The Effect of Husbandry and Original Location on the Fouling of Transplanted Panels
Abstract
1. Introduction
2. Materials and Methods
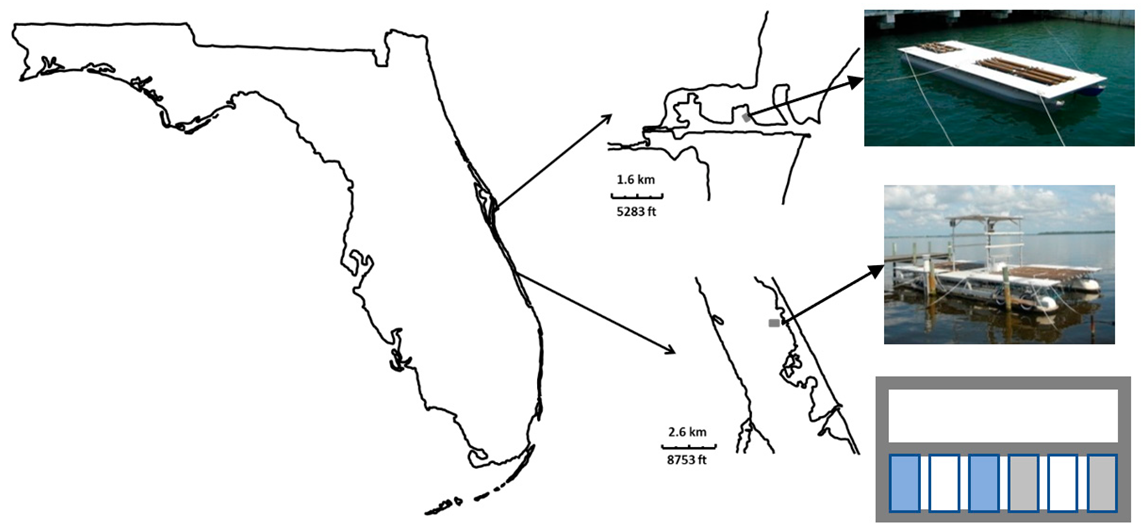
2.1. Study Sites
2.2. Panel Preparation
2.3. Field Testing
2.4. Statistical Analysis
3. Results
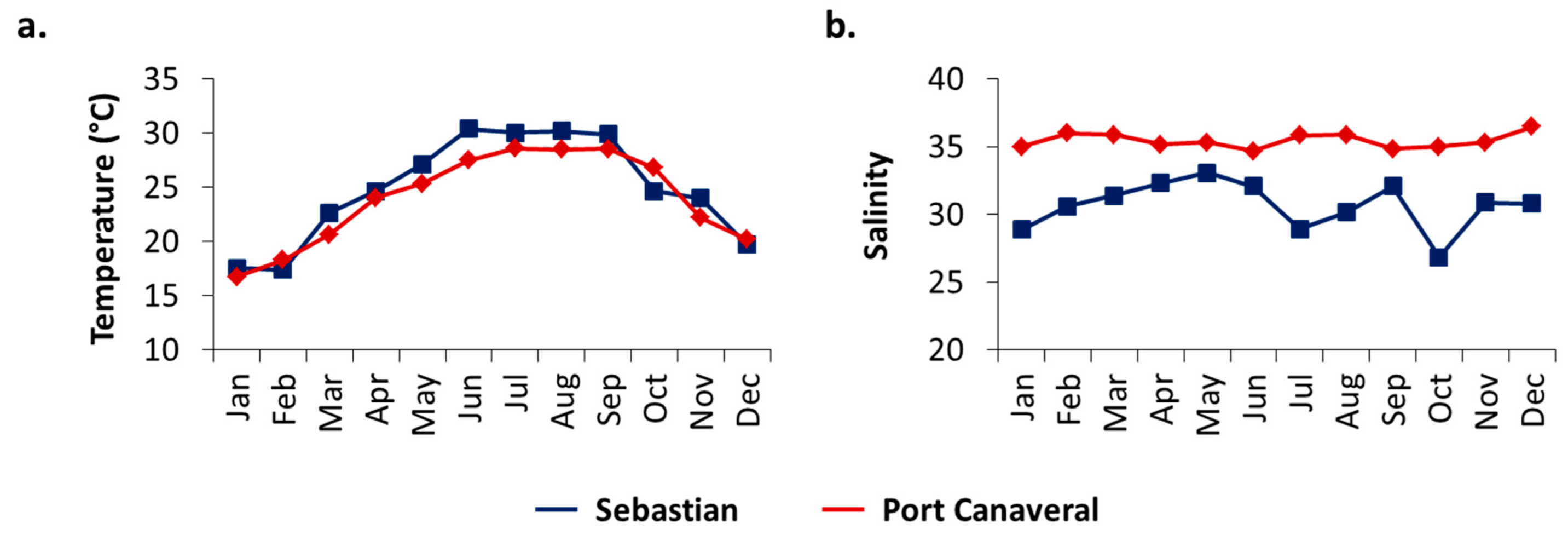
3.1. Salinity and Temperature
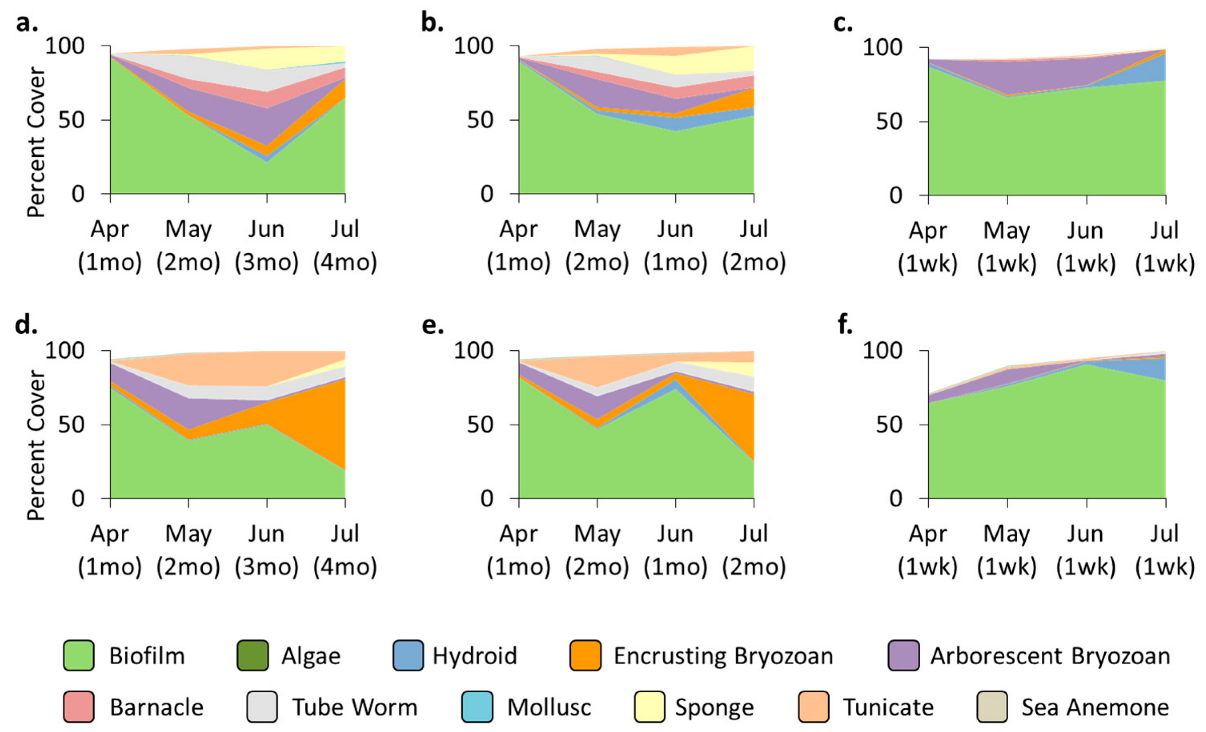
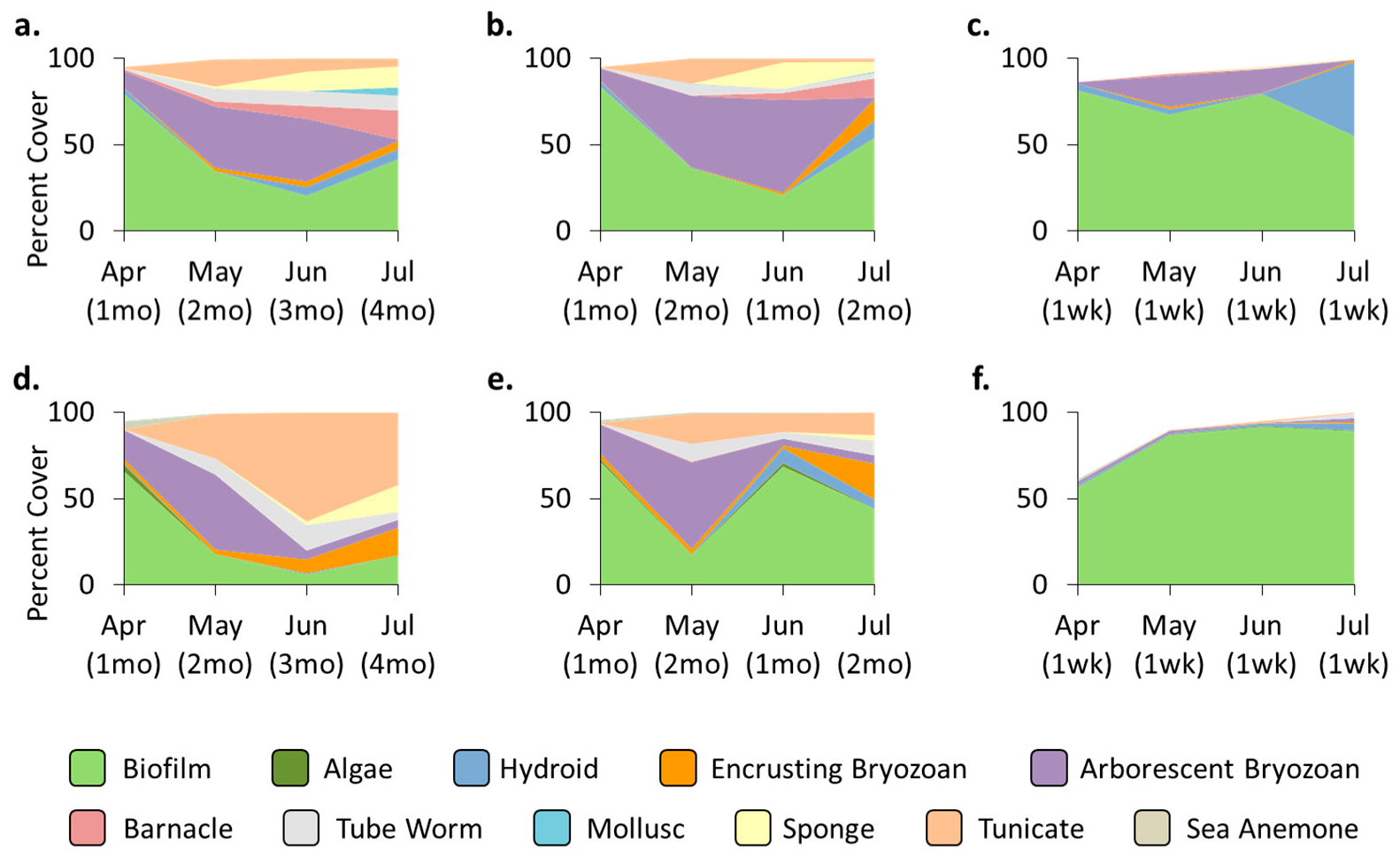
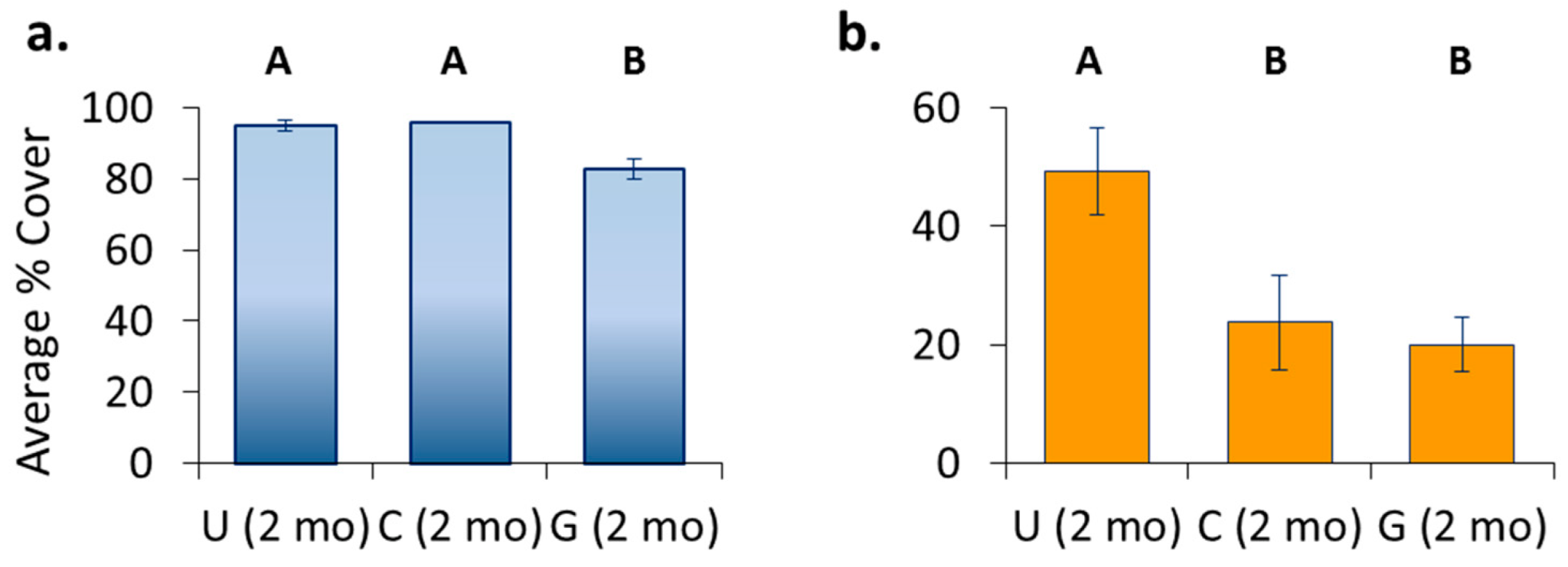
3.2. Community—Pre-Transplant
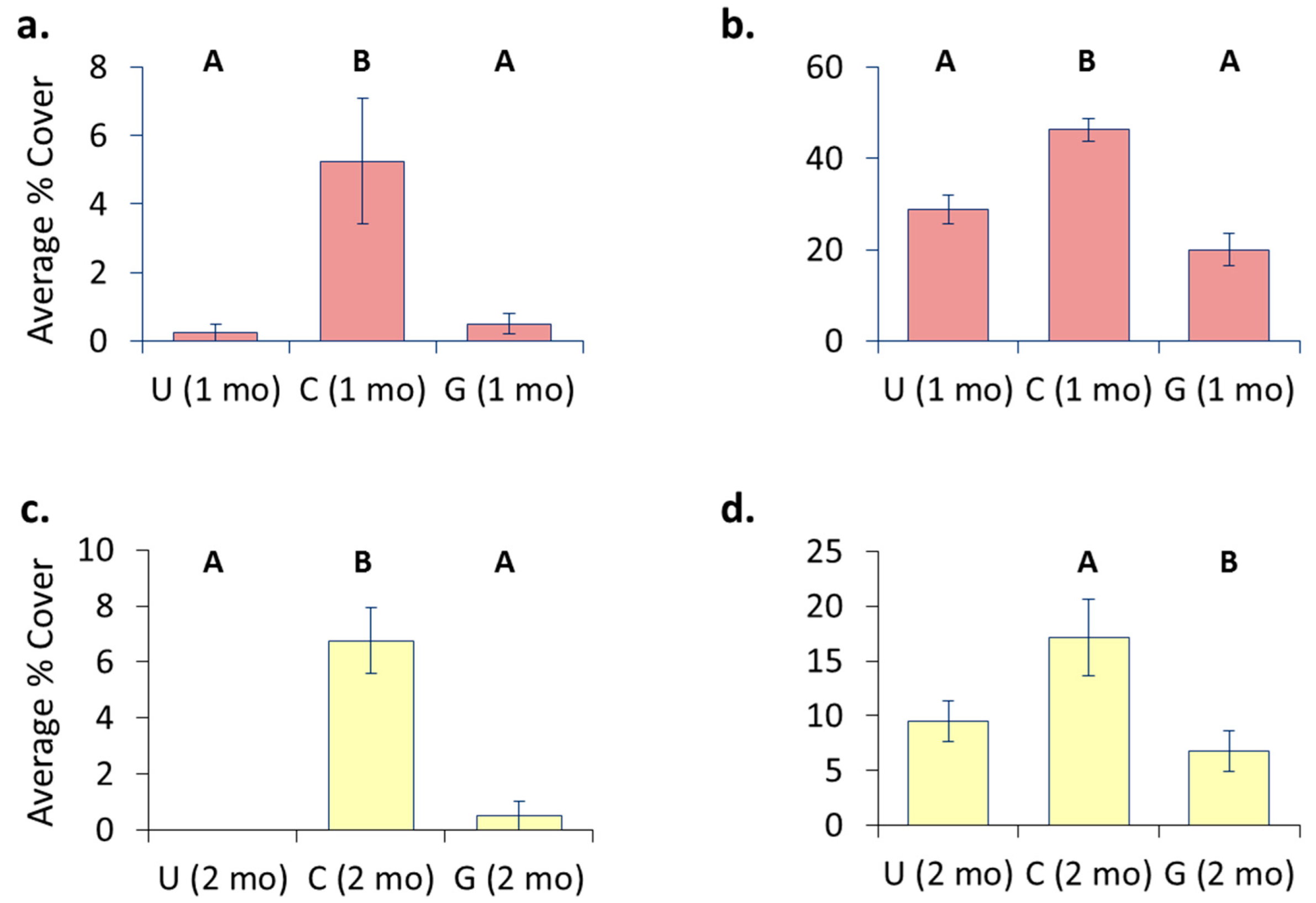
3.3. Treatment Effects—Pre-Transplant
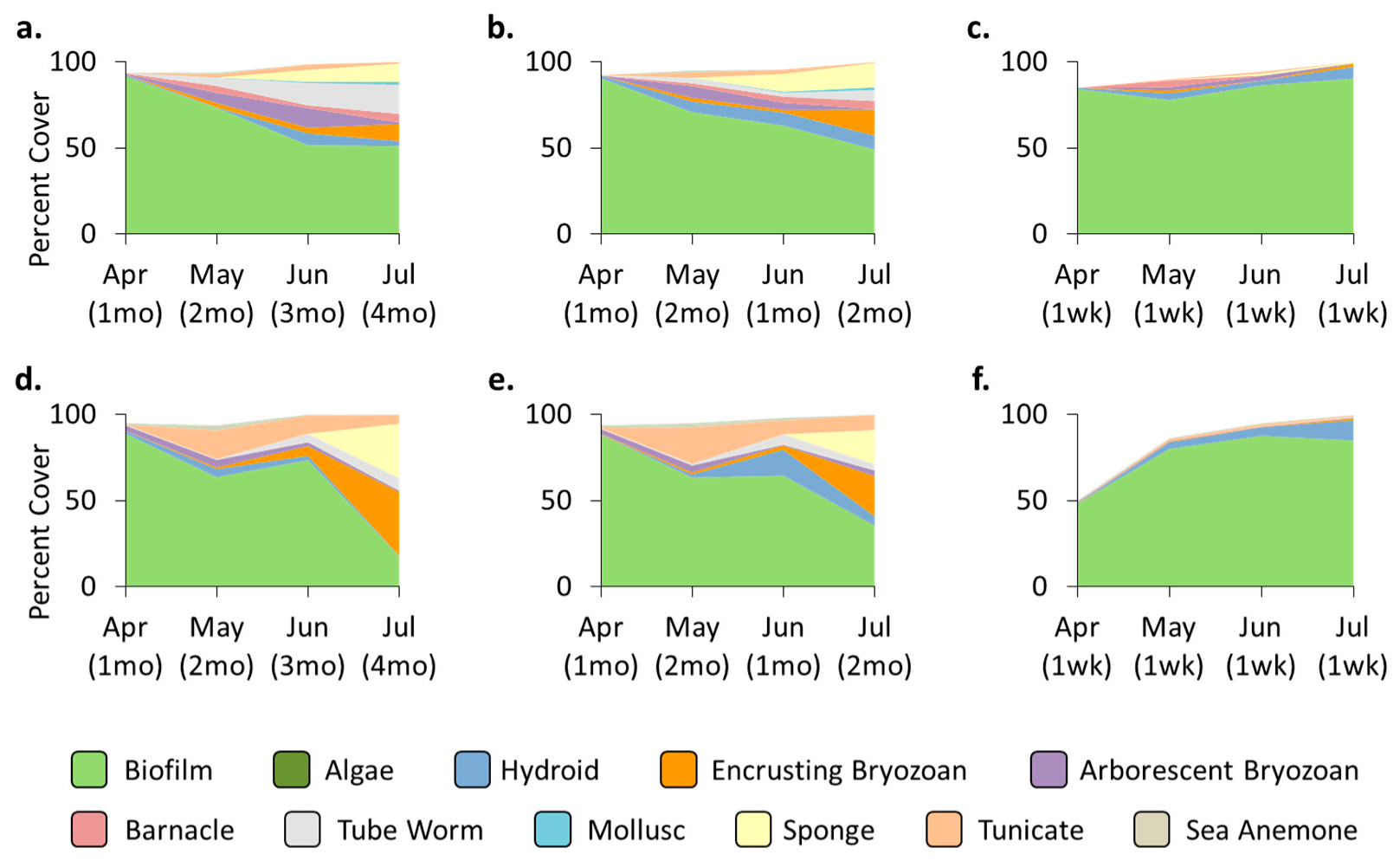
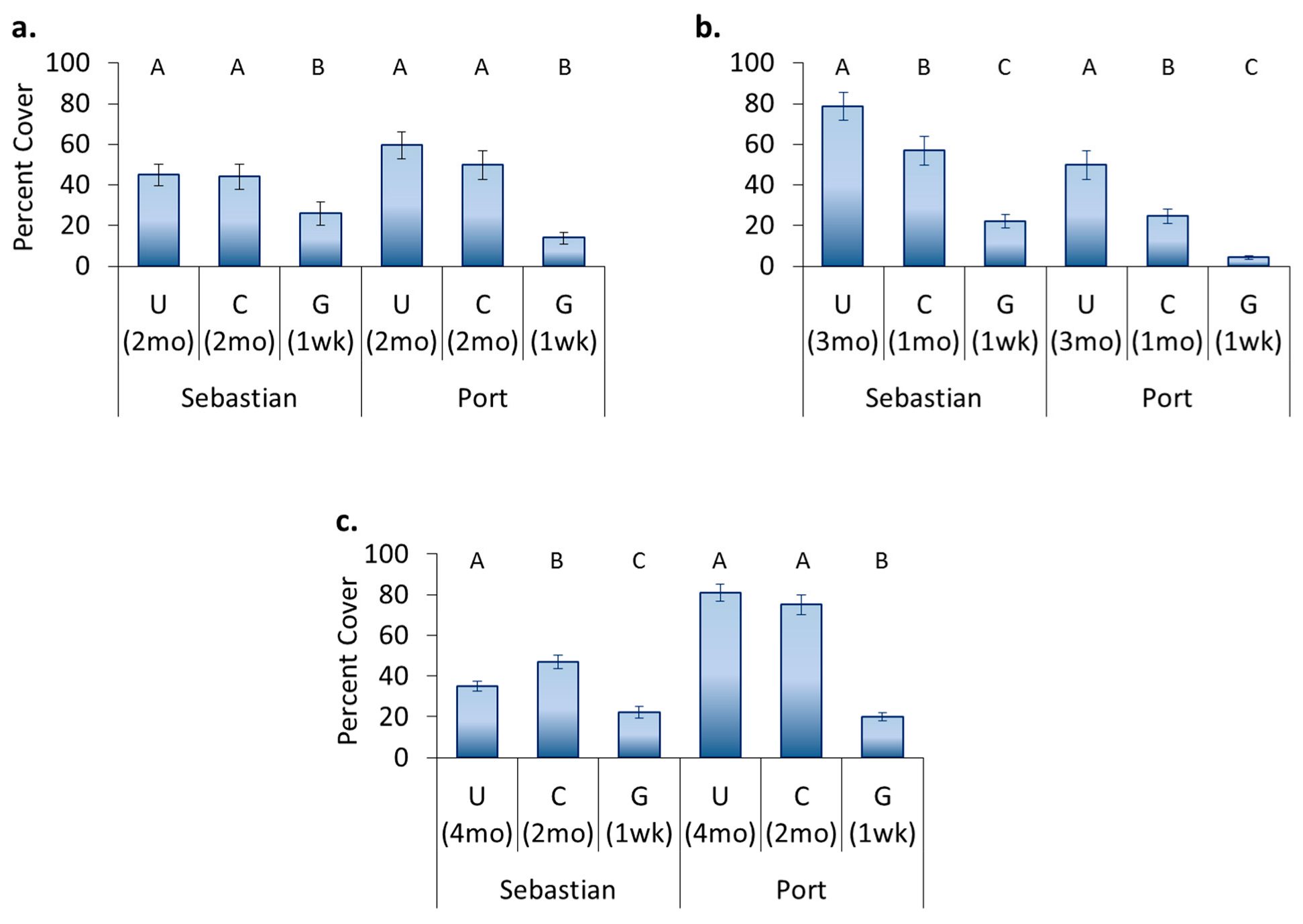
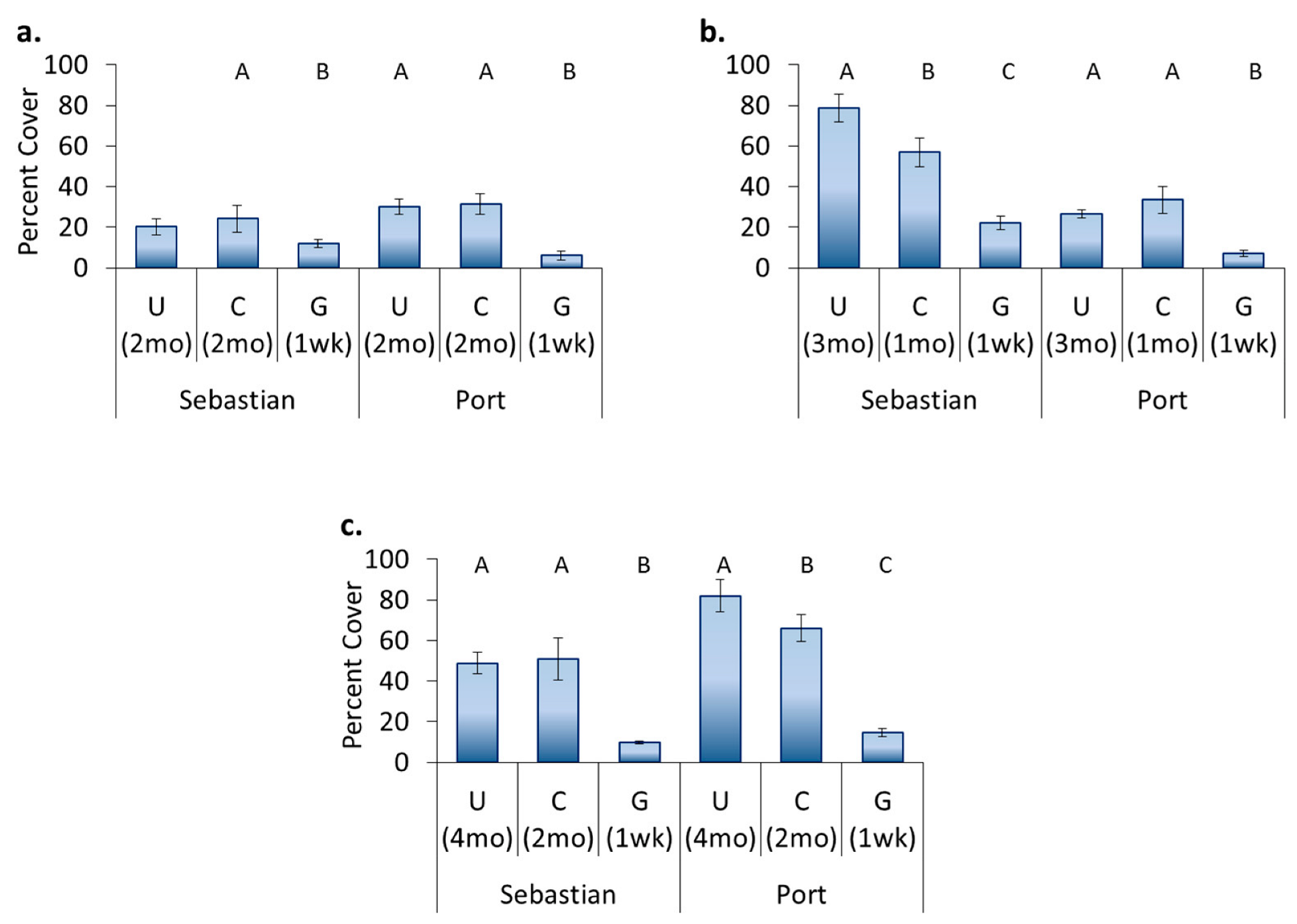
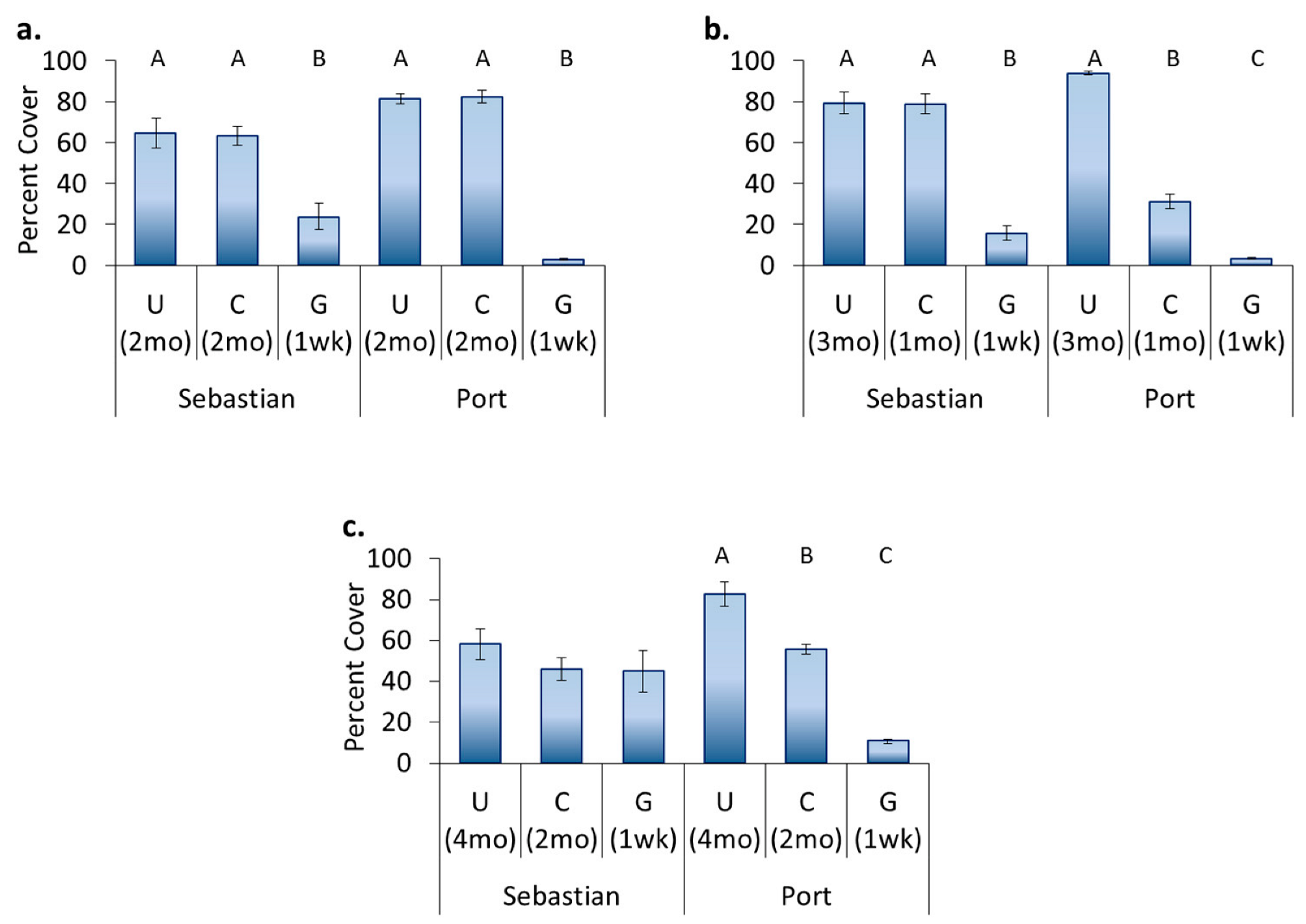
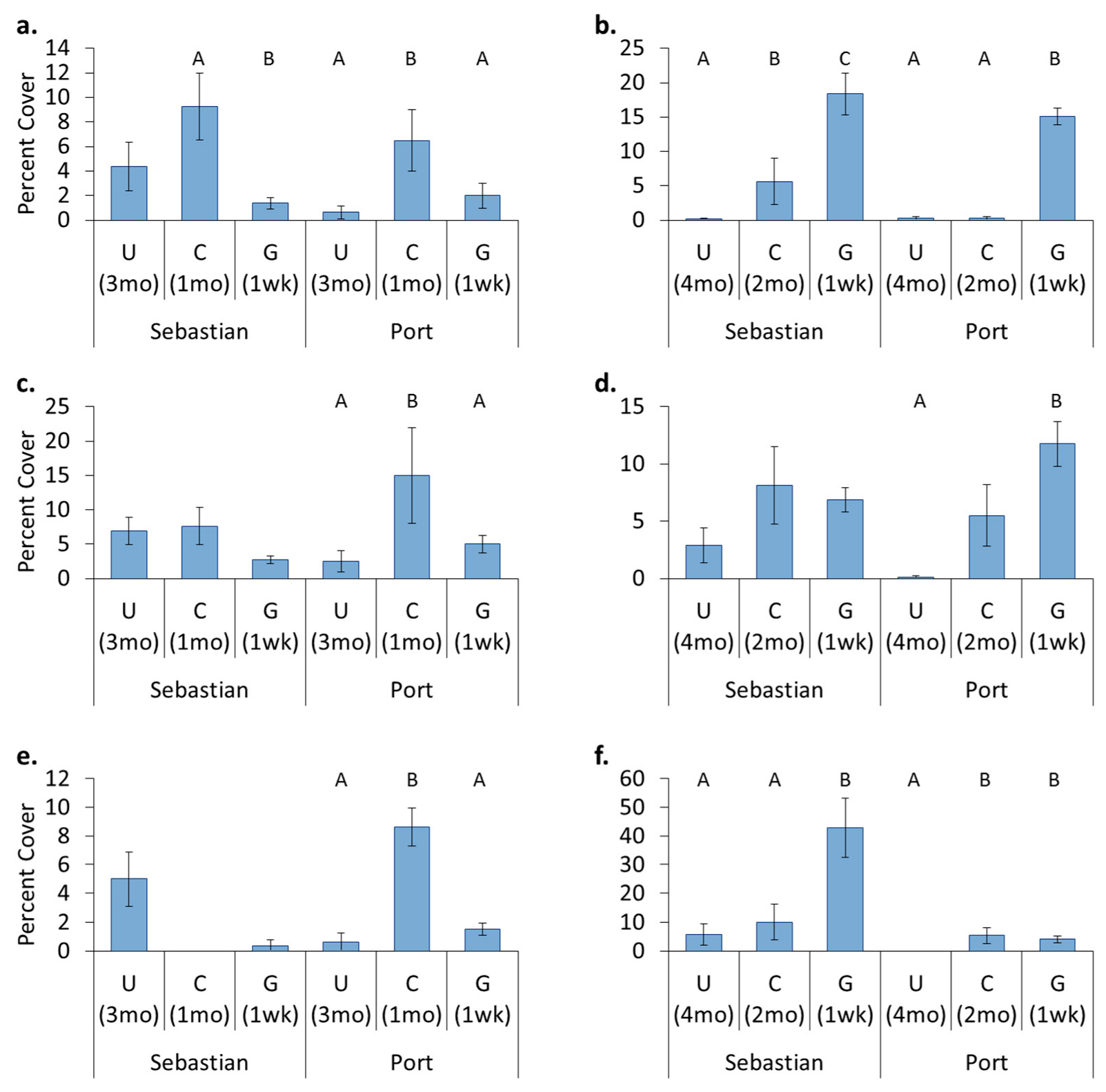
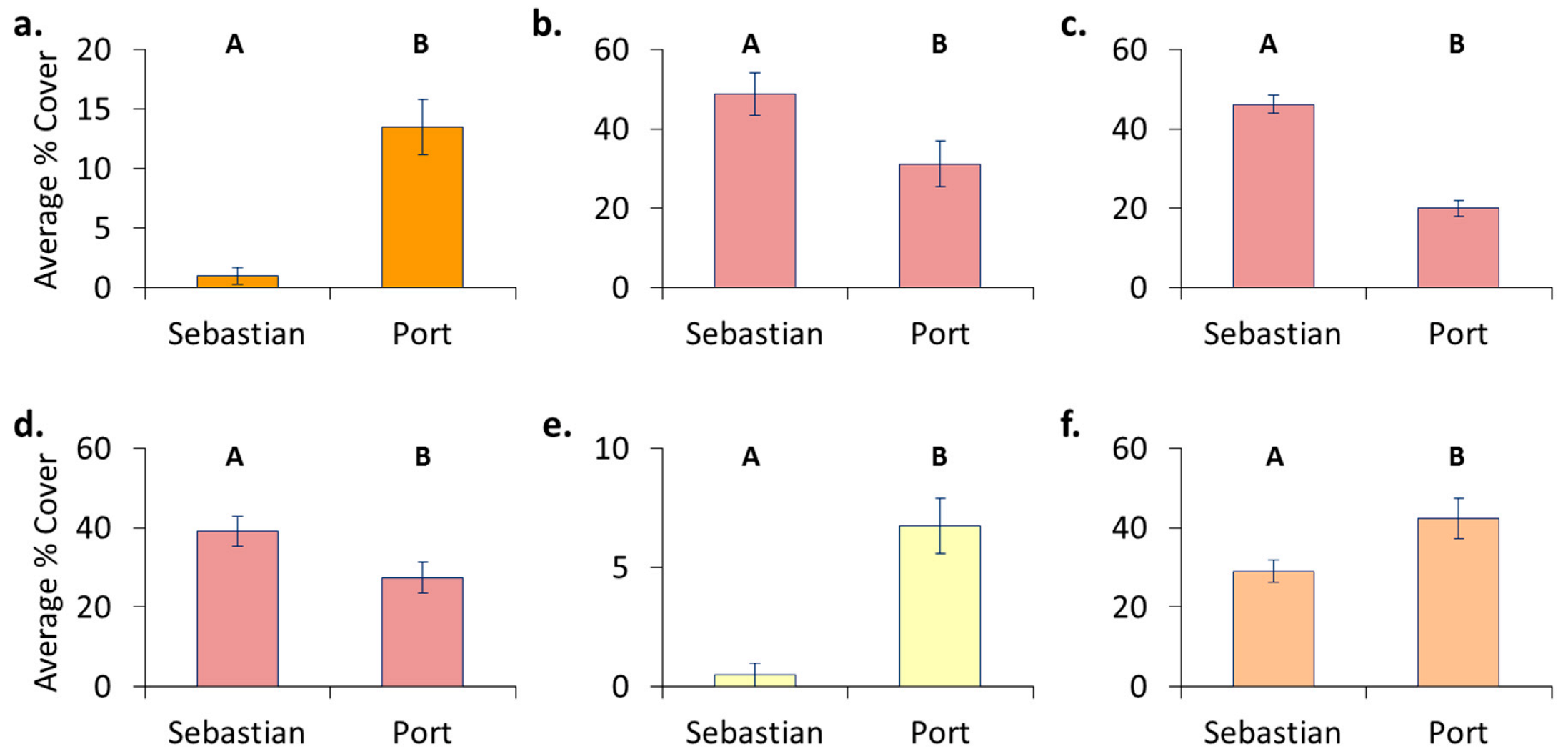
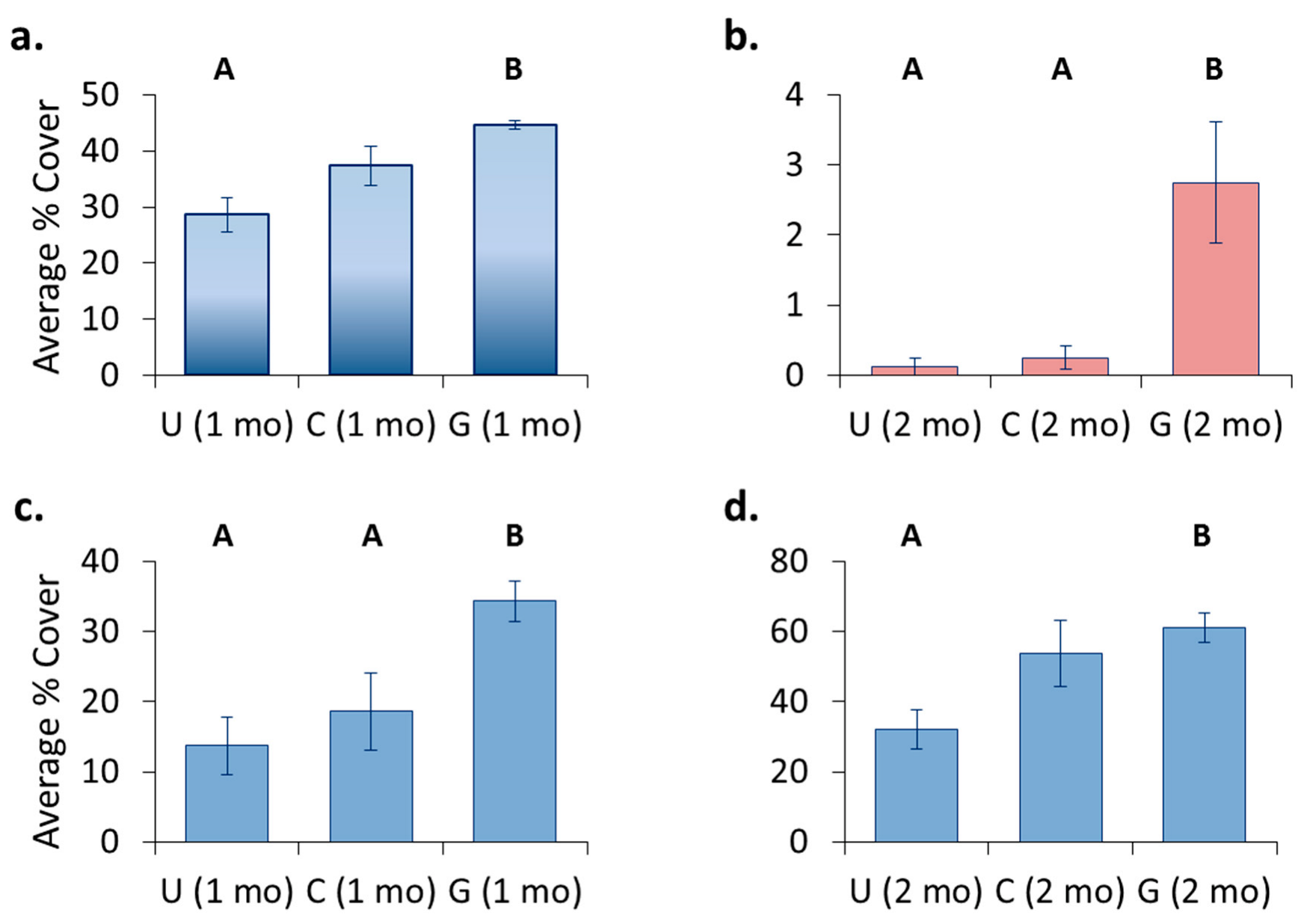
3.4. Location Effects—Post-Transplant
3.5. Treatment Effects—Post-Transplant
4. Discussion
5. Conclusions
- -
- Hull husbandry affects fouling accumulation with groomed surfaces generally having the lowest cover.
- -
- Original location affects recolonization after transplant. Encrusting bryozoans, barnacles, and tunicates all had significantly higher cover on panels which came from a location where these organisms originally dominated, whereas sponges had higher cover on panels that stayed in place.
- -
- Hull husbandry affects recolonization after transplant and the discontinuance of treatment. Encrusting bryozoans preferred surfaces that had been freely fouling, barnacles and sponges preferred surfaces that had been cleaned, and hydroids showed a continuing preference for surfaces that had been groomed.
- -
- Grooming maintains surfaces free of macrofouling for long periods of time [17,48]. Once grooming stops, hydroids and potentially other taxa colonize these surfaces preferentially. The best way to lower the risk of transporting and introducing NISs is to continue grooming. This maintains hulls in a clean condition, which is on target to become an international biosecurity requirement [7,16].
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bax, N.; Williamson, A.; Aguero, M.; Gonzalez, E.; Geeves, W. Marine invasion alien species: A threat to global biodiversity. Mar. Policy 2013, 27, 313–323. [Google Scholar] [CrossRef]
- Dafforn, K.; Glasby, T.; Johnston, E. Differential effects of tributyltin and copper antifoulants on recruitment of non-indigenous species. Biofouling 2008, 24, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, L. NAFTA and member country strategies for maritime trade and invasive species. J. Environ. Manag. 2008, 89, 308–321. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, G.; Forrest, B. Management options for vessel hull fouling: An overview of risks posed by in-water cleaning. ICES J. Mar. Sci. 2008, 65, 811–815. [Google Scholar] [CrossRef]
- Piola, R.; Johnston, E. The potential for translocation of marine species via small-scale disruption to antifouling surfaces. Biofouling 2008, 24, 145–155. [Google Scholar] [CrossRef]
- Hopkins, G.; Forrest, B. A preliminary assessment of biofouling and non-indigenous species associated with commercial slow-moving vessels arriving in New Zealand. Biofouling 2010, 26, 613–621. [Google Scholar] [CrossRef]
- IMO. Guidelines for the Control and Management of Ships’ Biofouling to Minimize the Transfer of Invasive Aquatic Species. MEPC 62/24/Add.1. 2011. Available online: https://wwwcdn.imo.org/localresources/en/KnowledgeCentre/IndexofIMOResolutions/MEPCDocuments/MEPC.207(62).pdf (accessed on 3 March 2012).
- Sylvester, F.; Kalaci, O.; Leung, B.; Lacoursiere-Roussel, A.; Murray, C.; Choi, F.; Bravo, M.; Therriault, T.; MacIsaac, H. Hull fouling as an invasion vector: Can simple models explain a complex problem? J. Appl. Ecol. 2011, 48, 415–423. [Google Scholar] [CrossRef]
- Mack, R.; Simberloff, D.; Lonsdale, W.; Evans, H.; Clout, M.; Bazzaz, F. Biotic invasions: Causes, epidemiology, global consequences, and control. Ecol. Appl. 2000, 10, 689–710. [Google Scholar] [CrossRef]
- Floerl, O.; Inglis, G.; Hayden, B. A risk-based predictive tool to prevent accidental introductions of nonindigenous marine species. Envi. Manag. 2005, 35, 765–778. [Google Scholar] [CrossRef]
- Hewitt, C.; Campbell, M. Mechanisms for the prevention of marine bioinvasions for better biosecurity. Mar. Pollut. Bull. 2007, 55, 395–401. [Google Scholar] [CrossRef]
- Hayes, K.; Sliwa, C. Identifying potential marine pests—A deductive approach applied to Australia. Mar. Pollut. Bull. 2003, 46, 91–98. [Google Scholar] [CrossRef]
- Campbell, M.; Hewitt, C. Assessing the port to port risk of vessel movements vectoring non-indigenous marine species within and across domestic Australian borders. Biofouling 2011, 27, 631–644. [Google Scholar] [CrossRef]
- McClay, T.; Zabin, C.; Davidson, I.; Young, R.; Elam, D. Vessel Biofouling Prevention and Management Options Report; Coast Guard New London ct Research and Development Center: New London, CT, USA, 2015. [Google Scholar]
- Scianni, C.; Georgiades, E. Vessel in-water cleaning or treatment: Identification of environmental risks and science needs for evidence-based decision making. Front. Mar. Sci. 2019, 26, 467. [Google Scholar] [CrossRef]
- Georgiades, E.; Kluza, D.; Bates, T.; Lubarsky, K.; Brunton, J.; Growcott, A.; Smith, T.; McDonald, S.; Gould, B.; Parker, N.; et al. Regulating vessel biofouling to support New Zealand’s marine biosecurity system—A blue print for evidence-based decision making. Front. Mar. Sci. 2020, 19, 390. [Google Scholar] [CrossRef]
- Ralston, E.; Gardner, H.; Hunsucker, K.; Swain, G. The effect of grooming on five commercial antifouling coatings. Front. Mar. Sci. 2022, 9, 836555. [Google Scholar] [CrossRef]
- Dafforn, K.; Lewis, J.; Johnston, E. Antifouling strategies: History and regulation, ecological impacts and mitigation. Mar. Pollut. Bull. 2011, 62, 453–465. [Google Scholar] [CrossRef]
- Schultz, M.; Bendick, J.; Holm, E.; Hertel, W. Economic impact of biofouling on a naval surface ship. Biofouling 2011, 27, 87–98. [Google Scholar] [CrossRef]
- Davidson, I.; Scianni, C.; Hewitt, C.; Everett, R.; Holm, E.; Tamburri, M. Assessing the drivers of ship biofouling management—Aligning industry and biosecurity goals. Biofouling 2016, 32, 411–428. [Google Scholar] [CrossRef]
- Hunsucker, K.; Vora, G.; Hunsucker, J.; Gardner, H.; Leary, D.; Kim, S.; Lin, B. Biofilm community structure and the associated drag penalties of a groomed fouling release ship hull coating. Biofouling 2018, 34, 162–172. [Google Scholar] [CrossRef]
- Hunsucker, K.; Ralston, E.; Gardner, H.; Swain, G. Specialized grooming as a mechanical method to prevent marine invasive species recruitment and transport on ship hulls. In Impacts of Invasive Species on Coastal Environments: Coasts in Crisis; Makowski, C., Finkl, C., Eds.; Springer: Cham, Switzerland, 2019; pp. 247–265. [Google Scholar]
- Marsland, A.; Moss, B. The effects of underwater scrubbing on ship-fouling algae. BSRA Rep. 1975, 42. [Google Scholar]
- Davidson, I.; McCann, L.; Sytsma, M.; Ruiz, G. Interrupting a multi-species bioinvasion vector: The efficacy of in-water cleaning for removing biofouling on obsolete vessels. Mar. Pollut. Bull. 2008, 56, 1538–1544. [Google Scholar] [CrossRef] [PubMed]
- Nickels, J.; Bobbie, R.; Lott, D.; Martz, R.; Benson, P.; White, D. Effect of manual brush cleaning on biomass and community structure of microfouling film formed on aluminum and titanium surfaces exposed to rapidly flowing seawater. Appl. Environ. Microbiol. 1981, 41, 1442–1453. [Google Scholar] [CrossRef]
- Floerl, O.; Inglis, G.; Marsh, H. Selectivity in vector management: An investigation of the effectiveness of measures used to prevent transport of non-indigenous species. Biol. Invasions 2005, 7, 459–475. [Google Scholar] [CrossRef]
- Cologer, C. Six year interaction of underwater cleaning with copper based antifouling paints on Navy ships. Nav. Eng. J. 1984, 96, 200–208. [Google Scholar] [CrossRef]
- Bando, K. The roles of competition and disturbance in a marine invasion. Biol. Invasions 2006, 8, 755–763. [Google Scholar] [CrossRef]
- Hearin, J.; Hunsucker, K.; Swain, G.; Gardner, H.; Stephens, A.; Lieberman, K. Analysis of mechanical grooming at various frequencies on a large scale test panel coated with a fouling release coating. Biofouling 2016, 32, 561–569. [Google Scholar] [CrossRef]
- Pech, D.; Ardisson, P.; Bourget, E. Settlement of a tropical marine epibenthic assemblage on artificial panels: Influence of substratum heterogeneity and complexity scales. Estuar. Coast. Shelf Sci. 2002, 55, 743–750. [Google Scholar] [CrossRef]
- Chabot, R.; Bourget, E. Influence of substratum heterogeneity and settled barnacle density on the settlement of cyprid larvae. Mar. Biol. 1988, 97, 45–56. [Google Scholar] [CrossRef]
- Crisp, D. Overview of research on marine invertebrate larvae, 1940–1980. In Marine Biodeterioration: And Interdisciplinary Study; Costlow, J., Tipper, R., Eds.; Naval Institute Press: Annapolis, MD, USA, 1984. [Google Scholar]
- Callow, M.; Jennings, A.; Brennan, A.; Seegert, C.; Gibson, A.; Wilson, L.; Feinberg, A.; Baney, R.; Callow, J. Microtopographic cues for settlement of zoospores of the green fouling alga Enteromorpha. Biofouling 2002, 18, 237–245. [Google Scholar] [CrossRef]
- Birkeland, C.; Randall, R. Facilitation of coral recruitment by echinoid excavations. Proc. 4th Int. Coral Reef Symp 1981, 1, 695–698. [Google Scholar]
- Highsmith, R. Burrowing by the bivalve mollusk Lithophaga curta in the living reef coral Montipora berryi and a hypothesis of reciprocal larval recruitment. Mar. Biol. 1980, 56, 155–162. [Google Scholar] [CrossRef]
- Maldonado, M.; Uriz, M. Microrefuge exploitation by subtidal encrusting sponges: Patterns of settlement and post-settlement survival. Mar. Ecol. Prog. Ser. 1998, 174, 141–150. [Google Scholar] [CrossRef]
- Walters, L.; Wethey, D. Settlement and early post-settlement survival of sessile marine invertebrates on topographically complex surfaces: The importance of refuge dimensions and adult morphology. Mar. Ecol. Prog. Ser. 1996, 137, 161–171. [Google Scholar] [CrossRef]
- Hopkins, G.; Forrest, B.; Piola, R.; Gardner, J. Factors affecting survivorship of defouled communities and the effect of fragmentation on establishment success. J. Exp. Mar. Biol. Ecol. 2011, 396, 233–243. [Google Scholar] [CrossRef]
- Woods, C.; Floerl, O.; Jones, L. Biosecurity risks associated with in-water and shore-based marine vessel hull cleaning operations. Mar. Pollut. Bull. 2012, 64, 1392–1401. [Google Scholar]
- Bullard, S.; Sedlack, B.; Reinhardt, J.; Litty, C.; Gareau, K.; Whitlatch, R. Fragmentation of colonial ascidians: Differences in reattachment capability among species. J. Exp. Mar. Biol. Ecol. 2007, 342, 166–168. [Google Scholar] [CrossRef]
- Floerl, O.; Coutts, A. Potential ramification of the global economic crisis on human-mediated dispersal of marine non-indigenous species. Mar. Pollut. Bull. 2009, 58, 1595–1598. [Google Scholar]
- Cologer, C.; Bohlander, G.; Preiser, H. Review of underwater cleaning methods and their interaction on Navy anti-fouling paint systems. J. Coat. Technol. 1977, 49, 51–60. [Google Scholar]
- Borchardt, J. Grooming the fleet. Mech. Eng. 2010, 132, 33–35. [Google Scholar] [CrossRef]
- Tribou, M.; Swain, G. The use of proactive in-water grooming to improve the performance of ship hull antifouling coatings. Biofouling 2010, 26, 47–56. [Google Scholar] [CrossRef]
- Hearin, J.; Hunsucker, K.; Swain, G.; Stephens, A.; Gardner, H.; Lieberman, K.; Harper, M. Analysis of long-term mechanical grooming on a large-scale test panel coated with an antifouling and a fouling-release coating. Biofouling 2015, 31, 625–638. [Google Scholar] [CrossRef] [PubMed]
- Hunsucker, K.; Braga, C.; Erdogan, C.; Gardner, H.; Hearin, J.; Ralston, E. The advantages of proactive in-water hull grooming from a biologist’s perspective. In In Proceedings of the HullPIC 18. 3rd Hull Performance & Insight Conference, Redworth, UK, 12–14 March 2018; pp. 210–222. [Google Scholar]
- Swain, G.; Erdogan, C.; Foy, L.; Gardner, H.; Harper, M.; Hearin, J.; Hunsucker, K.; Hunsucker, J.; Lieberman, K.; Nanney, M.; et al. Proactive in-water ship hull grooming as a method to reduce the environmental footprint of ships. Front. Mar. Sci. 2022, 8, 2017. [Google Scholar] [CrossRef]
- Ralston, E.; Swain, G. The ghost of fouling communities past: The effect of original community on subsequent recruitment. Biofouling 2014, 30, 459–471. [Google Scholar] [CrossRef] [PubMed]
- Kolle, S.; Ahanotu, O.; Meeks, A.; Stafslien, S.; Kreder, M.; Vanderwal, L.; Cohen, L.; Waltz, G.; Lim, C.; Slocum, D.; et al. On the mechanism of marine fouling-prevention performance of oil-containing silicone elastomers. Sci. Rep. 2022, 12, 11799. [Google Scholar] [CrossRef]
- Hunsucker, K.; Swain, G. In situ measurements of diatom adhesion to silicone-based ship hull coatings. J. Appl. Phycol. 2016, 28, 269–277. [Google Scholar] [CrossRef]
- Lejars, M.; Margaillan, A.; Bressy, C. Fouling Release Coatings: A nontoxic alternative to biocidal antifouling coatings. Chem. Rev. 2012, 8, 4347–4390. [Google Scholar] [CrossRef]
- Tribou, M.; Swain, G. The effects of grooming on a copper ablative coatin: A six year study. Biofouling 2017, 33, 494–504. [Google Scholar] [CrossRef]
- Clark, G.; Johnston, E. Propagule pressure and disturbance interact to overcome biotic resistance of marine invertebrate communities. Oikos 2009, 118, 1679–1686. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ralston, E.; Swain, G. The Effect of Husbandry and Original Location on the Fouling of Transplanted Panels. J. Mar. Sci. Eng. 2023, 11, 478. https://doi.org/10.3390/jmse11030478
Ralston E, Swain G. The Effect of Husbandry and Original Location on the Fouling of Transplanted Panels. Journal of Marine Science and Engineering. 2023; 11(3):478. https://doi.org/10.3390/jmse11030478
Chicago/Turabian StyleRalston, Emily, and Geoffrey Swain. 2023. "The Effect of Husbandry and Original Location on the Fouling of Transplanted Panels" Journal of Marine Science and Engineering 11, no. 3: 478. https://doi.org/10.3390/jmse11030478
APA StyleRalston, E., & Swain, G. (2023). The Effect of Husbandry and Original Location on the Fouling of Transplanted Panels. Journal of Marine Science and Engineering, 11(3), 478. https://doi.org/10.3390/jmse11030478







