Response of Foraminifera to Anthropogenic Nicotine Pollution of Cigarette Butts: An Experimental Approach
Abstract
:1. Introduction
2. Materials and Methods
2.1. Foraminiferal Species
2.2. Experimental Design
2.3. High Performance Liquid Chromatography Analysis (HPLC)
2.4. Fourier Transform Infrared Analyses
3. Results
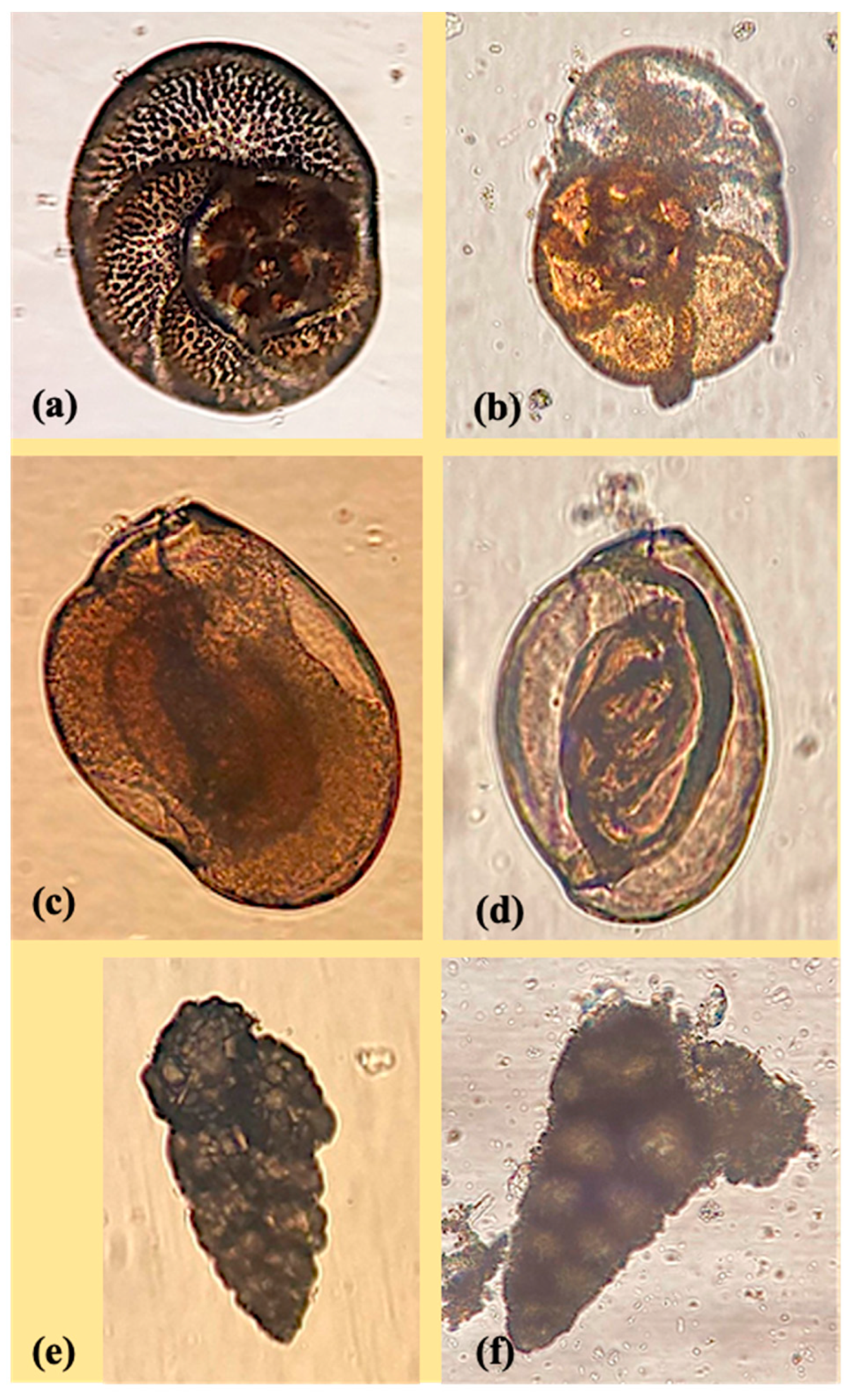
3.1. Foraminiferal Acute 48 h Toxicological Tests
3.2. High Performance Liquid Chromatography
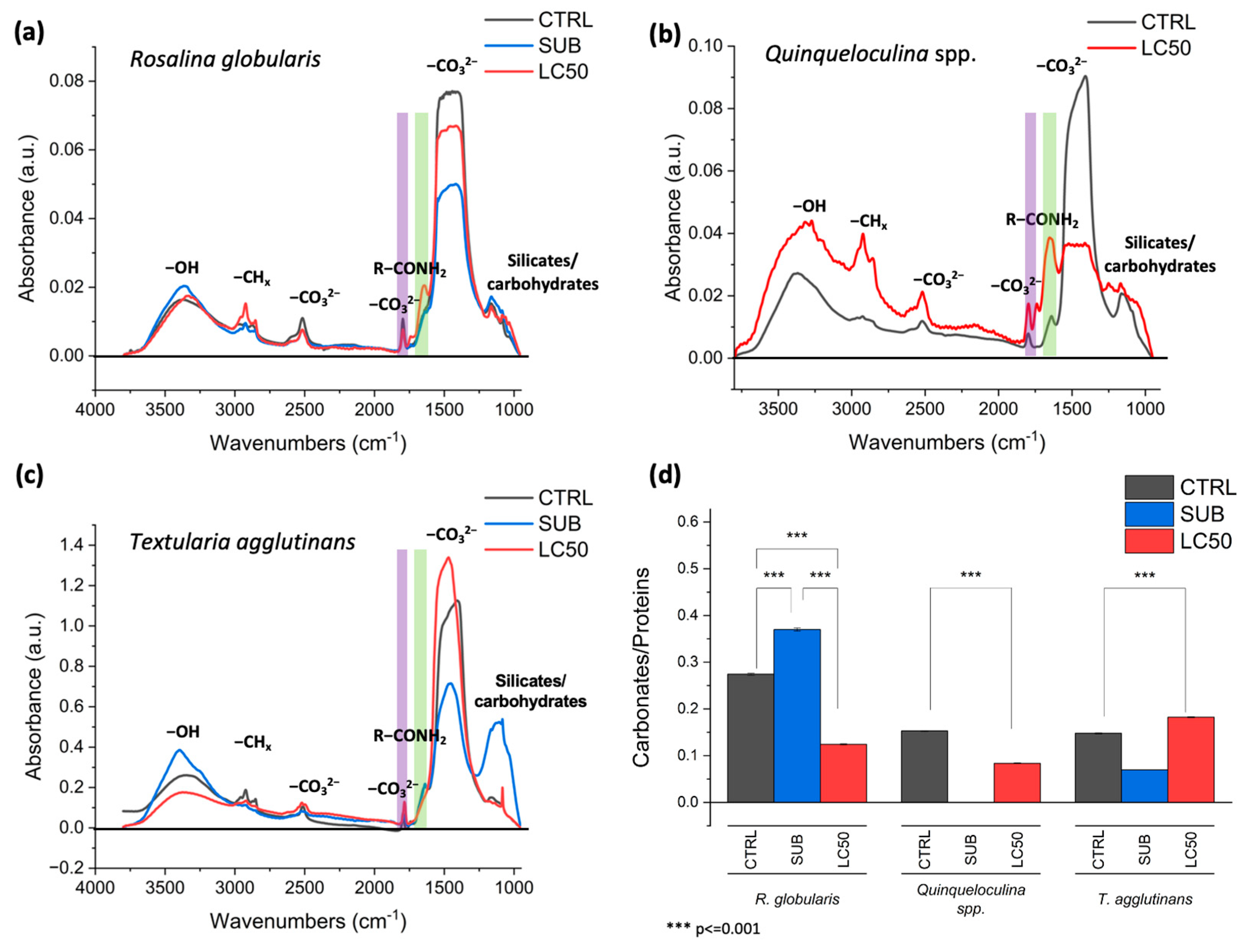
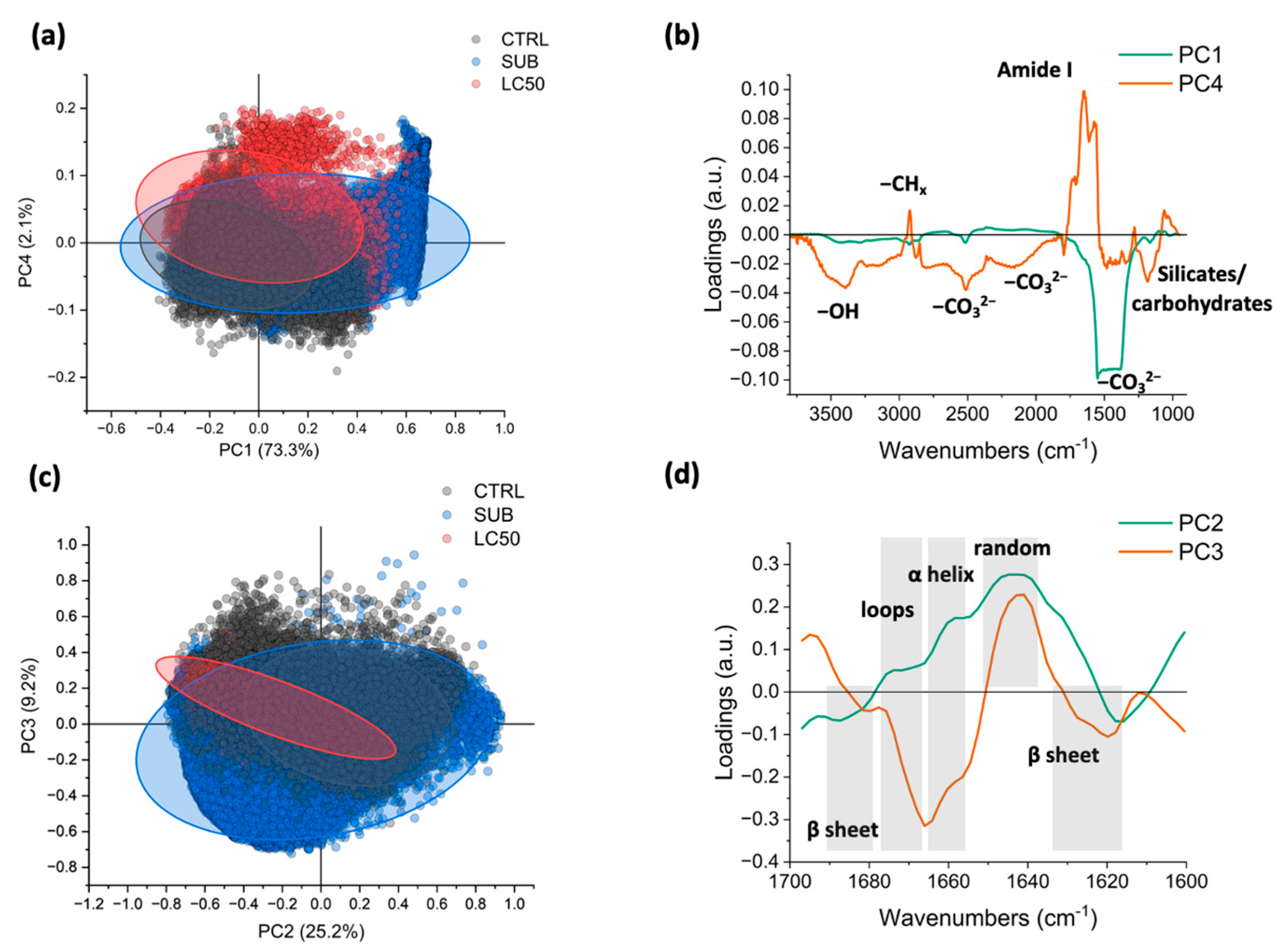
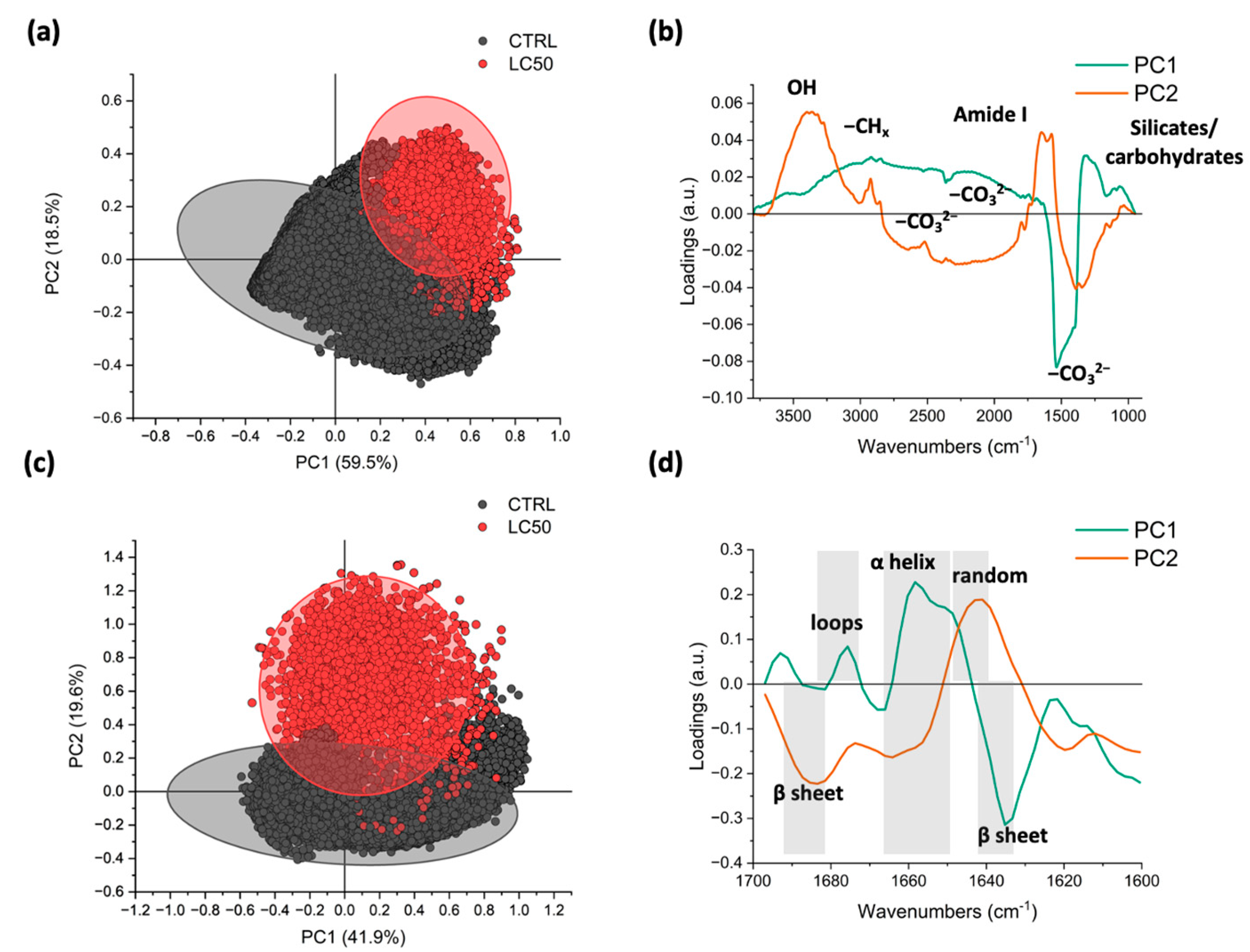
3.3. Fourier Transform Infrared (FTIR)
4. Discussion
4.1. Nicotine and Its Effect on the Foraminiferal Vitality
4.2. Nicotine and Its Effect on Foraminiferal Macromolecular Composition
4.3. Nicotine and Its Effect on Foraminiferal Decalcification
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
- High Performance Liquid Chromatography analysis (HPLC)
- Nicotine salt (nicotine hemisulfate salt, 35% w/v in H2O, Sigma-Aldrich, St. Louis, MO, USA) standard was made with 20 mM in buffer A.
- Free base nicotine (≥99% (TLC), liquid, Sigma-Aldrich, St. Louis, MO, USA) base standard was made with 20 mM in buffer A.
- Cotinine (≥98%, Sigma-Aldrich, St. Louis, MO, USA) standard was made with 20 nM in buffer A.
- Gradient curve
- 40% methanol
- T = 45.9 °C
- Mix 1 = nicotine salt (5 µL) + cotinine (5 µL) + mobile phase B with 18% methanol (990 µL)
- Mix 2 = nicotine salt (5 µL) + cotinine (5 µL) + mobile phase B with 18% methanol (990 µL)
- Mix 3 = nicotine salt (5 µL) + cotinine (5 µL) + pure nicotine (5 µL) + mobile phase B with 18% methanol (958 µL).
- (1)
- Lyophilization.
- (2)
- Mixing for 10 min, adding to each sample 4 mL of mobile phase (2 mL of phase A and 2 mL of phase B) with 20% methanol.
- (3)
- Sonication for 1 min, keeping the samples on ice.
- (4)
- Centrifuging for 10 min (at 22,000 rpm) and supernatant was utilized for HPLC analysis.
- Synthetic nicotine determination
Appendix B
| Foraminiferal Species | Replicates | mL Nicotine (HSO4-) | Area (mAu) | nmoles | Initial Mmoles of Synthetic Nicotine in Solution Samples | Final Mmoles of Synthetic Nicotine in Solution Samples | Nicotine Recovery Rate (%) |
| Rosalina globularis | Control | 0 | 0 | 0 | 0 | 0 | 0 |
| R1—sublethal | 2.24 | 641.041 | 2.93 | 2.1 | 0.4 | 18.3 | |
| R1—sublethal | 2.24 | 1111.953 | 5.08 | 2.1 | 0.7 | 31.8 | |
| R1—sublethal | 2.24 | 595.455 | 2.72 | 2.1 | 0.4 | 17.0 | |
| R1—LC50 | 4.2 | 1030.853 | 4.71 | 4.0 | 0.6 | 15.7 | |
| R1—LC50 | 4.2 | 902.317 | 4.12 | 4.0 | 0.5 | 13.7 | |
| R1—LC50 | 4.2 | 1269.829 | 5.8 | 4.0 | 0.8 | 19.3 | |
| Quinqueloculina spp. | Control | 0 | 0 | 0 | 0 | 0 | 0 |
| R1—sublethal | 4.46 | 901.549 | 4.12 | 4.2 | 0.5 | 12.9 | |
| R1—sublethal | 4.46 | 945.711 | 4.32 | 4.2 | 0.6 | 13.6 | |
| R1—sublethal | 4.46 | 1139.925 | 5.21 | 4.2 | 0.7 | 16.4 | |
| R1—LC50 | 16.0 | 3509.625 | 16.04 | 15.2 | 2.1 | 14.0 | |
| R1—LC50 | 16.0 | 3906.988 | 17.86 | 15.2 | 2.4 | 15.6 | |
| R1—LC50 | 16.0 | 3535.302 | 16.16 | 15.2 | 2.2 | 14.1 | |
| Textularia agglutinans | Control | 0 | 0 | 0 | 0 | 0 | 0 |
| R1—sublethal | 4.46 | 743.195 | 3.40 | 4.2 | 0.5 | 10.7 | |
| R1—sublethal | 4.46 | 1107.947 | 5.06 | 4.2 | 0.7 | 15.9 | |
| R1—sublethal | 4.46 | 1058.257 | 4.84 | 4.2 | 0.6 | 15.2 | |
| R1—LC50 | 12.5 | 2990.652 | 13.67 | 11.9 | 1.8 | 15.3 | |
| R1—LC50 | 12.5 | 2746.864 | 12.56 | 11.9 | 1.7 | 14.1 | |
| R1—LC50 | 12.5 | 2658.851 | 12.15 | 11.9 | 1.6 | 13.6 |
References
- Cau, A.; Avio, C.G.; Dessì, C.; Moccia, D.; Pusceddu, A.; Regoli, F.; Cannas, R.; Follesa, M.C. Benthic Crustacean Digestion Can Modulate the Environmental Fate of Microplastics in the Deep Sea. Environ. Sci. Technol. 2020, 54, 4886–4892. [Google Scholar] [CrossRef] [PubMed]
- Asensio-Montesinos, F.; Anfuso, G.; Williams, A.T. Beach Litter Distribution along the Western Mediterranean Coast of Spain. Mar. Pollut. Bull. 2019, 141, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Becherucci, M.E.; Rosenthal, A.F.; Pon, J.P.S. Marine Debris in Beaches of the Southwestern Atlantic: An Assessment of Their Abundance and Mass at Different Spatial Scales in Northern Coastal Argentina. Mar. Pollut. Bull. 2017, 119, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Wilson, S.P.; Verlis, K.M. The Ugly Face of Tourism: Marine Debris Pollution Linked to Visitation in the Southern Great Barrier Reef, Australia. Mar. Pollut. Bull. 2017, 117, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Araújo, M.C.B.; Costa, M.F. A Critical Review of the Issue of Cigarette Butt Pollution in Coastal Environments. Environ. Res. 2019, 172, 137–149. [Google Scholar] [CrossRef]
- Li, L.; Yang, D.C.; Chen, C.-H. Metabolic Reprogramming: A Driver of Cigarette Smoke-Induced Inflammatory Lung Diseases. Free Radic. Biol. Med. 2021, 163, 392–401. [Google Scholar] [CrossRef]
- Torkashvand, J.; Farzadkia, M.; Sobhi, H.R.; Esrafili, A. Littered Cigarette Butt as a Well-Known Hazardous Waste: A Comprehensive Systematic Review. J. Hazard. Mater. 2020, 383, 121242. [Google Scholar] [CrossRef]
- Ockene, I.S.; Miller, N.H. Cigarette Smoking, Cardiovascular Disease, and Stroke: A Statement for Healthcare Professionals from the American Heart Association. Circulation 1997, 96, 3243–3247. [Google Scholar] [CrossRef]
- Dobaradaran, S.; Soleimani, F.; Akhbarizadeh, R.; Schmidt, T.C.; Marzban, M.; BasirianJahromi, R. Environmental Fate of Cigarette Butts and Their Toxicity in Aquatic Organisms: A Comprehensive Systematic Review. Environ. Res. 2021, 195, 110881. [Google Scholar] [CrossRef]
- Rebischung, F.; Chabot, L.; Biaudet, H.; Pandard, P. Cigarette Butts: A Small but Hazardous Waste, According to European Regulation. Waste Manag. 2018, 82, 9–14. [Google Scholar] [CrossRef]
- Bonanomi, G.; Maisto, G.; De Marco, A.; Cesarano, G.; Zotti, M.; Mazzei, P.; Libralato, G.; Staropoli, A.; Siciliano, A.; De Filippis, F. The Fate of Cigarette Butts in Different Environments: Decay Rate, Chemical Changes and Ecotoxicity Revealed by a 5-Years Decomposition Experiment. Environ. Pollut. 2020, 261, 114108. [Google Scholar] [CrossRef]
- Lucia, G.; Giuliani, M.E.; d’Errico, G.; Booms, E.; Benedetti, M.; Di Carlo, M.; Fattorini, D.; Gorbi, S.; Regoli, F. Toxicological Effects of Cigarette Butts for Marine Organisms. Environ. Int. 2023, 171, 107733. [Google Scholar] [CrossRef]
- Quéméneur, M.; Chifflet, S.; Akrout, F.; Bellaaj-Zouari, A.; Belhassen, M. Impact of Cigarette Butts on Microbial Diversity and Dissolved Trace Metals in Coastal Marine Sediment. Estuar. Coast. Shelf Sci. 2020, 240, 106785. [Google Scholar] [CrossRef]
- Green, D.S.; Kregting, L.; Boots, B. Effects of Cigarette Butts on Marine Keystone Species (Ulva lactuca L. and Mytilus edulis L.) and Sediment Microphytobenthos. Mar. Pollut. Bull. 2021, 165, 112152. [Google Scholar] [CrossRef]
- Booth, D.J.; Gribben, P.; Parkinson, K. Impact of Cigarette Butt Leachate on Tidepool Snails. Mar. Pollut. Bull. 2015, 95, 362–364. [Google Scholar] [CrossRef] [PubMed]
- Wright, S.L.; Rowe, D.; Reid, M.J.; Thomas, K.V.; Galloway, T.S. Bioaccumulation and Biological Effects of Cigarette Litter in Marine Worms. Sci. Rep. 2015, 5, 14119. [Google Scholar] [CrossRef] [PubMed]
- Soleimani, F.; Dobaradaran, S.; Vazirizadeh, A.; Mohebbi, G.; Ramavandi, B.; De-la-Torre, G.E.; Nabipour, I.; Schmidt, T.C.; Novotny, T.E.; Maryamabadi, A. Chemical Contents and Toxicity of Cigarette Butts Leachates in Aquatic Environment: A Case Study from the Persian Gulf Region. Chemosphere 2023, 311, 137049. [Google Scholar] [CrossRef]
- Caridi, F.; Sabbatini, A.; Birarda, G.; Costanzi, E.; De Giudici, G.; Galeazzi, R.; Medas, D.; Mobbili, G.; Ricciutelli, M.; Ruello, M.L. Cigarette Butts, a Threat for Marine Environments: Lessons from Benthic Foraminifera (Protista). Mar. Environ. Res. 2020, 162, 105150. [Google Scholar] [CrossRef]
- Matta, S.G.; Balfour, D.J.; Benowitz, N.L.; Boyd, R.T.; Buccafusco, J.J.; Caggiula, A.R.; Craig, C.R.; Collins, A.C.; Damaj, M.I.; Donny, E.C. Guidelines on Nicotine Dose Selection for in Vivo Research. Psychopharmacology 2007, 190, 269–319. [Google Scholar] [CrossRef] [PubMed]
- Keul, N.; Schmidt, S. Calcification in Foraminifera Largely Supported by Ion Channels-Biomineralization Pathways and Their Effect on Trace Elemental Composition. In AGU Fall Meeting Abstracts; American Geophysical Union: Washington, DC, USA, 2018; Volume 2018, p. PP43A-02. [Google Scholar]
- Birarda, G.; Buosi, C.; Caridi, F.; Casu, M.A.; De Giudici, G.; Di Bella, L.; Medas, D.; Meneghini, C.; Pierdomenico, M.; Sabbatini, A. Plastics, (Bio)Polymers and Their Apparent Biogeochemical Cycle: An Infrared Spectroscopy Study on Foraminifera. Environ. Pollut. 2021, 279, 116912. [Google Scholar] [CrossRef] [PubMed]
- Murray, J.W. Ecology and Applications of Benthic Foraminifera; Cambridge University Press: Cambridge, UK, 2006. [Google Scholar]
- Ross, B.J.; Hallock, P. Challenges in Using CellTracker Green on Foraminifers That Host Algal Endosymbionts. PeerJ 2018, 6, e5304. [Google Scholar] [CrossRef] [PubMed]
- Bernhard, J.M.; Ostermann, D.R.; Williams, D.S.; Blanks, J.K. Comparison of Two Methods to Identify Live Benthic Foraminifera: A Test between Rose Bengal and CellTracker Green with Implications for Stable Isotope Paleoreconstructions. Paleoceanography 2006, 21, PA4210. [Google Scholar] [CrossRef]
- Ross, B. Responses to Chemical Exposure by Foraminifera: Distinguishing Dormancy from Mortality; University of South Florida: Tampa, FL, USA, 2012. [Google Scholar]
- Massadeh, A.M.; Gharaibeh, A.A.; Omari, K.W. A single-step extraction method for the determination of nicotine and cotinine in Jordanian smokers’ blood and urine samples by RP-HPLC and GC–MS. J. Chromatogr. Sci. 2009, 47, 170–177. [Google Scholar]
- Konar, S.K. Histopathological Effects of the Insecticides, Heptachlor and Nicotine, on the Gills of the Catfish, Heteropneustes Fossilis. Jpn. J. Ichthyol. 1969, 15, 156–159. [Google Scholar]
- Konar, S.K. Toxicity of Nicotine to Aquatic Life. Indian J. Fish. Ernakulam 1977, 24, 124–128. [Google Scholar]
- Chang, P.-H.; Chiang, C.-H.; Ho, W.-C.; Wu, P.-Z.; Tsai, J.-S.; Guo, F.-R. Combination Therapy of Varenicline with Nicotine Replacement Therapy Is Better than Varenicline Alone: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. BMC Public. Health 2015, 15, 1–8. [Google Scholar] [CrossRef]
- Cecchini, M.; Changeux, J.-P. The Nicotinic Acetylcholine Receptor and Its Prokaryotic Homologues: Structure, Conformational Transitions & Allosteric Modulation. Neuropharmacology 2015, 96, 137–149. [Google Scholar]
- de Nooijer, L.J.; Toyofuku, T.; Oguri, K.; Nomaki, H.; Kitazato, H. Intracellular PH Distribution in Foraminifera Determined by the Fluorescent Probe HPTS. Limnol. Oceanogr. Methods 2008, 6, 610–618. [Google Scholar] [CrossRef]
- De Nooijer, L.J.; Toyofuku, T.; Kitazato, H. Foraminifera Promote Calcification by Elevating Their Intracellular PH. Proc. Natl. Acad. Sci. USA 2009, 106, 15374–15378. [Google Scholar] [CrossRef]
- Zamora-Duran, M.A.; Aronson, R.B.; Leichter, J.J.; Flannery, J.A.; Richey, J.N.; Toth, L.T. Imprint of Regional Oceanography on Foraminifera of Eastern Pacific Coral Reefs. J. Foraminifer. Res. 2020, 50, 279–290. [Google Scholar] [CrossRef]
- Erez, J. The Source of Ions for Biomineralization in Foraminifera and Their Implications for Paleoceanographic Proxies. Rev. Mineral. Geochem. 2003, 54, 115–149. [Google Scholar] [CrossRef]
- de Nooijer, L.J.; Spero, H.J.; Erez, J.; Bijma, J.; Reichart, G.-J. Biomineralization in Perforate Foraminifera. Earth-Sci. Rev. 2014, 135, 48–58. [Google Scholar] [CrossRef]
- Barth, A. Infrared Spectroscopy of Proteins. Biochim. Biophys. Acta (BBA)-Bioenerg. 2007, 1767, 1073–1101. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.; Zhao, H.; Liu, T.; Li, L.; Cheng, E.; Zhi, S.; Kong, L.; Yao, H.-W.; Li, J. Cigarette Smoke Reduces Fatty Acid Catabolism, Leading to Apoptosis in Lung Endothelial Cells: Implication for Pathogenesis of COPD. Front. Pharmacol. 2019, 10, 941. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Lin, Y.; Luna, K.; Logue, A.; Yoon, A.J.; Haptonstall, K.P.; Moheimani, R.; Choroomi, Y.; Nguyen, K.; Tran, E. Electronic and Tobacco Cigarettes Alter Polyunsaturated Fatty Acids and Oxidative Biomarkers. Circ. Res. 2021, 129, 514–526. [Google Scholar] [CrossRef] [PubMed]
- Ciacci, C.; Grimmelpont, M.V.; Corsi, I.; Bergami, E.; Curzi, D.; Burini, D.; Bouchet, V.M.; Ambrogini, P.; Gobbi, P.; Ujiié, Y. Nanoparticle-Biological Interactions in a Marine Benthic Foraminifer. Sci. Rep. 2019, 9, 19441. [Google Scholar] [CrossRef] [PubMed]
- Le Cadre, V.; Debenay, J.-P. Morphological and Cytological Responses of Ammonia (Foraminifera) to Copper Contamination: Implication for the Use of Foraminifera as Bioindicators of Pollution. Environ. Pollut. 2006, 143, 304–317. [Google Scholar] [CrossRef]
- Murphy Jr, G.; Rouse, R.L.; Polk, W.W.; Henk, W.G.; Barker, S.A.; Boudreaux, M.J.; Floyd, Z.E.; Penn, A.L. Combustion-Derived Hydrocarbons Localize to Lipid Droplets in Respiratory Cells. Am. J. Respir. Cell Mol. Biol. 2008, 38, 532–540. [Google Scholar] [CrossRef]
- Boren, J.; Brindle, K.M. Apoptosis-Induced Mitochondrial Dysfunction Causes Cytoplasmic Lipid Droplet Formation. Cell Death Differ. 2012, 19, 1561–1570. [Google Scholar] [CrossRef]
- Al-Saffar, N.M.S.; Titley, J.C.; Robertson, D.; Clarke, P.A.; Jackson, L.E.; Leach, M.O.; Ronen, S.M. Apoptosis Is Associated with Triacylglycerol Accumulation in Jurkat T-Cells. Br. J. Cancer 2002, 86, 963–970. [Google Scholar] [CrossRef]
- Schmitz, J.E.; Kettunen, M.I.; Hu, D.-E.; Brindle, K.M. 1H MRS-Visible Lipids Accumulate during Apoptosis of Lymphoma Cells in Vitro and in Vivo. Magn. Reson. Med. Off. J. Int. Soc. Magn. Reson. Med. 2005, 54, 43–50. [Google Scholar] [CrossRef]
- Cristea, I.M.; Degli Esposti, M. Membrane Lipids and Cell Death: An Overview. Chem. Phys. Lipids 2004, 129, 133–160. [Google Scholar] [CrossRef] [PubMed]
- Crimi, M.; Degli Esposti, M. Apoptosis-Induced Changes in Mitochondrial Lipids. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2011, 1813, 551–557. [Google Scholar] [CrossRef] [PubMed]
- Pati, P.; Patra, P.K. Benthic foraminiferal responses to coastal pollution: A review. Int. J. Geol. 2012, 2, 42–56. [Google Scholar]
- Loeblich, A.R.; Tappan, H. Foraminiferal Classification and Evolution. Geol. Soc. India 1964, 5, 5–40. [Google Scholar]
- Traverse, A. Paleopalynology; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2007; Volume 28. [Google Scholar]
- Banner, F.T. Test Structure, Organic Skeleton and Extrathalamous Cytoplasm of Ammonia Brunnich. J. Foraminifer. Res. 1973, 3, 49–69. [Google Scholar] [CrossRef]
- Sabbatini, A.; Morigi, C.; Nardelli, M.P.; Negri, A. Foraminifera. In The Mediterranean Sea; Goffredo, S., Dubinsky, Z., Eds.; Springer Netherlands: Dordrecht, The Netherland, 2014; pp. 237–256. ISBN 978-94-007-6703-4. [Google Scholar]
- Krall, E.A.; Dawson-Hughes, B. Smoking Increases Bone Loss and Decreases Intestinal Calcium Absorption. J. Bone Min. Res. 1999, 14, 215–220. [Google Scholar] [CrossRef]
- Tanaka, H.; Tanabe, N.; Kawato, T.; Nakai, K.; Kariya, T.; Matsumoto, S.; Zhao, N.; Motohashi, M.; Maeno, M. Nicotine Affects Bone Resorption and Suppresses the Expression of Cathepsin K, MMP-9 and Vacuolar-Type H+-ATPase D2 and Actin Organization in Osteoclasts. PLoS ONE 2013, 8, e59402. [Google Scholar] [CrossRef]
- Costa-Rodrigues, J.; Rocha, I.; Fernandes, M.H. Complex Osteoclastogenic Inductive Effects of Nicotine over Hydroxyapatite. J. Cell Physiol. 2018, 233, 1029–1040. [Google Scholar] [CrossRef]
- Tanaka, H.; Tanabe, N.; Suzuki, N.; Shoji, M.; Torigoe, H.; Sugaya, A.; Motohashi, M.; Maeno, M. Nicotine Affects Mineralized Nodule Formation by the Human Osteosarcoma Cell Line Saos-2. Life Sci. 2005, 77, 2273–2284. [Google Scholar] [CrossRef]
- De Goeyse, S.; Webb, A.E.; Reichart, G.-J.; De Nooijer, L.J. Carbonic Anhydrase Is Involved in Calcification by the Benthic Foraminifer Amphistegina lessonii. Biogeosciences 2021, 18, 393–401. [Google Scholar] [CrossRef]
- Toyofuku, T.; Matsuo, M.Y.; De Nooijer, L.J.; Nagai, Y.; Kawada, S.; Fujita, K.; Reichart, G.-J.; Nomaki, H.; Tsuchiya, M.; Sakaguchi, H.; et al. Proton Pumping Accompanies Calcification in Foraminifera. Nat. Commun. 2017, 8, 14145. [Google Scholar] [CrossRef] [PubMed]
- Zeebe, R.E.; Ridgwell, A.; Zachos, J.C. Anthropogenic Carbon Release Rate Unprecedented during the Past 66 Million Years. Nat. Geosci. 2016, 9, 325–329. [Google Scholar] [CrossRef]
- Prazeres, M.; Uthicke, S.; Pandolfi, J.M. Ocean Acidification Induces Biochemical and Morphological Changes in the Calcification Process of Large Benthic Foraminifera. Proc. R. Soc. B 2015, 282, 20142782. [Google Scholar] [CrossRef]
- Byrne, M.; Fitzer, S. The Impact of Environmental Acidification on the Microstructure and Mechanical Integrity of Marine Invertebrate Skeletons. Conserv. Physiol. 2019, 7, coz062. [Google Scholar] [CrossRef]


| Species | Rosalina globularis | Quinqueloculina spp. | Textularia agglutinans | References |
|---|---|---|---|---|
| CTRL | 0 | 0 | 0 | |
| SUBLETHAL | ||||
| CBs (mg/L) | 1 | 2 | 2 | [18] |
| Synthetic Nicotine (mg/L) | 1.96 | 3.91 | 3.91 | Current study |
| LC50 | ||||
| CBs (mg/L) | 1.9 | 7.2 | 5.6 | [18] |
| Synthetic Nicotine (mg/L) | 3.72 | 14.11 | 10.98 | Current study |
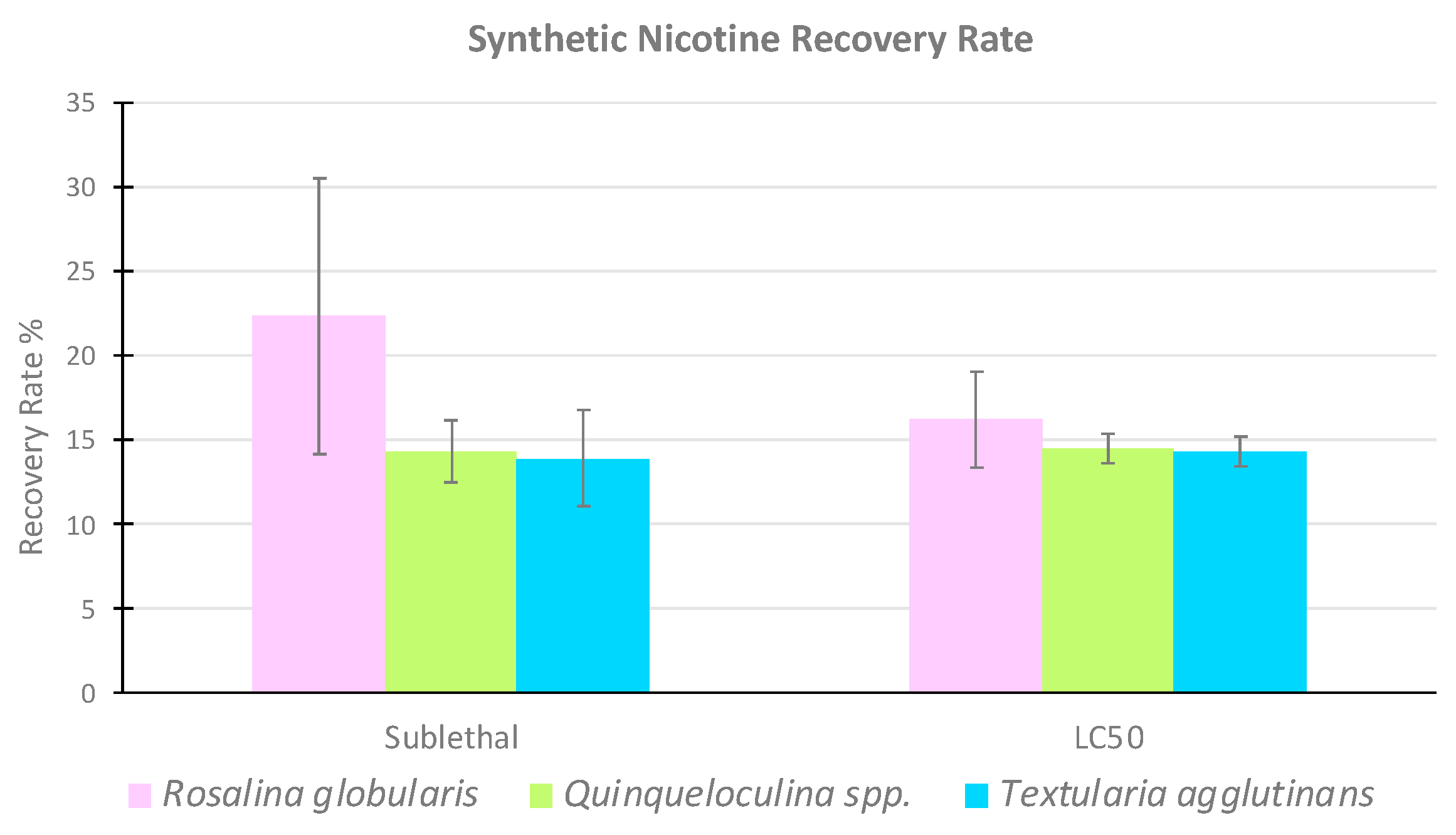
| Foraminiferal Species | Replicates | Synthetic Nicotine Recovery Rate (%) | ||
|---|---|---|---|---|
| Rosalina globularis | CTRL | Sublethal | LC50 | |
| R1 | 0 | 18.3 | 15.7 | |
| R2 | 0 | 31.8 | 13.7 | |
| R3 | 0 | 17.0 | 19.3 | |
| Medium value | 22.36 | 16.23 | ||
| Standard deviation | 8.20 | 2.84 | ||
| Quinqueloculina spp. | CTRL | Sublethal | LC50 | |
| R1 | 0 | 12.9 | 14.0 | |
| R2 | 0 | 13.6 | 15.6 | |
| R3 | 0 | 16.4 | 14.1 | |
| Medium value | 14.3 | 14.5 | ||
| Standard deviation | 1.85 | 0.90 | ||
| Textularia agglutinans | CTRL | Sublethal | LC50 | |
| R1 | 0 | 10.7 | 15.3 | |
| R2 | 0 | 15.9 | 14.1 | |
| R3 | 0 | 15.2 | 13.6 | |
| Medium value | 13.93 | 14.33 | ||
| Standard deviation | 2.82 | 0.87 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sabbatini, A.; Caridi, F.; Birarda, G.; Costanzi, E.; Amici, A.; Mobbili, G.; Buosi, C.; De Giudici, G.; Medas, D.; Negri, A. Response of Foraminifera to Anthropogenic Nicotine Pollution of Cigarette Butts: An Experimental Approach. J. Mar. Sci. Eng. 2023, 11, 1951. https://doi.org/10.3390/jmse11101951
Sabbatini A, Caridi F, Birarda G, Costanzi E, Amici A, Mobbili G, Buosi C, De Giudici G, Medas D, Negri A. Response of Foraminifera to Anthropogenic Nicotine Pollution of Cigarette Butts: An Experimental Approach. Journal of Marine Science and Engineering. 2023; 11(10):1951. https://doi.org/10.3390/jmse11101951
Chicago/Turabian StyleSabbatini, Anna, Francesca Caridi, Giovanni Birarda, Elisa Costanzi, Adolfo Amici, Giovanna Mobbili, Carla Buosi, Giovanni De Giudici, Daniela Medas, and Alessandra Negri. 2023. "Response of Foraminifera to Anthropogenic Nicotine Pollution of Cigarette Butts: An Experimental Approach" Journal of Marine Science and Engineering 11, no. 10: 1951. https://doi.org/10.3390/jmse11101951
APA StyleSabbatini, A., Caridi, F., Birarda, G., Costanzi, E., Amici, A., Mobbili, G., Buosi, C., De Giudici, G., Medas, D., & Negri, A. (2023). Response of Foraminifera to Anthropogenic Nicotine Pollution of Cigarette Butts: An Experimental Approach. Journal of Marine Science and Engineering, 11(10), 1951. https://doi.org/10.3390/jmse11101951









