The Effect of Salinity on the Strength Behavior of Hydrate-Bearing Sands
Abstract
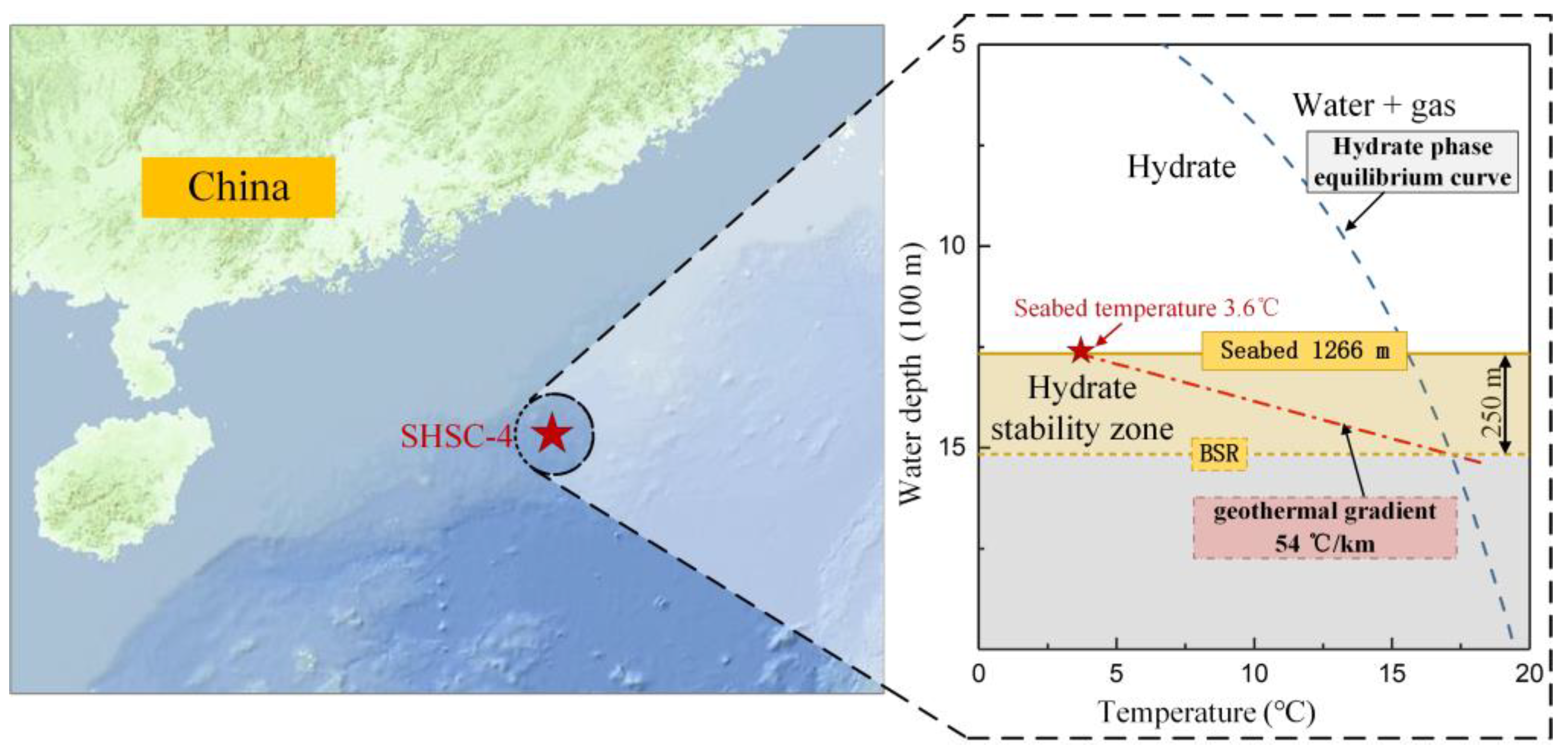
1. Introduction
2. Experiment
2.1. Experimental Apparatus
2.2. Sample Remolding
2.3. Triaxial Tests (Water-Saturated Samples)
2.4. Triaxial Tests (Gas-Saturated Samples)
3. Results and Discussion
3.1. The Effect of Salinity under Water-Saturated Conditions
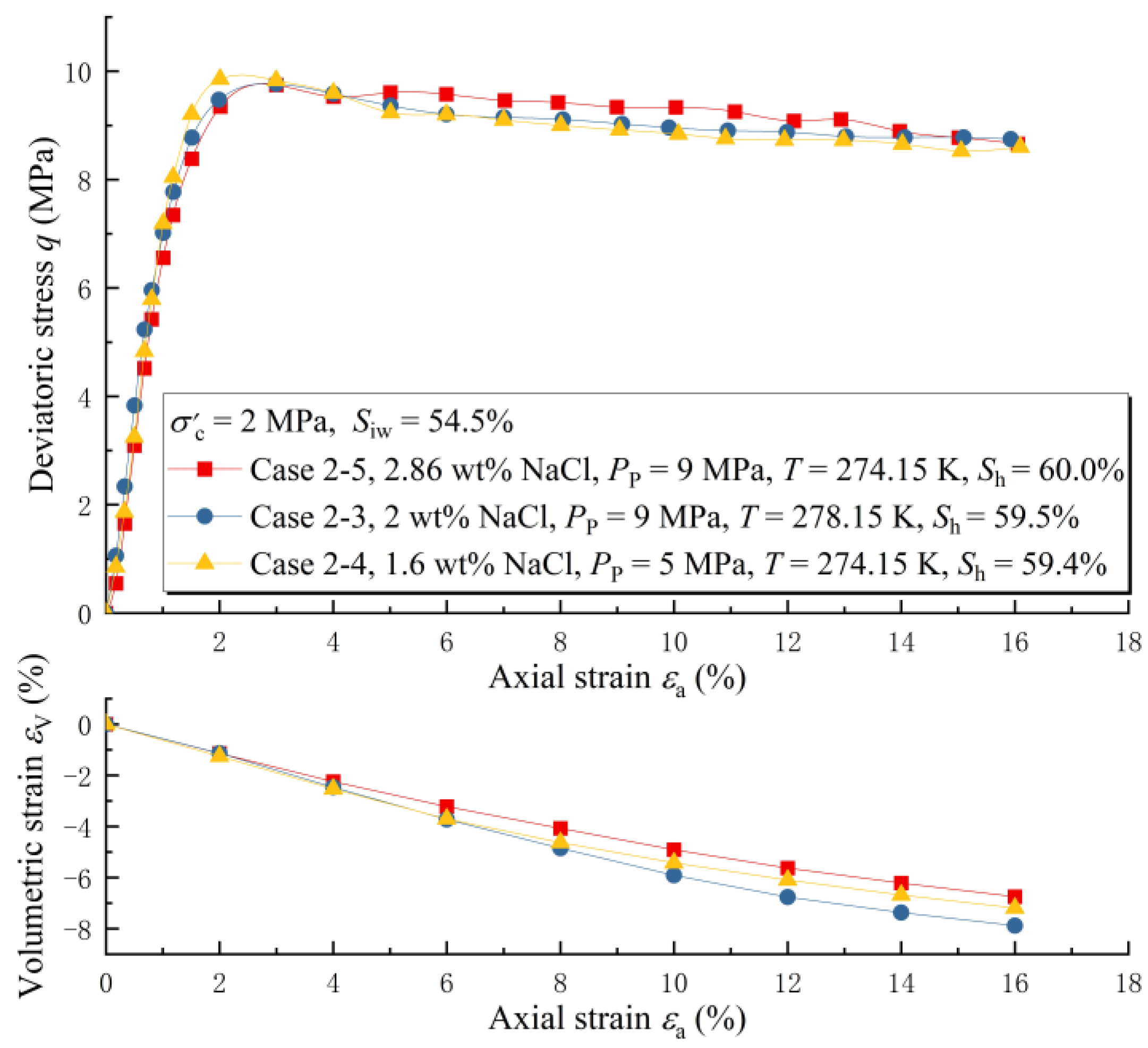
3.2. The Effect of Salinity under Unsaturated Conditions
3.3. Variation Law of MHBS Strength under Saline Conditions
4. Conclusions
- When the T-PP conditions are in the stable zone of the hydrate, the strength of water-saturated MHBSs decreases with the increasing pore water salinity. Meanwhile, with increasing pore water salinity, the dilatancy of samples will be weakened.
- The strength of unsaturated MHBSs is also negatively correlated with salinity. However, unsaturated samples are more significantly influenced by salinity than water-saturated samples.
- The difference in salinity sensitivity between gas-saturated sample strength and water-saturated sample strength is caused by the change in residual pore water salinity during hydrate synthesis (salt precipitation during hydrate synthesis).
- For triaxial tests of unsaturated samples, the T-PP can be close to the hydrate phase equilibrium curve by adding NaCl. Furthermore, when the T and PP conditions of sandy MHBSs are on the hydrate phase equilibrium curve, the mechanical performance of the samples will show a high degree of consistency.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sloan, E.D.; Koh, C.A. Clathrate Hydrates of Natural Gases, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Song, Y.; Yang, L.; Zhao, J.; Liu, W.; Yang, M.; Li, Y.; Liu, Y.; Li, Q. The Status of Natural Gas Hydrate Research in China: A Review. Renew. Sustain. Energy Rev. 2014, 31, 778–791. [Google Scholar] [CrossRef]
- Englezos, P. Clathrate Hydrates. Ind. Eng. Chem. Res. 1993, 32, 1251–1274. [Google Scholar] [CrossRef]
- Li, X.S.; Xu, C.G.; Zhang, Y.; Ruan, X.K.; Li, G.; Wang, Y. Investigation into Gas Production from Natural Gas Hydrate: A Review. Appl. Energy 2016, 172, 286–322. [Google Scholar] [CrossRef]
- Piñero, E.; Marquardt, M.; Hensen, C.; Haeckel, M.; Wallmann, K. Estimation of the Global Inventory of Methane Hydrates Estimation of the Global Inventory of Methane Hydrates in Marine Sediments Using Transfer Functions Estimation of the Global Inventory of Methane Hydrates. Biogeosciences 2012, 10, 959–975. [Google Scholar] [CrossRef]
- Yamamoto, K. Overview and Introduction: Pressure Core-Sampling and Analyses in the 2012e2013 MH21 Offshore Test of Gas Production from Methane Hydrates in the Eastern Nankai Trough. Mar. Pet. Geol. 2014, 66, 296–309. [Google Scholar] [CrossRef]
- Schoderbek, D.; Farrell, H.; Hester, K.; Howard, J.; Raterman, K.; Silpngarmlert, S.; Martin, K.L.; Smith, B.; Klein, P. ConocoPhillips Gas Hydrate Production Test Final Technical Report Oil & Natural Gas Technology; U.S. Department of Energy Office of Scientific and Technical Information: Washington, DC, USA, 2008. [Google Scholar]
- Moridis, G.J.; Silpngarmlert, S.; Reagan, M.T.; Collett, T.; Zhang, K. Gas Production from a Cold, Stratigraphically-Bounded Gas Hydrate Deposit at the Mount Elbert Gas Hydrate Stratigraphic Test Well, Alaska North Slope: Implications of Uncertainties. Mar. Pet. Geol. 2011, 28, 517–534. [Google Scholar] [CrossRef]
- Kurihara, M.; Sato, A.; Funatsu, K.; Ouchi, H. Analysis of Production Data for 2007/2008 Mallik Gas Hydrate Production Tests in Canada. In Proceedings of the International Oil and Gas Conference and Exhibition in China, Beijing, China, 8–10 June 2010. [Google Scholar]
- Makogon, Y.F.; Omelchenko, R.Y. Commercial Gas Production from Messoyakha Deposit in Hydrate Conditions. J. Nat. Gas Sci. Eng. 2013, 11, 1–6. [Google Scholar] [CrossRef]
- Santamarina, J.C.; Dai, S.; Terzariol, M.; Jang, J.; Waite, W.F.; Winters, W.J.; Nagao, J.; Yoneda, J.; Konno, Y.; Fujii, T.; et al. Hydro-Bio-Geomechanical Properties of Hydrate-Bearing Sediments from Nankai Trough. Mar. Pet. Geol. 2015, 66, 434–450. [Google Scholar] [CrossRef]
- Konno, Y.; Fujii, T.; Sato, A.; Akamine, K.; Naiki, M.; Masuda, Y.; Yamamoto, K.; Nagao, J. Key Findings of the World’s First off Shore Methane Hydrate Production Test off the Coast of Japan: Toward Future Commercial Production. Energy Fuels 2017, 31, 2607–2616. [Google Scholar] [CrossRef]
- Yoneda, J.; Oshima, M.; Kida, M.; Kato, A.; Konno, Y.; Jin, Y.; Tenma, N. Consolidation and Hardening Behavior of Hydrate-Bearing Pressure-Core Sediments Recovered from the Krishna–Godavari Basin, Offshore India. Mar. Pet. Geol. 2019, 108, 512–523. [Google Scholar] [CrossRef]
- Bochu, Y. Preliminary Exploration of Gas Hydrate in the Northern Margin of the South China Sea. Mar. Geol. Quat. Geol. 1998, 18, P744.4. [Google Scholar]
- Wu, N.; Hydrate, G.; Geological, G.M.; Yang, S.; Marine, G.; Survey, G.; Zhang, H.; Liang, J.; Wang, H.; Marine, G.; et al. OTC 20485 Gas Hydrate System of Shenhu Area, Northern South China Sea: Wire-Line Logging, Geochemical Results and Preliminary Resources Estimates. In Proceedings of the Offshore Technology Conference, Houston, TX, USA, 3–6 May 2010. [Google Scholar]
- Wu, N.; Zhang, H.; Yang, S.; Zhang, G.; Liang, J.; Lu, J.; Su, X.; Schultheiss, P.; Holland, M.; Zhu, Y. Gas Hydrate System of Shenhu Area, Northern South China Sea: Geochemical Results. J. Geol. Res. 2011, 2011, 370298. [Google Scholar] [CrossRef]
- Wu, N.; Yang, S.; Zhang, H.; Liang, J.; Wang, H.; Su, X.; Fu, S. Preliminary Discussion on Gas Hydrate Reservoir System of Shenhu Area, North Slope of South China Sea. In Proceedings of the International Conference on Gas Hydrates (ICGH), Vancouver, BC, Canada, 6–10 July 2008. [Google Scholar]
- Qin, X.; Liang, Q.; Ye, J.; Yang, L.; Qiu, H.; Xie, W.; Liang, J.; Lu, J.; Lu, C.; Lu, H.; et al. The Response of Temperature and Pressure of Hydrate Reservoirs in the First Gas Hydrate Production Test in South China Sea. Appl. Energy 2020, 278, 115649. [Google Scholar] [CrossRef]
- Ye, J.L.; Qin, X.W.; Xie, W.W.; Lu, H.L.; Ma, B.J.; Qiu, H.J.; Liang, J.Q.; Lu, J.A.; Kuang, Z.G.; Lu, C.; et al. The Second Natural Gas Hydrate Production Test in the South China Sea. China Geol. 2020, 3, 197–209. [Google Scholar] [CrossRef]
- Tupsakhare, S.S.; Kattekola, S.; Castaldi, M.J. An Application of the Results from the Large-Scale Thermal Stimulation Method of Methane Hydrate Dissociation to the Field Tests. Ind. Eng. Chem. Res. 2017, 56, 4588–4599. [Google Scholar] [CrossRef]
- Kamath, V.; Mutallk, P.; Slra, M.; Patll, S. Experimental Study of Brine Injection and Depressurization Methods for Dissociation of Gas Hydrates. SPE Form. Eval. 1991, 6, 477–484. [Google Scholar] [CrossRef]
- Wang, Y.; Feng, J.C.; Li, X.S.; Zhang, Y.; Chen, Z.Y. Fluid Flow Mechanisms and Heat Transfer Characteristics of Gas Recovery from Gas-Saturated and Water-Saturated Hydrate Reservoirs. Int. J. Heat Mass Transf. 2018, 118, 1115–1127. [Google Scholar] [CrossRef]
- Jung, J.W.; Santamarina, J.C. CH4-CO2 Replacement in Hydrate-Bearing Sediments: A Pore-Scale Study. Geochem. Geophys. Geosyst. 2010, 11, Q0AA13. [Google Scholar] [CrossRef]
- Zhou, S.; Zhao, J.; Li, Q.; Chen, W.; Zhou, J.; Wei, N. Optimal Design of the Engineering Parameters for the First Global Trial Production of Marine Natural Gas Hydrates through Solid Fluidization. Nat. Gas Ind. B 2018, 5, 118–131. [Google Scholar] [CrossRef]
- Zhou, S.; Chen, W.; Li, Q.; Zhou, J.; Shi, H. Research on the Solid Fluidzation Well Testing and Production for Shallow Non-Diagenetic Natural Gas Hydrate in Deep Water Area. China Offshore Oil Gas 2017, 29, 1–8. [Google Scholar] [CrossRef]
- Yun, T.S.; Santamarina, C.J.; Ruppel, C. Mechanical Properties of Sand, Silt, and Clay Containing Tetrahydrofuran Hydrate. J. Geophys. Res. Solid Earth 2007, 112, e2006JB004484. [Google Scholar] [CrossRef]
- Wu, P.; Li, Y.; Sun, X.; Liu, W.; Song, Y. Pore-scale 3D Morphological Modeling and Physical Characterization of Hydrate-bearing Sediment Based on Computed Tomography. J. Geophys. Res. Solid Earth 2020, 125, e2020JB020570. [Google Scholar] [CrossRef]
- Wu, P.; Li, Y.; Liu, W.; Liu, Y.; Wang, D.; Song, Y. Microstructure Evolution of Hydrate-Bearing Sands during Thermal Dissociation and Ensued Impacts on the Mechanical and Seepage Characteristics. J. Geophys. Res. Solid Earth 2020, 125, e2019JB019103. [Google Scholar] [CrossRef]
- Park, S. Effect of Large Void Formation on Strength of Cemented Glass Beads. Eng. Geol. 2012, 126, 75–81. [Google Scholar] [CrossRef]
- Luo, T.; Li, Y.; Madhusudhan, B.N.; Sun, X.; Song, Y. Deformation Behaviors of Hydrate-Bearing Silty Sediment Induced by Depressurization and Thermal Recovery. Appl. Energy 2020, 276, 115468. [Google Scholar] [CrossRef]
- Hyodo, M.; Yoneda, J.; Yoshimoto, N.; Nakata, Y. Mechanical and Dissociation Properties of Methane Hydrate-Bearing Sand in Deep Seabed. Soils Found. 2013, 53, 299–314. [Google Scholar] [CrossRef]
- Ning, F.; Yu, Y.; Kjelstrup, S.; Vlugt, T.J.H.; Glavatskiy, K. Mechanical Properties of Clathrate Hydrates: Status and Perspectives. Energy Environ. Sci. 2012, 5, 6779–6795. [Google Scholar] [CrossRef]
- Brown, H.E.; Holbrook, W.S.; Hornbach, M.J.; Nealon, J. Slide Structure and Role of Gas Hydrate at the Northern Boundary of the Storegga Slide, Offshore Norway. Mar. Geol. 2006, 229, 179–186. [Google Scholar] [CrossRef]
- Glasby, G.P. Potential Impact on Climate of the Exploitation of Methane Hydrate Deposits Offshore. Mar. Pet. Geol. 2003, 20, 163–175. [Google Scholar] [CrossRef]
- Boswell, R. Is Gas Hydrate Energy within Reach? Science 2009, 325, 957–958. [Google Scholar] [CrossRef]
- Kvenvolden, K.A. Gas Hydrate and Humans. Ann. N. Y. Acad. Sci. 2000, 921, 17–22. [Google Scholar] [CrossRef]
- Miyazaki, K.; Masui, A.; Tenma, N.; Ogata, Y.; Aoki, K. Study on Mechanical Behavior for Methane Hydrate Sediment Based on Constant Strain-Rate Test and Unloading-Reloading Test under Triaxial Compression. Int. J. Offshore Polar Eng. 2010, 20, 61–67. [Google Scholar]
- Hyodo, M.; Li, Y.; Yoneda, J.; Nakata, Y.; Yoshimoto, N.; Nishimura, A.; Song, Y. Mechanical Behavior of Gas-Saturated Methane Hydrate-Bearing Sediments. J. Geophys. Res. Solid Earth 2013, 118, 5185–5194. [Google Scholar] [CrossRef]
- Durham, W.B.; Kirby, S.H.; Stern, L.A.; Zhang, W. The Strength and Rheology of Methane Clathrate Hydrate. J. Geophys. Res. Solid Earth 2003, 108, e2002JB001872. [Google Scholar] [CrossRef]
- Wang, L.; Zhao, J.; Sun, X.; Wu, P.; Shen, S.; Liu, T.; Li, Y. Comprehensive Review of Geomechanical Constitutive Models of Gas Hydrate-Bearing Sediments. J. Nat. Gas Sci. Eng. 2021, 88, 103755. [Google Scholar] [CrossRef]
- Li, Y.; Wang, L.; Xie, Y.; Wu, P.; Liu, T.; Huang, L.; Zhang, S.; Song, Y. Deformation Characteristics of Methane Hydrate-Bearing Clayey and Sandy Sediments during Depressurization Dissociation. Energy 2023, 275, 127527. [Google Scholar] [CrossRef]
- Wang, H.; Liu, W.; Wu, P.; Pan, X.; You, Z.; Lu, J.; Li, Y. Gas Recovery from Marine Hydrate Reservoir: Experimental Investigation on Gas Flow Patterns Considering Pressure Effect. Energy 2023, 275, 127482. [Google Scholar] [CrossRef]
- Cheng, F.; Wu, Z.; Sun, X.; Shen, S.; Wu, P.; Liu, W.; Chen, B.; Liu, X.; Li, Y. Compression-Induced Dynamic Change in Effective Permeability of Hydrate-Bearing Sediments during Hydrate Dissociation by Depressurization. Energy 2023, 264, 126137. [Google Scholar] [CrossRef]
- Wu, P.; Li, Y.; Yu, T.; Wu, Z.; Huang, L.; Wang, H.; Song, Y. Microstructure Evolution and Dynamic Permeability Anisotropy during Hydrate Dissociation in Sediment under Stress State. Energy 2023, 263, 126126. [Google Scholar] [CrossRef]
- Wang, H.; Wu, P.; Li, Y.; Liu, W.; Pan, X.; Li, Q.; He, Y.; Song, Y. Gas Permeability Variation during Methane Hydrate Dissociation by Depressurization in Marine Sediments. Energy 2023, 263, 125749. [Google Scholar] [CrossRef]
- Wu, P.; Li, Y.; Wang, L.; Sun, X.; Wu, D.; He, Y.; Li, Q.; Song, Y. Hydrate-Bearing Sediment of the South China Sea: Microstructure and Mechanical Characteristics. Eng. Geol. 2022, 307, 106782. [Google Scholar] [CrossRef]
- Li, Y.; Wu, P.; Liu, W.; Sun, X.; Cui, Z.; Song, Y. A Microfocus X-Ray Computed Tomography Based Gas Hydrate Triaxial Testing Apparatus. Rev. Sci. Instrum. 2019, 90, 055105. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Wei, H.; Peng, L.; Wei, C.; Ning, F. An Easy and Efficient Way to Evaluate Mechanical Properties of Gas Hydrate-Bearing Sediments: The Direct Shear Test. J. Pet. Sci. Eng. 2017, 149, 56–64. [Google Scholar] [CrossRef]
- Liu, Z.; Sheng, D.; Fulong, N.; Li, P.; Houzhen, W.; Changfu, W. Strength Estimation for Hydrate-Bearing Sediments from Direct Shear Tests of Hydrate-Bearing Sand and Silt. Geophys. Res. Lett. 2018, 45, 715–723. [Google Scholar] [CrossRef]
- Yoneda, J.; Masui, A.; Tenma, N.; Nagao, J. Triaxial Testing System for Pressure Core Analysis Using Image Processing Technique. Rev. Sci. Instrum. 2013, 84, 2–9. [Google Scholar] [CrossRef]
- Wu, P.; Li, Y.; Sun, X.; Liu, W.; Song, Y. Mechanical Characteristics of Hydrate-Bearing Sediment: A Review. Energy Fuels 2020, 35, 1041–1057. [Google Scholar] [CrossRef]
- Priest, J.A.; Druce, M.; Roberts, J.; Schultheiss, P.; Nakatsuka, Y.; Suzuki, K. PCATS Triaxial: A New Geotechnical Apparatus for Characterizing Pressure Cores from the Nankai Trough, Japan. Mar. Pet. Geol. 2015, 66, 460–470. [Google Scholar] [CrossRef]
- Waite, W.F.; Santamarina, J.C.; Cortes, D.D.; Dugan, B.; Espinoza, D.N.; Germaine, J.; Jang, J.; Jung, J.W.; Kneafsey, T.J.; Shin, H.; et al. Physical Properties of Hydrate-Bearing Sediments. Rev. Geophys. 2009, 47, RG4003. [Google Scholar] [CrossRef]
- Academy, C.; Resources, G. A Preliminary Study of the Gas Hydrate Stability Zone in the South China Sea. Acta Geol. Sin. 2002, 76, 423–428. [Google Scholar] [CrossRef]
- Chaouachi, M.; Falenty, A.; Sell, K.; Enzmann, F.; Kersten, M.; Haberthur, D.; Kuhs, W.F. Microstructural Evolution of Gas Hydrates in Sedimentary Matrices Observed with Synchrotron X-Ray Computed Tomographic Microscopy. Geochem. Geophys. Geosyst. 2015, 16, 1711–1722. [Google Scholar] [CrossRef]
- Chaouachi, M.; Neher, S.H.; Falenty, A.; Kuhs, W.F. Time Resolved Coarsening of Clathrate Crystals: The Case of Gas Hydrates. Cryst. Growth Des. 2017, 17, 2458–2472. [Google Scholar] [CrossRef]
- Ma, S.; Zheng, J.; Tian, M.; Tang, D.; Yang, M. NMR Quantitative Investigation on Methane Hydrate Formation Characteristics under Different Driving Forces. Fuel 2020, 261, 116364. [Google Scholar] [CrossRef]
- Zhang, L.; Sun, M.; Wang, T.; Yang, L.; Zhang, X.; Zhao, J.; Song, Y. An In-Situ MRI Method for Quantifying Temperature Changes during Crystal Hydrate Growths in Porous Medium. J. Therm. Sci. 2022, 31, 1542–1550. [Google Scholar] [CrossRef]
- Li, J.F.; Ye, J.L.; Qin, X.W.; Qiu, H.J.; Wu, N.Y.; Lu, H.L.; Xie, W.W.; Lu, J.A.; Peng, F.; Xu, Z.Q.; et al. The First Offshore Natural Gas Hydrate Production Test in South China Sea. China Geol. 2018, 1, 5–16. [Google Scholar] [CrossRef]
- Hyodo, M.; Nakata, Y.; Yoshimoto, N.; Fukunaga, M.; Kubo, K.; Nanjo, Y.; Matsuo, T.; Hyde, A.F.L.; Nakamura, K. Triaxial Compressive Strength of Methane Hydrate. In Proceedings of the Twelfth International Offshore and Polar Engineering Conference, Kitakyushu, Japan, 26–31 May 2002; Volume 3, pp. 422–428. [Google Scholar]
- Shen, S.; Li, Y.; Sun, X.; Wang, L.; Song, Y. Analysis of the Mechanical Properties of Methane Hydrate-Bearing Sands with Various Pore Pressures and Confining Pressures. J. Nat. Gas Sci. Eng. 2021, 87, 103786. [Google Scholar] [CrossRef]
- Shen, S.; Sun, X.; Wang, L.; Song, Y.; Li, Y. Effect of Temperature on the Mechanical Properties of Hydrate-Bearing Sand under Different Confining Pressures. Energy Fuels 2021, 35, 4106–4117. [Google Scholar] [CrossRef]
- Shen, S.; Li, Y.; Sun, X.; Wang, L.; Song, Y. Mechanical Properties of Methane Hydrate-Bearing Sandy Sediments under Various Temperatures and Pore Pressures. J. Pet. Sci. Eng. 2021, 208, 109474. [Google Scholar] [CrossRef]
- Jiang, M.; Sun, R.; Arroyo, M.; Du, W. Salinity Effects on the Mechanical Behaviour of Methane Hydrate Bearing Sediments: A DEM Investigation. Comput. Geotech. 2021, 133, 104067. [Google Scholar] [CrossRef]
- Gambelli, A.M.; Tinivella, U.; Giovannetti, R.; Castellani, B.; Giustiniani, M.; Rossi, A.; Zannotti, M.; Rossi, F. Observation of the Main Natural Parameters Influencing the Formation of Gas Hydrates. Energies 2021, 14, 1803. [Google Scholar] [CrossRef]
- Gambelli, A.M.; Castellani, B.; Nicolini, A.; Rossi, F. Water Salinity as Potential Aid for Improving the Carbon Dioxide Replacement Process’ Effectiveness in Natural Gas Hydrate Reservoirs. Processes 2020, 8, 1298. [Google Scholar] [CrossRef]
- Wang, L.; Sun, X.; Shen, S.; Wu, P.; Liu, T.; Liu, W.; Zhao, J.; Li, Y. Undrained Triaxial Tests on Water-Saturated Methane Hydrate–Bearing Clayey-Silty Sediments of the South China Sea. Can. Geotech. J. 2021, 58, 351–366. [Google Scholar] [CrossRef]
- Wu, Z.; Liu, W.; Zheng, J.; Li, Y. Effect of Methane Hydrate Dissociation and Reformation on the Permeability of Clayey Sediments. Appl. Energy 2020, 261, 114479. [Google Scholar] [CrossRef]
- Jiang, M.; Zhu, F. DEM Investigation on Mechanical Properties of Methane Hydrate Bearing Soils under Different Temperatures and Pore-Water Pressures. Chin. J. Geotech. Eng. 2014, 36, 1761–1769. [Google Scholar] [CrossRef]
- Yang, L.; Falenty, A.; Chaouachi, M.; Haberthur, D.; Kuhs, W.F. Synchrotron X-ray Computed Microtomography Study on Gas Hydrate Decomposition in a Sedimentary Matrix. Geochem. Geophys. Geosyst. 2016, 17, 3717–3732. [Google Scholar] [CrossRef]
- Jung, J.W.; Santamarina, J.C. Hydrate Adhesive and Tensile Strengths. Geochem. Geophys. Geosyst. 2011, 12, Q08003. [Google Scholar] [CrossRef]
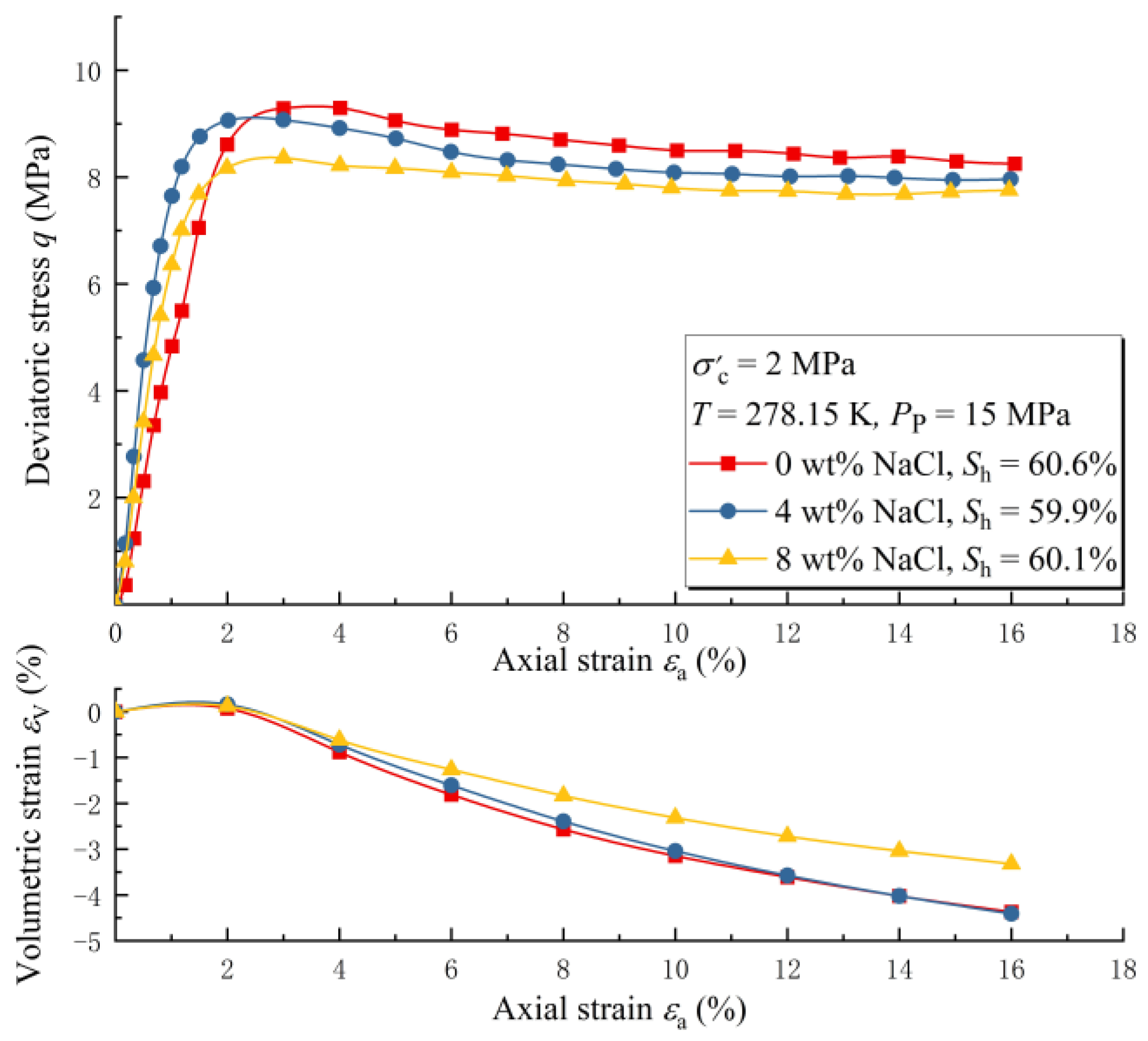

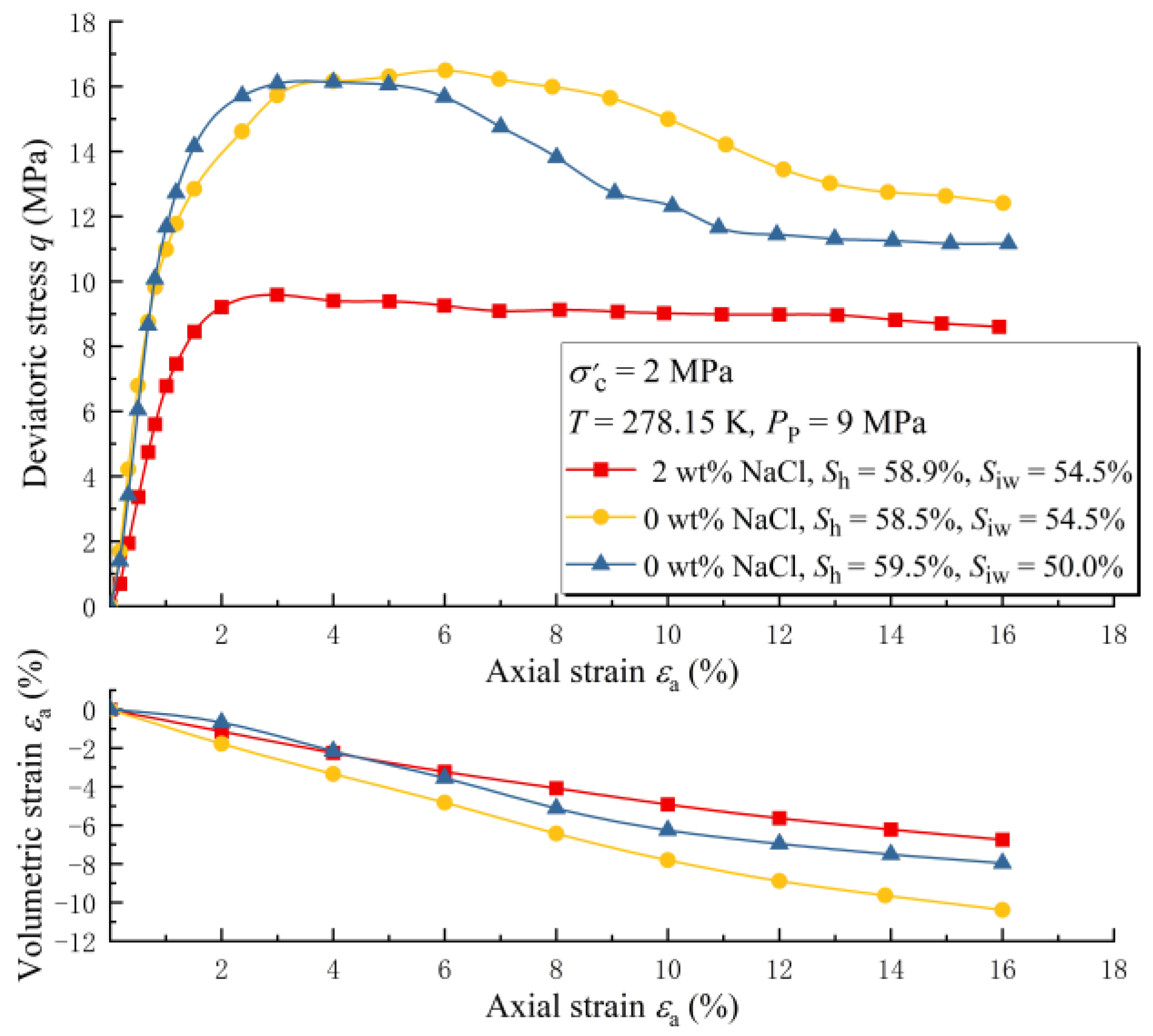
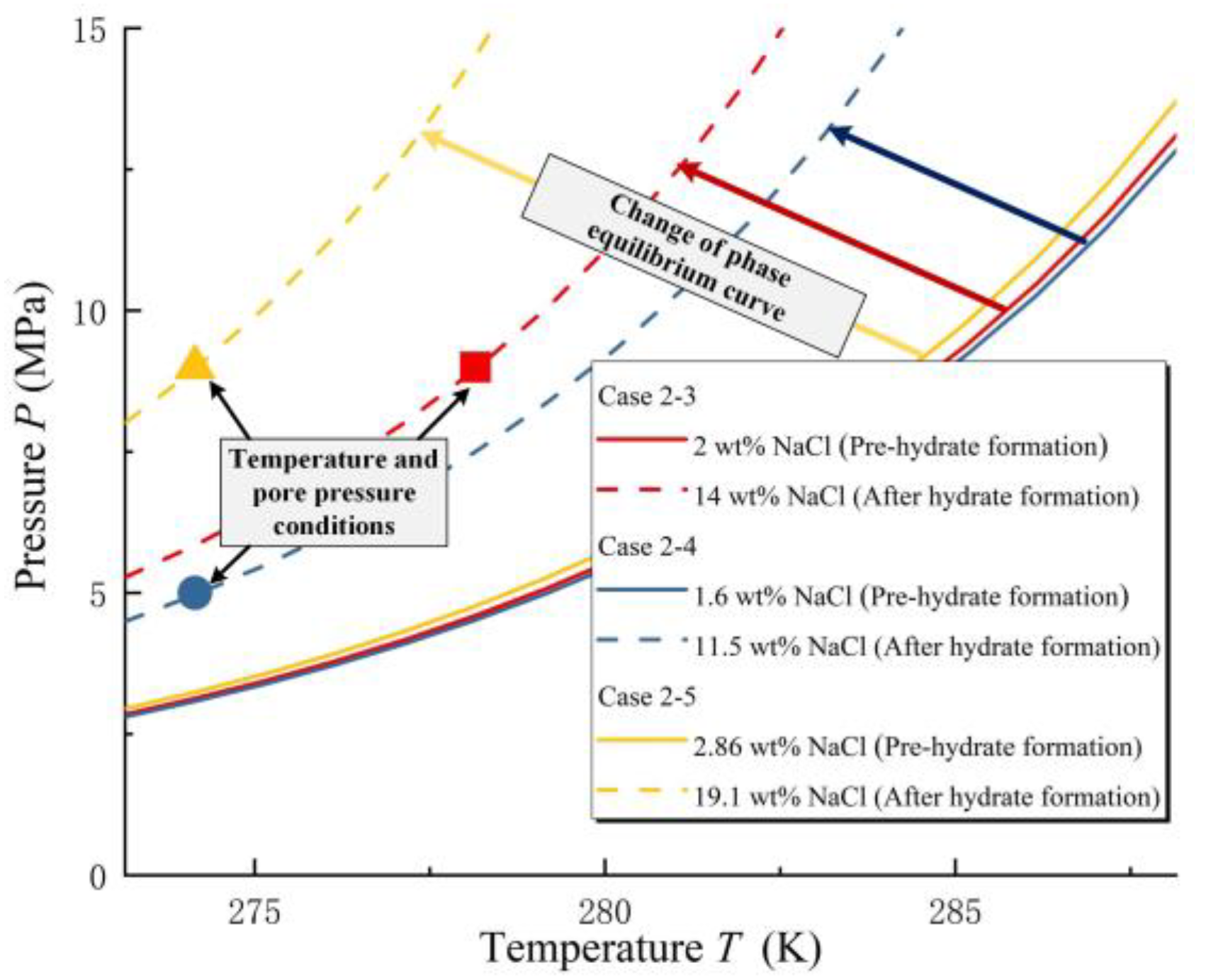
| Case Number | The Salinity of Pore Water (wt%) | Hydrate Saturation (%) |
|---|---|---|
| 1-1 | 0 | 60.6 |
| 1-2 | 4 | 59.9 |
| 1-3 | 8 | 60.1 |
| Case Number | Initial Water Saturation (%) | Initial Salinity (wt%) | Hydrate Saturation (%) | Temperature T (K) | Pore Pressure (MPa) |
|---|---|---|---|---|---|
| 2-1 | 50 | 0 | 58.5 | 278.15 | 9 |
| 2-2 | 54.5 | 0 | 58.9 | ||
| 2-3 | 54.5 | 2.00 | 59.5 | ||
| 2-4 | 54.5 | 1.60 | 59.4 | 274.15 | 5 |
| 2-5 | 54.5 | 2.86 | 60.0 | 274.15 | 9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, S.; Wang, L.; Ge, Y.; Chu, J.; Liang, H. The Effect of Salinity on the Strength Behavior of Hydrate-Bearing Sands. J. Mar. Sci. Eng. 2023, 11, 1350. https://doi.org/10.3390/jmse11071350
Shen S, Wang L, Ge Y, Chu J, Liang H. The Effect of Salinity on the Strength Behavior of Hydrate-Bearing Sands. Journal of Marine Science and Engineering. 2023; 11(7):1350. https://doi.org/10.3390/jmse11071350
Chicago/Turabian StyleShen, Shi, Lei Wang, Yang Ge, Jiawei Chu, and Huiyong Liang. 2023. "The Effect of Salinity on the Strength Behavior of Hydrate-Bearing Sands" Journal of Marine Science and Engineering 11, no. 7: 1350. https://doi.org/10.3390/jmse11071350
APA StyleShen, S., Wang, L., Ge, Y., Chu, J., & Liang, H. (2023). The Effect of Salinity on the Strength Behavior of Hydrate-Bearing Sands. Journal of Marine Science and Engineering, 11(7), 1350. https://doi.org/10.3390/jmse11071350







