Iron Curtain Formation in Coastal Aquifers: Insights from Darcy-Scale Experiments and Reactive Transport Modelling
Abstract
1. Introduction
- (1)
- Multifaceted formation processes: The iron curtain develops through a combination of abiotic and biotic drivers, including groundwater flow, kinetic reactions, microbial activity, and long-term mineral transformations [17,18,19,20]. Previous studies often examined these factors in isolation, limiting holistic understanding of how physical transport and geochemical reactions interact [14].
- (2)
- Pore-scale dynamics: At the pore scale, chemical reactions can alter flow pathways by reducing pore throat size, clogging pore spaces, and increasing particle surface area [21]. These microscale changes are difficult to scale up, creating challenges when integrating across representative elementary volumes (REVs) and continuum models [22].
- (3)
- Permeability reduction: Precipitate growth and increasing surface area generate tortuous flow paths that reduce permeability [23,24]. Because surface area (the total exposed area of mineral grains available for reactions) governs reactivity, its dynamic evolution strongly influences the rates and distribution of precipitation processes [25]. For example, a larger surface area increases contact between particles, fluids, and solutes, thereby accelerating reaction rates.
- (4)
- Impact of solid-phase minerals: Changes in porous media properties depend on the distribution of precipitates [26]. Under complete pore clogging, effective porosity and thus specific yield can approach zero, eliminating the interconnected pore space needed for fluid flow [27]. Some isolated pores may still remain, contributing to total porosity but not to flow. Modelling these scenarios is challenging, as most reactive transport models (RTMs) impose a porosity threshold below which fluid flow is assumed to cease [28].
- (5)
- (6)
- Hydrological variability: Fluctuating water tables promote alternating oxic–anoxic conditions, which intensify redox cycling and lead to spatial heterogeneity in Fe distribution [32,33]. Current models rarely couple these hydrological dynamics with geochemical reactions, limiting predictive accuracy.
2. Laboratory Experiment
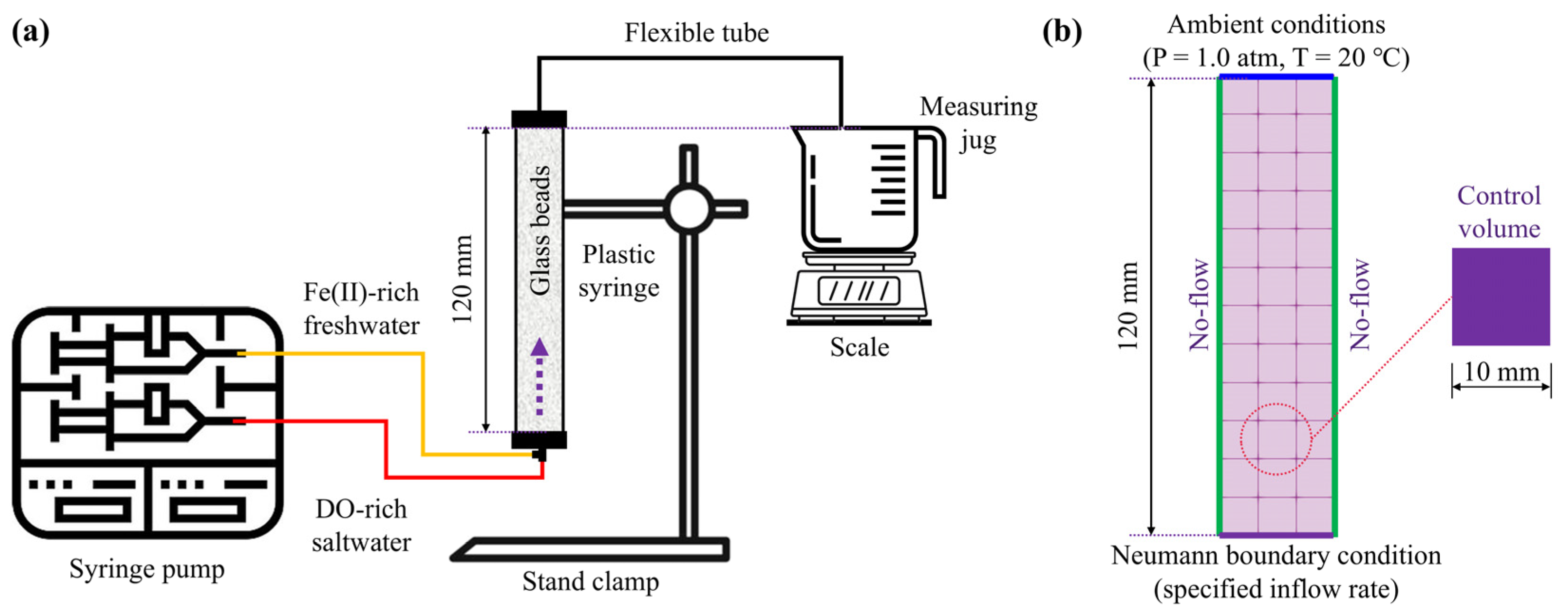
2.1. Apparatus and Settings
2.2. Solutions and Glass Beads
2.2.1. Freshwater and Saltwater
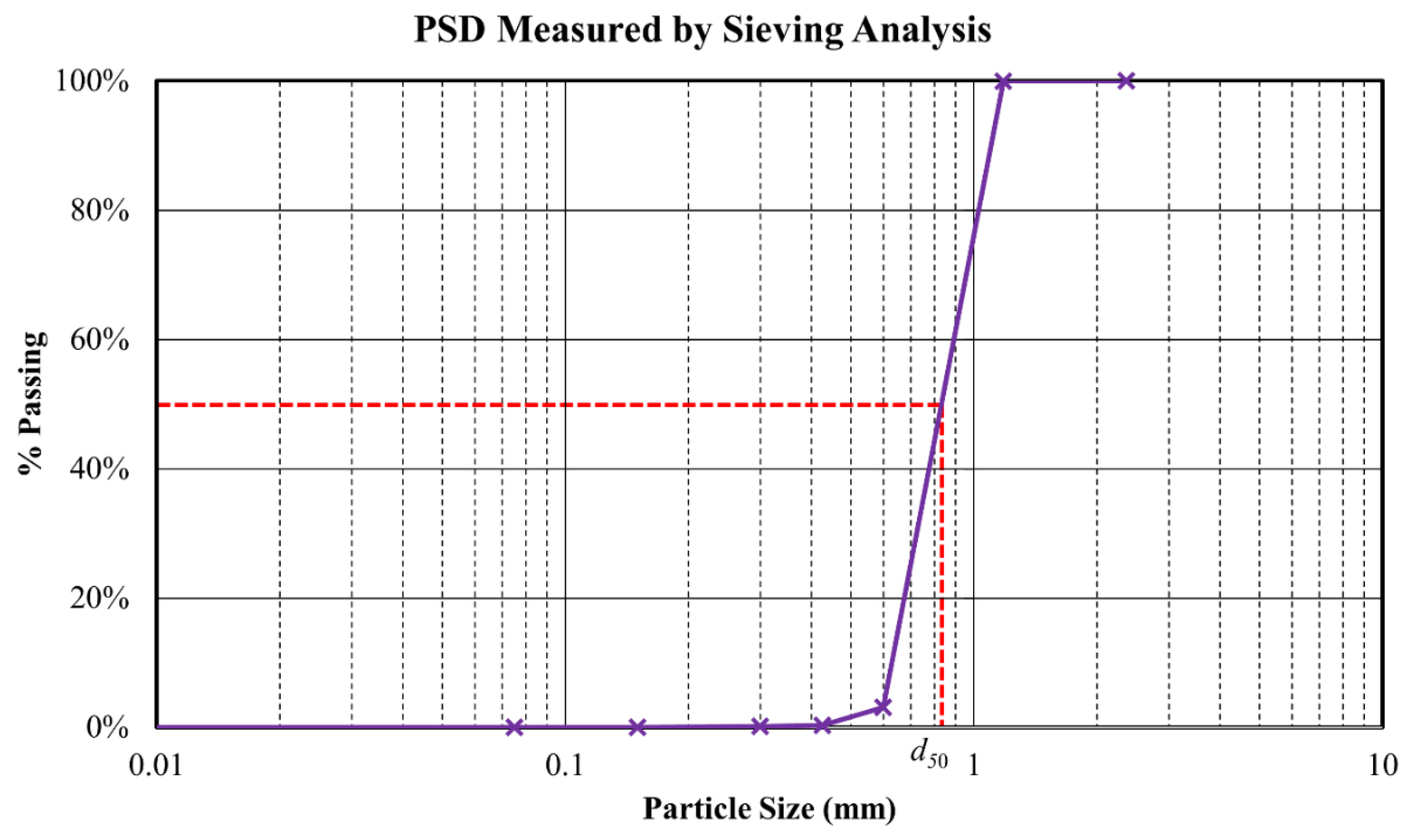
2.2.2. Glass Beads
2.3. Experimental Procedures
3. Numerical Modelling
3.1. Governing Equations and Theoretical Models
3.2. Chemical Reactions and Rate Laws
3.3. Boundary and Initial Conditions
3.4. Key Factors and Parametric Analysis
4. Experimental and Numerical Results
4.1. Temporal Change in Outflow Chemistry
- (1)
- Redox Potential (Eh): Measured in volts, Eh reflects the oxidation-reduction state of the solutions. Saltwater and Fe(II)-rich freshwater initially shared similar Eh values (~0.5 V), indicating comparable redox conditions.
- (2)
- pH Dynamics: Fe(II)-rich freshwater exhibited a significantly lower pH than saltwater, corresponding to a 40-fold increase in H+ activity. This acidity arises from the stability of Fe2+ and hydrolysis products (e.g., FeOH+), which release H+ ions.
- (3)
- Mixing Effects: When Fe(II)-rich freshwater mixed with oxygenated saltwater, Fe2+ oxidation occurred near the inlet. This process consumed H+ ions (e.g., via reactions in Equation (7)), raising the pH to 5.5–6.5 in the outflow.
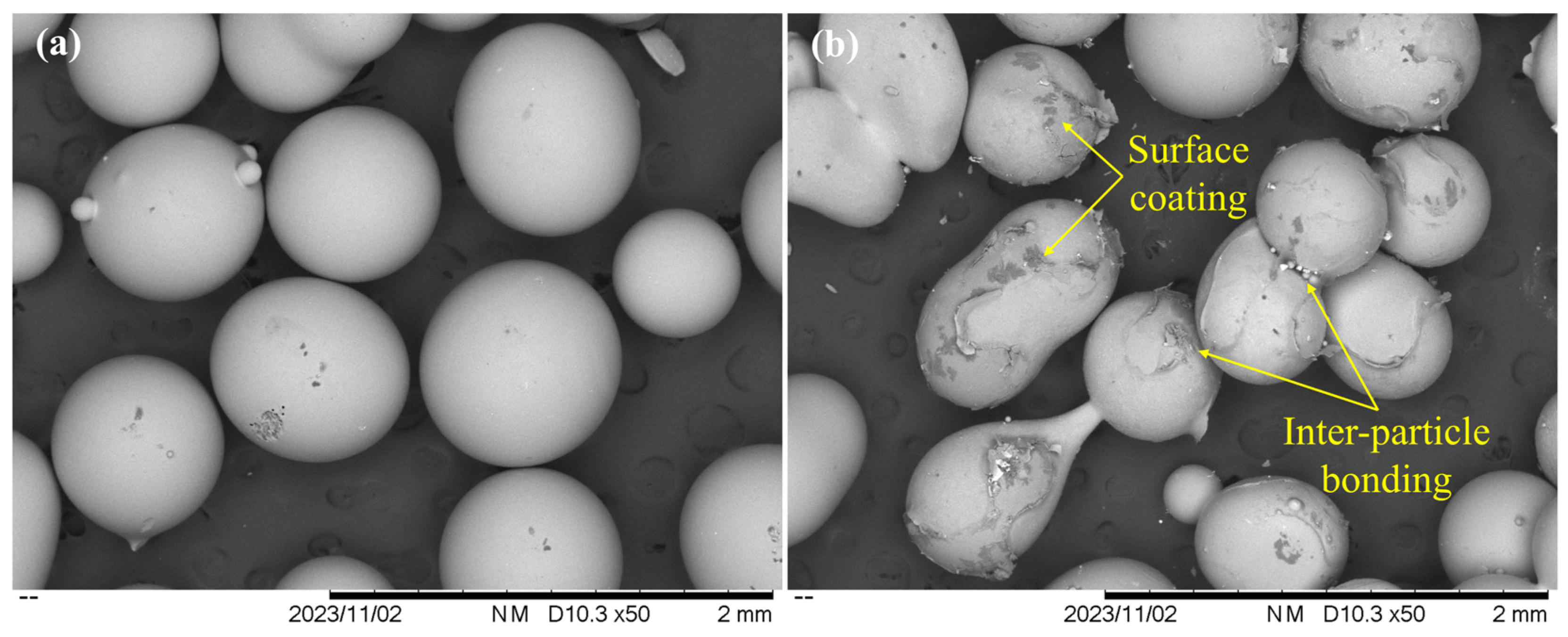
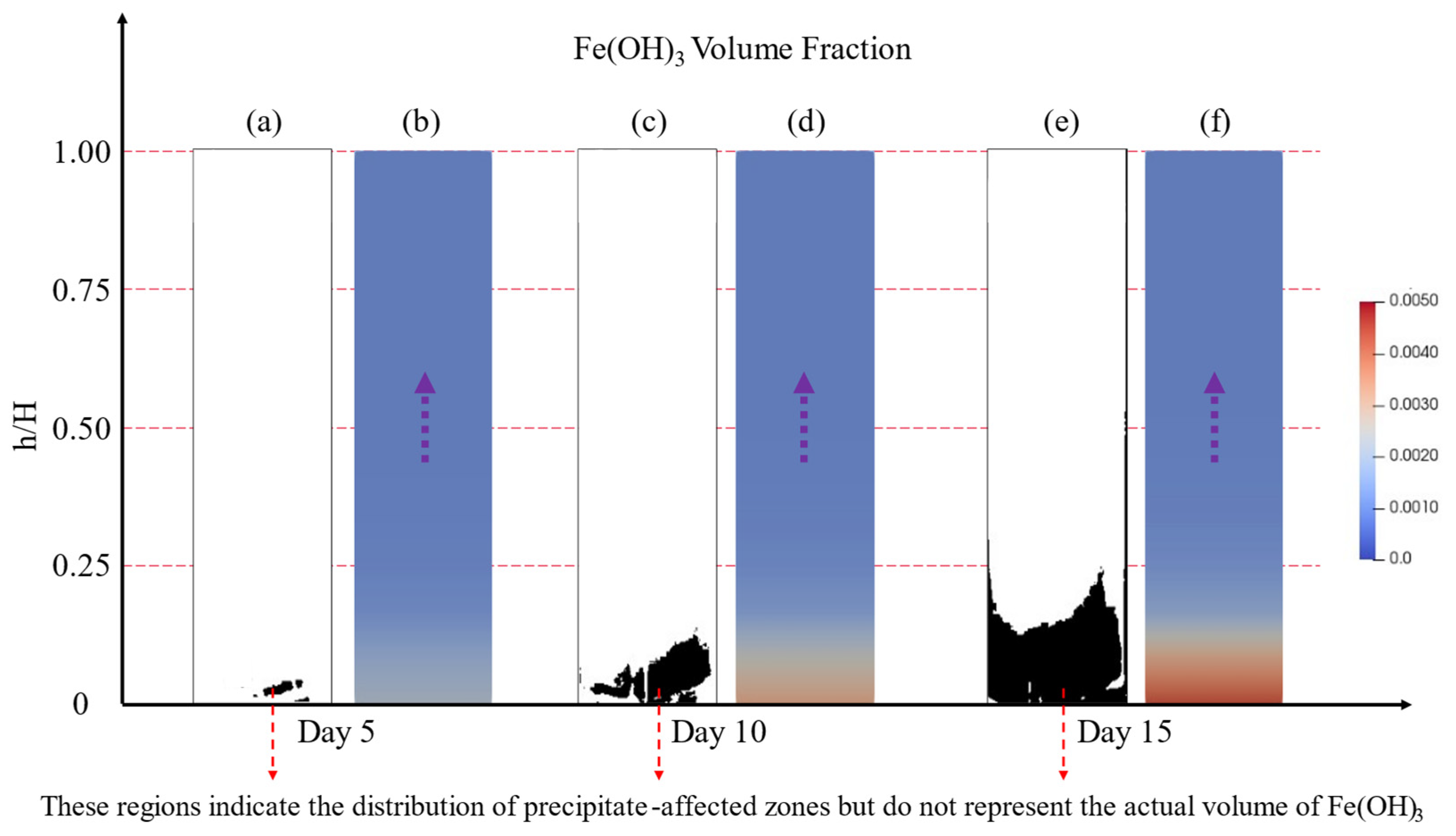
4.2. Spatial Variations in Pore Structure
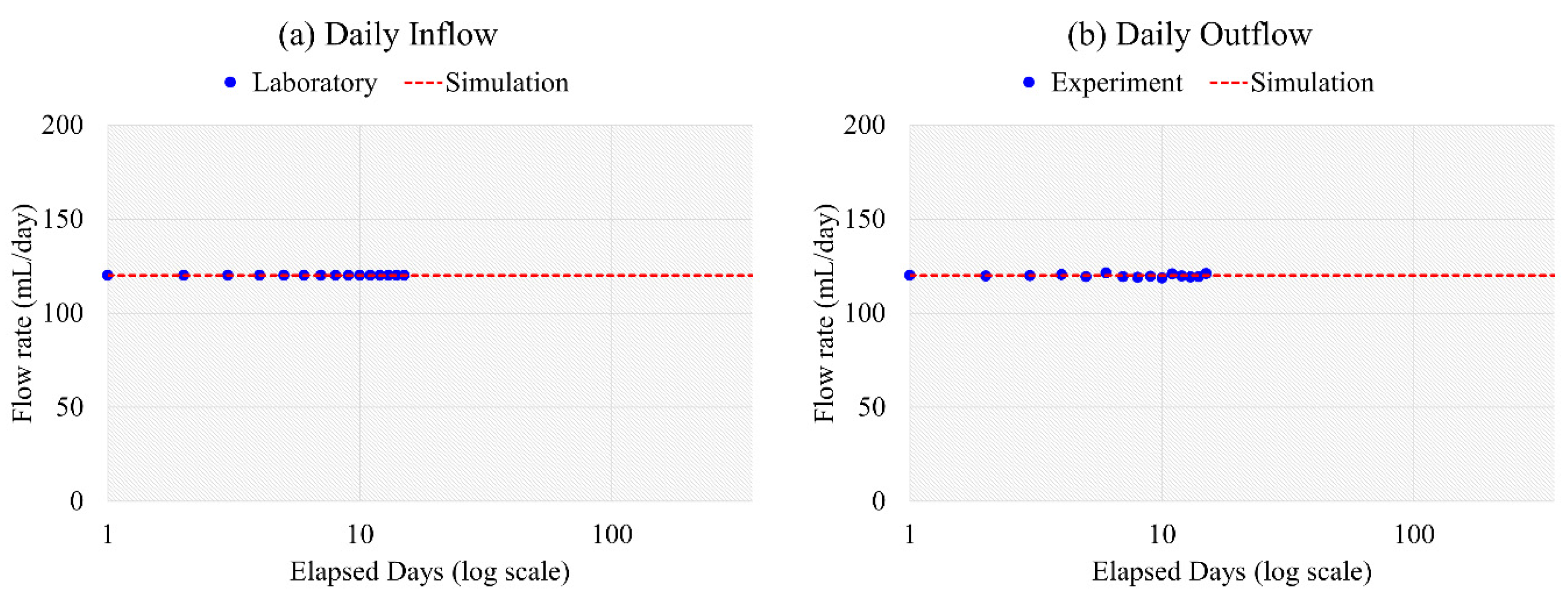
4.3. Model Calibration and Validation
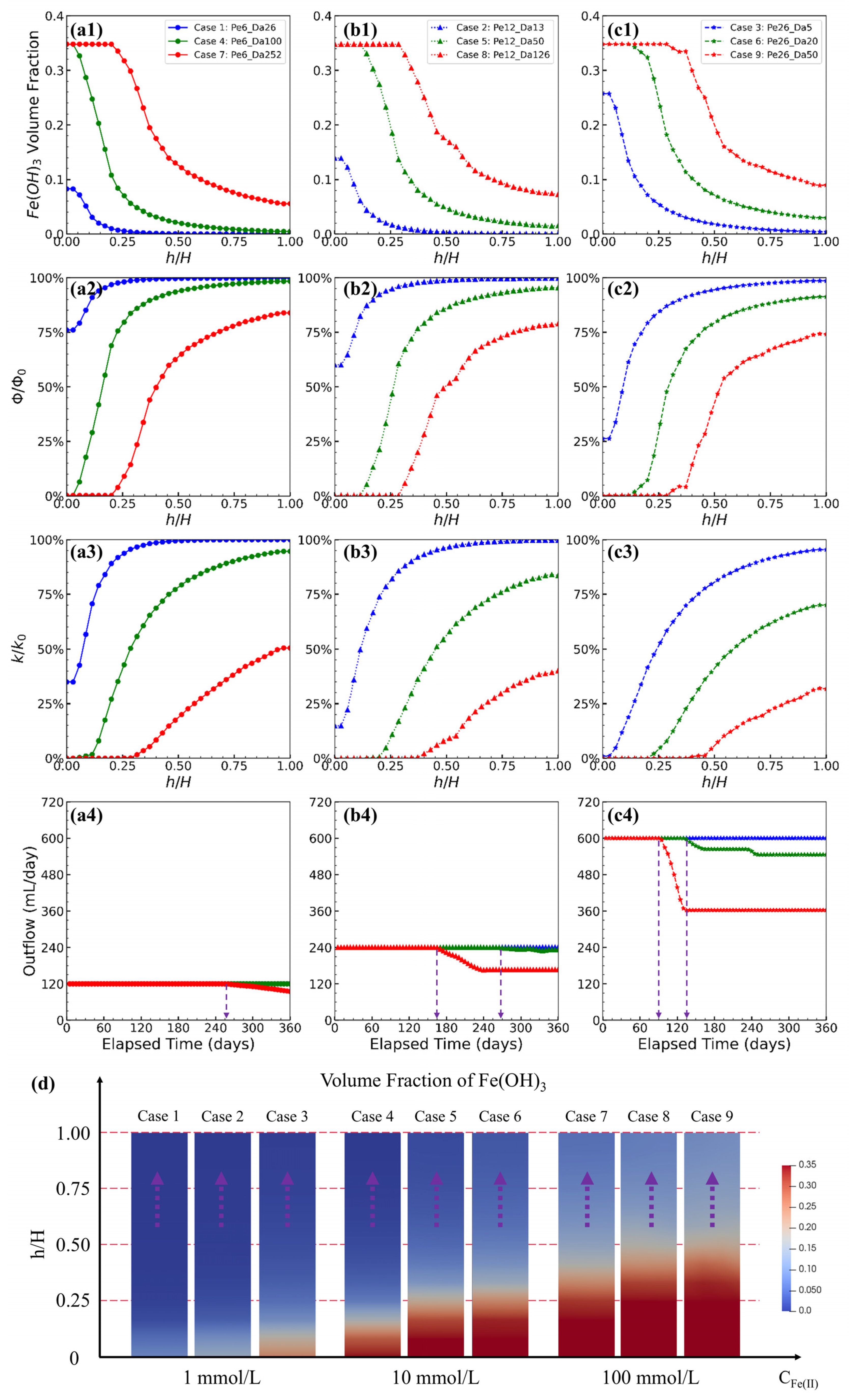
4.4. Pe Influence on Precipitation Front
4.5. Da Effect on Precipitation Patterns
5. Discussion and Implications
5.1. Dynamic Characteristics of Fe Oxides
5.2. Microbial and Auto-Catalytic Feedback
5.3. Upscaling and Practical Applications
5.4. Limitations and Future Directions
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Larrahondo, J.M.; Burns, S.E. Laboratory-Prepared Iron Oxide Coatings on Sands: Surface Characterization and Strength Parameters. J. Geotech. Geoenviron. Eng. 2014, 140, 04013052. [Google Scholar] [CrossRef]
- Viscarra Rossel, R.A.; Bui, E.N.; de Caritat, P.; McKenzie, N.J. Mapping Iron Oxides and the Color of Australian Soil Using Visible–near-Infrared Reflectance Spectra. J. Geophys. Res. 2010, 115, F04031. [Google Scholar] [CrossRef]
- Charette, M.A.; Allen, M.C. Precision Ground Water Sampling in Coastal Aquifers Using a Direct-Push, Shielded-Screen Well-Point System. Groundw. Monit. Remediat. 2006, 26, 87–93. [Google Scholar] [CrossRef]
- Spiteri, C.; Regnier, P.; Slomp, C.P.; Charette, M.A. pH-Dependent Iron Oxide Precipitation in a Subterranean Estuary. J. Geochem. Explor. 2006, 88, 399–403. [Google Scholar] [CrossRef]
- Lalonde, K.; Mucci, A.; Ouellet, A.; Gélinas, Y. Preservation of Organic Matter in Sediments Promoted by Iron. Nature 2012, 483, 198–200. [Google Scholar] [CrossRef] [PubMed]
- Linkhorst, A.; Dittmar, T.; Waska, H. Molecular Fractionation of Dissolved Organic Matter in a Shallow Subterranean Estuary: The Role of the Iron Curtain. Environ. Sci. Technol. 2017, 51, 1312–1320. [Google Scholar] [CrossRef]
- Zhang, D.; Guo, H.; Xiu, W.; Ni, P.; Zheng, H.; Wei, C. In-Situ Mobilization and Transformation of Iron Oxides-Adsorbed Arsenate in Natural Groundwater. J. Hazard. Mater. 2017, 321, 228–237. [Google Scholar] [CrossRef]
- Reckhardt, A.; Beck, M.; Seidel, M.; Riedel, T.; Wehrmann, A.; Bartholomä, A.; Schnetger, B.; Dittmar, T.; Brumsack, H.-J. Carbon, Nutrient and Trace Metal Cycling in Sandy Sediments: A Comparison of High-Energy Beaches and Backbarrier Tidal Flats. Estuar. Coast. Shelf Sci. 2015, 159, 1–14. [Google Scholar] [CrossRef]
- Charette, M.A.; Sholkovitz, E.R. Oxidative Precipitation of Groundwater-Derived Ferrous Iron in the Subterranean Estuary of a Coastal Bay. Geophys. Res. Lett. 2002, 29, 85-1–85-4. [Google Scholar] [CrossRef]
- Anwar, N.; Robinson, C.; Barry, D.A. Influence of Tides and Waves on the Fate of Nutrients in a Nearshore Aquifer: Numerical Simulations. Adv. Water Resour. 2014, 73, 203–213. [Google Scholar] [CrossRef]
- O’Connor, A.E.; Luek, J.L.; McIntosh, H.; Beck, A.J. Geochemistry of Redox-Sensitive Trace Elements in a Shallow Subterranean Estuary. Mar. Chem. 2015, 172, 70–81. [Google Scholar] [CrossRef]
- Paffrath, R.; Pahnke, K.; Behrens, M.K.; Reckhardt, A.; Ehlert, C.; Schnetger, B.; Brumsack, H.-J. Rare Earth Element Behavior in a Sandy Subterranean Estuary of the Southern North Sea. Front. Mar. Sci. 2020, 7, 424. [Google Scholar] [CrossRef]
- Porubsky, W.P.; Weston, N.B.; Moore, W.S.; Ruppel, C.; Joye, S.B. Dynamics of Submarine Groundwater Discharge and Associated Fluxes of Dissolved Nutrients, Carbon, and Trace Gases to the Coastal Zone (Okatee River Estuary, South Carolina). Geochim. Cosmochim. Acta 2014, 131, 81–97. [Google Scholar] [CrossRef]
- Robinson, C.E.; Xin, P.; Santos, I.R.; Charette, M.A.; Li, L.; Barry, D.A. Groundwater Dynamics in Subterranean Estuaries of Coastal Unconfined Aquifers: Controls on Submarine Groundwater Discharge and Chemical Inputs to the Ocean. Adv. Water Resour. 2018, 115, 315–331. [Google Scholar] [CrossRef]
- Roy, M.; Martin, J.B.; Cherrier, J.; Cable, J.E.; Smith, C.G. Influence of Sea Level Rise on Iron Diagenesis in an East Florida Subterranean Estuary. Geochim. Cosmochim. Acta 2010, 74, 5560–5573. [Google Scholar] [CrossRef]
- Trezzi, G.; Garcia-Orellana, J.; Rodellas, V.; Santos-Echeandia, J.; Tovar-Sánchez, A.; Garcia-Solsona, E.; Masqué, P. Submarine Groundwater Discharge: A Significant Source of Dissolved Trace Metals to the North Western Mediterranean Sea. Mar. Chem. 2016, 186, 90–100. [Google Scholar] [CrossRef]
- Houben, G.J.; Wachenhausen, J.; Guevara Morel, C.R. Effects of Ageing on the Hydraulics of Water Wells and the Influence of Non-Darcy Flow. Hydrogeol. J. 2018, 26, 1285–1294. [Google Scholar] [CrossRef]
- McAllister, S.M.; Barnett, J.M.; Heiss, J.W.; Findlay, A.J.; MacDonald, D.J.; Dow, C.L.; Luther, G.W.; Michael, H.A.; Chan, C.S. Dynamic Hydrologic and Biogeochemical Processes Drive Microbially Enhanced Iron and Sulfur Cycling within the Intertidal Mixing Zone of a Beach Aquifer: Fe and S Cycling in a Beach Aquifer. Limnol. Oceanogr. 2015, 60, 329–345. [Google Scholar] [CrossRef]
- Sevinç Şengör, S.; Spycher, N.F.; Ginn, T.R.; Sani, R.K.; Peyton, B. Biogeochemical Reactive–Diffusive Transport of Heavy Metals in Lake Coeur d’Alene Sediments. Appl. Geochem. 2007, 22, 2569–2594. [Google Scholar] [CrossRef]
- Shen, C.; Zhang, C.; Xin, P.; Kong, J.; Li, L. Salt Dynamics in Coastal Marshes: Formation of Hypersaline Zones. Water Resour. Res. 2018, 54, 3259–3276. [Google Scholar] [CrossRef]
- Stack, A.G. Precipitation in Pores: A Geochemical Frontier. Rev. Mineral. Geochem. 2015, 80, 165–190. [Google Scholar] [CrossRef]
- Seigneur, N.; Mayer, K.U.; Steefel, C.I. Reactive Transport in Evolving Porous Media. Rev. Mineral. Geochem. 2019, 85, 197–238. [Google Scholar] [CrossRef]
- Cao, W.; Hu, N.; Yan, G.; Hofmann, H.; Scheuermann, A. Permeability–Porosity Model Considering Oxidative Precipitation of Fe(II) in Granular Porous Media. J. Hydrol. 2024, 636, 131346. [Google Scholar] [CrossRef]
- Saripalli, K.P.; Serne, R.J.; Meyer, P.D.; McGrail, B.P. Prediction of Diffusion Coefficients in Porous Media Using Tortuosity Factors Based on Interfacial Areas. Groundwater 2002, 40, 346–352. [Google Scholar] [CrossRef]
- Soulaine, C.; Tchelepi, H.A. Micro-Continuum Approach for Pore-Scale Simulation of Subsurface Processes. Transp. Porous Media 2016, 113, 431–456. [Google Scholar] [CrossRef]
- Tenthorey, E.; Fitzgerald, J. Feedbacks between Deformation, Hydrothermal Reaction and Permeability Evolution in the Crust: Experimental Insights. Earth Planet. Sci. Lett. 2006, 247, 117–129. [Google Scholar] [CrossRef]
- Hommel, J.; Coltman, E.; Class, H. Porosity–Permeability Relations for Evolving Pore Space: A Review with a Focus on (Bio-)Geochemically Altered Porous Media. Transp. Porous Media 2018, 124, 589–629. [Google Scholar] [CrossRef]
- Xie, M.; Mayer, K.U.; Claret, F.; Alt-Epping, P.; Jacques, D.; Steefel, C.; Chiaberge, C.; Simunek, J. Implementation and Evaluation of Permeability-Porosity and Tortuosity-Porosity Relationships Linked to Mineral Dissolution-Precipitation. Comput. Geosci. 2015, 19, 655–671. [Google Scholar] [CrossRef]
- Amoako, K.; Saban, R.C.; Böttcher, M.E.; Waska, H. Sources and Processing of Dissolved Organic Matter in a Microtidal Subterranean Estuary. Estuaries Coasts 2025, 48, 171. [Google Scholar] [CrossRef]
- Peng, K.; Yan, L.; Xie, X.; Deng, Y.; Gan, Y.; Li, Q.; Zhang, Y.; Tang, X. Quantifying the Fate of Biogenic Elements in Mangrove Aquifers: Insights from Reactive Transport Modeling under Saltwater-Freshwater Mixing. Water Res. 2025, 278, 123381. [Google Scholar] [CrossRef] [PubMed]
- Caschetto, M.; Colombani, N.; Mastrocicco, M.; Petitta, M.; Aravena, R. Nitrogen and Sulphur Cycling in the Saline Coastal Aquifer of Ferrara, Italy. A Multi-Isotope Approach. Appl. Geochem. 2017, 76, 88–98. [Google Scholar] [CrossRef]
- Peng, K.; Yan, L.; Xie, X.; Deng, Y.; Gan, Y.; Zhang, Y. Hydrogeochemical Dynamics under Saltwater-Freshwater Mixing in a Mangrove Wetland over Tidal Cycles. Sci. Total Environ. 2024, 954, 176827. [Google Scholar] [CrossRef] [PubMed]
- Heiss, J.W.; Post, V.E.A.; Laattoe, T.; Russoniello, C.J.; Michael, H.A. Physical Controls on Biogeochemical Processes in Intertidal Zones of Beach Aquifers. Water Resour. Res. 2017, 53, 9225–9244. [Google Scholar] [CrossRef]
- Banzhaf, S.; Hebig, K.H. Use of Column Experiments to Investigate the Fate of Organic Micropollutants—A Review. Hydrol. Earth Syst. Sci. 2016, 20, 3719–3737. [Google Scholar] [CrossRef]
- Pavuluri, S.; Tournassat, C.; Claret, F.; Soulaine, C. Reactive Transport Modeling with a Coupled OpenFOAM®-PHREEQC Platform. Transp. Porous Media 2022, 145, 475–504. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhang, C.; Cao, W.; Hofmann, H.; Wang, T.; Li, L. Oxidative Precipitation of Fe(II) in Porous Media: Laboratory Experiment and Numerical Simulation. ACS EST Water 2023, 3, 963–973. [Google Scholar] [CrossRef]
- Soulaine, C.; Pavuluri, S.; Claret, F.; Tournassat, C. porousMedia4Foam: Multi-Scale Open-Source Platform for Hydro-Geochemical Simulations with OpenFOAM®. Environ. Model. Softw. 2021, 145, 105199. [Google Scholar] [CrossRef]
- Cao, W.; Yan, G.; Hofmann, H.; Scheuermann, A. A Novel Permeability–Tortuosity–Porosity Model for Evolving Pore Space and Mineral-Induced Clogging in Porous Medium. Geotechnics 2025, 5, 2. [Google Scholar] [CrossRef]
- Cao, W.; Yan, G.; Hofmann, H.; Scheuermann, A. A State-of-the-Art Perspective on Fe Precipitation in Porous Media: Hydrogeochemical Processes and Evolving Parameters. J. Mar. Sci. Eng. 2024, 12, 690. [Google Scholar] [CrossRef]
- Archie, G.E. The Electrical Resistivity Log as an Aid in Determining Some Reservoir Characteristics. Trans. AIME 1942, 146, 54–62. [Google Scholar] [CrossRef]
- Alhama, I.; García-Ros, G.; Icardi, M. Non-Stationary Contaminant Plumes in the Advective-Diffusive Regime. Mathematics 2021, 9, 725. [Google Scholar] [CrossRef]
- Van Der Grift, B.; Rozemeijer, J.C.; Griffioen, J.; Van Der Velde, Y. Iron Oxidation Kinetics and Phosphate Immobilization along the Flow-Path from Groundwater into Surface Water. Hydrol. Earth Syst. Sci. 2014, 18, 4687–4702. [Google Scholar] [CrossRef]
- Poonoosamy, J.; Soulaine, C.; Burmeister, A.; Deissmann, G.; Bosbach, D.; Roman, S. Microfluidic Flow-through Reactor and 3D Raman Imaging for in Situ Assessment of Mineral Reactivity in Porous and Fractured Porous Media. Lab. Chip 2020, 20, 2562–2571. [Google Scholar] [CrossRef]
- Cudennec, Y.; Lecerf, A. The Transformation of Ferrihydrite into Goethite or Hematite, Revisited. J. Solid State Chem. 2006, 179, 716–722. [Google Scholar] [CrossRef]
- Houben, G.J. Iron Oxide Incrustations in Wells. Part 1: Genesis, Mineralogy and Geochemistry. Appl. Geochem. 2003, 18, 927–939. [Google Scholar] [CrossRef]
- Algive, L.; Bekri, S.; Vizika, O. Pore-Network Modeling Dedicated to the Determination of the Petrophysical-Property Changes in the Presence of Reactive Fluid. SPE J. 2010, 15, 618–633. [Google Scholar] [CrossRef]
- Auffan, M.; Rose, J.; Bottero, J.-Y.; Lowry, G.V.; Jolivet, J.-P.; Wiesner, M.R. Towards a Definition of Inorganic Nanoparticles from an Environmental, Health and Safety Perspective. Nat. Nanotechnol. 2009, 4, 634–641. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Lee, B.; Bell, C.; Jun, Y.-S. Environmentally Abundant Anions Influence the Nucleation, Growth, Ostwald Ripening, and Aggregation of Hydrous Fe(III) Oxides. Langmuir 2012, 28, 7737–7746. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Axe, L. Synthesis and Characterization of Iron Oxide-Coated Silica and Its Effect on Metal Adsorption. J. Colloid Interface Sci. 2005, 282, 11–19. [Google Scholar] [CrossRef]
- Yan, G.; Cheng, Z.; Ma, Y.; Scheuermann, A.; Li, L. Applying Imaging Technique to Investigate Effects of Solute Concentrations and Gas Injection Rates on Gas Bubble Generation. Geofluids 2022, 2022, 1–26. [Google Scholar] [CrossRef]
- Hiemstra, T.; Mendez, J.C.; Li, J. Evolution of the Reactive Surface Area of Ferrihydrite: Time, pH, and Temperature Dependency of Growth by Ostwald Ripening. Environ. Sci. Nano 2019, 6, 820–833. [Google Scholar] [CrossRef]
- Cao, W.; Hofmann, H.; Yan, G.; Scheuermann, A. Porewater Exchange and Iron Transformation in a Coastal Groundwater System: A Field Investigation, Driving Mechanisms Analysis, and Conceptual Model. Front. Mar. Sci.—Sec Coast. Ocean Process. 2024, 11, 1385517. [Google Scholar] [CrossRef]
- Wu, Z.; Wang, S.; Ji, N. Distribution Character of Localized Iron Microniche in Lake Sediment Microzone Revealed by Chemical Image. Environ. Sci. Pollut. Res. 2019, 26, 35704–35716. [Google Scholar] [CrossRef]
- Baker, I.R.; Matzen, S.L.; Schuler, C.J.; Toner, B.M.; Girguis, P.R. Aerobic Iron-Oxidizing Bacteria Secrete Metabolites That Markedly Impede Abiotic Iron Oxidation. PNAS Nexus 2023, 2, pgad421. [Google Scholar] [CrossRef] [PubMed]
- Kügler, S.; Cooper, R.E.; Wegner, C.-E.; Mohr, J.F.; Wichard, T.; Küsel, K. Iron-Organic Matter Complexes Accelerate Microbial Iron Cycling in an Iron-Rich Fen. Sci. Total Environ. 2019, 646, 972–988. [Google Scholar] [CrossRef]
- Zhou, S.; Chen, S.; Yuan, Y.; Lu, Q. Influence of Humic Acid Complexation with Metal Ions on Extracellular Electron Transfer Activity. Sci. Rep. 2015, 5, 17067. [Google Scholar] [CrossRef]
- Sukekava, C.F.; Downes, J.; Filella, M.; Vilanova, B.; Laglera, L.M. Ligand Exchange Provides New Insight into the Role of Humic Substances in the Marine Iron Cycle. Geochim. Cosmochim. Acta 2024, 366, 17–30. [Google Scholar] [CrossRef]
- Costa, O.Y.A.; Raaijmakers, J.M.; Kuramae, E.E. Microbial Extracellular Polymeric Substances: Ecological Function and Impact on Soil Aggregation. Front. Microbiol. 2018, 9, 1636. [Google Scholar] [CrossRef]
- Harrison, A.L.; Dipple, G.M.; Power, I.M.; Mayer, K.U. The Impact of Evolving Mineral–Water–Gas Interfacial Areas on Mineral–Fluid Reaction Rates in Unsaturated Porous Media. Chem. Geol. 2016, 421, 65–80. [Google Scholar] [CrossRef]
- Jiang, Z.; Shi, M.; Shi, L. Degradation of Organic Contaminants and Steel Corrosion by the Dissimilatory Metal-Reducing Microorganisms Shewanella and Geobacter spp. Int. Biodeterior. Biodegrad. 2020, 147, 104842. [Google Scholar] [CrossRef]
- Zhong, H.; Lyu, H.; Wang, Z.; Tian, J.; Wu, Z. Application of Dissimilatory Iron-Reducing Bacteria for the Remediation of Soil and Water Polluted with Chlorinated Organic Compounds: Progress, Mechanisms, and Directions. Chemosphere 2024, 352, 141505. [Google Scholar] [CrossRef]
- Benaiges-Fernandez, R.; Palau, J.; Offeddu, F.G.; Cama, J.; Urmeneta, J.; Soler, J.M.; Dold, B. Dissimilatory Bioreduction of Iron(III) Oxides by Shewanella Loihica under Marine Sediment Conditions. Mar. Environ. Res. 2019, 151, 104782. [Google Scholar] [CrossRef]
- Brodersen, K.E.; Trevathan-Tackett, S.M.; Nielsen, D.A.; Connolly, R.M.; Lovelock, C.E.; Atwood, T.B.; Macreadie, P.I. Oxygen Consumption and Sulfate Reduction in Vegetated Coastal Habitats: Effects of Physical Disturbance. Front. Mar. Sci. 2019, 6, 14. [Google Scholar] [CrossRef]
- Antler, G.; Mills, J.V.; Hutchings, A.M.; Redeker, K.R.; Turchyn, A.V. The Sedimentary Carbon-Sulfur-Iron Interplay—A Lesson From East Anglian Salt Marsh Sediments. Front. Earth Sci. 2019, 7, 140. [Google Scholar] [CrossRef]
- Beam, J.P.; George, S.; Record, N.R.; Countway, P.D.; Johnston, D.T.; Girguis, P.R.; Emerson, D. Mud, Microbes, and Macrofauna: Seasonal Dynamics of the Iron Biogeochemical Cycle in an Intertidal Mudflat. Front. Mar. Sci. 2020, 7, 562617. [Google Scholar] [CrossRef]
- Snyder, M.; Taillefert, M.; Ruppel, C. Redox Zonation at the Saline-Influenced Boundaries of a Permeable Surficial Aquifer: Effects of Physical Forcing on the Biogeochemical Cycling of Iron and Manganese. J. Hydrol. 2004, 296, 164–178. [Google Scholar] [CrossRef]
- Alotaibi, M.D.; Patterson, B.M.; McKinley, A.J.; Reeder, A.Y.; Furness, A.J.; Donn, M.J. Fate of Benzotriazole and 5-Methylbenzotriazole in Recycled Water Recharged into an Anaerobic Aquifer: Column Studies. Water Res. 2015, 70, 184–195. [Google Scholar] [CrossRef] [PubMed]
- Luef, B.; Fakra, S.C.; Csencsits, R.; Wrighton, K.C.; Williams, K.H.; Wilkins, M.J.; Downing, K.H.; Long, P.E.; Comolli, L.R.; Banfield, J.F. Iron-Reducing Bacteria Accumulate Ferric Oxyhydroxide Nanoparticle Aggregates That May Support Planktonic Growth. ISME J. 2013, 7, 338–350. [Google Scholar] [CrossRef]
- Jeen, S.-W.; Mayer, K.U.; Gillham, R.W.; Blowes, D.W. Reactive Transport Modeling of Trichloroethene Treatment with Declining Reactivity of Iron. Environ. Sci. Technol. 2007, 41, 1432–1438. [Google Scholar] [CrossRef]
- Osorio-Leon, I.; Bouchez, C.; Chatton, E.; Lavenant, N.; Longuevergne, L.; Le Borgne, T. Hydrological and Geological Controls for the Depth Distribution of Dissolved Oxygen and Iron in Silicate Catchments. Water Resour. Res. 2023, 59, e2023WR034986. [Google Scholar] [CrossRef]
- Fitzsimmons, J.N.; Conway, T.M. Novel Insights into Marine Iron Biogeochemistry from Iron Isotopes. Annu. Rev. Mar. Sci. 2023, 15, 383–406. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhu, X.; Zhang, R.; Ren, J.; Wu, Y.; Liu, S.; Huang, D. Dissolved Fe in the East China Sea Under the Influences of Land Sources and the Boundary Current With Implications for Global Marginal Seas. Glob. Biogeochem. Cycles 2022, 36, e2021GB006946. [Google Scholar] [CrossRef]
- Steefel, C.I.; Appelo, C.A.J.; Arora, B.; Jacques, D.; Kalbacher, T.; Kolditz, O.; Lagneau, V.; Lichtner, P.C.; Mayer, K.U.; Meeussen, J.C.L.; et al. Reactive Transport Codes for Subsurface Environmental Simulation. Comput. Geosci. 2015, 19, 445–478. [Google Scholar] [CrossRef]
- Ladd, A.J.C.; Szymczak, P. Reactive Flows in Porous Media: Challenges in Theoretical and Numerical Methods. Annu. Rev. Chem. Biomol. Eng. 2021, 12, 543–571. [Google Scholar] [CrossRef] [PubMed]



| Category | Parameter | Value | Units |
|---|---|---|---|
| Porous media properties (Glass beads) | Dry density (ρd) | 1600 [a] | kg/m3 |
| Initial porosity (ϕ0) | 0.35 [a] | - | |
| Initial permeability (k0) | 7.0 × 10−11 [a] | m2 | |
| Median particle size (d50) | 0.83 [a] | mm | |
| Freshwater properties (Containing Fe(II)) | Fe(II) concentration (CFe(II)) | 0.001 | mol/L |
| Density (ρfw) | 1000 | kg/m3 | |
| pH | 4.0 [a] | - | |
| Saltwater properties (Containing DO) | DO concentration (CDO) | 2.25 × 10−4 [b] | mol/L |
| Density (ρsw) | 1025 | kg/m3 | |
| pH | 8.0 [a] | - | |
| Injection scheme and transport properties | Volumetric influx (Q) | 0.12 | L/day |
| Pore velocity (v = Q/Aϕ0) | 4.78 × 10−6 | m/s | |
| Vfw/Vsw [c] | 1.0 | - | |
| Duration (t) | 15 | day | |
| Diffusion coefficient (D0) | 1.0 × 10−9 [d] | m2/s | |
| Dispersion coefficient (αL) | 1.0 × 10−5 [d] | m | |
| Reaction rates and mineral parameters | Fe(II) oxidation rate constant (kox) | 2.46 × 1014 [d] | L3/mol3/s |
| Fe precipitation rate constant (kpr) | 5.0 × 10−6 [d] | mol/m2/s | |
| Mineral density (ρs) | 4370 [d] | kg/m3 | |
| Molar volume (Vm) | 0.024 [d] | L/mol | |
| Initial reactive surface area (S0) | 1.0 [d] | m−1 |
| Item | Case ID | Injection Scheme (mL/day) | Hydrochemistry (mmol/L) | Variables | ||||
|---|---|---|---|---|---|---|---|---|
| Vsw | Vfw | CFe(II) | CDO | pH * | Pe | Da (×10−2) | ||
| 1 | Fe1_Pe6_Da26 | 60 | 60 | 1 | 0.225 | 4.1 | 6 | 26 |
| 2 | Fe1_Pe12_Da13 | 120 | 120 | 1 | 0.225 | 4.1 | 12 | 13 |
| 3 | Fe1_Pe26_Da5 | 300 | 300 | 1 | 0.225 | 4.1 | 26 | 5 |
| 4 | Fe10_Pe6_Da100 | 60 | 60 | 10 | 0.225 | 3.7 | 6 | 100 |
| 5 | Fe10_Pe12_Da50 | 120 | 120 | 10 | 0.225 | 3.7 | 12 | 50 |
| 6 | Fe10_Pe26_Da20 | 300 | 300 | 10 | 0.225 | 3.7 | 26 | 20 |
| 7 | Fe100_Pe6_Da252 | 60 | 60 | 100 | 0.225 | 3.1 | 6 | 252 |
| 8 | Fe100_Pe12_Da126 | 120 | 120 | 100 | 0.225 | 3.1 | 12 | 126 |
| 9 | Fe100_Pe26_Da50 | 300 | 300 | 100 | 0.225 | 3.1 | 26 | 50 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, W.; Hofmann, H.; Scheuermann, A. Iron Curtain Formation in Coastal Aquifers: Insights from Darcy-Scale Experiments and Reactive Transport Modelling. J. Mar. Sci. Eng. 2025, 13, 1909. https://doi.org/10.3390/jmse13101909
Cao W, Hofmann H, Scheuermann A. Iron Curtain Formation in Coastal Aquifers: Insights from Darcy-Scale Experiments and Reactive Transport Modelling. Journal of Marine Science and Engineering. 2025; 13(10):1909. https://doi.org/10.3390/jmse13101909
Chicago/Turabian StyleCao, Wenran, Harald Hofmann, and Alexander Scheuermann. 2025. "Iron Curtain Formation in Coastal Aquifers: Insights from Darcy-Scale Experiments and Reactive Transport Modelling" Journal of Marine Science and Engineering 13, no. 10: 1909. https://doi.org/10.3390/jmse13101909
APA StyleCao, W., Hofmann, H., & Scheuermann, A. (2025). Iron Curtain Formation in Coastal Aquifers: Insights from Darcy-Scale Experiments and Reactive Transport Modelling. Journal of Marine Science and Engineering, 13(10), 1909. https://doi.org/10.3390/jmse13101909






