Description and Phylogenetic Analysis of Two New Species, Trissonchulus sinensis sp. nov. and Metachromadora sinica sp. nov. (Nematoda) from the South China Sea
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling and Morphological Analysis
2.2. Molecular Analysis
3. Results and Discussion
3.1. Taxonomy of Trissonchulus sinensis sp. nov.
3.1.1. Type Material
3.1.2. Type Locality and Habitat
3.1.3. Etymology
3.1.4. Measurements
3.1.5. Description of Trissonchulus sinensis sp. nov. (Figure 1, Figure 2 and Figure 3)
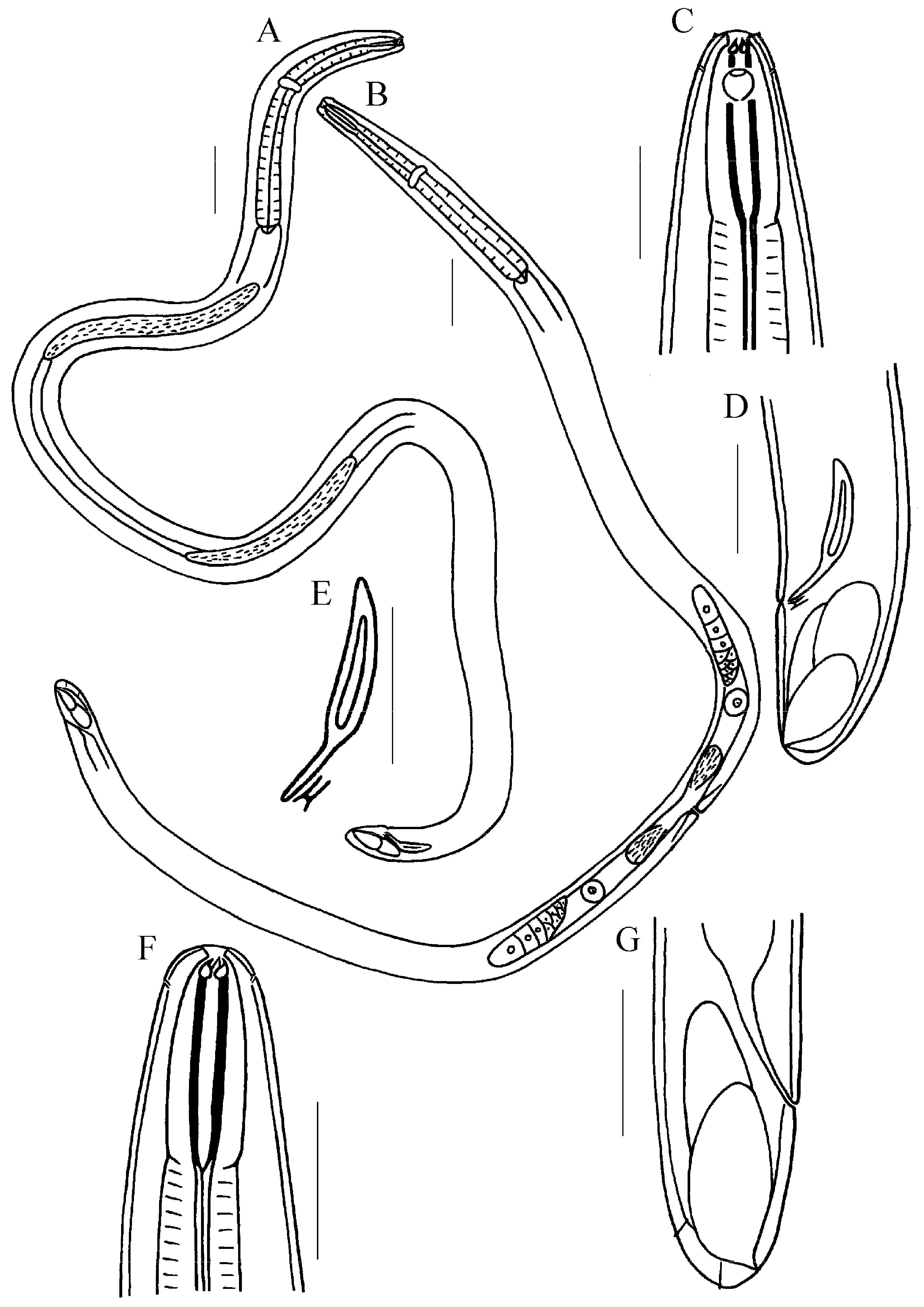
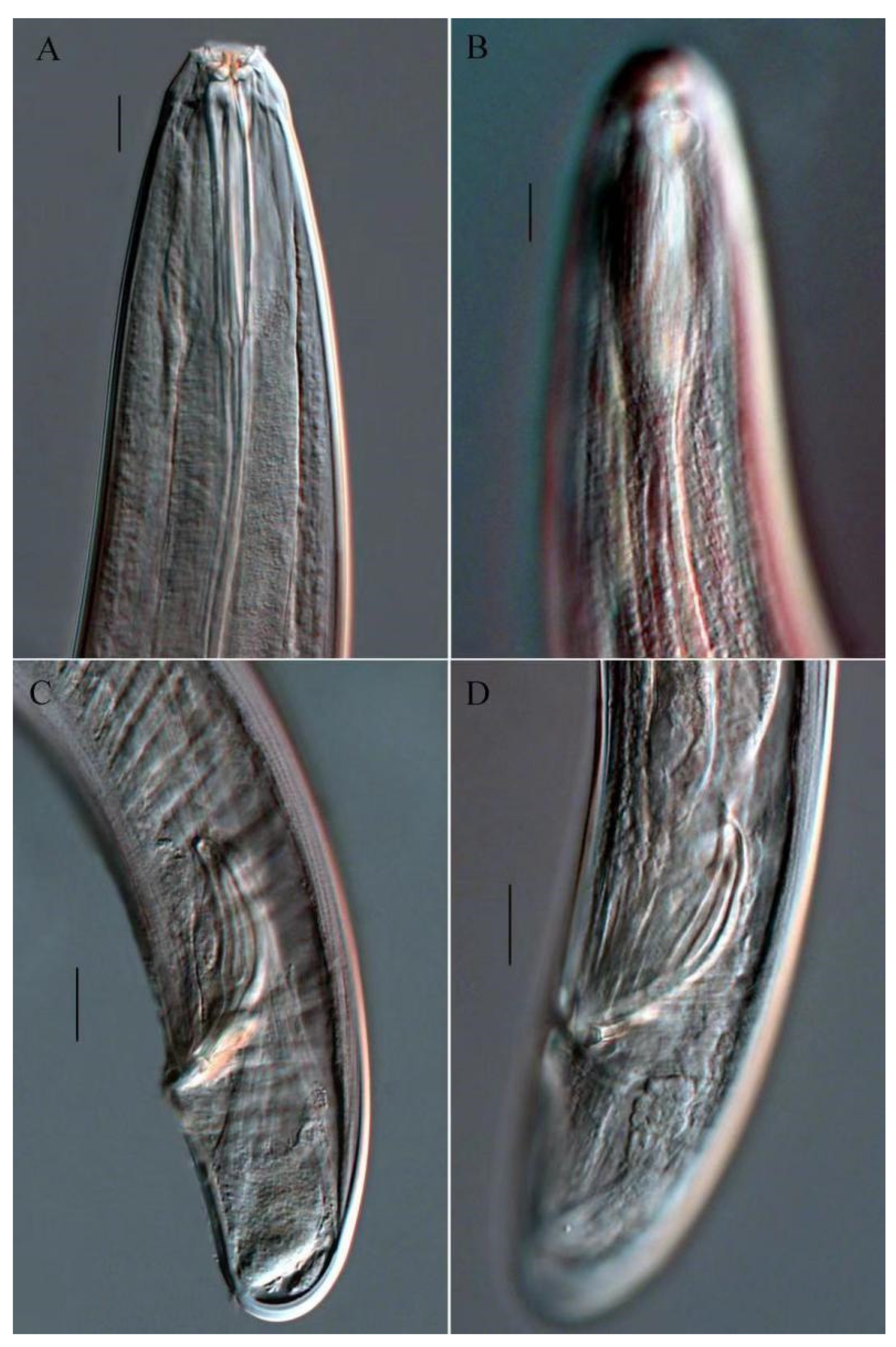
3.1.6. Differential Diagnosis and Discussion
3.2. Taxonomy of Metachromadora sinica sp. nov.
3.2.1. Type Material
3.2.2. Type Locality and Habitat
3.2.3. Etymology
3.2.4. Measurements
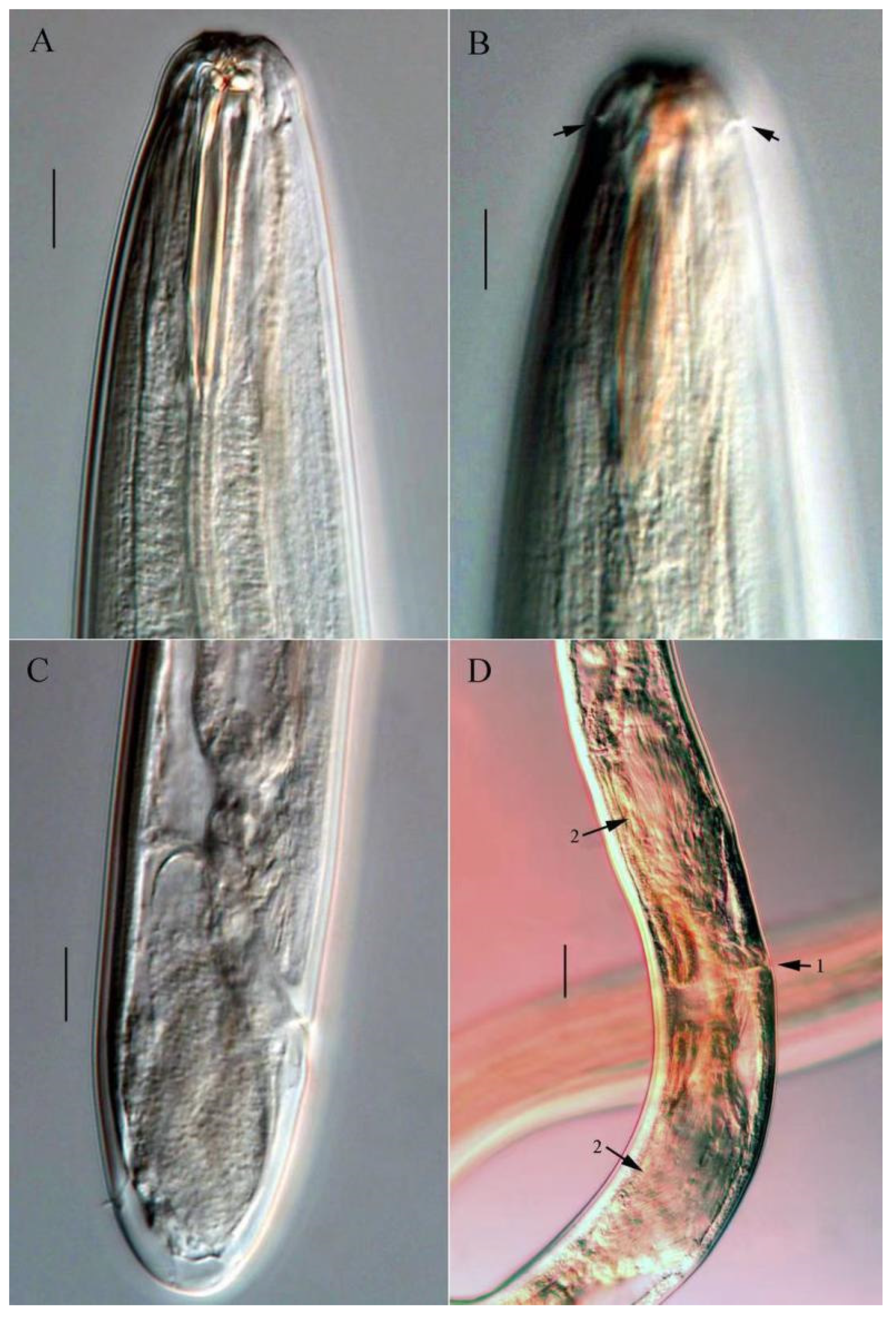
3.2.5. Description of Metachromadora sinica sp. nov. (Figure 4, Figure 5 and Figure 6)
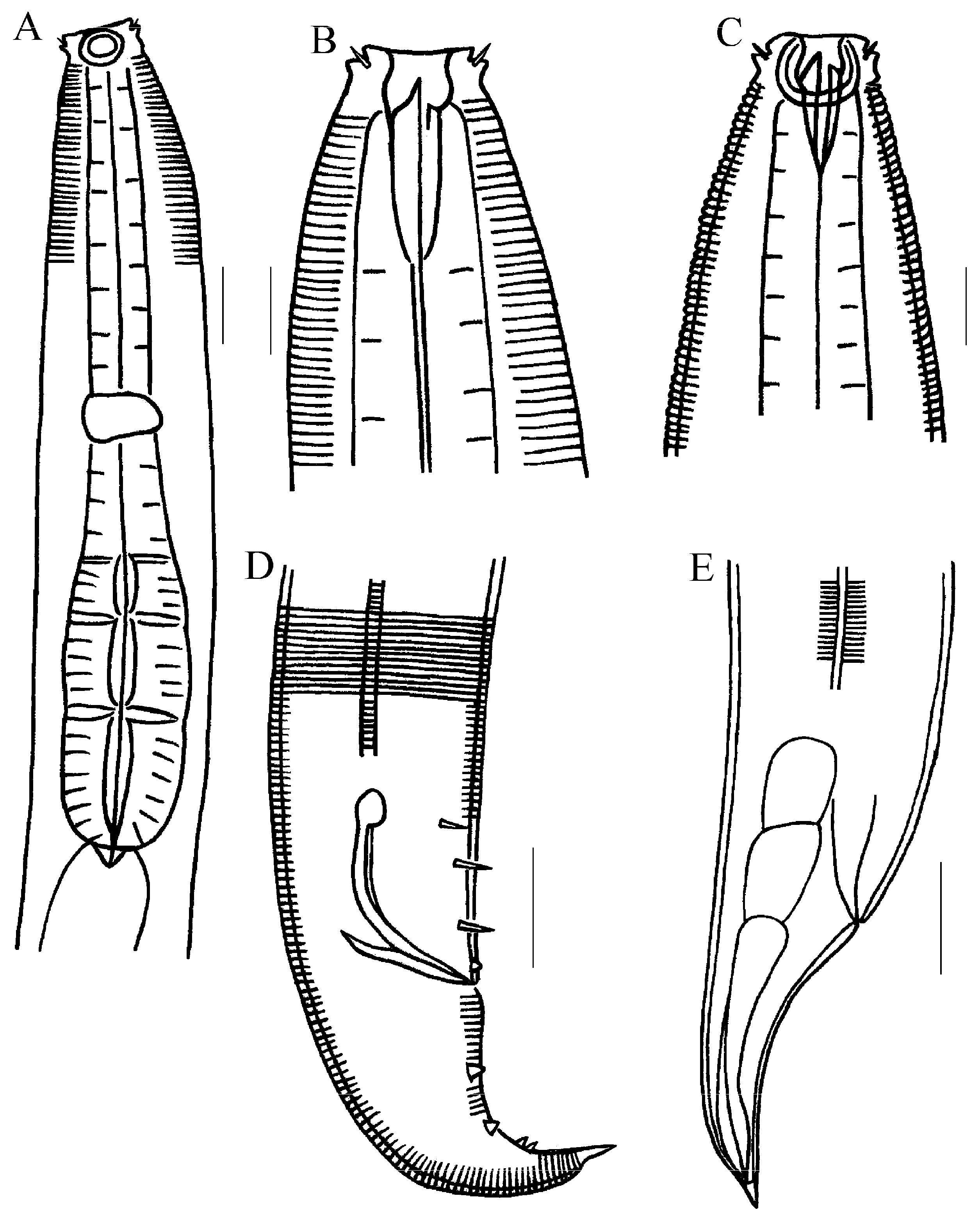
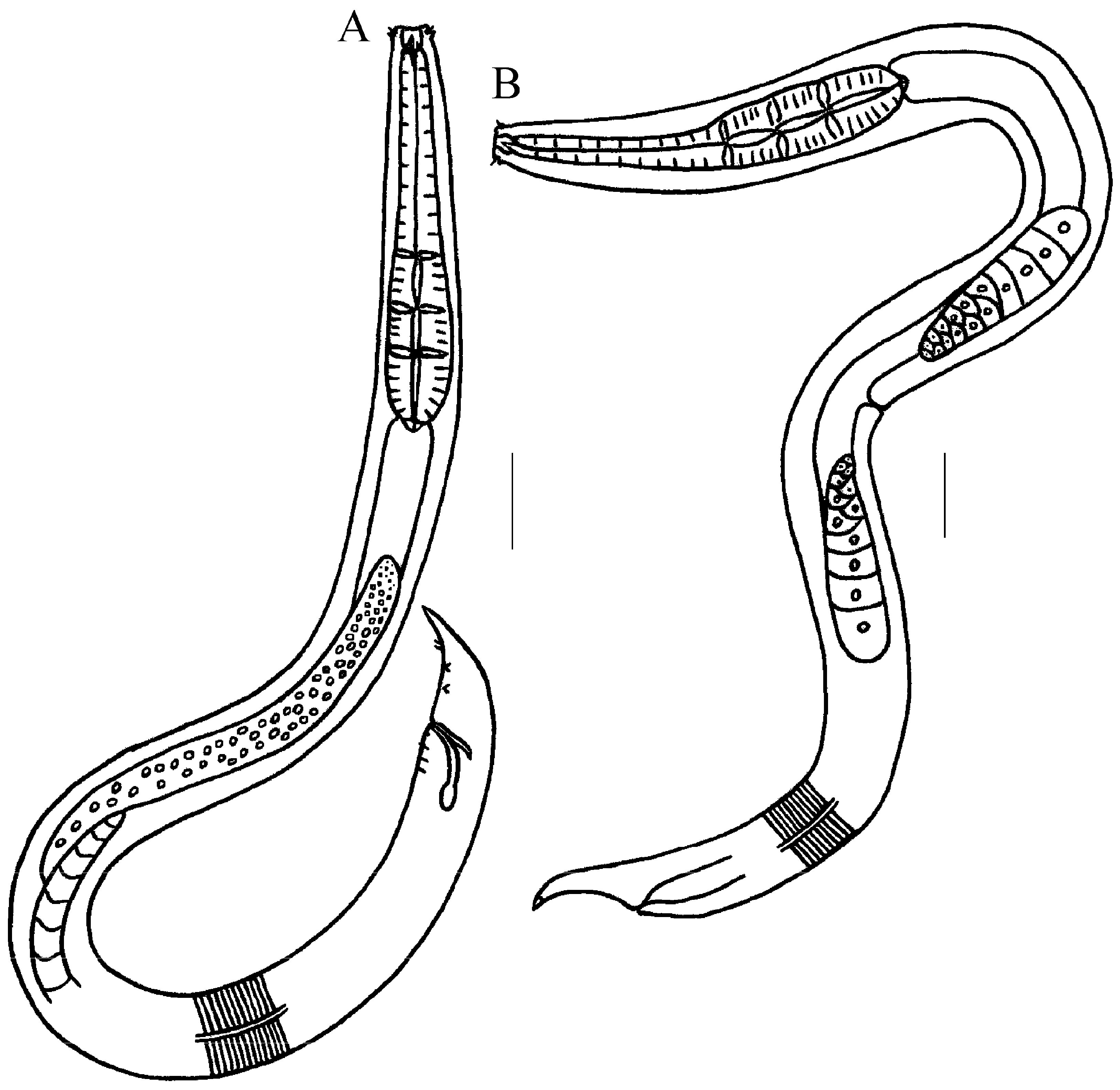

3.2.6. Differential Diagnosis and Discussion
3.3. Molecular Phylogenetic Analysis
3.3.1. Phylogenetic Analysis of Trissonchulus sinensis sp. nov
3.3.2. Phylogenetic Analysis of Metachromadora sinica sp. nov.
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Meldal, B.H.M.; Debenham, N.J.; De Ley, P.; De Ley, I.T.; Vanfleteren, J.R.; Vierstraete, A.R.; Bert, W.; Borgonie, G.; Moens, T.; Tyler, P.A.; et al. An improved molecular phylogeny of the Nematoda with special emphasis on marine taxa. Mol. Phylogenet. Evol. 2007, 42, 622–636. [Google Scholar] [CrossRef]
- Sun, L.Y.; Zhai, H.X.; Huang, Y. Three new species of the order Enoplida (Nematoda) from the Yellow Sea, China. J. Oceanol. Limnol. 2024, 43, 984–995. [Google Scholar] [CrossRef]
- Sun, X.Y.; Bai, R.B.; Zhai, H.X.; Huang, Y. Species list of free-living marine nematodes in the Yellow Sea, China. J. Liaocheng Univ. (Nat. Sci. Ed.) 2024, 37, 34–52. [Google Scholar]
- De Man, J.G. Onderzoekingen over vrij in de aarde levende Nematoden. Tijdschr. Ned. Dierkd. Ver. 1876, 2, 78–196. [Google Scholar]
- Tahseen, Q.; Mehdi, S.J. Taxonomy and relationships of a new and the first continental species of Trissonchulus Cobb, 1920 along with two species of Ironus (Nematoda: Ironidae) collected from Coal mines. Nematol. Mediterr. 2009, 37, 117–132. [Google Scholar]
- Schuurmans Stekhoven, J.H. Freilebende marine Nematoden des Mittelmeeres. IV. Freilebende marine Nematoden der Fischereigrunde bei Alexandrien. Zool. Jahrb. (Syst.) 1943, 76, 323–380. [Google Scholar]
- Chitwood, B.G. North American marine nematodes. Tex. J. Sci. 1951, 3, 617–672. [Google Scholar]
- Yeates, G.W. Studies on nematodes from dune sands: 3. Oncholaimidae, Ironidae, Alaimidae and Mononchidae. N. Z. J. Sci. 1967, 10, 299–321. [Google Scholar]
- Platt, H.M.; Warwick, R.M. Free-living marine nematodes. Part I. British Enoplids. In Synopses of the British Fauna (New Series); Kermack, D.M., Barnes, R.S.K., Eds.; Cambridge University Press: Cambridge, UK, 1983; Volume 28, pp. 169–171. [Google Scholar]
- Nemys: World Database of Nematodes. Available online: https://www.nemys.ugent.be (accessed on 1 October 2025).
- Chen, Y.Z.; Guo, Y.Q. Three new and two known free-living marine nematode species of the family Ironidae from the East China Sea. Zootaxa 2015, 4018, 151–175. [Google Scholar] [CrossRef]
- Nguyen, V.T.; Gagarin, V.G. Two new species of free-living marine nematodes (Nematoda: Enoplida) from the near-mouth area of the Yen River in Vietnam. Biol. Morya 2015, 41, 340–348. [Google Scholar]
- De Ward, C.T.P. New species of Hopperia (Nematoda, Comesomatidae) and Metachromadora (Nematoda, Desmodoridae) from Patagonia, Chubut, Argentina. Zootaxa 2004, 542, 1–15. [Google Scholar] [CrossRef]
- Vincx, M. Free-Living Marine Nematodes from the Southern Bight of the North Sea. Ph.D. Thesis, State University of Ghent, Gent, Belgium, 1989; p. 678. [Google Scholar]
- Timm, R.W. The marine nematodes of the Bay of Bengal. Proc. Pak. Acad. Sci. 1961, 1, 25–88. [Google Scholar]
- Furstenberg, J.P.; Vincx, M. Three new species of Chromadoropsis (Nematoda, Desmodoridae) from Southern Africa and the North Sea. S. Afr. J. Zool. 1988, 23, 215–223. [Google Scholar]
- Verschelde, D.; Muthumbi, A.; Vincx, M. Papillonema danieli gen. et sp.n. and Papillonema clavatum (Gerlach, 1957) comb.n. (Nematoda, Desmodoridae) from the Ceriops mangrove sediments of Gazi Bay, Kenya. Hydrobiologia 1995, 316, 225–237. [Google Scholar]
- Huang, S.; Yan, Y.L.; Su, F.; Huang, X.R.; Xia, D.D.; Jiang, X.X.; Dong, Y.H.; Lv, P.; Chen, F.Y.; Lv, Y.W. Research progress in gene editing technology. Front. Biosci. (Landmark Ed) 2021, 26, 916–927. [Google Scholar] [CrossRef]
- Lee, Y.C.; Ke, H.M.; Liu, Y.C.; Song, Y.S.; Chiu, Y.W.; Tang, P.; Chen, Y.R.; Chen, P.J.; Lu, H.H.S.; Lo, C.F.; et al. Single-worm long-read sequencing reveals genome diversity in free-living nematodes. Nucleic Acids Res. 2023, 51, 8035–8047. [Google Scholar] [CrossRef]
- De Ley, P.; Blaxter, M.L. A new system for Nematoda: Combining morphological characters with molecular trees, and translating clades into ranks and taxa. In Nematology Monographs and Perspectives; Cook, R., Hunt, D.J., Eds.; Brill: Leiden, The Netherlands, 2004; Volume 2, pp. 633–653. [Google Scholar]
- Bik, H.M.; Lambshead, P.J.D.; Thomas, W.K.; Lunt, D.H. Moving towards a complete molecular framework on the Nematoda: A focus on the Enoplida and early-branching clades. BMC Evol. Biol. 2010, 10, 353. [Google Scholar] [CrossRef]
- Meng, Z.; Guo, W.; Wang, C. Description of two new species, Sphaerolaimus obesus sp. nov. and Pseudosteineria articulata sp. nov. (Nematoda, Sphaerolaimoidea) from the Yellow Sea, China and phylogenetic analysis within superfamily Sphaerolaimoidea with combined ribosome DNA sequences. J. Oceanol. Limnol. 2025, 43, 1320–1333. [Google Scholar] [CrossRef]
- Venekey, V.; Gheller, P.F.; Kandratavicius, N.; Cunha, B.P.; Souza, G.F.; Fonseca, G.; Maria, T.F. The state of the art of Chromadoridae (Nematoda, Chromadorida): A historical review, diagnoses and comments about valid and dubious genera and a list of valid species. Zootaxa 2019, 4578, 1–67. [Google Scholar] [CrossRef] [PubMed]
- Leduc, D.; Fu, S.; Zhao, Z.Q. New nematode species from the continental slope of New Zealand (Chromadorea, Microlaimida, and Chromadorida), and unexpected placement of the genus Molgolaimus Ditlevsen, 1921. Mar. Biodivers. 2019, 49, 2267–2280. [Google Scholar] [CrossRef]
- Sun, J.; Huang, Y. Metachromadora parobscura sp. nov. and Molgolaimus longicaudatus sp. nov. (Nematoda, Desmodoridae) from Mangrove Wetlands of China. J. Mar. Sci. Eng. 2024, 12, 1621. [Google Scholar] [CrossRef]
- Huang, M.; Xu, K. Two new species of Promonhystera Wieser, 1956 (Nematoda: Monhysterida: Xyalidae) from the South China Sea. Zootaxa 2024, 5399, 130–140. [Google Scholar] [CrossRef]
- De Ley, I.T.; De Ley, P.; Vierstraete, A.; Waerebeek, K.V.; Vanfleteren, J.; Vierstraete, A.R.; Bert, W.; Borgonie, G. Phylogenetic analysis of Meloidogyne small subunit rDNA. J. Nematol. 2002, 34, 319–327. [Google Scholar]
- De Ley, P.; De Ley, I.T.; Morris, K.; Abebe, E.; Mundo-Ocampo, M.; Yoder, M.; Heras, J.; Waumann, D.; Rocha-Olivares, A.; Jay Burr, A.H.; et al. An integrated approach to fast and informative morphological vouchering of nematodes for applications in molecular barcoding. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2005, 360, 1945–1958. [Google Scholar] [CrossRef]
- Lanfear, R.; Frandsen, P.B.; Wright, A.M.; Senfeld, T.; Calcott, B. PartitionFinder 2: New methods for selecting partitioned models of evolution for molecular and morphological phylogenetic analyses. Mol. Biol. Evol. 2017, 34, 772–773. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Gao, F.; Jakovlić, I.; Zou, H.; Zhang, J.; Li, W.X.; Wang, G.T. PhyloSuite: An integrated and scalable desktop platform for streamlined molecular sequence data management and evolutionary phylogenetics studies. Mol. Ecol. Resour. 2020, 20, 348–355. [Google Scholar] [CrossRef]
- Xiang, C.; Gao, F.; Jakovlić, I.; Lei, H.; Hu, Y.; Zhang, H.; Zou, H.; Wang, G. Using PhyloSuite for molecular phylogeny and tree-based analyses. iMeta 2023, 2, e87. [Google Scholar] [CrossRef] [PubMed]
- Ronquist, F.; Teslenko, M.; van der Mark, P.; Ayres, D.L.; Darling, A.; Höhna, S.; Huelsenbeck, J.P. MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef]
- Schmidt-Rhaesa, A. (Ed.) Handbook of Zoology: Gastrotricha, Cycloneuralia and Gnathifera (Vol. Nematoda); De Gruyter: Berlin, Germany, 2014; p. 759. [Google Scholar]
- Inglis, W.G. Free-living nematodes from South Africa. Bull. Br. Mus. (Nat. Hist.) 1961, 7, 291–319. [Google Scholar]
- Wieser, W. Free-living marine nematodes. I. Enoplida. Acta Univ. Lund. 1953, 49, 1–155. [Google Scholar]
- Filipjev, I.N. Free-living marine nematodes of the vicinities of Sevastopol. Tr. Osoboi Zool. Labor. Sevastopol. Biol. St. RAN. 1918, Ser. 1, 218–219. [Google Scholar]
- Mordukhovich, V.V.; Semenchenko, A.A.; Fadeeva, N.P.; Zograf, J.K. One new genus and two new free-living deep-sea nematode species with discussion of phylogeny of the family Leptosomatodae Filipjev, 1916. Prog. Oceanogr. 2019, 178, 102160. [Google Scholar] [CrossRef]
- Platonova, T.A.; Mokievsky, V.O. Revision of the marine nematodes of the family Ironidae (Nematoda: Enoplida). Zoosystematica Ross. 1994, 3, 5–17. [Google Scholar]
- Lorenzen, S. The Phylogenetic Systematics of Freeliving Nematodes; The Ray Society: London, UK, 1994; p. 383. [Google Scholar]
- van Megen, H.; van den Elsen, S.; Holterman, M.; Karssen, G.; Mooyman, P.; Bongers, T.; Holovachov, O.; Bakker, J.; Helder, J. A phylogenetic tree of nematodes based on about 1200 full-length small subunit ribosomal DNA sequences. Nematology 2009, 11, 927–950. [Google Scholar] [CrossRef]
- Armenteros, M.; Rojas-Corzo, A.; Ruiz-Abierno, A.; Derycke, S.; Backeljau, T.; Decraemer, W. Systematics and DNA barcoding of free-living marine nematodes with emphasis on tropical desmodorids using nuclear SSU rDNA and mitochondrial COI sequences. Nematology 2014, 16, 979–989. [Google Scholar] [CrossRef]


| Characters | Holotype | Paratypes | |||||
|---|---|---|---|---|---|---|---|
| ♂1 | ♂2 | ♂3 | ♀1 | ♀2 | ♀3 | ♀4 | |
| Total body length | 2730 | 3051 | 2961 | 3252 | 2758 | 3124 | 2825 |
| Maximum body diameter | 72 | 73 | 67 | 82 | 69 | 77 | 72 |
| Head diameter | 22 | 25 | 26 | 27 | 21 | 22 | 20 |
| Depth of buccal cavity | 70 | 72 | 60 | 70 | 71 | 63 | 70 |
| Width of buccal cavity | 6 | 6 | 6 | 6 | 9 | 7 | 9 |
| Width of amphid | 10 | 11 | 9 | 12 | 10 | 12 | 10 |
| Body diameter at amphid | 29 | 32 | 28 | 32 | 28 | 32 | 29 |
| Amphid from the anterior end | 12 | 16 | 12 | 16 | 11 | 15 | 12 |
| Nerve ring from anterior end | 172 | 175 | 166 | 181 | 178 | 154 | 184 |
| Body diameter at never ring | 62 | 59 | 58 | 60 | 61 | 59 | 59 |
| Length of pharynx | 420 | 435 | 412 | 454 | 428 | 441 | 443 |
| Body diameter at pharyngeal base | 74 | 70 | 70 | 74 | 69 | 73 | 71 |
| Spicule length along arc | 65 | 62 | 63 | ||||
| Length of gubernaculum | 16 | 14 | 19 | ||||
| Vulva from anterior end | 1492 | 1431 | 1542 | 1455 | |||
| V% | 45.9% | 51.9% | 49.4% | 51.5% | |||
| Body diameter at cloaca or anus | 49 | 50 | 44 | 50 | 66 | 65 | 65 |
| Tail length | 63 | 63 | 61 | 62 | 60 | 66 | 58 |
| a | 37.9 | 41.7 | 44.5 | 39.9 | 40.0 | 40.6 | 39.2 |
| b | 6.5 | 7.0 | 7.2 | 7.2 | 6.4 | 7.1 | 6.4 |
| c | 43.3 | 48.5 | 48.5 | 52 | 56.3 | 47.3 | 48.7 |
| c′ | 1.3 | 1.3 | 1.4 | 1.2 | 1.1 | 1.4 | 1.2 |
| Morphological Characters | Holotype | Paratype | |||
|---|---|---|---|---|---|
| ♂1 | ♂2 | ♂3 | ♀1 | ♀2 | |
| a | 23.7 | 23.5 | 22.6 | 17.2 | 19.0 |
| b | 4.9 | 4.9 | 4.8 | 4.6 | 4.4 |
| c | 16.1 | 15.4 | 15.0 | 18.4 | 19.0 |
| c’ | 2.0 | 1.9 | 2.2 | 2.2 | 2.0 |
| Total body length | 998 | 988 | 973 | 1032 | 1047 |
| Maximum body diameter | 42 | 42 | 43 | 60 | 55 |
| Head diameter | 15 | 14 | 15 | 16 | 17 |
| Amphideal fovea width | 11 | 11 | 12 | 11 | 10 |
| Amphideal fovea c.b.d. | 17 | 18 | 17 | 21 | 20 |
| Amphideal fovea as % of c.b.d. | 64.7 | 61.1 | 70.6 | 52.3 | 50.0 |
| Pharynx length | 202 | 200 | 201 | 226 | 236 |
| Pharynx c.b.d. | 44 | 38 | 42 | 56 | 53 |
| Length of tripartite bulb | 74 | 82 | 74 | 105 | 103 |
| Bulb as % of pharynx length | 36.6 | 41.0 | 36.8 | 46.5 | 43.6 |
| Spicule length as arch | 47 | 48 | 50 | - | - |
| Gubernacular length | 27 | 28 | 27 | - | - |
| Cloacal/Anal body diameter | 31 | 33 | 30 | 26 | 27 |
| Distance from the first protuberance to cloaca | 34 | 30 | 36 | - | - |
| Distance from the second protuberance to cloaca | 37 | 33 | 39 | - | - |
| Tail length | 62 | 64 | 65 | 56 | 55 |
| Vulva from anterior end | - | - | - | 555 | 569 |
| Vulva c.b.d. | - | - | - | 47 | 48 |
| V% | - | - | - | 53.8 | 54.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, J.; Bai, R.; Huang, Y. Description and Phylogenetic Analysis of Two New Species, Trissonchulus sinensis sp. nov. and Metachromadora sinica sp. nov. (Nematoda) from the South China Sea. J. Mar. Sci. Eng. 2025, 13, 2085. https://doi.org/10.3390/jmse13112085
Sun J, Bai R, Huang Y. Description and Phylogenetic Analysis of Two New Species, Trissonchulus sinensis sp. nov. and Metachromadora sinica sp. nov. (Nematoda) from the South China Sea. Journal of Marine Science and Engineering. 2025; 13(11):2085. https://doi.org/10.3390/jmse13112085
Chicago/Turabian StyleSun, Jing, Ruobing Bai, and Yong Huang. 2025. "Description and Phylogenetic Analysis of Two New Species, Trissonchulus sinensis sp. nov. and Metachromadora sinica sp. nov. (Nematoda) from the South China Sea" Journal of Marine Science and Engineering 13, no. 11: 2085. https://doi.org/10.3390/jmse13112085
APA StyleSun, J., Bai, R., & Huang, Y. (2025). Description and Phylogenetic Analysis of Two New Species, Trissonchulus sinensis sp. nov. and Metachromadora sinica sp. nov. (Nematoda) from the South China Sea. Journal of Marine Science and Engineering, 13(11), 2085. https://doi.org/10.3390/jmse13112085






