massachusetts. Biol. Bull. 2002, 203, 255–256. [Google Scholar] [CrossRef]
- Díaz-Puga, M.A.; Pulido-Bosch, A.; Vallejos, A.; Sola, F.; Daniele, L.; Simón, M.; García, I. Impact of mine leachates on a carbonate aquifer (SE Spain). Mine Water Environ. 2021, 40, 225–234. [Google Scholar] [CrossRef]
- Burnett, W.C.; Bokuniewicz, H.; Huettel, M.; Moore, W.S.; Taniguchi, M. Groundwater and pore water inputs to the coastal zone. Biogeochemistry 2003, 66, 3–33. [Google Scholar] [CrossRef]
- Moore, W.S. The effect of submarine groundwater discharge on the ocean. Annu. Rev. Mar. Sci. 2010, 2, 59–88. [Google Scholar] [CrossRef]
- Sawyer, A.H.; Michael, H.A.; Schroth, A.W. From soil to sea: The role of groundwater in coastal critical zone processes. WIREs Water 2016, 3, 706–726. [Google Scholar] [CrossRef]
- Santos, I.R.; Chen, X.; Lecher, A.L.; Sawyer, A.H.; Moosdorf, N.; Rodellas, V.; Tamborski, J.; Cho, H.-M.; Dimova, N.; Sugimoto, R.; et al. Submarine groundwater discharge impacts on coastal nutrient biogeochemistry. Nat. Rev. Earth Environ. 2021, 2, 307–323. [Google Scholar] [CrossRef]
- Martinez-Ruiz, F.; Paytan, A.; Gonzalez-Muñoz, M.T.; Jroundi, F.; Abad, M.D.M.; Lam, P.J.; Bishop, J.K.B.; Horner, T.J.; Morton, P.L.; Kastner, M. Barite formation in the ocean: Origin of amorphous and crystalline precipitates. Chem. Geol. 2019, 511, 441–451. [Google Scholar] [CrossRef]
- Goldberg, E.D.; Arrhenius, G. Chemistry of pelagic sediments. Geochim. Cosmochim. Acta 1958, 13, 153–212. [Google Scholar] [CrossRef]
- Bishop, J.K.B. The barite-opal-organic carbon association in oceanic particulate matter. Nature 1988, 332, 341–343. [Google Scholar] [CrossRef]
- Bernstein, R.E.; Byrne, R.H.; Betzer, P.R.; Greco, A.M. Morphologies and transformations of celestite in seawater: The role of acantharians in the strontium and barium geochemistry. Geochim. Cosmochim. Acta 1992, 56, 3273–3279. [Google Scholar] [CrossRef]
- Bernstein, R.E.; Byrne, R.H.; Schijf, J. Acantharians: A missing link in the oceanic biogeochemistry of barium. Deep-Sea Res. I 1998, 45, 491–505. [Google Scholar] [CrossRef]
- Ganeshram, R.S.; François, R.; Commeau, J.; Brown-Leger, S.L. An experimental investigation of barite formation in seawater. Geochim. Cosmochim. Acta 2003, 67, 2599–2605. [Google Scholar] [CrossRef]
- Breheret, J.-G.; Brumsack, H.-J. Barite concretions as evidence of pauses in sedimentation in the Marnes Bleues Formation of Vocontian Basin (SE France). Sediment. Geol. 2000, 130, 205–228. [Google Scholar] [CrossRef]
- Morrow, D.W.; Krouse, H.R.; Ghent, E.D.; Taylor, G.C.; Dawson, K.R. A hypothesis concerning the origin of barite in Devonian carbonate rocks of northeastern British Columbia. Can. J. Earth Sci. 1978, 15, 1391–1406. [Google Scholar] [CrossRef]


| Aguadulce Site | Palmer Site | |||||
|---|---|---|---|---|---|---|
| Cliff | AG (Core) | PAL-1 (Core) | ||||
| g/Kg | wt% | g/Kg | wt% | g/Kg | wt% | |
| P | 0.41 | 0.20 | 5.46 | 1.24 | 0.33 | 0.25 |
| Ti | 0.11 | 0.05 | 0.32 | 0.07 | 0.83 | 0.64 |
| Mn | 140.40 | 67.93 | 0.43 | 0.10 | 102.58 | 79.43 |
| Fe | 1.74 | 0.84 | 390.99 | 88.60 | 18.62 | 14.42 |
| Co | 3.97 | 1.92 | 0.29 | 0.07 | 0.55 | 0.43 |
| Ni | 0.45 | 0.22 | 0.87 | 0.20 | 1.01 | 0.78 |
| Cu | 2.38 | 1.15 | 0.98 | 0.22 | 0.17 | 0.13 |
| Zn | 0.91 | 0.44 | 6.39 | 1.45 | 1.87 | 1.45 |
| As | 0.59 | 0.29 | 6.58 | 1.49 | 0.65 | 0.50 |
| Sr | 0.66 | 0.32 | 0.25 | 0.06 | 0.31 | 0.24 |
| Ba | 12.23 | 5.92 | 21.89 | 4.96 | 1.45 | 1.12 |
| Pb | 42.84 | 20.73 | 6.86 | 1.55 | 0.79 | 0.61 |
| Depth | Saturation Index | SW | |||||
|---|---|---|---|---|---|---|---|
| (m a.s.l.) | Calcite | Dolomite | Barite | Hematite | Pyrolusite | % | |
| AG | 1 | −0.43 | −0.59 | −0.04 | 13.87 | −14.06 | 0 |
| −1 | −0.13 | 0.02 | −0.14 | 15.07 | −12.77 | 0 | |
| −3 | −0.16 | −0.04 | −0.12 | 14.50 | −13.04 | 0 | |
| −5 | −0.10 | 0.18 | −0.12 | 14.24 | −12.80 | 3 | |
| −7 | −0.03 | 0.46 | −0.07 | 15.45 | −11.85 | 14 | |
| −9 | −0.16 | 0.40 | −0.07 | 14.04 | −13.56 | 51 | |
| −11 | −0.17 | 0.42 | −0.07 | 14.12 | −13.46 | 63 | |
| −13 | −0.16 | 0.47 | −0.10 | 14.36 | −13.29 | 69 | |
| −15 | −0.14 | 0.50 | −0.06 | 14.84 | −13.12 | 74 | |
| −17 | −0.10 | 0.59 | −0.05 | 15.02 | −13.18 | 75 | |
| −19 | −0.16 | 0.49 | −0.07 | 15.27 | −13.21 | 80 | |
| PAL-1 | −1 | −0.85 | −1.16 | 0.17 | 16.89 | −12.00 | 12 |
| −2 | 0.37 | 1.25 | −0.36 | 15.31 | −10.80 | 16 | |
| −3 | −0.32 | 0.03 | 0.04 | 14.27 | −11.64 | 39 | |
| −4 | 0.09 | 0.94 | −0.06 | 16.32 | −11.62 | 74 | |
| −7 | 0.25 | 1.33 | −0.18 | 16.50 | −10.64 | 99 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sola, F.; Mancuso, M.; Vallejos, Á. Diagenetic Barite Growths in the Mixing Zone of a Carbonate Coastal Aquifer. J. Mar. Sci. Eng. 2025, 13, 2090. https://doi.org/10.3390/jmse13112090
Sola F, Mancuso M, Vallejos Á. Diagenetic Barite Growths in the Mixing Zone of a Carbonate Coastal Aquifer. Journal of Marine Science and Engineering. 2025; 13(11):2090. https://doi.org/10.3390/jmse13112090
Chicago/Turabian StyleSola, Fernando, Malva Mancuso, and Ángela Vallejos. 2025. "Diagenetic Barite Growths in the Mixing Zone of a Carbonate Coastal Aquifer" Journal of Marine Science and Engineering 13, no. 11: 2090. https://doi.org/10.3390/jmse13112090
APA StyleSola, F., Mancuso, M., & Vallejos, Á. (2025). Diagenetic Barite Growths in the Mixing Zone of a Carbonate Coastal Aquifer. Journal of Marine Science and Engineering, 13(11), 2090. https://doi.org/10.3390/jmse13112090





