Distributional Range Shifts Caused by Glacial–Interglacial Cycles: A Review on Timing, Main Processes, and Patterns of Late Pleistocene Marine Dispersal by Invertebrates in the NE Atlantic
Abstract
1. Introduction
2. Materials and Methods
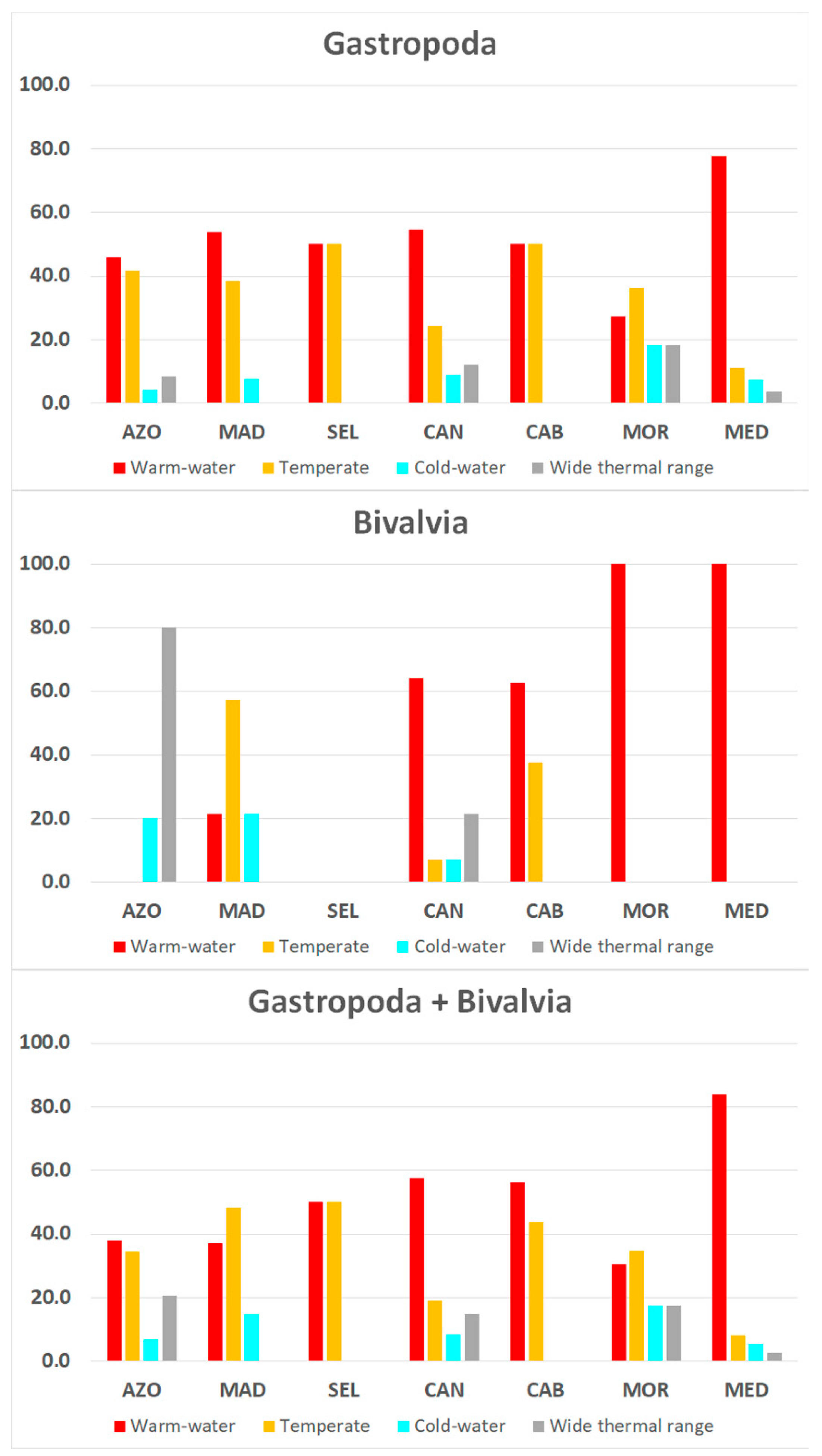
3. Results
4. Discussion
4.1. Modes of Larval Development and Long-Distance Dispersal of Shallow-Water Marine Species: The Main Processes
4.2. Relevance of the Fossil Record for Establishing the Times of Dispersal of Marine Species in the Macaronesian Archipelagos and in the Mediterranean
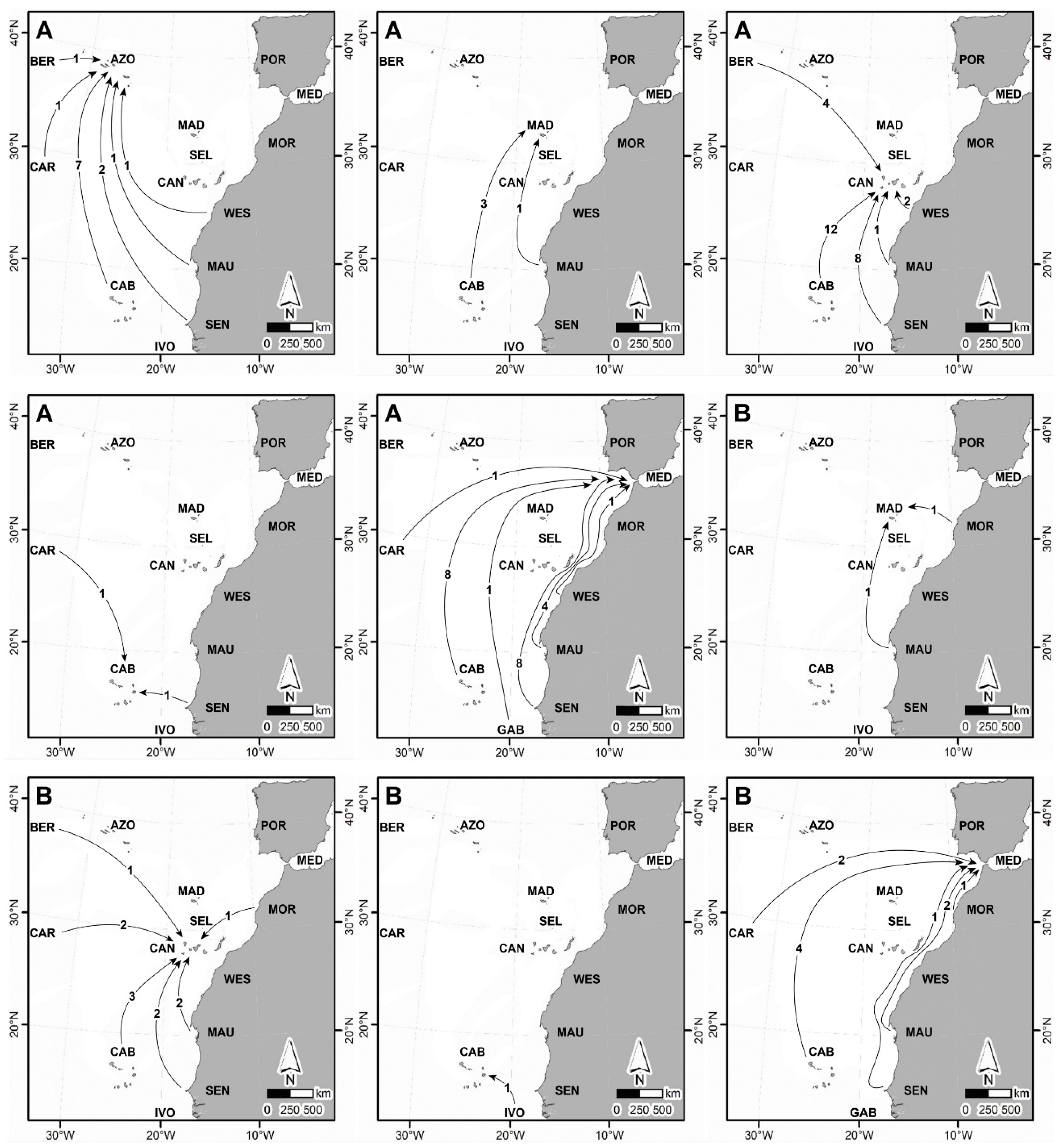
4.3. Main Routes of Dispersal in the East Atlantic During the MIS 5e
5. Conclusions
Supplementary Materials
Funding
Data Availability Statement
Conflicts of Interest
References
- Cronin, T.M. Geographical Isolation in Marine Species: Evolution and Speciation in Ostracoda, I. Dev. Palaeontol. Stratigr. 1988, 11, 871–889. [Google Scholar]
- Vermeij, G. Island life: A view from the sea. In Frontiers of Biogeography: New Directions in the Geography of Nature; Lomolino, M.W., Heaney, L.R., Eds.; Sinauer: Sunderland, MA, USA, 2004; pp. 239–254. [Google Scholar]
- Baptista, L.; Meimberg, H.; Ávila, S.P.; Santos, A.M.; Curto, M. Dispersal ability, habitat characteristics, and sea-surface circulation shape population structure of Cingula trifasciata (Gastropoda: Rissoidae) in the remote Azores Archipelago. BMC Ecol. Evol. 2021, 21, 128. [Google Scholar] [CrossRef]
- Sinigaglia, L.; Baptista, L.; Alves, C.; Feldmann, F.; Sacchetti, C.; Rupprecht, C.; Vijayan, T.; Martín-González, E.; Ávila, S.P.; Santos, A.M.; et al. Oceanic islands act as drivers for the genetic diversity of marine species: Cardita calyculata (Linnaeus, 1758) in the NE Atlantic as a case-study. BMC Ecol. Evol. 2024, 24, 138. [Google Scholar]
- Hachich, N.F.; Bonsall, M.B.; Arraut, E.M.; Barneche, D.R.; Lewinsohn, T.M.; Floeter, S.R. Island biogeography: Patterns of marine shallow-water organisms in the Atlantic Ocean. J. Biogeogr. 2015, 45, 1871–1882. [Google Scholar] [CrossRef]
- Hachich, N.F.; Bonsall, M.B.; Arraut, E.M.; Barneche, D.R.; Lewinsohn, T.M.; Floeter, S.R. Marine island biogeography. Response to comment on ‘Island biogeography: Patterns of marine shallow-water organisms’. J. Biogeogr. 2016, 43, 2517–2519. [Google Scholar] [CrossRef]
- Hachich, N.F.; Ferrari, D.S.; Quimbayo, J.P.; Pinheiro, H.T.; Floeter, S.R. Island biogeography of marine shallow-water organisms. Encycl. World’s Biomes 2020, 1, 61–75. [Google Scholar] [CrossRef]
- Pinheiro, H.T.; Bernardi, G.; Simon, T.; Joyeux, J.-C.; Macieira, R.M.; Gasparini, J.L.; Rocha, C.; Rocha, L.A. Island biogeography of marine organisms. Nature 2017, 82, 82–86. [Google Scholar] [CrossRef] [PubMed]
- Ávila, S.P.; Cordeiro, R.; Haroun, R.; Wirtz, P. Comment on ‘Island biogeography: Patterns of marine shallow-water organisms’ by Hachich et al., Journal of Biogeography (2015). J. Biogeog. 2016, 43, 2515–2516. [Google Scholar] [CrossRef][Green Version]
- Ávila, S.P.; Cordeiro, R.; Madeira, P.; Silva, L.; Medeiros, A.; Rebelo, A.C.; Melo, C.; Neto, A.I.; Haroun, R.; Monteiro, A.; et al. Global change impacts on large-scale biogeographic patterns of marine organisms on Atlantic oceanic islands. Mar. Pollut. Bull. 2018, 126, 101–112. [Google Scholar] [CrossRef]
- Ávila, S.P.; Melo, C.; Berning, B.; Sá, N.; Quartau, R.; Rijsdijk, K.F.; Ramalho, R.S.; Cordeiro, R.; De Sá, N.C.; Pimentel, A.; et al. Towards a “Sea-Level Sensitive” dynamic model: Impact of island ontogeny and glacio-eustasy on global patterns of marine island biogeography. Biol. Rev. 2019, 94, 1116–1142. [Google Scholar] [CrossRef]
- Ávila, S.P.; Santos, A.M.; Melo, C.S.; Porteiro, J.M.; Medeiros, A.M.; Baptista, L.; Pimentel, A.; Madeira, P.; Rebelo, A.C.; Hipólito, A.; et al. Extending the Sea-Level Sensitive dynamic model of marine island biogeography to include fusion-fission islands. Front. Biogeog. 2025, 18, e141200. [Google Scholar] [CrossRef]
- Scheltema, R.S. Larval dispersal as a means of genetic exchange between geographically separated populations of shallow-water benthic marine gastropods. Biol. Bull. Mar. Biol. Lab. Woods Hole Massachussets 1971, 140, 284–322. [Google Scholar] [CrossRef]
- Scheltema, R.S. The dispersal of larvae of shoal-water benthic invertebrate species over long distances by ocean currents. In Fourth Marine Biology Symposium; Crisp, D.J., Ed.; Cambridge University Press: Cambridge, UK, 1971; pp. 7–28. [Google Scholar]
- Scheltema, R.S. The relevance of passive dispersal for the biogeography of Caribbean mollusks. Am. Malacol. Bull. 1995, 11, 99–115. [Google Scholar]
- Scheltema, R.S. Dispersal of marine invertebrate organisms: Paleobiogeographic and biostratigraphic implications. In Concepts and Methods of Biostratigraphy; Kauffmann, R.G., Hazel, J.E., Eds.; Dowden, Hutchinson & Ross: Stroudsburg, PA, USA, 1977; pp. 73–108. [Google Scholar]
- Scheltema, R.S. Long distance dispersal by planktonic larvae of shoal-water benthic invertebrates among central Pacific Islands. Bull. Mar. Sci. 1986, 39, 241–256. [Google Scholar]
- Scheltema, R.S. On dispersal and planktonic larvae of benthic invertebrates: An eclectic overview and summary of problems. Bull. Marine Sci. 1986, 39, 290–322. [Google Scholar]
- Scheltema, R.S. Planktonic and non-planktonic development among prosobranch gastropods and its relationship to the geographic range of species. In Reproduction, Genetics and Distribution of Marine Organisms; Ryland, J.S., Tyler, P.A., Eds.; International Symposium Series; Olsen & Olsen: Hamburg, Germany, 1989; pp. 183–188. [Google Scholar]
- Jablonski, D.; Lutz, R.A. Larval ecology of marine benthic invertebrates: Paleobiological implications. Biol. Rev. 1983, 58, 21–89. [Google Scholar] [CrossRef]
- Scheltema, R.S.; Williams, I.P. Long-distance dispersal of planktonic larvae and the biogeography and evolution of some Polynesian and western Pacific mollusks. Bull. Marine Sci. 1983, 33, 545–565. [Google Scholar]
- Scheltema, R.S.; Williams, I.P.; Lobel, P.S. Retention around and long-distance dispersal between oceanic islands by planktonic larvae of benthic gastropod mollusca. Am. Malacol. Bull. 1996, 12, 67–75. [Google Scholar]
- Scheltema, R.S. On the relation between dispersal of pelagic larvae and the evolution of marine prosobranch gastropods. In Marine Organisms: Genetics, Ecology, and Evolution; Battaglia, B., Beardmore, J.A., Eds.; NATO Conference Series; Series IV: Marine Sciences; Plenum Press: New York, NY, USA, 1978; pp. 303–322. [Google Scholar]
- Scheltema, R.S. Dispersal of pelagic larvae and the zoogeography of Tertiary marine benthic gastropods. In Historical Biogeography, Plate Tectonics and the Changing Environment; Gray, J., Boucot, A., Eds.; Oregon State University Press: Corvallis, OR, USA, 1979; pp. 391–397. [Google Scholar]
- Jablonski, D.; Hunt, G. Larval ecology, geographic range, and species survivorship in Cretaceous mollusks: Organismic versus species-level explanations. Am. Nat. 2006, 168, 556–564. [Google Scholar] [CrossRef]
- Ávila, S.P. Oceanic islands, rafting, geographical range and bathymetry: A neglected relationship? Occas. Publ. Ir. Biogeogr. Soc. 2006, 9, 22–39. [Google Scholar]
- Ávila, S.P. Unravelling the patterns and processes of evolution of marine life in oceanic islands: A global framework. In Climate Change Perspectives from the Atlantic: Past, Present and Future; Fernández-Palacios, J.M., de Nascimento, L., Hernández, J., Clemente, S., González, A., Díaz-González, J.P., Eds.; Universidad de La Laguna, Tenerife: La Laguna, Spain, 2013; pp. 95–125. [Google Scholar]
- Ávila, S.P.; Rebelo, A.C.; Medeiros, A.; Melo, C.; Gomes, C.; Bagaço, L.; Madeira, P.; Borges, P.A.; Monteiro, P.; Cordeiro, R.; et al. Os fósseis de Santa Maria (Açores): 1. A jazida da Prainha; OVGA—Observatório Vulcanológico e Geotérmico dos Açores: Lagoa, Portugal, 2010; 103p, ISBN 978-989-8164-09-04. [Google Scholar]
- Meireles, R.P.; Quartau, R.; Ramalho, R.; Madeira, J.; Rebelo, A.C.; Zanon, V.; Ávila, S.P. Depositional processes on oceanic island shelves—evidence from storm-generated Neogene deposits from the mid-North Atlantic. Sedimentology 2013, 60, 1769–1785. [Google Scholar] [CrossRef]
- Baptista, L.; Santos, A.M.; Cabezas, M.P.; Cordeiro, R.; Melo, C.; Ávila, S.P. Intertidal or subtidal/circalittoral species: Who appeared first? A phylogenetic approach to the evolution of non-planktotrophic species in Atlantic Archipelagos. Mar. Biol. 2019, 166, 88. [Google Scholar] [CrossRef]
- Meco, J. Los Strombus Neógenos y Cuaternarios del Atlántico Euroafricano (Taxonomía, Biostratigrafía y Paleoecología). In Paleontología de Canarias. Tomo I. Excmo; Cabildo insular de Gran Canaria: Las Palmas de Gran Canaria, Spain, 1977; 142p. [Google Scholar]
- Meco, J. Neogastrópodos fósiles de las Canarias orientales. Anu. Estud. Atl. 1981, 27, 601–615. [Google Scholar]
- García-Talavera, F.; Kardas, S.J.; Richards, H.G. Quaternary marine mollusks from Tenerife, Canary Islands. Nautilus 1978, 92, 97–102. [Google Scholar]
- García Talavera, F.G.; Paredes, R.; Martín, M. Catálogo-Inventario: Yacimientos paleontológicos Provincia de Santa Cruz de Tenerife; Instituto de Estudios Canarios: La Laguna, Spain, 1989; 76p. [Google Scholar]
- García-Talavera, F. Fauna tropical en el Neotirreniense de Santa Maria (I. Azores). Lav. Soc. Ital. Di Malacol. 1990, 23, 439–443. [Google Scholar]
- García-Talavera, F. Sobre la presencia de Strombus latus Gmel. (Mollusca, Megagasteropoda) en el Cuaternario marino de Tenerife. In Homenaje al Prof. Dr. Telesforo Bravo., Secretariado de Publicaciones; Universidad de la Laguna: La Laguna, Spain, 1990; pp. 375–382. [Google Scholar]
- García-Talavera, F. Fauna malacológica del Cuaternario marino de Cabo Verde. Rev. Académica Canar. De Las Ciências 1999, 11, 9–25. [Google Scholar]
- Meco, J.; Petite-Maire, N.; Fontugne, M.; Shimmield, G.; Ramos, A.J. The Quaternary deposits in Lanzarote and Fuerteventura (eastern Canary Islands, Spain): An overview. In Climates of the Past; Meco, J., Petite-Maire, N., Eds.; IUGS-UNESCO-Universidad de Las Palmas de Gran Canaria: Las Palmas de Gran Canaria, Spain, 1997; pp. 123–136. [Google Scholar]
- Meco, J.; Gillou, H.; Carracedo, J.C.; Lomoschitz, A.; Ramos, A.J.G.; Rodríguez-Yánez, J.J. The maximum warmings of the Pleistocene world climate recorded in the Canary Islands. Palaeogeog. Palaeoclim. Palaeoecol. 2002, 185, 197–210. [Google Scholar] [CrossRef]
- Meco, J.; Ballester, J.; Betancort, J.F.; Cilleros, A.; Scaillet, S.; Guillou, H.; Carracedo, J.C.; Lomoschitz, A.; Petit-Maire, N.; Ramos, A.J.G.; et al. Historia geologica del clima en Canarias; Las Palmas de Gran Canaria: Las Palmas de Gran Canaria, Spain, 2008; 296p. [Google Scholar]
- Meco, J.; Lomoschitz, A.; Betancort, J.F.; Sendino, C. Comment on “Range expansion of tropical shallow-water marine molluscs in the NE Atlantic during the last interglacial (MIS 5e): Causes, consequences and utility of ecostratigraphic indicators for the Macaronesian archipelagos”, by C.S. Melo, E. Martín-González, C.M. da Silva, I. Galindo, A. González-Rodríguez, L. Baptista, A.C. Rebelo, P. Madeira, A. Voelker, M. Johnson, S.A. Arruda and S.P. Ávila, Quaternary Science Reviews 278 (2022), 107377. Quat. Sci. Rev. 2022, 288, 107534. [Google Scholar] [CrossRef]
- Callapez, P.; Soares, A.F. Late Quaternary warm marine mollusks from Santa Maria (Azores) paleoecologic and paleobiogeographic considerations. Ciências Terra 2000, 14, 313–322. [Google Scholar]
- García-Talavera, F.; Sánchez-Pinto, L. Moluscos marinos fósiles de Selvagem Pequenha e Ilheu de Fora (Islas Salvajes). Descripción de una nueva especie de neogasterópodo. Vieraea 2001, 13, 9–21. [Google Scholar]
- Ávila, S.P.; Amen, R.; Azevedo, J.M.N.; Cachão, M.; García-Talavera, F. Checklist of the Pleistocene marine molluscs of Prainha and Lagoinhas (Santa Maria Island, Azores). Açoreana. 2002, 9, 343–370. [Google Scholar]
- Ávila, S.P.; da Silva, C.M.; Schiebel, R.; Cecca, F.; Backeljau, T.; Martins, A.M.F. How did they get here? Palaeobiogeography of the Pleistocene marine molluscs of the Azores. Bull. Geol. Soc. Fr. 2009, 180, 201–213. [Google Scholar] [CrossRef]
- Ávila, S.P.; Madeira, P.; Zazo, C.; Kroh, A.; Kirby, M.; da Silva, C.M.; Cachão, M.; Martins, A.M.F. Palaeoecology of the Pleistocene (MIS 5.5) outcrops of Santa Maria Island (Azores) in a complex oceanic tectonic setting. Palaeogeog. Palaeoclim. Palaeoecol. 2009, 274, 18–31. [Google Scholar] [CrossRef]
- Ávila, S.P.; Melo, C.; Silva, L.; Ramalho, R.; Quartau, R.; Hipólito, A.; Cordeiro, R.; Rebelo, A.C.; Madeira, P.; Rovere, A.; et al. A review of the MIS 5e highstand deposits from Santa Maria Island (Azores, NE Atlantic): Palaeobiodiversity, palaeoecology and palaeobiogeography. Quat. Sci. Rev. 2015, 114, 126–148. [Google Scholar] [CrossRef]
- Ávila, S.P.; Landau, B.; Ávila, G.C.; Hipólito, A.; Uchman, A.; Johnson, M.E.; Madeira, P. A call to adress the taxonomic gap in the Pleistocene of Santa Maria Island (Azores Archipelago). Acta Geologica Polonica. 2025, 75, e40. [Google Scholar] [CrossRef]
- Ávila, S.P. Processos e Padrões de Dispersão e Colonização nos Rissoidae (Mollusca: Gastropoda) dos Açores. Ph.D. Thesis, Universidade dos Açores, Ponta Delgada, Portugal, 2005; p. 329. [Google Scholar]
- Cabero, A. Registro Costero de los Cambios Eustáticos y Climáticos Durante los Interglaciares Recientes Cuaternarios: Sur y Sureste Peninsular, Islas Baleares, Canarias y Cabo Verde. Ph.D. Thesis, University of Salamanca, Salamanca, Spain, 2009; p. 451. [Google Scholar]
- Cabero, A.; González-Delgado, J.Á.; Zazo, C.; Goy, J.L.; Dabrio, C.J.; Lario, J.; Bardají, T.; Hillaire-Marcel, C.; Ghaleb, B. Major migration of the Senegalese warm fauna in the Mediterranean during MIS 5. In Proceedings of the International Conference on Geoevents, Geological Heritage, and the Role of the IGCP, Caravaca de la Cruz, Spain, 15–18 September 2010; Lamolda, M.A., Díaz, E., Moreno, G.J., Maurrasse, F.J.-M.R., Meléndez, G., Paul, C.R.C., Tovar, F.J.R., Eds.; pp. 116–118. [Google Scholar]
- Martín-González, E.; González-Rodríguez, A.; Vera-Peláez, J.L.; Lozano-Francisco, M.d.C.; Castillo, C. Asociaciones de moluscos de los depósitos litorales del Pleistoceno Superior de Tenerife (Islas Canarias, España). Vieraea 2016, 44, 87–106. [Google Scholar]
- Martín-González, E.; Galindo, I.; Mangas, J.; Romero-Ruiz, C.; Sánchez, N.; González- Rodríguez, A.; Coello, J.J.; Márquez, A.; de Vera, A.; Vegas, J.; et al. Revisión de los depósitos costeros del estadio isotópico marino 5e (MIS5e) de Fuerteventura (islas Canarias). Vieraea 2019, 4, 667–688. [Google Scholar] [CrossRef]
- Melo, C.S.; Martín-González, E.; da Silva, C.M.; Galindo, I.; González-Rodríguez, A.; Baptista, L.; Rebelo, A.C.; Madeira, P.; Voelker, A.H.L.; Johnson, M.E.; et al. Range expansion of tropical shallow-water marine molluscs in the NE Atlantic during the Last Interglacial (MIS 5e): Causes, consequences and utility of ecostratigraphic indicators for the Macaronesian archipelagos. Quat. Sci. Rev. 2022, 278, 107377. [Google Scholar] [CrossRef]
- Melo, C.S.; Martín-González, E.; da Silva, C.M.; Galindo, I.; González-Rodríguez, A.; Baptista, L.; Rebelo, A.C.; Madeira, P.; Voelker, A.H.L.; Johnson, M.E.; et al. Reply to the comment by Meco et al. on “Range expansion of tropical shallow-water marine molluscs in the NE Atlantic during the last interglacial (MIS 5e): Causes, consequences and utility of ecostratigraphic indicators for the Macaronesian archipelagos”. Quat. Sci. Rev. 2022, 28, 107535. [Google Scholar]
- Lecointre, G. Recherches sur le Néogène et le Quaternaire marin de la côte atlantique du Maroc. Notes Mémoires Serv. Géologique Maroc. 1952, 99, 173–198. [Google Scholar]
- Lecointre, G. Recherches sur le Néogène et le Quaternaire marin de la côte atlantique du Maroc. Tome III, Les acquisitions nouvelles durant la période de 1952 à 1962 (stratigraphie et paléontologie). Notes Mémoires Serv. Géologique Maroc. 1963, 174, 1–75. [Google Scholar]
- Lecointre, G. Le Quaternaire marin de l’Afrique du nord-ouest. Quaternaria 1965, 7, 9–28. [Google Scholar]
- Ortlieb, L. Recherches sur les formations Plio-Quaternaires du littoral Ouest-Saharien (28°30′–20°40′ lat. N). Travaux Documents de l’O.R.S.T.O.M. 1975, 48, 1–267. [Google Scholar]
- Brebion, P. Etude biostratigraphique et paléoécologique du Quaternaire marocain. Ann. Paléontologie Invertébrés 1979, 65, 1–42. [Google Scholar]
- Brebion, P.; Hoang, C.T.; Weisrock, A. L’intérêt des coupes d’Agadir-Port pour l’étude du Pléistocène supérieur marin du Maroc. Bull. Muséum Natl. D’histoire Nat. Paris 1984, 4, 129–151. [Google Scholar]
- Weisrock, A.; Occhietti, S.; Hoang, C.; Lauriat-Rage, A.; Brebion, P.; Pichet, P. Les séquences littorales pléistocènes de l’Atlas atlantique entre Cap Rhir et Agadir, Maroc. Quaternaire 1999, 10, 227–244. [Google Scholar] [CrossRef]
- Plaziat, J.-C.; Aberkan, M.; Ahmamou, M.; Choukri, A. The quaternary deposits of Morocco. In The geology of Morocco; Michar, A., Ed.; Springer: Berlin/Heidelberg, Germany, 2008; pp. 359–379. [Google Scholar]
- Chakroun, A.; Chahid, D.; Boudad, L.; Campmas, E.; Lenoble, A.; Nespoulet, R.; El Hajraoui, M.-A. The Pleistocene of Rabat (Morocco): Mollusks, coastal environments and human behavior. Afr. Archaeol. Rev. 2017, 34, 493–510. [Google Scholar] [CrossRef]
- Melo, C.S.; da Silva, C.M.; Scarponi, D.; González, E.; Rolán, E.; Rojas, A.; Martinez, S.; Silva, L.; Johnson, M.; Rebelo, A.C.; et al. Palaeobiogeography of NE Atlantic archipelagos during the Last Interglacial (MIS 5e): A molluscan approach to the conundrum of Macaronesia as a marine biogeographic unit. Quat. Sci. Rev. 2023, 319, 108313. [Google Scholar] [CrossRef]
- La Perna, R.; De Santis, V.; Caldara, M. Two West African molluscan species (Gastropoda, Acteonidae) from MIS 5.5 in the Taranto area (Southern Italy). Riv. Ital. Paleontol. Stratigr. 2025, 131, 515–527. [Google Scholar] [CrossRef] [PubMed]
- Psarras, C.; Thiavaiou, D.; Quillévéré, F.; Conée, J.-J.; Moissette, P.; Philippon, M.; Fietzke, J.; Bosch, D.; Condomines, M.; Bruguier, O.; et al. Integrating Aegean Last Interglacial faunas into the Mediterranean palaeobiogeographic framework: New evidence from Karpathos (Greece). J. Quat. Sci. in press. in press.
- Ávila, S.P. Atlantic and Mediterranean Gastropoda: Geographical Distribution and Functional Traits Database. Version 1.1. 2025. Available online: https://www.researchgate.net/publication/385907293_Atlantic_and_Mediterranean_Gastropoda_geographical_distribution_and_functional_traits_database_Version_11 (accessed on 17 August 2025).
- Ávila, S.P. Atlantic and Mediterranean Bivalvia: Geographical Distribution and Functional Traits Database. Version 1.0. 2024. Available online: https://www.researchgate.net/publication/387085775_Atlantic_and_Mediterranean_Bivalvia_geographical_distribution_and_functional_traits_database_Version_10 (accessed on 23 August 2025).
- Chahid, D.; Boudad, L.; Lenoble, A.; El Hmaidi, A.; Chakroun, A.; Jacobs, Z. Nouvelles données morphostratigraphiques et géochronologiques sur le cordon littoral externe (SIM 5-c) de Rabat–Témara, Maroc. Géomorphologie 2016, 22, 253–264. [Google Scholar]
- Chakroun, A.; Zaghbib-Turki, D. Facies and fauna proxies used to reconstruct the MIS 5 and MIS 7 coastal environments in eastern Tunisia. Geol. Q. 2017, 61, 186–204. [Google Scholar] [CrossRef][Green Version]
- Hoffman, J.S.; Clark, P.U.; Parnell, A.C.; He, F. Regional and global sea-surface temperatures during the last interglaciation. Science 2017, 355, 276–279. [Google Scholar] [CrossRef] [PubMed]
- Gignoux, M. Les formations marines pliocènes et quaternaires de l’Italie du sud et de la Sicile. Ann. L’université Lyon 1913, 36, 1–693. [Google Scholar]
- Carlqvist, S. The biota of long-distance dispersal. I. Principles of dispersal and evolution. Quart. Rev. Biol. 1966, 41, 247–270. [Google Scholar] [CrossRef]
- van den Hoek, C. The possible significance of long-range dispersal for the biogeography of seaweeds. Helgoländer Meeresunters. 1987, 41, 261–272. [Google Scholar] [CrossRef]
- Jokiel, P.L. Long-distance dispersal by rafting: Reemergence of an old hypothesis. Endeav. New Ser. 1990, 14, 66–73. [Google Scholar] [CrossRef]
- Nikula, R.; Spencer, H.G.; Waters, J.M. Passive rafting is a powerful driver of transoceanic gene flow. Biol. Lett. 2013, 9, 20120821. [Google Scholar] [CrossRef]
- Afonso, P.; Porteiro, F.M.; Fontes, J.; Tempera, F.; Morato, T.; Cardigos, F.; Santos, R.S. 2013. New and rare coastal fishes in the Azores islands: Occasional events or tropicalization process? J. Fish Biol. 2013, 83, 272–294. [Google Scholar] [CrossRef] [PubMed]
- González, J.A.; Espino, F.; González-Lorenzo, J.G. Changes in biogeographic patterns of coastal fishes: Indicators of tropicalization in the Canary Islands over the last 40 years. Mar. Environ. Res. 2025, 205, 107002. [Google Scholar] [CrossRef]
- Barcelos, L.M.D.; Barreiros, J.P. Pinniped (Carnivora, Phocidae) occurrences in the Azores Archipelago (NE Atlantic). Biodivers. Data J. 2022, 10, e96342. [Google Scholar] [CrossRef]
- Rees, W.J. The aerial dispersal of Mollusca. J. Molluscan Stud. 1965, 36, 269–282. [Google Scholar] [CrossRef]
- Wesselingh, F.P.; Cadée, G.C.; Renema, W. Flying high: On the airborne dispersal of aquatic organisms as illustrated by the distribution histories of the gastropod genera Tryonia and Planorbarius. Geol. Mijnb. 1999, 7, 165–174. [Google Scholar] [CrossRef]
- Gittenberger, E.; Groenenberg, D.; Kokshoorn, B.; Preece, R. Molecular trails from hitch-hiking snails. Nature 2006, 43, 409. [Google Scholar] [CrossRef]
- Sousa, W.P. Size-dependent predation on the saltmarsh snail Cerithidea californica Haldeman. J. Exper. Mar. Biol. and Ecol. 1993, 166, 19–37. [Google Scholar] [CrossRef]
- Burger, J.; Gochfeld, M.; Ridgely, R. Migratory behavior of Franklin’s gulls (Larus pipixcan) in Peru. Energy Power Eng. 2010, 2, 143–147. [Google Scholar] [CrossRef]
- Edgar, G.J. Dispersal of fauna and floral propagules associated with drifting Macrocystis pyrifera plants. Mar. Biol. 1987, 95, 599–610. [Google Scholar]
- Helmuth, B.; Veit, R.R.; Holberton, R. Long-distance dispersal of a subantarctic brooding bivalve (Gaimardia trapesina) by kelp-rafting. Mar. Biol. 1994, 120, 421–426. [Google Scholar] [CrossRef]
- Ingólfsson, A. Floating clumps of seaweed around Iceland: Natural microcosms and a means of dispersal for shore fauna. Mar. Biol. 1995, 122, 13–21. [Google Scholar] [CrossRef]
- Ingólfsson, A. Dynamics of macrofaunal communities of floating seaweed clumps off western Iceland: A study patches on the surface of the sea. J. Exp. Mar. Biol. Ecol. 1998, 231, 119–137. [Google Scholar] [CrossRef]
- Thiel, M. Rafting of benthic macrofauna: Important factors determining the temporal succession of the assemblage on detached macroalgae. Hydrobiologia 2003, 503, 49–57. [Google Scholar] [CrossRef]
- Thiel, M.; Gutow, L. The ecology of rafting in the marine environment. II. The rafting organisms and community. Oceanog. Mar. Biol. Ann. Rev. 2005, 43, 279–418. [Google Scholar]
- Alsos, I.G.; Eidesen, P.B.; Ehrich, D.; Skrede, I.; Westergaard, K.; Jacobsen, G.H.; Landvik, J.Y.; Taberlet, P.; Brochmann, C. Frequent long-distance plant colonization in the changing Arctic. Science 2007, 316, 1606–1609. [Google Scholar] [CrossRef]
- Fraser, C.I.; Nikula, R.; Waters, J.M. Oceanic rafting by a coastal community. Proc. R. Soc. B 2011, 278, 649–655. [Google Scholar] [CrossRef]
- Gutow, L.; Beermann, J.; Buschbaum, C.; Rivadeneira, M.M.; Thiel, M. Castaways can’t be choosers—Homogenization of rafting assemblages on floating seaweeds. J. Sea Res. 2014, 95, 161–171. [Google Scholar] [CrossRef]
- Doong, D.J.; Chuang, H.C.; Shieh, C.L.; Hu, J.H. Quantity, distribution, and impacts of coastal driftwood triggered by a typhoon. Mar. Pollution Bull. 2011, 62, 1446–1454. [Google Scholar] [CrossRef] [PubMed]
- Garden, C.J.; Craw, D.; Waters, J.M.; Smith, A. Rafting rocks reveal marine biological dispersal: A case study using clasts from beach-cast macroalgal holdfasts. Estuar. Coast. Shelf Sci. 2011, 95, 388–394. [Google Scholar] [CrossRef]
- Johansen, S.; Hytteborn, H. A contribution to the discussion of biota dispersal with drift ice and driftwood in the North Atlantic. J. Biogeogr. 2001, 28, 105–115. [Google Scholar] [CrossRef]
- Baptista, L.; dos Santos, A.M.; Melo, C.S.; Rebelo, A.C.; Madeira, P.; Cordeiro, R.; Botelho, A.Z.; Hipólito, A.; Pombo, J.; Voelker, A.H.L.; et al. Untangling the origin of the newcomer Phorcus sauciatus (Mollusca: Gastropoda) in a remote northeast Atlantic archipelago. Mar. Biol. 2021, 168, 9. [Google Scholar] [CrossRef]
- Frazier, J.; Margaritoulis, D.; Muldoon, K.; Potter, C.W.; Rosewater, J.; Ruckeschel, C.; Sales, S. Epizoan communities on marine turtles. I. Bivalves and gastropod Mollusca. Mar. Ecol. Prog. Ser. Z. N. I. 1985, 6, 127–140. [Google Scholar] [CrossRef]
- Jokiel, P.L. Long-distance dispersal of reef corals by rafting. Coral Reefs. 1984, 3, 69–76. [Google Scholar]
- Bryan, S.E.; Cook, A.; Evans, J.P.; Colls, P.W.; Wells, M.G.; Lawrence, M.G.; Jell, J.S.; Greig, A.; Leslie, R. Pumice rafting and faunal dispersion during 2001–2002 in the southwest Pacific: Record of a dacitic submarine explosive eruption from Tonga. Earth Planet Sci. Lett. 2004, 227, 135–154. [Google Scholar] [CrossRef]
- Bryan, S.E.; Cook, A.G.; Evans, J.P.; Hebden, K.; Hurrey, L.; Colls, P.; Jell, J.S.; Weatherley, D.; Firn, J. Rapid, long-distance dispersal by pumice rafting. PLoS ONE 2012, 7, e40583. [Google Scholar] [CrossRef]
- Highsmith, R.C. Floating and larval rafting as potential dispersal mechanisms in brooding invertebrates. Mar. Ecol. Prog. Ser. 1985, 25, 169–179. [Google Scholar]
- Parker, T.; Tunnicliffe, V. Dispersal strategies of the biota on an oceanic seamount: Implications for ecology and biogeography. Biol. Bull. 1994, 187, 336–345. [Google Scholar] [CrossRef]
- Cornelius, P.F.S. The Azores hydroid fauna and its origin, with discussion of rafting and medusa suppression. Arquipélago 1992, 10, 75–99. [Google Scholar]
- Knight-Jones, P.; Knight-Jones, E.W. Systematics, ecology and distribution of Southern Hemisphere spirorbids. In First International Polychaete Conference; Hutchings, P.A., Ed.; Linnean Society: Sydney, Australia, 1984; pp. 196–210. [Google Scholar]
- Madeira, P.; Kroh, A.; Cordeiro, R.; Martins, A.M.F.; Ávila, S.P. The echinoderm fauna of the Azores (NE Atlantic Ocean). Zootaxa 2019, 4639, 1–231. [Google Scholar] [CrossRef]
- Svavarsson, J. Limnoria borealis (Isopoda, Flabellifera) and its commensal, Caecijaera borealis (Isopoda, Asellota), found in Icelandic waters. Sarsia 1982, 67, 223–226. [Google Scholar] [CrossRef]
- Peck, S.B. Diversity and zoogeography of the non-oceanic Crustacea of the Galapagos-Islands, Ecuador (Excluding Terrestrial Isopoda). Can. J. Zool.-Rev. Can. Zool. 1994, 72, 54–69. [Google Scholar]
- Gili, C.; Martinell, J. Relationship between species longevity and larval ecology in nassariid gastropods. Lethaia 1994, 27, 291–299. [Google Scholar] [CrossRef]
- Friend, D.S.; Anderson, B.M.; Allmon, W.D. Geographic contingency, not species sorting, dominates macroevolutionary dynamics in an extinct clade of neogastropods (Volutospina; Volutidae). Paleobio 2021, 47, 236–250. [Google Scholar] [CrossRef]
- Freitas, R.; Romeiras, M.; Silva, L.; Cordeiro, R.; Madeira, P.; González, J.A.; Wirtz, P.; Falcón, J.M.; Brito, A.; Floeter, S.R.; et al. Restructuring of the “Macaronesia” biogeographic unit: A marine multi-taxon biogeographical approach. Sci. Rep. 2019, 9, 15792. [Google Scholar] [CrossRef] [PubMed]
- Shackleton, S.; Baggenstos, D.; Menking, J.A.; Dyonisius, M.N.; Bereiter, B.; Bauska, T.K.; Rhodes, R.H.; Brook, E.J.; Petrenko, V.V.; McConnell, J.R.; et al. Global ocean heat content in the Last Interglacial. Nat. Geosci. 2020, 13, 77–81. [Google Scholar] [CrossRef]
- Zbyszewsky, G.; Ferreira, O.d.V. La faune marine des basses plages quaternaires de Praia et Prainha dans l’ile de Santa Maria (Açores). Comun. Dos Serviços Geológicos Port. 1961, 45, 467–478. [Google Scholar]
- Ávila, S.P.; Cordeiro, R.; Rodrigues, A.R.; Rebelo, A.C.; Melo, C.; Madeira, P.; Pyenson, N.D. Fossil mysticeti from the Pleistocene of Santa Maria island, Azores (NE Atlantic ocean), and the prevalence of fossil cetaceans on oceanic islands. Palaeontol. Electron. 2015, 18.2.27A, 1–12. [Google Scholar]
- Ávila, S.P.; Azevedo, J.M.N.; Madeira, P.; Cordeiro, R.; Melo, C.S.; Baptista, L.; Torres, P.; Johnson, M.E.; Vullo, R. Pliocene and Late-Pleistocene actinopterygian fishes from Santa Maria Island (Azores: NE Atlantic Ocean): Systematics, palaeoecology and palaeobiogeography. Geol. Mag. 2020, 157, 1526–1542. [Google Scholar] [CrossRef]
- Estevens, M.; Ávila, S.P. Fossil whales from the Azores. Açoreana 2007, (Suppl. 5), 140–161. [Google Scholar]
- Madeira, P.; Kroh, A.; Martins, A.M.F.; Ávila, S.P. The marine fossils from Santa Maria Island (Azores, Portugal): An historical overview. Açoreana Supl. 2007, 5, 5–73. [Google Scholar]
- Madeira, P.; Kroh, A.; Cordeiro, R.; Meireles, R.; Ávila, S.P. The fossil echinoids of Santa Maria Island, Azores (northern Atlantic Ocean). Acta Geol. Pol. 2011, 61, 243–264. [Google Scholar]
- Hyžný, M.; Melo, C.S.; Ramalho, R.S.; Cordeiro, R.; Madeira, P.; Baptista, L.; Rebelo, A.C.; Gómez, C.; Torres, P.; Uchman, A.; et al. Pliocene and Late Pleistocene (MIS 5e) decapod crustacean crabs from Santa Maria Island (Azores Archipelago: NE Atlantic): Systematics, palaeoecology and palaeobiogeography. J. Quat. Sci. 2021, 36, 91–109. [Google Scholar] [CrossRef]
- Rebelo, A.C.; Rasser, M.W.; Ramalho, R.S.; Johnson, M.E.; Melo, C.S.; Uchman, A.; Quartau, R.; Berning, B.; Neto, A.I.; Mendes, A.R.; et al. Pleistocene coralline algal build-ups on a mid-ocean rocky shore—insights into the MIS 5e record of the Azores. Palaeobiogeog. Palaeoclim Palaeoecol. 2021, 579, 110598. [Google Scholar]
- Ávila, S.P.; Madeira, P.; Mendes, N.; Rebelo, A.; Medeiros, A.; Gomes, C.; García-Talavera, F.; da Silva, C.M.; Cachão, M.; Hillaire-Marcel, C.; et al. Mass extinctions in the Azores during the last glaciation: Fact or myth? J. Biogeogr. 2008, 35, 1123–1129. [Google Scholar] [CrossRef]
- Gerber, J.; Hemmen, J.; Gröh, K. Eine pleistozäne marine Molluskenfauna von Porto Santo (Madeira-Archipel). Mitteilungen Der Dtsch. Malakozool. Ges. Heft. 1989, 44–45, 10–30. [Google Scholar]
- Montesinos, M.; Ramos, A.J.G.; Lomoschitz, A.; Coca, J.; Redondo, A.; Betancort, J.F.; Meco, J. Extralimital Senegalese species during marine isotope stages 5.5 and 11 in the Canary Islands (29° N): Sea Surface temperature estimates. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2014, 410, 153–163. [Google Scholar] [CrossRef]
- González-Rodríguez, A.; Melo, C.S.; Galindo, I.; Mangas, J.; Sánchez, N.; Coello, J.; Lozano-Francisco, M.C.; Johnson, M.E.; Romero, C.; Vegas, J.; et al. Historia geológica y reconstrucción paleobiológica de los depósitos paleontológicos de la playa de El Confital (Gran Canaria, Islas Canarias). Cuad. Del Mus. Geomin. 2018, 27, 491–499. [Google Scholar]
- Zazo, C.; Goy, J.L.; Hillaire-Marcel, C.; Dabrio, C.J.; González-Delgado, J.A.; Cabero, A.; Bardají, T.; Ghaleb, B.; Soler, V. Sea level changes during the last and present interglacials in Sal Island (Cape Verde archipelago). Glob. Planet. Change 2010, 72, 302–317. [Google Scholar] [CrossRef]
- Ávila, S.P.; Paris, R.; Ramalho, R.S.; Melo, C.S.; Martín-González, E.; Rolán, E.; Madeira, P.; Ávila, G.C.; Porteiro, J.M.; Medeiros, A.M.; et al. Mega-tsunami deposits and range expansion of cold-temperate marine species towards the tropics in glacial times. Front. Biogeog. 2025, 18, e138319. [Google Scholar] [CrossRef]
- Cabero, A.; González-Delgado, J.A.; Zazo, C.; Goy, J.L.; Dabrio, C.J.; Lario, J.; Bardají, T.; Hillaire-Marcel, C.; Ghaleb, B. Distribution of warm Senegalese species during MIS 5 in the Atlantic-Mediterranean linkage area: Coastal settings. In Decoding the Last Interglacial in Western Mediterranean; INQUA Project 0911-CMP Commission: Sardinia, Italy, 2010; pp. 15–16. [Google Scholar]
- Rohling, E.J.; Braun, K.; Grant, K.; Kucera, M.; Roberts, A.P.; Siddall, M.; Trommer, G. Comparison between Holocene and Marine Isotope Stage-11 sea-level histories. Earth Planet. Sci. Lett. 2010, 291, 97–105. [Google Scholar] [CrossRef]
- Govin, A.; Capron, E.; Tzedakis, P.C.; Verheyden, S.; Ghabelb, B.; Hillaire-Marcel, C.; St-Onge, G.; Stoner, J.S.; Bassinot, F.; Bazin, L.; et al. Sequence of events from the onset to the demise of the Last Interglacial: Evaluating strengths and limitations of chronologies used in climatic archives. Quat. Sci. Rev. 2015, 129, 1–36. [Google Scholar] [CrossRef]
- Past Interglacials Working Group of PAGES. Interglacials of the last 800,000 years. Rev. Geophys. 2016, 54, 162–219. [Google Scholar]
- Raymo, M.; Mitrovica, J.X. Collapse of Polar Ice Sheets During the Stage 11 Interglacial. Nature 2012, 483, 453–456. [Google Scholar] [CrossRef]
- Dutton, A.; Carlson, A.E.; Long, A.J.; Milne, G.A.; Clark, P.U.; DeConto, R.; Horton, B.P.; Rahmstorf, S.; Raymo, M.E. Sea-level rise due to polar ice-sheet mass loss during past warm periods. Science 2015, 349, aaa4019. [Google Scholar] [CrossRef]
- Hearty, P.J.; Tormey, B.R. Sea-level change and superstorms: Geologic evidence from the last interglacial (MIS 5e) in the Bahamas and Bermuda offers ominous prospects for a warming Earth. Mar. Geol. 2017, 390, 347–365. [Google Scholar] [CrossRef]
- Clark, P.U.; Huybers, P. Interglacial and future sea level. Geophys. Res. Lett. 2009, 462, 856–857. [Google Scholar]
- Maréchal, C.; Boutier, A.; Mélières, M.-A.; Clauzel, T.; Betancort, J.F.; Lomoschitz, A.; Meco, J.; Fourel, F.; Barral, A.; Amiot, R.; et al. Last interglacial Sea Surface warming during the Sea-level highstand in the Canary Islands: Implications for the Canary current and the upwelling off African coast. Quat. Sci. Rev. 2020, 234, 106246. [Google Scholar] [CrossRef]
- Milanković, M. Kanon der Erdbestrahlung und seine Anwendung auf das Eiszeitenproblem. R. Serbian Acad. Spec. Publ. 1941, 133, 1–633. [Google Scholar]
- Cita Sironi, M.B.; Capotondi, L.; Asioli, A. The Tyrrhenian stage in the Mediterranean: Definition, usage and recognition in the deep-sea record. A proposal. Accad. Naz. Lincei Rend. Cl. Sci. Fis. Mat. Nat. 2005, 9, 297–310. [Google Scholar]
- Issel, A. Lembi fossiliferi quaternari recenti osservati nella Sardegna meridionale dal prof. D. Lovisato. Rend. Della Accad. Naz. Dei Lincei 1914, 23 (Suppl. 5), 759–770. [Google Scholar]
- Cuerda Barceló, J. Fauna marina del Tirreniense de la bahía de Palma (Mallorca). Bolletí De La Soc. D’història Nat. De Les Balear. 1957, 3, 3–75. [Google Scholar]
- Cuerda Barceló, J. Moluscos Marinos y Salobres del Pleistoceno balear. Caja de Baleares ‘Sa Nostra’ Mallorca: Mallorca, Spain, 1987; 421p. [Google Scholar]
- Cuerda Barceló, J. Los Tiempos Cuaternarios en Baleares, 2nd ed.; Conselleria de Cultura, Educacio i Esports del Govern Balear: Palma, Spain, 1989; 305p. [Google Scholar]
- Hillaire-Marcel, C.; Gariepy, C.; Ghaleb, B.; Goy, J.L.; Zazo, C.; Cuerda Barceló, J. U-series measurements in Tyrrhenian deposits from Mallorca. Further evidence for two last-interglacial high sea levels in the Balearic islands. Quat. Sci. Rev. 1996, 15, 53–62. [Google Scholar] [CrossRef]
- Gàsser, Z. Jaciments paleontològics marins del Miocè i Quaternari d’es Ram (Formentera, Illes Pitiüses). Boll. Soc. Hist. Nat. Balears. 2002, 45, 87–92. [Google Scholar]
- Vicens, D. El registre paleontològic dels dipòsits litorals Quaternaris a l’illa de Mallorca (Illes Balears, Mediterrània Occidental). Ph.D. Thesis, University of the Balearic Islands, Palma, Spain, 2015; 1011p. [Google Scholar]
- del Valle, L.; Pons, G.X.; Fornos, J.J. Upper Pleistocene Marine Levels of the Es Copinar–Es Estufadors (Formentera, Balearic Islands, West Mediterranean). Quaternary 2025, 8, 38. [Google Scholar] [CrossRef]
- Galili, E.; Şevketoğlu, M.; Salamon, A.; Zviely, D.; Mienis, H.K.; Rosen, B.; Moshkovitz, S. Late Quaternary beach deposits and archaeological relicts on the coasts of Cyprus, and the possible implications of sea-level changes and tectonics on the early populations. Geol. Soc. Lond. Spec. Publ. 2016, 411, 179–218. [Google Scholar] [CrossRef]
- Hegab, O.A.; Hesman El Asmar, M. Last interglacial stratigraphy in the burg El-Arab region of the northwestern coast of Egypt. Quat. Int. 1995, 29, 23–30. [Google Scholar] [CrossRef]
- Barriere, J. Le rivage Tyrrhénien de L’étang de Bages et de Sigean (Aude). Bull. l’Assoc. Française Pour L’etude Quat. 1966, 4, 251–283. [Google Scholar]
- Anapliotis, K.A. Les couches à Strombes à l’île Armathia (région de Cassos). Proc. Acad. Athens 1963, 38, 137–142. [Google Scholar]
- Angelier, J.; Gigout, M.; Hogrel, M. A propos du gisement Tyrrhénien d’Arvi (Crete): Cadre stratigraphique, faune, esquisse paléoécologique. Ann. Géologiques Des Pays Helléniques 1977, 28, 471–488. [Google Scholar]
- Issar, A.; Picard, L. Sur le Tyrrhenien des cotes d’Israel et du Liban. Bull. L’association Française Pour L’étude Du Quat. 1969, 6, 35–41. [Google Scholar] [CrossRef]
- Gignoux, M. Les couches à Strombus bubonius (LMK) dans la Méditerranée occidentale. Comptes-Rendus De L’académie Des Sci. Paris. 1911, 15, 1–339. [Google Scholar]
- Blanc, A.C. Una spiaggia pleistocenica a Strombus bubonius presso Palidoro (Roma). Rend. Dell’accademia Naz. Dei Lincei 1936, 23, 200–204. [Google Scholar]
- Trevisan, L.; di Napoli, E. Tirreniano, Siciliano e Calabriano nella Sicilia Sud-Occidentale. Note di Stratigrafia, Paleontologia e Morfologia. G. Di Sci. Nat. Ed Econ. 1938, 39, 1–39. [Google Scholar]
- Fabiani, R. Tracce di Tirreniano (strati a Strombus bubonius Lk) entro la citta di Palermo. Boll. Della Soc. Di Sci. Nat. Ed Econ. Palermo 1941, 19, 1–7. [Google Scholar]
- Segre, A.G. Molluschi del Tirreniano di Porto Torres e di Golfo Aranci (Sardegna). Boll. Del Serv. Geol. D’italia 1952, 73, 269–290. [Google Scholar]
- Segre, A.G. Il Tirreniano del Golfo di Terranova Pausania (Olbia) e la sua fauna malacologica. Boll. Del Serv. Geol. D’italia 1954, 76, 43–84. [Google Scholar]
- Comaschi-Caria, A. Nuovi lembi del quaternario in Sardegna. In Rendiconti Seminari della Facoltà di Scienze dell’Università di Cagliari; University of Cagliari: Cagliari, Italy, 1954; Volume 24, pp. 1–4. [Google Scholar]
- Malatesta, A. Risultati del rilevamento del Foglio 192 (Alghero-Isola di Sardegna): Fossili delle spiagge tirreni,ane. Bull. Serv. Geol. Ital. 1954, 76, 9–17. [Google Scholar]
- Bonifay, E.; Mars, P. Le Tyrrhenien dans le cadre de la chronologie Quaternaire Mediterraneenne. Bull. Société Géologique Fr. 1959, 7, 62–78. [Google Scholar] [CrossRef]
- Ruggieri, G.; Buccheri, G.; Rendina, M. Segnalazione di Tirreniano fossilifero a Trapani. Riv. Mineraria Sicil. 1968, 112–114, 216–219. [Google Scholar]
- Bonfiglio, L. Il Tirreniano di Bovetto e Ravagnese presso Reggio Calabria. Quaternaria 1972, 6, 137–148. [Google Scholar]
- Ozer, A.; Ulzega, A. Livret-guide de l’excursion-table ronde sur le Tyrrhenian de Sardaigne. In Annales de Géographie; Armand Colin: Cagliari, France, 1980; 88p. [Google Scholar]
- Vazzana, A. Le conchiglie del periodo Tirreniano nei dintorni di Reggio Calabria. La Conchiglia 1988, 20, 25–26. [Google Scholar]
- Abate, B.; Ferruzza, G.; Incandela, A.; Renda, P. Ritrovamento di depositi a Strombus bubonius Lmk (Tirreniano) nell’isola di Favignana. Riv. Mineraria Sicil. 1992, 162, 37–46. [Google Scholar]
- Buccheri, G.; Renda, P.; Morreale, C.; Sorrentino, G. Il Tirreniano dell’Isola di Lampedusa (Arcipelago Pelagiano, Agrigento, Italia). Boll. Società Geol. Ital. 1999, 118, 361–373. [Google Scholar]
- Santagati, P.; Perri, E.; Bernasconi, M.P.; Borrelli, M.; Guerrieri, S.; Critelli, S. MIS 5e sea surface temperature estimation; a multi-proxy approach using a marine macrofossil assemblage (Mar Piccolo, Gulf of Taranto, Southern Italy). J. Palaeogeogr. 2024, 13, 327–350. [Google Scholar] [CrossRef]
- Nalin, R.; Bracchi, V.A.; Basso, D.; Massari, F. Persististrombus latus (Gmelin) in the upper Pleistocene deposits of the marine terraces of the Crotone peninsula (southern Italy). Italian J. Geosci. 2012, 131, 95–101. [Google Scholar]
- Amorosi, A.; Antonioli, F.; Bertini, A.; Marabini, S.; Mastronuzzi, G.; Montagna, P.; Negri, A.; Rossi, V.; Scarponi, D.; Taviani, M.; et al. The Middle-Late Quaternary Fronte Section (Taranto, Italy): An exceptionally preserved marine record of the Last Interglacial. Glob. Planet. Change 2014, 119, 23–38. [Google Scholar] [CrossRef]
- Fleisch, H.J.; Comati, P.R.; Elouard, P. Gisement a Strombus bubonius Lmk. (Tyrrhenien) a Naame (Liban). Quaternaria 1973, 15, 217–237. [Google Scholar]
- Lario, C.J.; Zazo, C.; Goy, J.L.; Hoyos, M.; Hillaire-Marcel, C. Episodios marinos del último Interglacial (Estadio isotópico 5) del litoral de Málaga (SE penínsular). In Elementos de los paisajes de la província de Málaga, González, J.M., Ferre Bueno, E., Eds.; (coord. Senciales), Servicio de publicaciones, Universidad de Málaga: Málaga, Spain, 1999; pp. 63–75. [Google Scholar]
- Vera-Peláez, J.L.; Lozano-Francisco, M.C.; Ramos Fernández, J.R.; Cortés Sánchez, M. Moluscos del Tirreniense (Pleistoceno Superior) de la Playa la Araña-Cala del Moral (Málaga). Rev. Española Paleontol. 2004, 19, 251–259. [Google Scholar] [CrossRef]
- Bardaji, T.; Goy, J.L.; Zazo, C.; Hillaire-Marcel, C.; Dabrio, C.J.; Cabero, A.; Ghaleb, B.; Silva, P.G.; Lario, J. Sea level and climate changes during OIS 5e in the Western Mediterranean. Geomorphology 2009, 104, 22–37. [Google Scholar] [CrossRef]
- Castany, G. Le niveau à strombes de Tunisie. Sa place dans la chronologie préhistorique du Quaternaire. Compte-Rendu Somm. Des Séances Société Géologique Fr. 1954, 55–56. [Google Scholar]
- Castany, G. Données nouvelles sur la stratigraphie du Quaternaire de Jerba. Bull. de la Soc. des Sci, Nat. de Tunisie. 1955, 8, 135–144. [Google Scholar]
- Castany, G. Données nouvelles sur le Quaternaire marin de Monastir (Tunisie orientale). Comptes-Rendus De L’académie Des Sci. Paris 1956, 242, 533–536. [Google Scholar]
- Herm, D.; Karray, R.; Pascoff, R.; Sanlaville, P. Sur deux depots a Strombus bubonius du golfe de Tunis. Bull. Soc. Géologique Fr. 1975, 17 (Suppl. 1), 21–22. [Google Scholar]
- Ozer, A.; Paskoff, R.; Sanlaville, P.; Ulzega, A. Essai de corrélation du Pléistocène supérieur de la Sardaigne et de la Tunisie. Comptes Rendus L’academie Des Sci. Paris Série D 1980, 291, 801–804. [Google Scholar]
- Chakroun, A. Étude sédimentologique et paléontologique des affleurements du Quaternaire le long de la côte Nord-orientale de la Tunisie. Ph.D. Thesis, Université de Tunis, Tunis, Tunisie, 2006; 400p. [Google Scholar]
- Chakroun, A.; Zaghbib Turki, D.; Moncef Turki, M. The Upper Pleistocene deposits in Rafraf (North eastern Tunisia): New data on the Persististrombus latus level. Arabian Jounal of Geosciences 2016, 9, 1–13. [Google Scholar]
- Chakroun, A.; Zaghbib-Turki, D.; Miskovsky, J.-C.; Davaud, E. Two Tyrrhenian transgressive cycles in coastal deposits of the Cap Bon Peninsula. Quaternaire 2009, 20, 215–226. [Google Scholar] [CrossRef]
- Chakroun, A.; Zaghbib-Turki, D.; Turki, M.-M. Synthèse sur le Pléistocène de la zone côtière de Tunisie. Mémoire Du Serv. Géologique De L’algérie 2021, 22, 109–128. [Google Scholar]
- de Porta, J.; Martinell, J. El Tyrrheniense Catalan, Sintesis y Nuevas Aportaciones; Department de Paleontologia Universitat de Barcelona: Barcelona, Spain, 1981; 27p. [Google Scholar]
- Lisiecki, L.E.; Raymo, M.E. A Pliocene-Pleistocene stack of 57 globally distributed benthic δ18O records. Paleoceanography 2005, 20, PA1003. [Google Scholar] [CrossRef]
- Meco, J. Los niveles con “Strombus” de Jandia (Fuerteventura, Islas Canarias). Anu. Estud. Atl. 1975, 21, 643–660. [Google Scholar]
- Meco, J.; Ballester, J.; Betancourt, J.F.; Cilleros, A.; Scaillet, S.; Guillou, H.; Carracedo, J.C.; Lomoschitz, A.; Petit-Maire, N.; Ramos, A.J.G.; et al. Paleoclimatologia del Neógeno en las Islas Canarias: Geliense, Pleistoceno y Holoceno; Ministerio de Medio Ambiente, Universidad de las Palmas de Gran Canaria: Las Palmas, Spain, 2006; p. 203. [Google Scholar]
- Taviani, M. Unpersisting Persististrombus: A Mediterranean story. Vieraea 2014, 42, 9–18. [Google Scholar] [CrossRef]
- Muhs, D.R.; Meco, J.; Simmons, K.R. Uranium-series ages of corals, sea level history, and palaeozoogeography, Canary Islands, Spain: An exploratory study for two Quaternary interglacial periods. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2014, 394, 99–118. [Google Scholar] [CrossRef]
- Versteegh, G.J.M.; Zonneveld, K.A.F.; Hefter, J.; Romero, O.E.; Fischer, G.; Mollenhauer, G. Performance of temperature and productivity proxies based on long-chain alkane-1, mid-chain diols at test: A 5-year sediment trap record from the Mauritanian upwelling. Biogeosciences 2022, 19, 1587–1610. [Google Scholar] [CrossRef]
- Romero, O.E.; Baumann, K.-H.; Zonneveld, K.A.F.; Donner, B.; Hefter, J.; Hamady, B.; Pospelova, V.; Fischer, G. Flux variability of phyto- and zooplankton communities in the Mauritanian coastal upwelling between 2003 and 2008. Biogeosciences 2020, 17, 187–214. [Google Scholar] [CrossRef]
- Lovecchio, E.; Gruber, N.; Münnich, M. Mesoscale contribution to the long-range offshore transport of organic carbon from the Canary Upwelling System to the open North Atlantic. Biogeosciences 2018, 15, 5061–5091. [Google Scholar] [CrossRef]
- Gabric, A.J.; Garcia, L.; Camp, L.V.; Nykjaer, L.; Eifler, W.; Schrimpf, W. Offshore export of shelf production in the Cape Blanc (Mauritania) giant filament as derived from coastal zone color scanner imagery. J. Geophys. Res. 1993, 98, 4697–4712. [Google Scholar] [CrossRef]
- Hildenbrand, A.; Weis, D.; Madureira, P.; Marques, F.O. Recent plate reorganization at the Azores triple junction: Evidence from combined geochemical and geochronological data on Faial, S. Jorge and Terceira volcanic islands. Lithos 2014, 210–211, 27–39. [Google Scholar] [CrossRef]
- Cornu, S.; Pätzold, J.; Bard, E.; Meco, J.; Cuerda-Barcelo, J. Paleotemperature of the last interglacial period based on δ18O of Strombus bubonius from the western Mediterranean Sea. Palaeogeogr. Palaeoclimatol. Palaeoecol. 1993, 103, 1–20. [Google Scholar] [CrossRef]
- Hevia-Cruz, F.; Sheldon, N.D.; Hildenbrand, A.; Hren, M.T.; Marques, F.O.; Carlut, J.; Chabauz, F. Regional variations of the Azores High across glacial-interglacial timescales. Paleoceanogr. Palaeoclimatol. 2024, 39, e2023PA004810. [Google Scholar] [CrossRef]



| Class | MIS 5e Extinct Species | Site | Geological Timespan | |||||
|---|---|---|---|---|---|---|---|---|
| Eocene | Oligocene | Miocene | Pliocene | Pleistocene | Recent | |||
| GAS | Acteon bovettensis G. Seguenza, 1880 † | MED | 1 | 1 | ||||
| GAS | Alvania calliope Chirli & U. Linse, 2011 † | MED | 1 | |||||
| GAS | Alvania curta (Dujardin, 1837) † | MED | 1 | |||||
| GAS | Alvania mariae (A. d’Orbigny, 1852) † | MED | 1 | |||||
| GAS | Alvania unica Amati & Quaggiotto, 2019 † | MED | 1 | |||||
| GAS | Cancilla alligata (Defrance, 1825) † | MED | 1 | |||||
| GAS | Capulus laevis (Bronn, 1831) † | MED | 1 | |||||
| GAS | Cerithium diblasii † | MED | 1 | |||||
| GAS | Clathromangelia clathrata (Serres, 1829) † | MED | 1 | 1 | ||||
| GAS | Clathromangelia quadrillum (Dujardin, 1837) † | MED | 1 | |||||
| GAS | Episcomitra fusiformis (Brocchi, 1814) † | MED | 1 | |||||
| GAS | Dizoniopsis bilineata (M. Hörnes, 1848) † | MED | 1 | 1 | ||||
| GAS | Fusinus rudis (R. A. Philippi, 1844) † | MED | 1 | 1 | ||||
| GAS | Helminthia triplicata (Brocchi, 1814) † | MED | 1 | |||||
| GAS | Jujubinus bullula (P. Fischer, 1877) † | MED | 1 | |||||
| GAS | Metula deshayesi (Michelotti, 1847) † | MED | 1 | 1 | ||||
| GAS | Ocinebrina scalaris (Brocchi, 1814) † | MED | 1 | 1 | ||||
| GAS | Petaloconchus intortus (Lamarck, 1818) † | MED | 1 | 1 | 1 | 1 | ||
| BIV | Europicardium multicostatum (Brocchi, 1814) † | MED | 1 | |||||
| BIV | Glycymeris inflata (Brocchi, 1814) † | MED | 1 | 1 | ||||
| BIV | Macoma obliqua (J. Sowerby, 1817) † | MED | 1 | |||||
| BIV | Megaxinus transversus (Bronn, 1831) † | MED | 1 | 1 | ||||
| BIV | Plicatula mytilina (R. A. Philippi, 1836) † | MED | 1 | 1 | ||||
| BIV | Pycnodonte squarrosa de Serres 1843 † | MED | 1 | 1 | ||||
| GAS | Acanthina dontelei Garcia-Talavera & Sanchez-Pinto, 2002 † | SEL | 1 | |||||
| GAS | Acanthina dontelei Garcia-Talavera & Sanchez-Pinto, 2002 † | CAN | 1 | |||||
| GAS | Spinucella plessisi (Lecointre, 1952) † | CAN | ||||||
| BIV | Lucina columbella Lamarck, 1818 † | CAN | 1 | |||||
| AZO | MAD | SEL | CAN | CAB | MOR | MED | |
|---|---|---|---|---|---|---|---|
| Gastropoda | 22 | 13 | 2 | 32 | 6 | 22 | 25 |
| Bivalvia | 5 | 9 | 0 | 13 | 6 | 1 | 10 |
| Total | 27 | 22 | 2 | 45 | 12 | 23 | 35 |
| Region | Class | Species/Taxa | Most Probable Source/Origin | ||
|---|---|---|---|---|---|
| Range Expansion Towards Higher Latitudes | Range Expansion Towards Lower Latitudes | Longitudinal Range Expansion | |||
| AZO | GAS | Claremontiella nodulosa | CAB/SEN/BER | ||
| AZO | GAS | Conus ambiguus | CAB/MAU | ||
| AZO | GAS | Conus ermineus | CAB/WES/CAR * | ||
| AZO | GAS | Conus miruchae | CAB | ||
| AZO | GAS | Conus roeckeli | CAB | ||
| AZO | GAS | Conus venulatus | CAB | ||
| AZO | GAS | Zonaria picta | CAB/SEN | ||
| MAD | BIV | Cardium sp. | MAU | MOR * | |
| MAD | GAS | Acliceratia carinata | MAU | ||
| MAD | GAS | Conus sp. | CAB | ||
| MAD | GAS | Thais nodosa | CAB | ||
| MAD | GAS | Zebina vitrea | CAR | ||
| CAN | BIV | Acar olivercoseli | CAB/SEN | ||
| CAN | BIV | Brachidontes puniceus | CAB/MAU | ||
| CAN | BIV | Cardium sp. | MAU | MOR * | |
| CAN | BIV | Chlamys sp. | CAR | ||
| CAN | BIV | Codakia sp. | CAR | BER | |
| CAN | BIV | Ctena eburnea | CAB/SEN | ||
| CAN | GAS | Cassis sp. | CAB/BER * | ||
| CAN | GAS | Cheilea equestris | CAB/BER * | ||
| CAN | GAS | Claremontiella nodulosa | CAB/SEN/BER * | ||
| CAN | GAS | Conus ermineus | CAB | WES | |
| CAN | GAS | Conus guinaicus | SEN | ||
| CAN | GAS | Cumia intertexta | SEN | ||
| CAN | GAS | Harpa doris | CAB/SEN | ||
| CAN | GAS | Heliacus cylindricus | CAB/BER * | ||
| CAN | GAS | Hexaplex rosarium | CAB/SEN | ||
| CAN | GAS | Nerita senegalensis | CAB/SEN | ||
| CAN | GAS | Prunum olivaeforme | MAU | ||
| CAN | GAS | Thais nodosa | CAB | ||
| CAN | GAS | Thetystrombus latus | CAB/SEN | ||
| CAN | GAS | Thylacodes arenarius | CAB | ||
| CAN | GAS | Vaughtia gruveli | CAB | WES | |
| CAN | GAS | Zonaria zonaria | SEN | ||
| CAB | BIV | Saccostrea cuccullata | IVO | SEN | |
| CAB | GAS | Fasciolaria sp. | CAR | ||
| CAB | GAS | Inermicosta inermicosta | SEN | ||
| MED | BIV | Acar olivercoseli | CAB/SEN | ||
| MED | BIV | Anadara geissei | CAB/MAU | ||
| MED | BIV | Arcopsis afra | CAB/MOR | ||
| MED | BIV | Brachidontes puniceus | CAB/MAU | ||
| MED | BIV | Dendostrea cristata | CAR | ||
| MED | BIV | Parvilucina crenella | CAR | ||
| MED | GAS | Acteon maltzani | CAB/SEN | ||
| MED | GAS | Acteon senegalensis | MAU | ||
| MED | GAS | Conus ermineus | CAB/WES/CAR * | ||
| MED | GAS | Conus guinaicus | SEN | ||
| MED | GAS | Epitonium trochoides | MAU | ||
| MED | GAS | Hexaplex rosarium | CAB/SEN | ||
| MED | GAS | Imbricariopsis carbonacea | CAB/SEN | ||
| MED | GAS | Simnia senegalensis | CAB/SEN | ||
| MED | GAS | Thetystrombus latus | CAB/SEN | ||
| MED | GAS | Turbonilla kerstinae | CAB/MAU | ||
| MED | GAS | Turbonilla secernenda | SEN | ||
| MED | GAS | Xenophora senegalensis | CAB/SEN | ||
| MED | GAS | Zonaria petitiana | MAU | ||
| MED | GAS | Zonaria angolensis | GAB | ||
| MOR | BIV | Crassatina contraria | MAU | ||
| MOR | GAS | Anachis aurantia | SEN | ||
| MOR | GAS | Cymbium gracile | WES | ||
| MOR | GAS | Hexaplex angularis | SEN | ||
| Archipelago/Island | # spp. | p | np | % spp. with Unknown Mode of Larval Development | Age (Ma) |
|---|---|---|---|---|---|
| Azores | 224 | 79 | 81 | 28.6 | 6 |
| Madeira | 332 | 98 | 101 | 40.1 | 18.8 |
| Selvagens | 156 | 45 | 73 | 24.4 | 29.5 |
| Canaries | 697 | 180 | 214 | 43.5 | 25 |
| Cabo Verde | 524 | 130 | 144 | 47.7 | 15.8 |
| Bermuda | 423 | 150 | 76 | 46.6 | 47 |
| São Tomé and Príncipe | 369 | 88 | 80 | 54.5 | 9 |
| Saint Helena Island | 37 | 7 | 10 | 54.1 | 15 |
| Fernando de Noronha Island | 152 | 62 | 60 | 19.7 | 12.3 |
| Trindade and Martim Vaz | 115 | 50 | 47 | 15.7 | 3.7 |
| Genus | Total Number of Species | Archipelagos/Islands | % of Endemic Insular Species | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AZO | MAD | SEL | CAN | CAB | BER | STP | STH | ASC | TRD | ASP | FNO | FAL | |||
| Alvania Risso, 1826 | 136 | 7 | 1 | 5 | 8 | 2 | 3 | 19.1 | |||||||
| Ammonicera Vayssière, 1893 | 16 | 2 | 12.5 | ||||||||||||
| Anachis H. Adams & A. Adams, 1853 | 21 | 2 | 1 | 14.3 | |||||||||||
| Barleeia W. Clark, 1853 | 12 | 3 | 1 | 33.3 | |||||||||||
| Bittium Leach, 1847 | 15 | 1 | 5 | 40.0 | |||||||||||
| Botryphallus Ponder, 1990 | 3 | 1 | 1 | 66.7 | |||||||||||
| Caecum J. Fleming, 1813 | 83 | 2 | 2 | 3 | 6 | 1 | 16.9 | ||||||||
| Chrysallida P. P. Carpenter, 1856 | 22 | 10 | 45.5 | ||||||||||||
| Conus Linnaeus, 1758 | 247 | 1 | 49 | 1 | 1 | 21.1 | |||||||||
| Coralliophila H. Adams & A. Adams, 1853 | 23 | 1 | 1 | 1 | 13.0 | ||||||||||
| Crisilla Monterosato, 1917 | 36 | 4 | 6 | 27.8 | |||||||||||
| Diodora J. E. Gray, 1821 | 40 | 1 | 2 | 1 | 10.0 | ||||||||||
| Eatonina Thiele, 1912 | 11 | 2 | 18.2 | ||||||||||||
| Euthria J. E. Gray, 1850 | 14 | 13 | 92.9 | ||||||||||||
| Fissurella Bruguière, 1789 | 29 | 6 | 1 | 1 | 2 | 34.5 | |||||||||
| Gibberula Swainson, 1840 | 116 | 1 | 10 | 3 | 4 | 3 | 18.1 | ||||||||
| Gibbula Risso, 1826 | 20 | 1 | 4 | 25.0 | |||||||||||
| Hastula H. Adams & A. Adams, 1853 | 22 | 1 | 2 | 1 | 18.2 | ||||||||||
| Jujubinus Monterosato, 1884 | 23 | 1 | 3 | 1 | 21.7 | ||||||||||
| Manzonia Brusina, 1870 | 28 | 2 | 1 | 1 | 7 | 7 | 1 | 67.9 | |||||||
| Marginella Lamarck, 1799 | 30 | 10 | 33.3 | ||||||||||||
| Metaxia Monterosato, 1884 | 15 | 2 | 1 | 20.0 | |||||||||||
| Mirpurina Ortea, Moro & Espinosa, 2019 | 12 | 11 | 91.7 | ||||||||||||
| Mitrella Risso, 1826 | 36 | 4 | 5 | 25.0 | |||||||||||
| Mitromorpha P. P. Carpenter, 1865 | 27 | 1 | 4 | 4 | 33.3 | ||||||||||
| Muricopsis Bucquoy & Dautzenberg, 1882 | 19 | 4 | 21.1 | ||||||||||||
| Odostomia J. Fleming, 1813 | 63 | 2 | 1 | 3 | 1 | 2 | 14.3 | ||||||||
| Onoba H. Adams & A. Adams, 1852 | 35 | 1 | 1 | 1 | 1 | 11.4 | |||||||||
| Parviturbo Pilsbry & T. L. McGinty, 1945 | 14 | 1 | 2 | 21.4 | |||||||||||
| Patella Linnaeus, 1758 | 13 | 1 | 1 | 1 | 23.1 | ||||||||||
| Phorcus Risso, 1826 | 10 | 1 | 1 | 20.0 | |||||||||||
| Plesiocystiscus G. A. Coovert & H. K. Coovert, 1995 | 15 | 1 | 3 | 3 | 46.7 | ||||||||||
| Pradoxa F. Fernandes & Rolán, 1993 | 4 | 4 | 100.0 | ||||||||||||
| Pseudoscilla O. Boettger, 1902 | 5 | 1 | 1 | 40.0 | |||||||||||
| Putzeysia Sulliotti, 1889 | 3 | 1 | 2 | 100.0 | |||||||||||
| Pyrgocythara Woodring, 1928 | 10 | 1 | 10.0 | ||||||||||||
| Rissoa Desmarest, 1814 | 37 | 1 | 3 | 1 | 13.5 | ||||||||||
| Rissoella J.E. Gray, 1847 | 31 | 3 | 1 | 1 | 16.1 | ||||||||||
| Schwartziella G. Nevill 1881 | 37 | 19 | 3 | 1 | 62.2 | ||||||||||
| Volvarina Hinds, 1844 | 266 | 1 | 1 | 12 | 6 | 1 | 1 | 1 | 8.6 | ||||||
| TOTAL | 25 | 5 | 3 | 57 | 174 | 8 | 48 | 12 | 4 | 6 | 1 | 1 | 3 | ||
| Gastropoda | AZO | MAD | SEL | CAN | CAB | MOR | MED |
| MIS 5e | 125 | 53 | 18 | 182 | 53 | 44 | 378 |
| Present-day | 225 | 333 | 156 | 699 | 528 | 164 | 1069 |
| MIS 5e/Present-day (%) | 55.6 | 15.9 | 11.5 | 26.0 | 10.0 | 26.8 | 35.4 |
| Bivalvia | AZO | MAD | SEL | CAN | CAB | MOR | MED |
| MIS 5e | 28 | 29 | 2 | 79 | 30 | 22 | 181 |
| Present-day | 150 | 135 | 39 | 233 | 130 | 287 | 374 |
| MIS 5e/Present-day (%) | 18.7 | 21.5 | 5.1 | 33.9 | 23.1 | 7.7 | 48.4 |
| Bivalve Species/Taxa | MIS 5e Geographic Range Expansion to: | SUBSTRATE | ||||
|---|---|---|---|---|---|---|
| Hard Grounds | Gravel, Pebbles | Coarse Sand | Fine Sand | Sandy Mud-Muddy Sand | ||
| Acar olivercoseli | CAN/MED | 1 | ||||
| Anadara geissei | MED | 1 | ||||
| Arcopsis afra | MED | 1 | ||||
| Brachidontes puniceus | CAN/MED | 1 | ||||
| Cardium sp. | MAD/CAN | 1 | 1 | |||
| Chlamys sp. | CAN | 1 | 1 | 1 | ||
| Codakia sp. | CAN | 1 | 1 | |||
| Crassatina contraria | MOR | |||||
| Ctena eburnea | CAN | 1 | 1 | |||
| Dendostrea cristata | MED | 1 | ||||
| Parvilucina crenella | MED | 1 | 1 | |||
| Saccostrea cuccullata | CAB | 1 | 1 | |||
| Gastropod Species/Taxa | MIS 5e Geographic Range Expansion to: | SUBSTRATE | ||||
|---|---|---|---|---|---|---|
| Hard Ground | Gravel, Pebbles | Coarse Sand | Fine Sand | Sandy Mud-Muddy Sand | ||
| Acliceratia carinata | MAD | |||||
| Acteon maltzani | MED | |||||
| Acteon senegalensis | MED | |||||
| Anachis aurantia | MOR | 1 | ||||
| Aporrhais senegalensis | MED | |||||
| Cassis sp. | CAN | 1 | 1 | 1 | ||
| Cheilea equestres | CAN | 1 | ||||
| Claremontiella nodulosa | AZO/CAN | 1 | ||||
| Conus sp. | MAD | 1 | ||||
| Conus ambiguus | AZO | 1 | 1 | |||
| Conus ermineus | AZO/CAN/MED | 1 | 1 | |||
| Conus guinaicus | CAN/MED | |||||
| Conus miruchae | AZO | |||||
| Conus roeckeli | AZO | |||||
| Conus venulatus | AZO | |||||
| Cumia intertexta | CAN | |||||
| Cymbium gracile | MOR | 1 | ||||
| Epitonium trochoides | MED | 1 | ||||
| Fasciolaria sp. | CAB | |||||
| Gemophos viverratus | AZO/MAD/MED | 1 | ||||
| Harpa doris | CAN | 1 | ||||
| Heliacus cylindricus | CAN | 1 | 1 | 1 | ||
| Hexaplex angularis | MOR | 1 | ||||
| Hexaplex rosarium | CAN/MED | 1 | ||||
| Imbricariopsis carbonacea | MED | |||||
| Inermicosta inermicosta | CAB | 1 | ||||
| Monoplex trigonus | MED | 1 | 1 | |||
| Nerita senegalensis | CAN | 1 | ||||
| Prunum olivaeforme | CAN | |||||
| Simnia senegalensis | MED | 1 | ||||
| Terebra corrugata | MED | 1 | ||||
| Thais nodosa | MAD/CAN | 1 | ||||
| Thetystrombus latus | CAN/MED | 1 | 1 | |||
| Thylacodes arenarius | CAN | |||||
| Turbonilla kerstinae | MED | |||||
| Turbonilla secernenda | MED | |||||
| Vaughtia gruveli | CAN | |||||
| Xenophora senegalensis | MED | 1 | ||||
| Zebina vítrea | MAD | |||||
| Zonaria angolensis | MED | 1 | ||||
| Zonaria petitiana | MED | 1 | ||||
| Zonaria picta | AZO | 1 | ||||
| Zonaria zonaria | CAN | 1 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ávila, S.P. Distributional Range Shifts Caused by Glacial–Interglacial Cycles: A Review on Timing, Main Processes, and Patterns of Late Pleistocene Marine Dispersal by Invertebrates in the NE Atlantic. J. Mar. Sci. Eng. 2025, 13, 2024. https://doi.org/10.3390/jmse13112024
Ávila SP. Distributional Range Shifts Caused by Glacial–Interglacial Cycles: A Review on Timing, Main Processes, and Patterns of Late Pleistocene Marine Dispersal by Invertebrates in the NE Atlantic. Journal of Marine Science and Engineering. 2025; 13(11):2024. https://doi.org/10.3390/jmse13112024
Chicago/Turabian StyleÁvila, Sérgio P. 2025. "Distributional Range Shifts Caused by Glacial–Interglacial Cycles: A Review on Timing, Main Processes, and Patterns of Late Pleistocene Marine Dispersal by Invertebrates in the NE Atlantic" Journal of Marine Science and Engineering 13, no. 11: 2024. https://doi.org/10.3390/jmse13112024
APA StyleÁvila, S. P. (2025). Distributional Range Shifts Caused by Glacial–Interglacial Cycles: A Review on Timing, Main Processes, and Patterns of Late Pleistocene Marine Dispersal by Invertebrates in the NE Atlantic. Journal of Marine Science and Engineering, 13(11), 2024. https://doi.org/10.3390/jmse13112024






