Assessment of the Ecotoxicity of Marine Sediments from the Eastern Kamchatka Using Bioassays
Abstract
1. Introduction
2. Materials and Methods
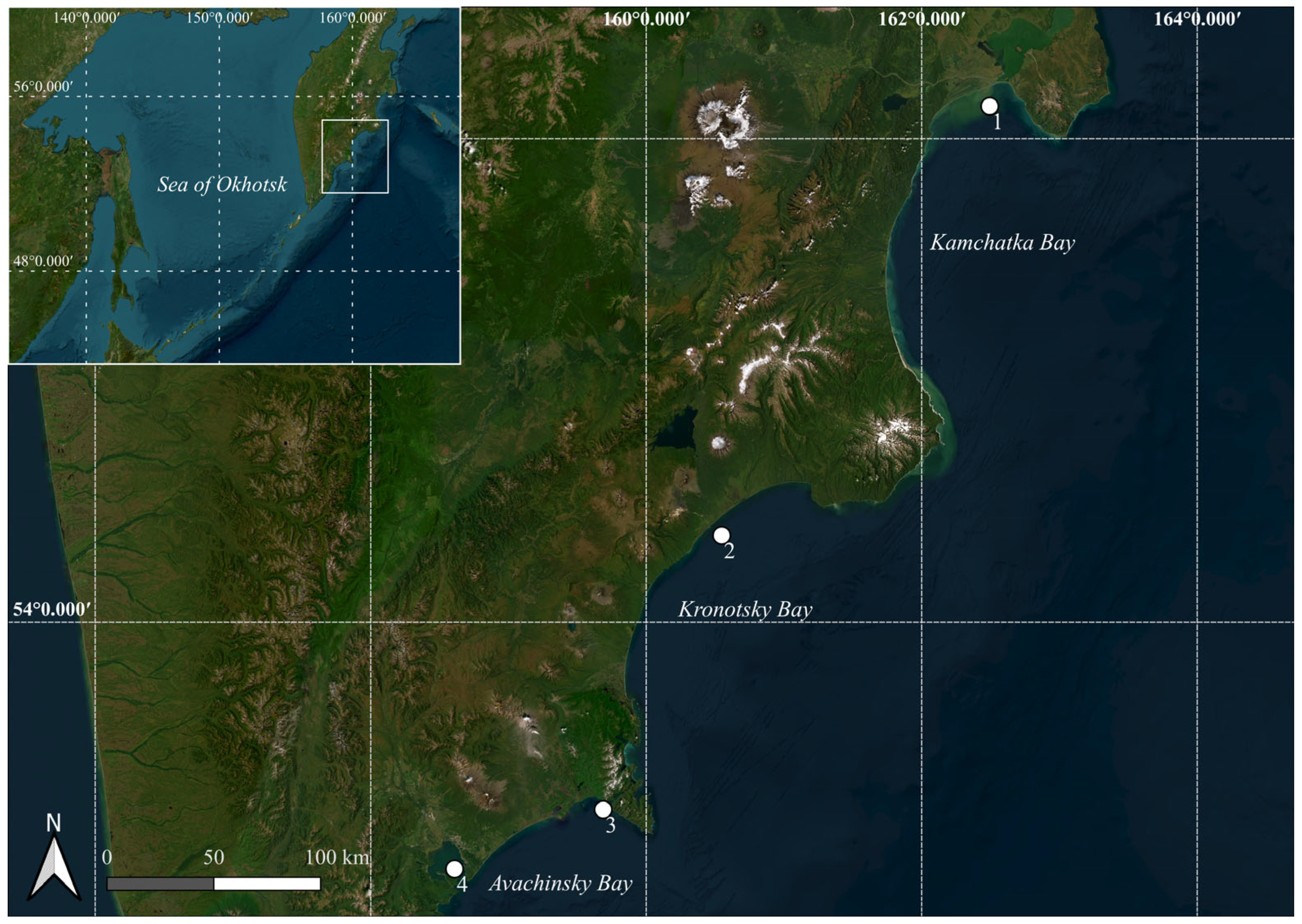
2.1. Sampling
2.2. Biotest Conditions
2.3. Statistical Analysis
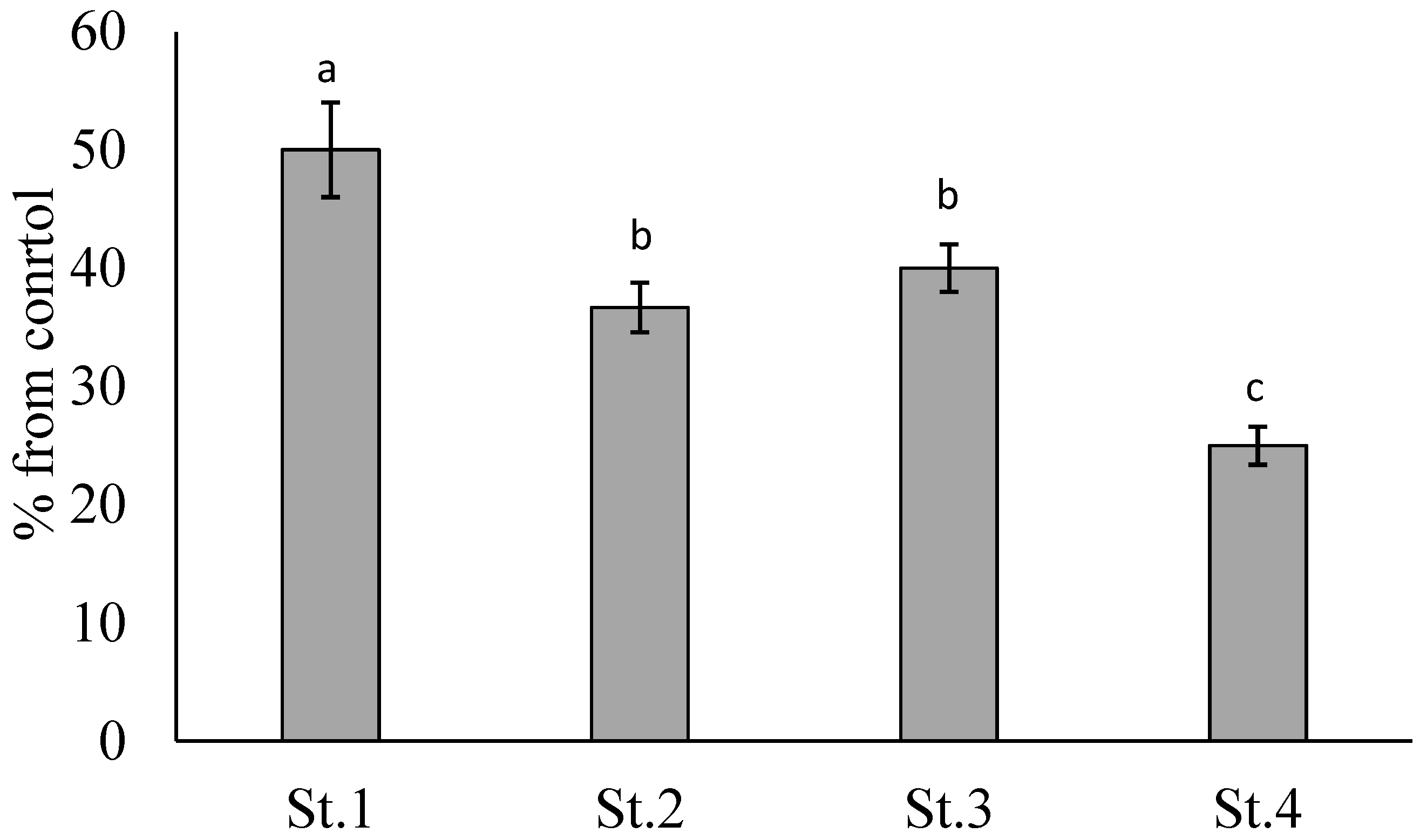
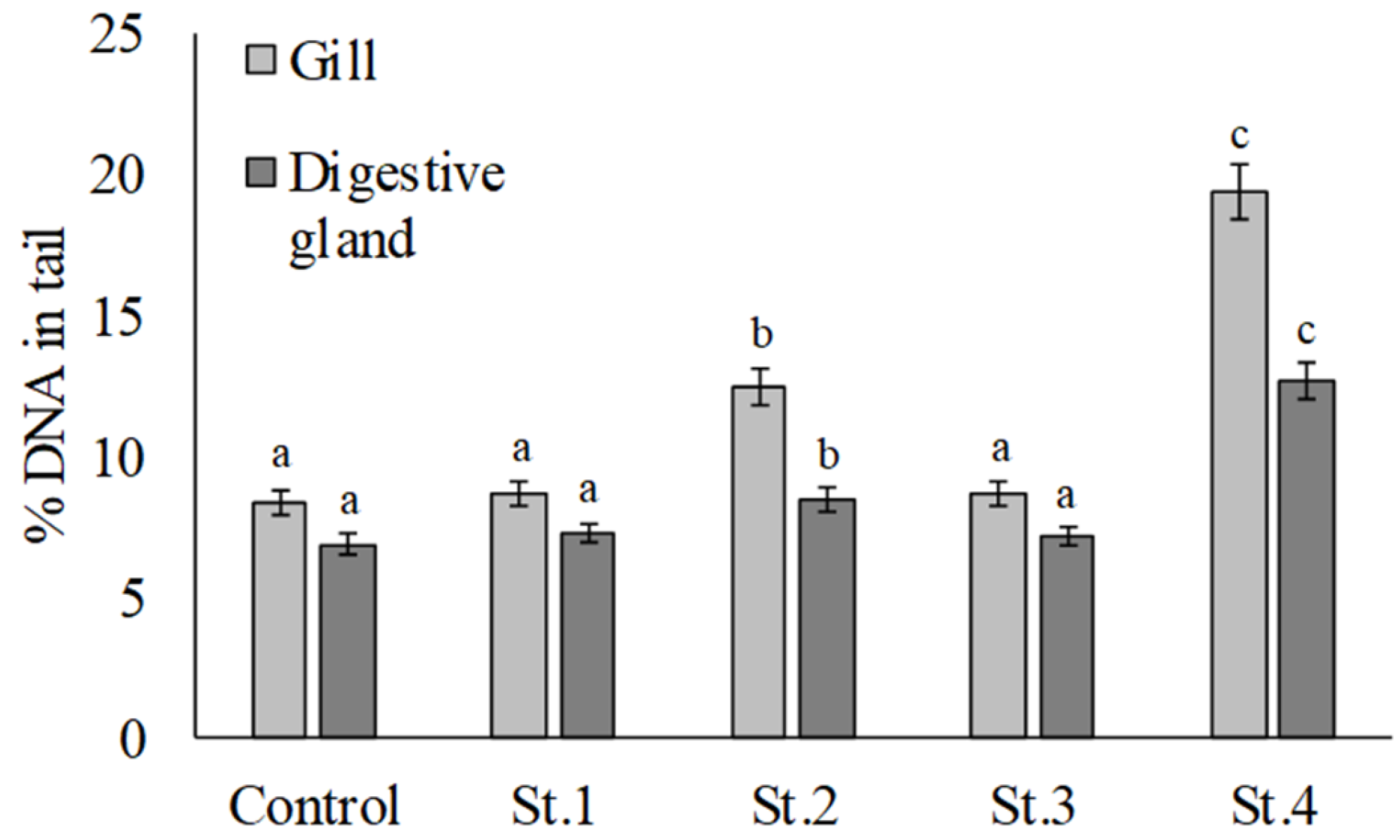
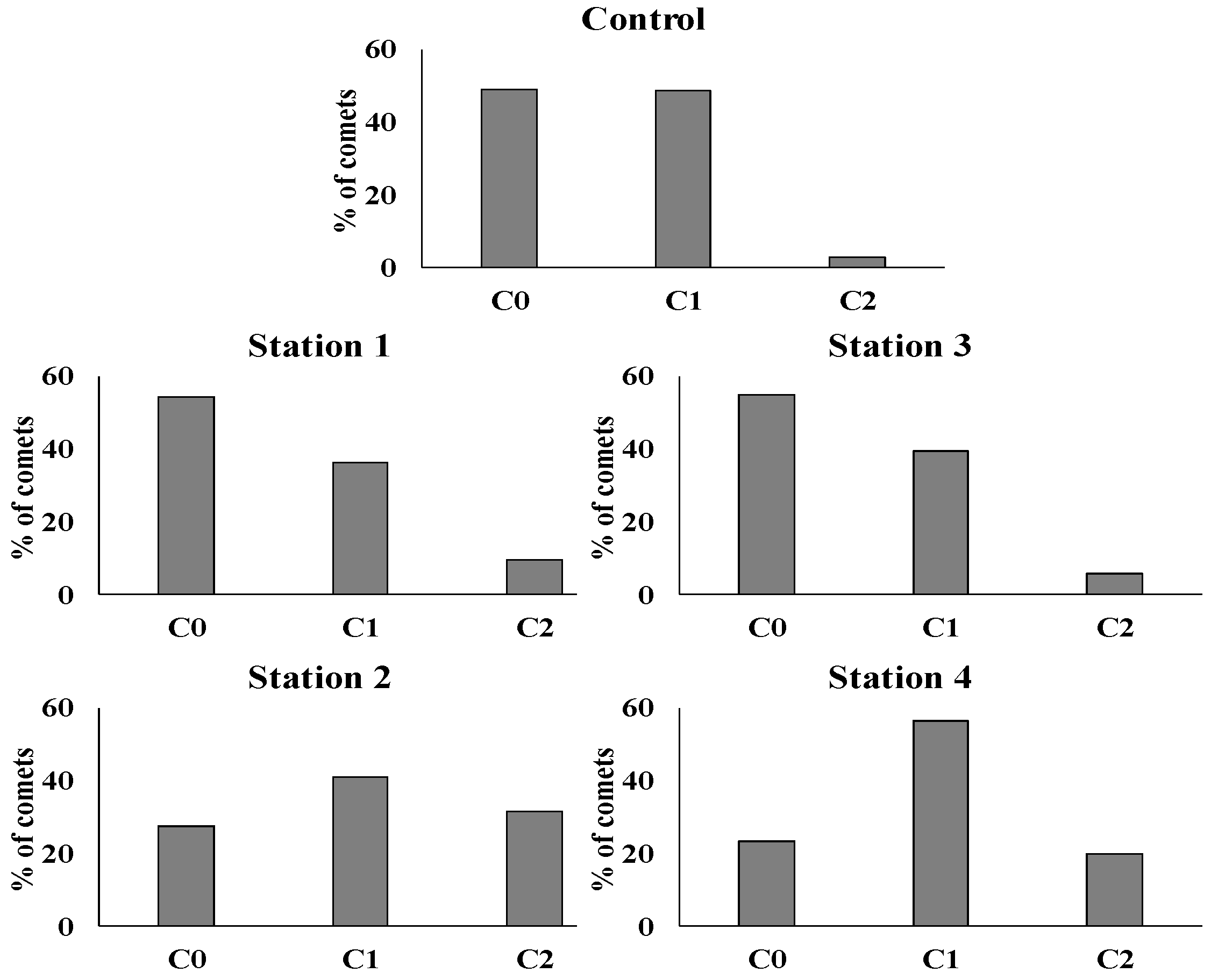
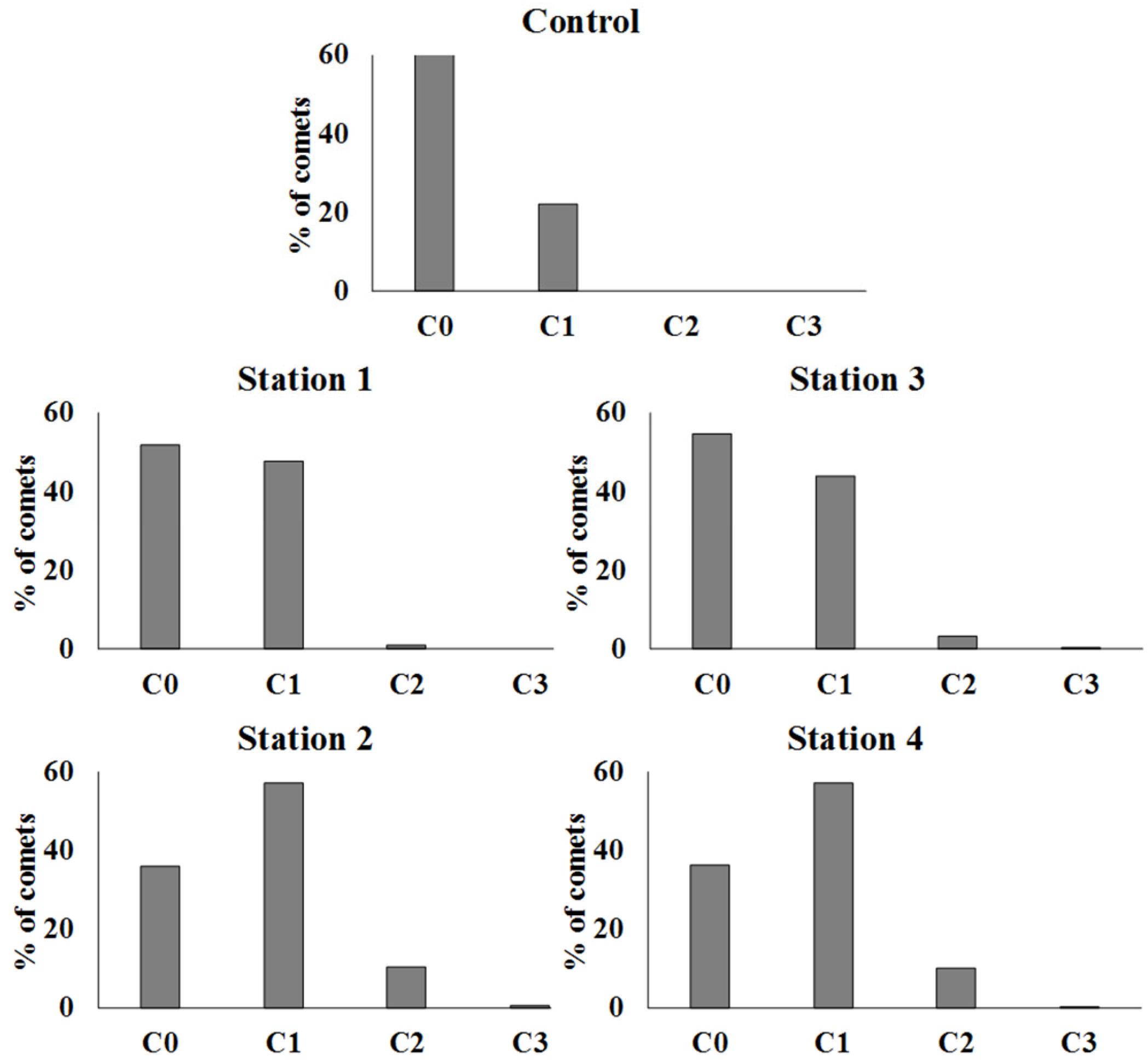
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chapman, P.M. Determining when contamination is pollution—Weight of evidence determinations for sediments and effluents. Environ. Int. 2007, 33, 492–501. [Google Scholar] [CrossRef] [PubMed]
- Benedetti, M.; Gorbi, S.; Fattorini, D.; D’Errico, G.; Piva, F.; Pacitti, D.; Regoli, F. Environmental hazards from natural hydrocarbons seepage: Integrated classification of risk from sediment chemistry, bioavailability and biomarkers responses in sentinel species. Environ. Pollut. 2014, 185, 116–126. [Google Scholar] [CrossRef] [PubMed]
- Mennillo, E.; Adeogun, A.O.; Arukwe, A. Quality screening of the Lagos lagoon sediment by assessing the cytotoxicity and toxicological responses of rat hepatoma H4IIE and fish PLHC-1 cell-lines using different extraction approaches. Environ. Res. 2020, 182, 108986. [Google Scholar] [CrossRef] [PubMed]
- Hidayati, N.V.; Syakti, A.D.; Nuning, V.; Asia, L.; Khabouchi, I.; Torre, F.; Widowati, I.; Sabdono, A.; Doumenq, P.; Syakti, A.D. Ecological risk assessment of persistent organic pollutants (POPs) in surface sediments from aquaculture system. Chemosphere 2021, 263, 128372. [Google Scholar] [CrossRef]
- Kammann, U.; Riggers, J.C.; Theobald, N.; Steinhart, H. Genotoxic potential of marine sediments from the North Sea. Mutat. Res. 2000, 467, 161–168. [Google Scholar] [CrossRef]
- Coughlan, B.M.; Hartl, M.G.J.; O’Reilly, S.J.; Sheehan, D.; Morthersill, C.; van Pelt, F.N.A.M.; O’Halloran, J.; O’Brien, N.M. Detecting genotoxicity using the Comet assay following chronic exposure of Manila clam Tapes semidecussatus to polluted estuarine sediments. Mar. Pollut. Bull. 2002, 44, 1359–1365. [Google Scholar] [CrossRef]
- Costa, P.M.; Pinto, M.; Vicente, A.M.; Gonçalves, C.; Rodrigo, A.P.; Louro, H.; Costa, M.H.; Caeiro, S.; Silva, M.J. An integra-tive assessment to determine the genotoxic hazard of estuarine sediments: Combining cell and whole-organism responses. Front. Genet. 2014, 5, 437. [Google Scholar] [CrossRef]
- Guidi, P.; Bernardeschi, M.; Scarcelli, V.; Cantafora, E.; Benedetti, M.; Falleni, A.; Frenzilli, G. Lysosomal, genetic and chromosomal damage in haemocytes of the freshwater bivalve (Unio pictorum) exposed to polluted sediments from the River Cecina (Italy). Chem. Ecol. 2017, 33, 516–527. [Google Scholar] [CrossRef]
- Chen, G.; White, P.A. The Mutagenic Hazards of Aquatic Sediments: A Review. Mutat. Res. 2004, 567, 151–225. [Google Scholar] [CrossRef]
- Charry, M.P.; Keesing, V.; Costello, M.; Tremblay, L.A. Assessment of the ecotoxicity of urban estuarine sediment using benthic and pelagic copepod bioassays. PeerJ 2018, 6, e4936. [Google Scholar] [CrossRef]
- Costa, P.M.; Neuparth, T.S.; Caeiro, S.; Lobo, J.; Martins, M.; Ferreira, A.M.; Caetano, M.; Vale, C.; Ángel DelValls, T.; Costa, M.H. Assessment of the genotoxic potential of contaminated estuarine sediments in fish peripheral blood: Laboratory versus in situ studies. Environ. Res. 2011, 111, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Pinto, M.; Costa, P.M.; Louro, H.; Costa, M.H.; Lavinha, J.; Caeiro, S.; Silva, M.J. Determining oxidative and non-oxidative genotoxic effects driven by estuarine sediment contaminants on a human hepatoma cell line. Sci. Total Environ. 2014, 478, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Chapman, P.M.; Anderson, B.; Carr, S.; Engle, V.; Green, R.; Hameedi, J.; Harmon, M.; Haverland, P.; Hyland, J.; Ingersoll, C.; et al. General guidelines for using the sediment quality triad. Mar. Pollut. Bull. 1997, 34, 368–372. [Google Scholar] [CrossRef]
- Rigaud, S.; Di Giorgio, C.; Radakovitch, O.; Garnier, J.; De Méo, M. Genotoxicity of sediment extracts of the Berre lagoon (France). Chemosphere 2012, 88, 937–944. [Google Scholar] [CrossRef] [PubMed]
- Kammann, U.; Biselli, S.; Hühnerfuss, H.; Reineke, N.; Theobald, N.; Vobach, M.; Wosniok, W. Genotoxic and teratogenic potential of marine sediment extracts investigated with comet assay and zebrafish test. Environ. Pollut. 2004, 132, 279–287. [Google Scholar] [CrossRef]
- do Nascimento Monte, C.; de Castro Rodrigues, A.P.; de Freitas, A.R.; Braz, B.F.; Freire, A.S.; Cordeiro, R.C.; Santelli, R.E.; Machado, W.T.V. Ecological risks associated to trace metals of contaminated sediments from a densely urbanized tropical eutrophic estuary. Environ. Monit. Assess. 2021, 193, 767. [Google Scholar] [CrossRef]
- Giorgioa, C.; Malleretb, L.; Gueydon-Morinb, C.; Rigaudc, S.; Méoa, M. Comparison of two extraction procedures for the assessment of sediment genotoxicity: Implication of polar organic compounds. Mutat. Res. 2011, 725, 1–12. [Google Scholar] [CrossRef]
- Hylland, K.; Burgeot, T.; Martínez-Gomez, C.; Lang, T.; Robinson, C.D.; Svavarsson, J.; Thain, J.E.; Vethaak, A.D.; Gubbins, M.J. How can we quantify impacts of contaminants in marine ecosystems? Mar. Environ. Res. 2017, 124, 2–10. [Google Scholar] [CrossRef]
- Baran, A.; Klimkowicz-Pawlas, A.; Ukalska-Jaruga, A. Distribution of polycyclic aromatic hydrocarbons (PAHs) in the bottom sediments of a dam reservoir, their interaction with organic matter and risk to benthic fauna. J. Soils Sediments 2021, 21, 2418–2431. [Google Scholar] [CrossRef]
- Mazur, M.A.; Zhuravel, E.V. Assessment of the Toxicity of Bottom Sediments from Coastal Areas of Peter the Great Gulf (Sea of Japan). Contemp. Probl. Ecol. 2022, 15, 699–708. [Google Scholar] [CrossRef]
- Šrut, M.; Traven, L.; Štambuk, A.; Kralj, S.; Žaja, R.; Mićović, V.; Klobučar, G. Genotoxicity of marine sediments in the fish hepatoma cell line PLHC-1 as assessed by the Comet assay. Toxicol. Vitr. 2011, 25, 308–314. [Google Scholar] [CrossRef] [PubMed]
- Kilemade, M.F.; Hartl, M.G.; Sheehan, D.; Mothersill, C.; Van Pelt, F.N.; O’Halloran, J.; O’Brien, N.M. Genotoxicity of field-collected inter-tidal sediments from Cork Harbor, Ireland, to juvenile turbot (Scophthalmus maximus L.) as measured by the Comet assay. Environ. Mol. Mutagen. 2004, 44, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Martins, M.; Costa, P.M. The comet assay in Environmental Risk Assessment of marine pollutants: Applications, assets and handicaps of surveying genotoxicity in non-model organisms. Mutagenesis 2015, 30, 89–106. [Google Scholar] [CrossRef]
- Li, Q.; Chen, L.; Liu, L. Embryotoxicity and genotoxicity evaluation of sediments from Yangtze River estuary using zebrafish (Danio rerio) embryos. Environ. Sci. Pollut. Res. 2016, 23, 4908–4918. [Google Scholar] [CrossRef] [PubMed]
- Orlova, T.Y.; Aleksanin, A.I.; Lepskaya, E.V.; Efimova, K.V.; Selina, M.S.; Morozova, T.V.; Stonik, I.V.; Kachur, V.A.; Karpenko, A.A.; Vinnikov, K.A.; et al. A massive bloom of Karenia species (Dinophyceae) off the Kamchatka coast, Russia, in the fall of 2020. Harmful Algae 2022, 120, 102337. [Google Scholar] [CrossRef]
- Alexanin, A.; Kachur, V.; Khramtsova, A.; Orlova, T. Methodology and Results of Satellite Monitoring of Karenia Microalgae Blooms, That Caused the Ecological Disaster off Kamchatka Peninsula. Remote Sens. 2023, 15, 1197. [Google Scholar] [CrossRef]
- Morozov, T.B.; Sanamyan, N.P.; Sanamyan, K.E. Consequences of the Impacts of a Harmful Algal Bloomon Benthic Invertebrates in the Eastern Kamchatka Shelf. Rus. J. Mar. Biol. 2024, 50, 319–334. [Google Scholar] [CrossRef]
- Zhuravel, E.V.; Mazur, M.A.; Abdrakhmanova, O.T.; Tunina, M.A. Application of a Test Battery for Assessing the Toxicity of Marine Sediments in Vostok Bay (Peter the Great Bay, Sea of Japan). Russ. J. Mar. Biol. 2024, 50, 203–211. [Google Scholar] [CrossRef]
- Slobodskova, V.V.; Dovzhenko, N.V.; Kukla, S.P.; Chelomin, V.P.; Mazur, A.A. Express Diagnosis and Prediction of Remote Mass Mortality of Scallop Mizuhopecten yessoensis in Mariculture Farms Using Biomarkers. J. Mar. Sci. Eng. 2024, 12, 1151. [Google Scholar] [CrossRef]
- Cavaş, T.; Könen, S. In vivo genotoxicity testing of the amnesic shellfish poison (domoic acid) in piscine erythrocytes using the micronucleus test and the comet assay. Aquat. Toxicol. 2008, 90, 154–159. [Google Scholar] [CrossRef]
- Kukla, S.P.; Slobodskova, V.V.; Zhuravel, E.V.; Mazur, A.A.; Chelomin, V.P. Exposure of adult sand dollars (Scaphechinus mirabilis) (Agassiz, 1864) to copper oxide nanoparticles induces gamete DNA damage. Environ. Sci. Pollut. Res. 2022, 29, 39451–39460. [Google Scholar] [CrossRef]
- Chelomin, V.P.; Slobodskova, V.V.; Kukla, S.P.; Zhuravel, E.V.; Chernyaev, A.P. Genotoxic Effects of Exposure to Water-Soluble Fraction of Diesel Fuel in Sand Dollar Scaphechinus mirabilis Gametes. Toxics 2023, 11, 29. [Google Scholar] [CrossRef]
- Mazur, M.; Zhuravel, E.; Kovekovdova, L. Pollution and Potential Ecological Risk Evaluation of Heavy Metals and Arsenic in Surface Marine Sediments of the Coastal Vostok Bay (Peter the Great Bay, Sea of Japan, Russia). Pollution 2025, 11, 510–524. [Google Scholar] [CrossRef]
- Faggio, C.; Pagano, M.; Alampi, R.; Vazzana, I.; Felice, M.R. Cytotoxicity, haemolymphatic parameters, and oxidative stress following exposure to sub-lethal concentrations of quaternium-15 in Mytilus galloprovincialis. Aquat. Toxicol. 2016, 180, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Lacaze, E.; Devaux, A.; Jubeaux, G.; Mons, R.; Gardette, M.; Bony, S.; Garric, J.; Geffard, O. DNA damage in Gammarus fossarum sperm as a biomarker of genotoxic pressure: Intrinsic variability and reference level. Sci. Total Environ. 2011, 409, 3230–3236. [Google Scholar] [CrossRef] [PubMed]
- Châtel, A.; Bruneau, M.; Lièvre, C.; Goupil, A.; Mouneyrac, C. Spermatozoa: A relevant biological target for genotoxicity assessment of contaminants in the estuarine bivalve Scrobicularia plana. Mar. Pollut. Bull. 2017, 116, 488–490. [Google Scholar] [CrossRef]
- Thomas, M.C.; Flores, F.; Kaserzon, S.; Fisher, R.; Negri, A.P. Toxicity of ten herbicides to the tropical marine microalgae Rhodomonas salina. Sci. Rep. 2020, 10, 7612. [Google Scholar] [CrossRef]
- Shaliutina, O.; Materiienko, A.; Shaliutina-Kolešová, A.; Gazo, I. Using fish spermatozoa in in vitro toxicity tests: A potential toxicology tool. Aquaculture 2021, 539, 736647. [Google Scholar] [CrossRef]
- Lin, Z.; Zhou, W.; Ke, Z.; Wu, Z. Ecotoxicity of perfluorooctanoic acid and perfluorooctane sulfonate on aquatic plant Vallisneria natans. Environ. Sci. Pollut. Res. Int. 2024, 31, 26646–26664. [Google Scholar] [CrossRef]
- Aitken, R.J.; Baker, M.A. Oxidative stress, sperm survival and fertility control. Mol. Cell. Endocrinol. 2006, 250, 66–69. [Google Scholar] [CrossRef]
- Lewis, C.; Galloway, T. Genotoxic damage in polychaetes: A study of species and cell-type sensitivities. Mutat. Res. 2008, 654, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Lacaze, E.; Geffard, O.; Bony, S.; Devaux, A. Genotoxicity assessment in the amphipod Gammarus fossarum by use of the alkaline Comet assay. Mutat. Res. 2010, 700, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Ferraro, A.; Siciliano, A.; Spampinato, M.; Morello, R.; Trancone, G.; Race, M.; Guida, M.; Fabbricino, M.; Spasiano, D.; Fratino, U. A multi-disciplinary approach based on chemical characterization of foreshore sediments, ecotoxicity assessment and statistical analyses for environmental monitoring of marine-coastal areas. Mar. Environ. Res. 2024, 202, 106780. [Google Scholar] [CrossRef] [PubMed]
- Brinkmann, M.; Eichbaum, K.; Reininghaus, M.; Koglin, S.; Kammann, U.; Baumann, L.; Segner, H.; Zennegg, M.; Buchinger, S.; Reifferscheid, G.; et al. Towards science-based sediment quality standards-Effects of field-collected sediments in rainbow trout (Oncorhynchus mykiss). Aquat. Toxicol. 2015, 166, 50–62. [Google Scholar] [CrossRef]
- Carotenuto, Y.; Vitiello, V.; Gallo, A.; Libralato, G.; Trifuoggi, M.; Toscanesi, M.; Lofrano, G.; Esposito, F.; Buttino, I. Assessment of the relative sensitivity of the copepods Acartia tonsa and Acartia clausi exposed to sediment-derived elutriates from the Bagnoli-Coroglio industrial area. Mar. Environ. Res. 2020, 155, 104878. [Google Scholar] [CrossRef]
- Cheney, C.; Pothier, M.; Thomas, P.J.; Sarma, S.N.; Poulain, A.J.; Blais, J.M. Paleoecotoxicology: Developing methods to assess the toxicity of lake sediment records influenced by legacy gold mining. Aquat. Toxicol. 2022, 250, 106248. [Google Scholar] [CrossRef]
- Li, J.; He, X.; Wei, J.; Tang, Q.; Bao, Y.; Gao, X.; Mao, Z.; Tang, X.; Huang, P.; Wu, S. Sedimentation substantially buries more carbon than nitrogen or phosphorus in the Three Gorges Reservoir, China. Environ. Res. 2025, 285, 122490. [Google Scholar] [CrossRef]
- Moore, M.N.; Allen, J.I.; McVeigh, A.; Shaw, J. Lysosomal and autophagic reactions as predictive indicators of environmental impact in aquatic animals. Autophagy 2006, 2, 217–220. [Google Scholar] [CrossRef]
- Brown, R.J.; Galloway, T.S.; Lowe, D.; Browne, M.A.; Dissanayake, A.; Jones, M.B.; Depledge, M.H. Differential sensitivity of three marine invertebrates to copper assessed using multiple biomarkers. Aquat. Toxicol. 2004, 66, 267–278. [Google Scholar] [CrossRef]
- Mamaca, E.; Bechmann, R.K.; Torgrimsen, S.; Aas, E.; Bjørnstad, A.; Baussant, T.; Floch, S.L. The neutral red lysosomal retention assay and Comet assay on haemolymph cells from mussels (Mytilus edulis) and fish (Symphodus melops) exposed to styrene. Aquat. Toxicol. 2005, 75, 191–201. [Google Scholar] [CrossRef]
- Guidi, P.; Frenzilli, G.; Benedetti, M.; Bernardeschi, M.; Falleni, A.; Fattorini, D.; Regoli, F.; Scarcelli, V.; Nigro, M. Antioxidant, genotoxic and lysosomal biomarkers in the freshwater bivalve (Unio pictorum) transplanted in a metal polluted river basin. Aquat. Toxicol. 2010, 100, 75–83. [Google Scholar] [CrossRef]
- Istomina, A.; Mazur, A.; Chelomin, V.; Kukla, S.; Slobodskova, V.; Zvyagintsev, A.; Fedorets, Y.; Yelovskaya, O.; Kolosova, L. The Integrated Response of Biomarkers in the Assessment of the Quality of the Marine Environment Based on the Example of the Bivalve Mollusk Mytilus trossulus (Gould, 1850). Russ. J. Mar. Biol. 2021, 47, 185–192. [Google Scholar] [CrossRef]
- Regoli, F.; Frenzilli, G.; Bocchetti, R.; Annarumma, F.; Scarcelli, V.; Fattorini, D.; Nigro, M. Time-course variations of oxyradical metabolism, DNA integrity and lysosomal stability in mussels, Mytilus galloprovincialis, during a field translocation experiment. Aquat. Toxicol. 2004, 68, 167–178. [Google Scholar] [CrossRef]
- Werner, I.; Teh, S.; Datta, S.; Lu, X.; Young, T. Biomarker responses in Macoma nasuta (Bivalvia) exposed to sediments from northern San Francisco Bay. Mar. Environ. Res. 2004, 58, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Gómez, C.; Robinson, C.D.; Burgeot, T.; Gubbins, M.; Halldorsson, H.P.; Albentosa, M.; Bignell, J.P.; Hylland, K.; Vethaak, A.D. Biomarkers of general stress in mussels as common indicators for marine biomonitoring programmes in Europe. Mar. Environ. Res. 2017, 124, 70–80. [Google Scholar] [CrossRef]
- Zhao, C.; Li, X.; Luo, S.; Chang, Y. Assessments of lysosomal membrane responses to stresses with neutral red retention assay and its potential application in the improvement of bivalve aquaculture. Afr. J. Biotechnol. 2011, 10, 13968–13973. [Google Scholar] [CrossRef]
- Jiang, W.J.; Hu, C.; Lai, F.; Pang, W.; Yi, X.; Xu, Q.; Wang, H.; Zhou, J.; Zhu, H.; Zhong, C.; et al. Assessing base-resolution DNA mechanics on the genome scale. Nucleic Acids Res. 2023, 51, 9552–9566. [Google Scholar] [CrossRef] [PubMed]
- Marques, M.B.; Luvizotto-Santos, R.; Hauser-Davis, R.A. Genetic damage in elasmobranchs: A review. Environ. Toxicol. Pharmacol. 2025, 113, 104607. [Google Scholar] [CrossRef]
- Smith, M.A.; Fernandez-Triana, J.; Roughley, R.; Hebert, P.D. DNA barcode accumulation curves for understudied taxa and areas. Mol. Ecol. Resour. 2009, 9, 208–216. [Google Scholar] [CrossRef]
- Williams, T.; Davies, I.; Wu, H.; Diab, A.; Webster, L.; Viant, M.; Chipman, J.; Leaver, M.; George, S.; Moffat, C.; et al. Molecular responses of European flounder (Platichthys flesus) chronically exposed to contaminated estuarine sediments. Chemosphere 2014, 108, 152–158. [Google Scholar] [CrossRef]
- Jeong, H.J.; Lee, H.J.; Hong, S.; Khim, J.S.; Shim, W.J.; Kim, G.B. DNA damage caused by organic extracts of contaminated sediment, crude, and weathered oil and their fractions recovered up to 5 years after the 2007 Hebei Spirit oil spill off Korea. Mar. Pollut. Bull. 2015, 95, 452–457. [Google Scholar] [CrossRef]
- Xiongyi, M.; Xueqin, W.; Yupei, H.; Xiqian, Z.; Xiaohua, Z. The sources of bioavailable toxic metals in sediments regulated their aggregated form, environmental responses and health risk—A case study in Liujiang River Basin, China. Water Res. 2025, 278, 123369. [Google Scholar] [CrossRef]
- Sazykina, M.; Barabashin, T.; Konstantinova, E.; Al-Rammahi, A.A.K.; Pavlenko, L.; Khmelevtsova, L.; Karchava, S.; Klimova, M.; Mkhitaryan, I.; Sazykin, I. Non-corresponding contaminants in marine surface sediments as a factor of ARGs spread in the Sea of Azov. Mar. Pollut. Bull. 2022, 184, 114196. [Google Scholar] [CrossRef]
- Fuentes Molina, N.; L’opez P’erez, T.M.; Puerta Cerpa, Y.D. Molecular mechanisms of microplastic toxicity in coastal sediments of La Guajira Colombia and emerging ecological risks. Case Stud. Chem. Environ. 2025, 11, 101108. [Google Scholar] [CrossRef]
- Semkin, P.; Pavlova, G.; Lobanov, V.; Barabanshchikov, Y.; Kukla, S.; Sagalaev, S.; Shvetsova, M.; Shkirnikova, E.; Tishchenko, P.; Tibenko, E.; et al. Nutrient Flux under the Influence of Melt Water Runoff from Volcanic Territories and Ecosystem Response of Vilyuchinskaya and Avachinskaya Bays in Southeastern Kamchatka. J. Mar. Sci. Eng. 2023, 11, 1299. [Google Scholar] [CrossRef]
- Cieszynska-Semenowicz, M.; Rogowska, J.; Ratajczyk, W.; Ratajczyk, J.; Wolska, L. Toxicity studies of elemental sulfur in marine sediments. Int. J. Sediment Res. 2018, 33, 191–197. [Google Scholar] [CrossRef]
- Karjalainen, J.; Hu, X.; Mäkinen, M.; Karjalainen, A.; Järvistö, J.; Järvenpää, K.; Sepponen, M.; Leppänen, M.T. Sulfate sensitivity of aquatic organism in soft freshwaters explored by toxicity tests and species sensitivity distribution. Ecotoxicol. Environ. Saf. 2023, 258, 114984. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Slobodskova, V.V.; Chelomin, V.P.; Kukla, S.P.; Mazur, A.A.; Dovzhenko, N.V.; Istomina, A.A.; Zhuravel, E.V. Assessment of the Ecotoxicity of Marine Sediments from the Eastern Kamchatka Using Bioassays. J. Mar. Sci. Eng. 2025, 13, 1891. https://doi.org/10.3390/jmse13101891
Slobodskova VV, Chelomin VP, Kukla SP, Mazur AA, Dovzhenko NV, Istomina AA, Zhuravel EV. Assessment of the Ecotoxicity of Marine Sediments from the Eastern Kamchatka Using Bioassays. Journal of Marine Science and Engineering. 2025; 13(10):1891. https://doi.org/10.3390/jmse13101891
Chicago/Turabian StyleSlobodskova, Valentina Vladimirovna, Victor Pavlovich Chelomin, Sergey Petrovich Kukla, Andrey Alexandrovich Mazur, Nadezhda Vladimirovna Dovzhenko, Aleksandra Anatolyevna Istomina, and Elena Vladimirovna Zhuravel. 2025. "Assessment of the Ecotoxicity of Marine Sediments from the Eastern Kamchatka Using Bioassays" Journal of Marine Science and Engineering 13, no. 10: 1891. https://doi.org/10.3390/jmse13101891
APA StyleSlobodskova, V. V., Chelomin, V. P., Kukla, S. P., Mazur, A. A., Dovzhenko, N. V., Istomina, A. A., & Zhuravel, E. V. (2025). Assessment of the Ecotoxicity of Marine Sediments from the Eastern Kamchatka Using Bioassays. Journal of Marine Science and Engineering, 13(10), 1891. https://doi.org/10.3390/jmse13101891





