An Integrative Taxonomic Survey of Benthic Foraminiferal Species (Protista, Rhizaria) from the Eastern Clarion-Clipperton Zone
Abstract
1. Introduction
2. Materials and Methods
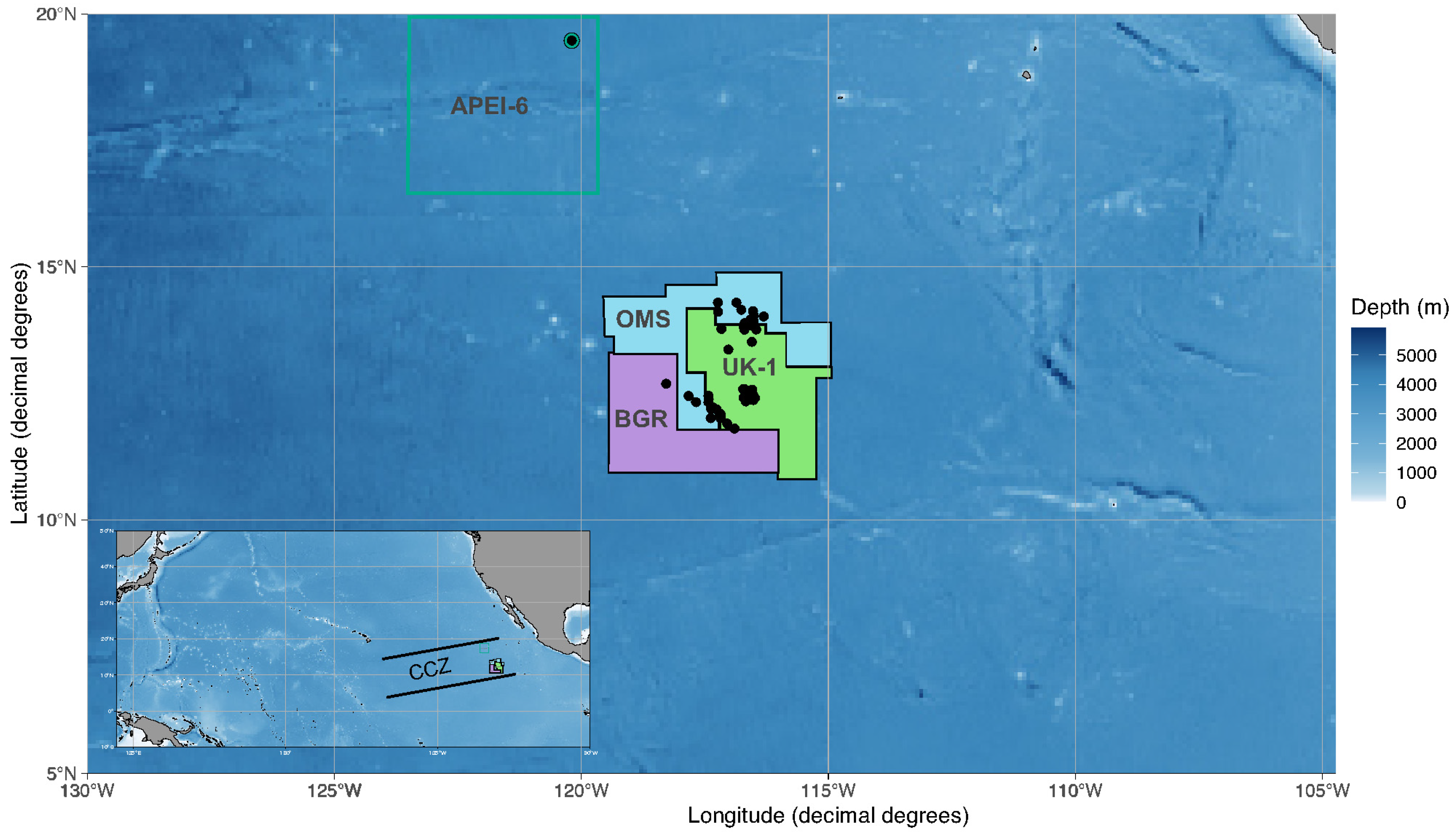
2.1. Sample Collection and Ship-Board Processing
2.2. Preparation for Analyses
2.3. DNA Extraction, PCR Amplification and Sanger Sequencing
2.4. High-Throughput Sequencing (HTS) and Analysis
2.5. Phylogenetic Analysis
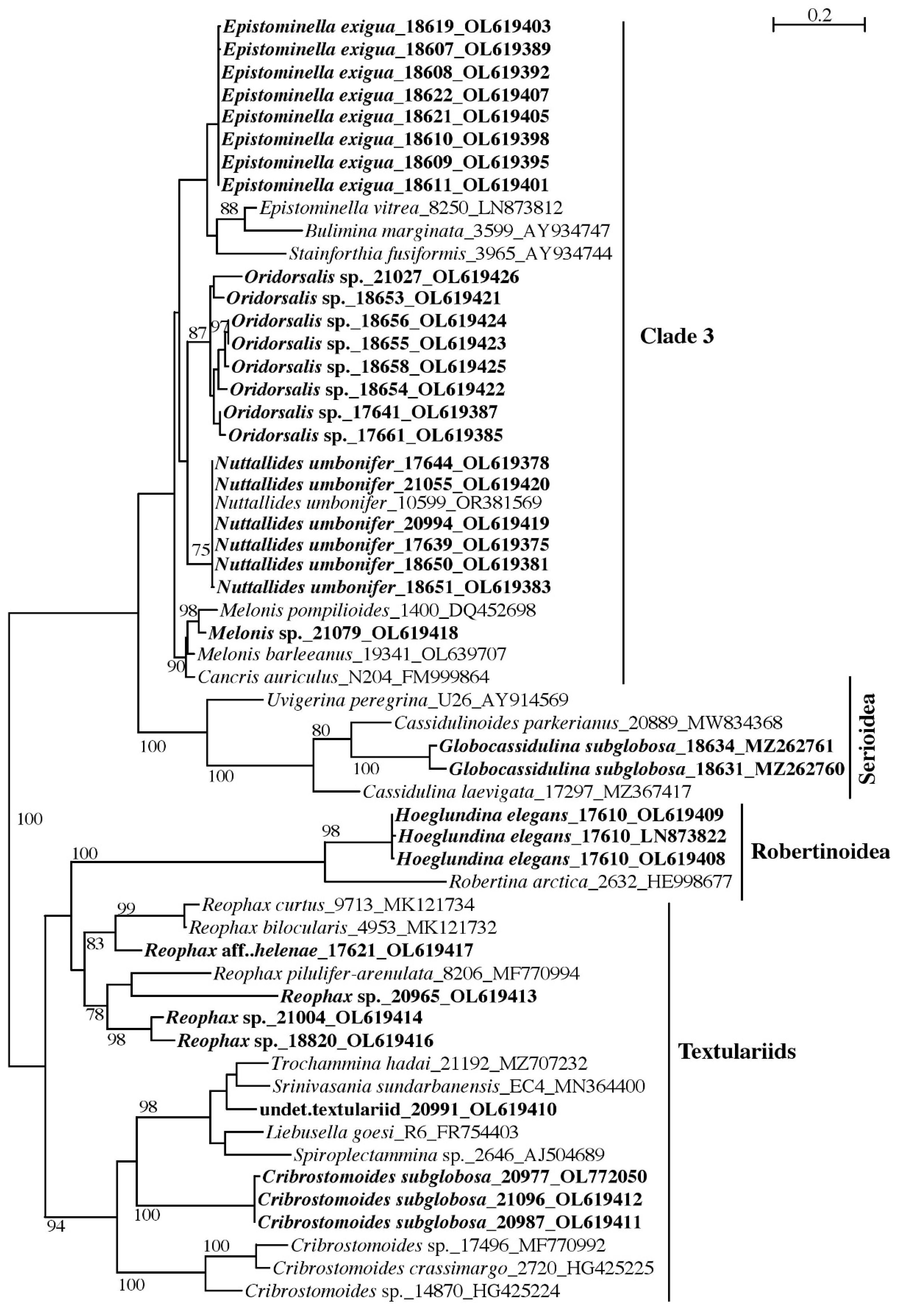
2.5.1. Sanger Sequences
2.5.2. HTS Sequences
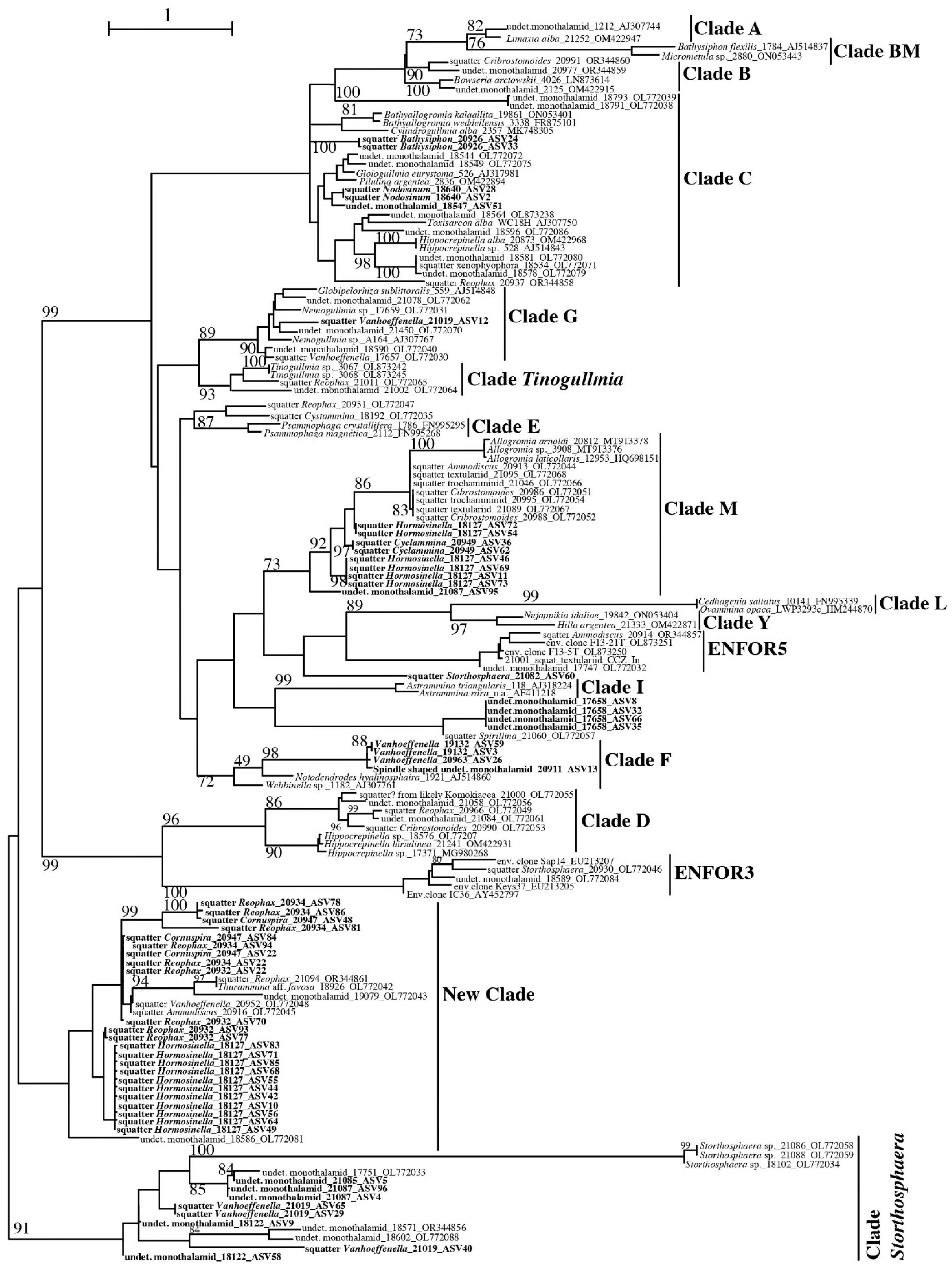

3. Results
3.1. Sanger Sequencing
3.1.1. Monothalamea
Monothalamea: Clade C
Monothalamea: ENFOR5
Monothalamea: Clade M
Monothalamea: Clade D and ENFOR3
Monothalamea: Clade Storthosphaera and New Clade
Monothalamea: Clade G, Tinogullmia and ENFOR5
3.1.2. Globothalamea
Globothalamea—Rotaliida and Robertinida
Globothalamea—Textulariida
3.2. High-Throughput Sequencing
3.2.1. Monothalamea
Monothalamea: Squatters
Monothalamea: Clade F and Clade V
3.2.2. Globothalamea
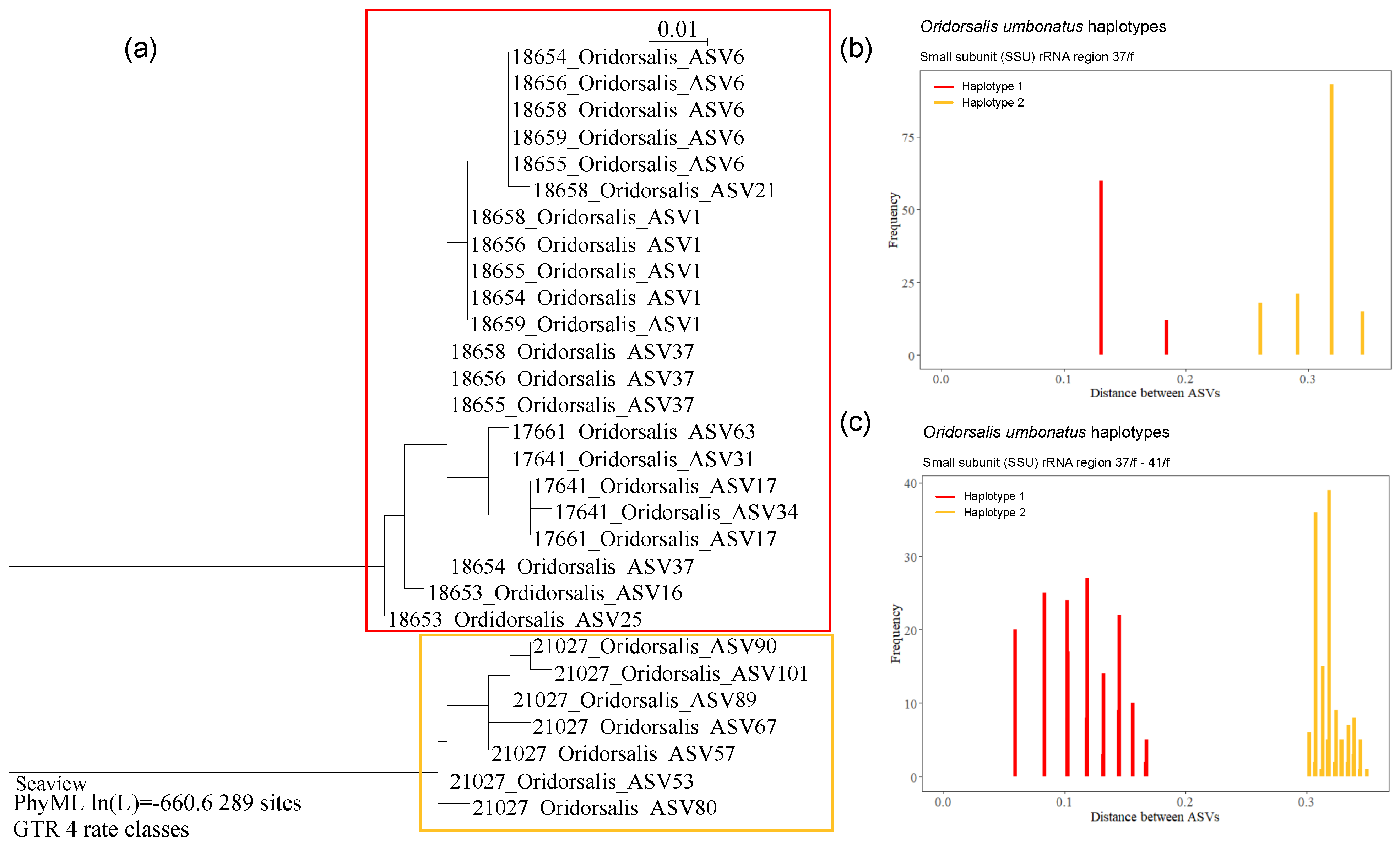
Globothalamea—Oridorsalis umbonatus
Globothalamea—Textulariida
4. Discussion
4.1. Calcareous Globothalamea
4.2. Polymorphism in Oridorsalis umbonatus
4.3. New Storthosphaera Clade
4.4. Cribrostomoides subglobosa (Cushman, 1910)
4.5. Squatters
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Cruise | Year | Area | Station | Deployment | Latitude (°N) | Longitude (°W) | Depth (m) |
|---|---|---|---|---|---|---|---|
| AB01 | 2013 | UK-1 | B-K-E | EB01 | 13°50′13.9″ | −116°33′30.4″ | 4182 |
| AB01 | 2013 | UK-1 | C | EB02 | 13°45′30.0″ | −116°41′54.7″ | 4070 |
| AB01 | 2013 | UK-1 | D | MC04 | 13°57′47.8″ | −116°34′05.7″ | 4084 |
| AB01 | 2013 | UK-1 | F | MC06 | 13°48′42.1″ | −116°42′36.1″ | 4076 |
| AB01 | 2013 | UK-1 | H | MC09 | 13°53′18.0″ | −116°41′23.9″ | 4150 |
| AB01 | 2015 | UK-1 | I | MC08 | 13°45′41.9″ | −116°27′36.0″ | 4111 |
| AB01 | 2013 | UK-1 | J | MC11 | 13°54′06.2″ | −116°35′24.1″ | 4166 |
| AB01 | 2013 | UK-1 | K | MC10 | 13°51.801 | −116°32.799 | 4053 |
| AB02 | 2015 | UK-1 | U01 | MC01 | 12°24′58.5″ | −116°42′53.4″ | 4126 |
| AB02 | 2015 | UK-1 | U02 | MC02 | 12°22′01.3″ | −116°31′01.3″ | 4166 |
| AB02 | 2016 | UK-1 | U03 | MC03 | 12°24′24.5″ | −116°29′05.1″ | 4148 |
| AB02 | 2015 | UK-1 | U04 | MC05 | 12°34′44.3″ | −116°43′25.5″ | 4235 |
| AB02 | 2015 | UK-1 | U05 | MC04 | 12°22′15.8″ | −116°36′49.1″ | 4163 |
| AB02 | 2015 | UK-1 | U06 | MC06 | 12°34′44.6″ | −116°41′16.9″ | 4234 |
| AB02 | 2015 | UK-1 | U07 | MC13 | 12°27′03.4″ | −116°35′40.1″ | 4129 |
| AB02 | 2015 | UK-1 | U09 | MC14 | 12°27′07.4″ | −116°30′44.3″ | 4198 |
| AB02 | 2015 | UK-1 | U10 | MC15 | 12°34′11.1″ | −116°32′19.8″ | 4226 |
| AB02 | 2015 | UK-1 | U12 | MC16 | 12°25′11.4″ | −116°37′28.8″ | 4160 |
| AB02 | 2015 | OMS | S02 | MC10 | 12°04′54.4″ | −117°10′41.8″ | 4072 |
| AB02 | 2015 | OMS | S03 | MC08 | 12°10′52.0″ | −117°15′39.4″ | 4114 |
| AB02 | 2015 | OMS | S04 | MC09 | 12°00′33.7″ | −117°10′41.5″ | 4148 |
| AB02 | 2015 | OMS | S05 | MC11 | 12°13′02.2″ | −117°19′31.6″ | 4106 |
| AB02 | 2015 | OMS | S08 | MC22 | 12°11′24.5″ | −117°22′17.0″ | 4179 |
| AB02 | 2015 | OMS | S09 | MC19 | 12°05′59.9″ | −117°11′47.9″ | 4082 |
| AB02 | 2015 | OMS | S11 | MC23 | 12°00′33.1″ | −117°22′49.3″ | 4152 |
| AB02 | 2015 | APEI-06 | APEI | BC29 | 19°28′20.5″ | −120°11′29.7″ | 4115 |
| RC01 | 2020 | OMS | BC001 | 14°1′7.979″ | −116°18′22.4″ | 4111 | |
| RC01 | 2020 | OMS | BC002 | 14°17′31.6″ | −116°51′37.3″ | 4144 | |
| RC01 | 2020 | OMS | BC004 | 14°17′31.1″ | −117°13′58.9″ | 4155 | |
| RC01 | 2020 | OMS | BC005 | 14°6′38.2″ | −117°13′54.2″ | 4200 | |
| RC01 | 2020 | OMS | BC007 | 14°9′1.13″ | −116°45′38.9″ | 4206 | |
| RC01 | 2020 | OMS | BC009 | 14°2′13.1″ | −116°30′28.9″ | 4138 | |
| RC01 | 2020 | OMS | BC015 | 14°7′34.917″ | −116°31′20.4″ | 4116 | |
| RC01 | 2020 | UK-1 | BC017 | 13°55′56.7″ | −116°30′39.9″ | 4144 | |
| RC01 | 2020 | UK-1 | BC020 | 13°46′18.3″ | −117°9′41.9″ | 4031 | |
| RC01 | 2020 | UK-1 | BC028 | 12°20′19.6″ | −116°40′8.6″ | 4158 | |
| RC01 | 2020 | UK-1 | BC033 | 12°23′47.06″ | −116°33′28.249″ | 4183 | |
| RC01 | 2020 | OMS | BC035 | 12°27′12.3″ | −117°25′30.9″ | 4149 | |
| RC01 | 2020 | OMS | BC036 | 12°26′45.5″ | −117°49′41.0″ | 4196 | |
| RC01 | 2020 | OMS | BC041 | 12°19′31.136″ | −117°40′34.605″ | 4137 | |
| RC01 | 2020 | OMS | BC043 | 12°19′34.9″ | −117°25′29.7″ | 4157 | |
| RC01 | 2020 | OMS | BC045 | 12°23′6.3348 | −117°25′13.9 | 4131 | |
| RC01 | 2020 | OMS | BC036 | 13°21′54.0″ | −117°1′22.0″ | 4200 | |
| BIONOD | 2016 | BGR | 17KG | BC17 | 11°48′10.2′′ | −116°53′51.6′′ | 4142 |
| BIONOD | 2016 | BGR | 24KG | BC24 | 11°53′58′′ | −117°02′44′′ | 4144 |
| BIONOD | 2016 | BGR | 56KG | BC56 | 12°41′22′′ | −118°16′52′′ | 4234 |
| Isolate | Taxon | Accession Numbers | Cruise | Locality Information | Photograph |
|---|---|---|---|---|---|
| Clade A | |||||
| 1212 | Indet. monothalamid | AJ307744 | Antarctica, New Harbour | ||
| 21252 | Limaxia alba | OM422947 | GBR, In column South Georgia | ||
| Clade B | |||||
| 2125 | Indet. monothalamid | OM422915 | Antarctica, New Harbour | ||
| 4026 | Bowseria arctowskii | LN873614 | Antarctica, Ross Ice Shelf | ||
| 20977 | Indet. monothalamid | OR344859 | RC01 | Depl. BC36, Sp. RC1661 | No photo |
| 20991 | Textulariid squatter | OR344860 | RC01 | Depl. BC002, Sp. RC0136 | |
| Clade BM | |||||
| 1784 | Bathysiphon flexilis | AJ514837 | Sweden, Gullmarsfjord | ||
| 2880 | Micrometula sp. | ON053443 | Norway, Svalbard | ||
| Clade C | |||||
| 526 | Gloiogullmia eurystoma | AJ317981 | Sweden, Tjaerno | ||
| 528 | Hippocrepinella sp. | AJ514843 | Sweden, Tjaerno | ||
| 20873 | Hippocrepinella alba | OM422968 | GBR, South Georgia | ||
| 2357 | Cylindrogullmia alba | MK748305 | Sweden, Tjaerno | ||
| 2836 | Pilulina argentea | OM422894 | Norway, Svalbard | ||
| 3338 | Bathyallogromia weddellensis | FR875101 | Weddell Sea, abyssal | ||
| 19861 | Bathyallogromia kalaallita | ON053401 | Greenland, Nuuk Fjord | ||
| 18534 * | xenophyophore squatter | OL772071 | AB01 | St. I, Depl. MC08 | |
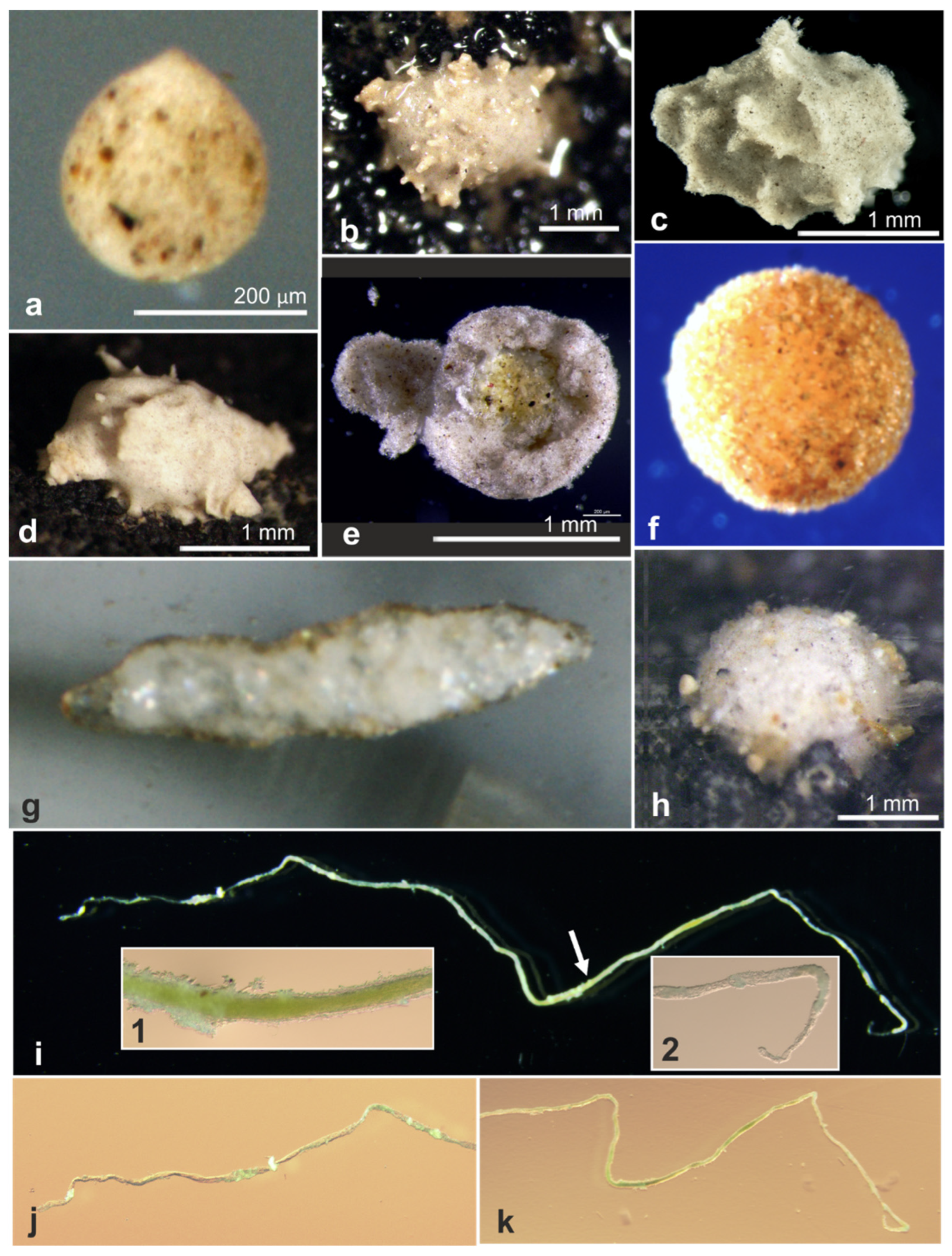
| 18544 * | Indet. monothalamid | OL772072, OL772073, OL772074 | AB02 | St. U02, Depl. MC02 | Figure A1d |
| 18549 * | Indet. monothalamid | OL772075, OL772076 | AB02 | St. U05, Depl. MC04 | Figure A1f |
| 18564 * | Indet. monothalamid | OL873238 | AB02 | St. U05, Depl. MC04 | Figure A1e |
| 18578 * | Indet. monothalamid | OL772079 | AB02 | St. S09, Depl. MC19 | Figure A1g |
| 18581 * | Indet. monothalamid | OL772080 | AB02 | St. S09, Depl. MC19 | Figure A1c |
| 18596 * | Indet. monothalamid | OL772086, OL772087 | AB02 | St. U12, Depl. MC16 | Figure A1h |
| 18791 | Indet. monothalamid | OL772038 | AB02 | St. U04, Depl. MC05 | Figure A1b |
| 18793 | Indet. monothalamid | OL772039 | AB02 | St. U04, Depl. MC06 | Figure A1a |
| 20937 | Reophax squatter | OR344858 | RC01 | Depl. BC028, Sp. RC1260 | |
| WC18H | Toxisarcon alba | AJ307750 | Scotland, Loch Linnhe | ||
| Clade D | |||||
| 17371 | Hippocrepinella sp. | MG980268 | Chile, Patagonia | ||
| 18576 * | Hippocrepinella sp. | OL772077, OL772078 | AB02 | St. U12, Depl. MC16 | Figure A3k |
| 21241 | Hippocrepinella hirudinea | OM422931 | GBR, South Georgia | ||
| 20966 | Reophax squatter | OL772049 | RC01 | Depl. BC36, Sp. RC1661 | |
| 20990 | Cribrostomoides squatter | OL772053 | RC01 | Depl. BC002, Sp. RC0136 | |
| 21000 | Komoki squatter | OL772055 | RC01 | Depl. BC007, Sp. RC0317 | Figure A3a,b |
| 21058 | Indet. monothalamid | OL772056 | RC01 | Depl. BC020, Sp. RC0755 | Figure A3i,j |
| 21084 | Indet. monothalamid | OL772061 | RC01 | Depl. BC043, Sp. RC1773 | Figure A3e,f |
| Clade E | |||||
| 1786 | Psammophaga crystallifera | FN995295 | Sweden, Gullmarsfjord | ||
| 2112 | Psammophaga magnetica | FN995268 | Antarctica, New Harbour | ||
| Clade F | |||||
| 1182 | Webbinella sp. | AJ307761 | Antarctica, New Harbour | ||
| 1921 | Notodendrodes hyalinosphaira | AJ514860 | Antarctica, New Harbour | ||
| Clade G | |||||
| 559 | Globipelorhiza sublittoralis | AJ514848 | Sweden, Tjaerno | ||
| 17657 | Vanhoeffenella squatter | OL772030 | AB01 | St. C, Depl. EB02 | |
| 17659 | Nemogullmia sp. | OL772031 | AB01 | St. B, Depl. EB01 | Figure A4i–k |
| 18590 | Indet. Monothalamid | OL772040 | AB02 | St. S02, Depl. MC10 | No photo |
| 21078 | Indet. Monothalamid | OL772062 | RC01 | RC1211, Depl. BC028 | Figure A6h |
| 21450 | Indet. Monothalamid | OL772070 | RC01 | Depl. BC004, Sp. RC1211 | Figure A2e |
| A164 | Nemogullmia sp. | AJ307767 | Antarctica, New Harbour | ||
| Clade I | |||||
| 118 | Astrammina triangularis | AJ318224 | Antarctica, New Harbour | ||
| n.a. | Astrammina rara | AF411218 | Antarctica, Explorers Cove | ||
| Clade L | |||||
| 10141 | Cedhagenia saltatus | FN995339 | Ukraine, Balaklava Bay | ||
| LWP3_29_3c | Ovammina opaca | HM244870 | USA, Sapelo Island | ||
| Clade M | |||||
| 12953 | Allogromia laticollaris | HQ698151 | USA, Cold Spring Harbour, strain CSH | ||
| 3908 | Allogromia sp. | MT913376 | Antarctica, Ross Ice Shelf | ||
| 20812 | Allogromia arnoldi | MT913378 | Cyprus | ||
| 20913 | Ammodiscus squatter | OL772044 | RC01 | Depl. BC004, Sp. RC0234 | |
| 20986 | Cribrostomoides squatter | OL772051 | RC01 | Depl. BC002, Sp. RC0136 | |
| 20988 | Cribrostomoides squatter | OL772052 | RC01 | Depl. BC002, Sp. RC0136 | |
| 20995 | Trochamminid squatter | OL772054 | RC01 | Depl. BC002, Sp. RC0143 | |
| 21046 | Trochamminid squatter | OL772066 | RC01 | Depl. BC009, Sp. RC0471 | |
| 21089 | Textulariid squatter | OL772067 | RC01 | Depl. BC015, Sp. RC0903 | |
| 21095 | Textulariid squatter | OL772068 | RC01 | Depl. BC015, Sp. RC0903 | |
| Clade Y | |||||
| 19842 | Nujappikia idaliae | ON053404 | Greenland, Nuuk Fjord | ||
| 21333 | Hilla argentea | OM422871 | GBR, South Georgia | ||
| Clade Storthosphaera | |||||
| 17751 | Indet. Monothalamid | OL772033 | AB01 | St. C, Depl. EB02 | Figure A4a |
| 18102 | Storthosphaera sp. | OL772034 | AB01 | St. F, Depl. MC06 | Figure A4d |
| 21086 | Storthosphaera sp. | OL772058 | RC01 | Depl. BC045, Sp. RC1876 | Figure A4b |
| 21088 | Storthosphaera sp. | OL772059 | RC01 | Depl. BC045, Sp. RC1888 | Figure A4c |
| 18571 | Indet. Monothalamid | OR344856 | AB02 | no photo | |
| 18602 * | Indet. Monothalamid | OL772088, OL772089 | AB02 | St. SO8, Depl. MC22 | Figure A4a |
| New Clade | |||||
| 18586 * | Indet. Monothalamid | OL772081, OL772082, OL772083 | AB02 | St. APEI-06, Depl. BC29 | Figure A4g |
| 18926 | Thurammina aff. favosa | OL772042 | Bionod | Bionod, 126 | Figure A4f |
| 19079 | Indet. Monothalamid | OL772043 | AB02 | St. U03, Depl. MC03 | no photo |
| 20916 | Ammodiscus squatter | OL772045 | RC01 | Depl. BC028, Sp. RC1265 | |
| 20952 | Vanhoeffenella squatter | OL772048 | RC01 | Depl. BC028, Sp. RC1272 | |
| 21094 | Th. aff. favosa—Reophax squatter | OR344861 | RC01 | Depl. BC015, Sp. RC0903 | |
| Clade Tinogullmia | |||||
| 3067 | Tinogullmia sp. | OL873242 | Antarctica, New Harbour | ||
| 3068 | Tinogullmia sp. | OL873245 | Antarctica, New Harbour | ||
| 21002 | likely komoki squatter | OL772064 | RC01 | Depl. BC001, Sp. RC0401 | Figure A2a,b |
| 21011 | Reophax squatter | OL772065 | RC01 | Depl. BC007, Sp. RC0328 | |
| ENFOR3 | |||||
| 18589 * | Indet. Monothalamid | OL772084, OL772085 | AB02 | St. S03, Depl. MC08 | Figure A3c,d |
| 20930 | Storthosphaera squatter | OL772046 | RC01 | Depl. BC028, Sp. RC0674 | Figure A3g,h |
| env.clone Sap14 | Indet. Monothalamid | EU213207 | USA, Sapelo Island | ||
| env.clone Keys37 | Indet. Monothalamid | EU213205 | USA, Florida, Tennessee Reef | ||
| env.clone IC36 | Indet. Monothalamid | AY452797 | Antarctica, Explorers Cove | ||
| ENFOR5 | |||||
| 17747 | Indet. Monothalamid | OL772032 | AB01 | St. C, Depl. EB02 | Figure A2c,d |
| 20914 | Ammodiscus squatter | OR344857 | RC01 | Depl. BC004, Sp. RC0234 | |
| 21001 | Textulariid squatter | OL772063 | RC01 | Depl. BC007, Sp. RC0319 | |
| env. clone F13-5T | Indet. Monothalamid | OL873250 | Southern Ocean, 2997m | ||
| env. clone F13-21T | Indet. Monothalamid | OL873251 | Southern Ocean, 2997m | ||
| Separate lineages | |||||
| 18192 | Cy pauciloculata squatter | OL772035 | AB02 | St. U06, Depl. MC06 | |
| 20931 | Reophax squatter | OL772047 | RC01 | Depl. BC028, Sp. RC1260 | |
| 21060 | Spirillina squatter | OL772057 | RC01 | Depl. BC020, Sp. RC0804 | |
| Species | Isolate | Accession Numbers | Cruise | Locality Information | Figures |
|---|---|---|---|---|---|
| Bulimina marginata | 3599 | AY934747 | Norway, Oslofjord | ||
| Cancris auriculus | N204 | FM999864 | Namibia, Atlantic Ocean | ||
| Cassidulina laevigata | 17297 | MZ367417 | Chile, Beagle Channel | ||
| Cassidulinoides parkerianus | 20889 | MW834368 | GBR, South Georgia | ||
| Cribrostomoides crassimargo | 2720 | HG425225 | Svalbard | ||
| Cribrostomoides sp. | 17496 | MF770992 | New Zealand | ||
| Cribrostomoides sp. | 14870 | HG425224 | Southern Ocean, 2779 m | ||
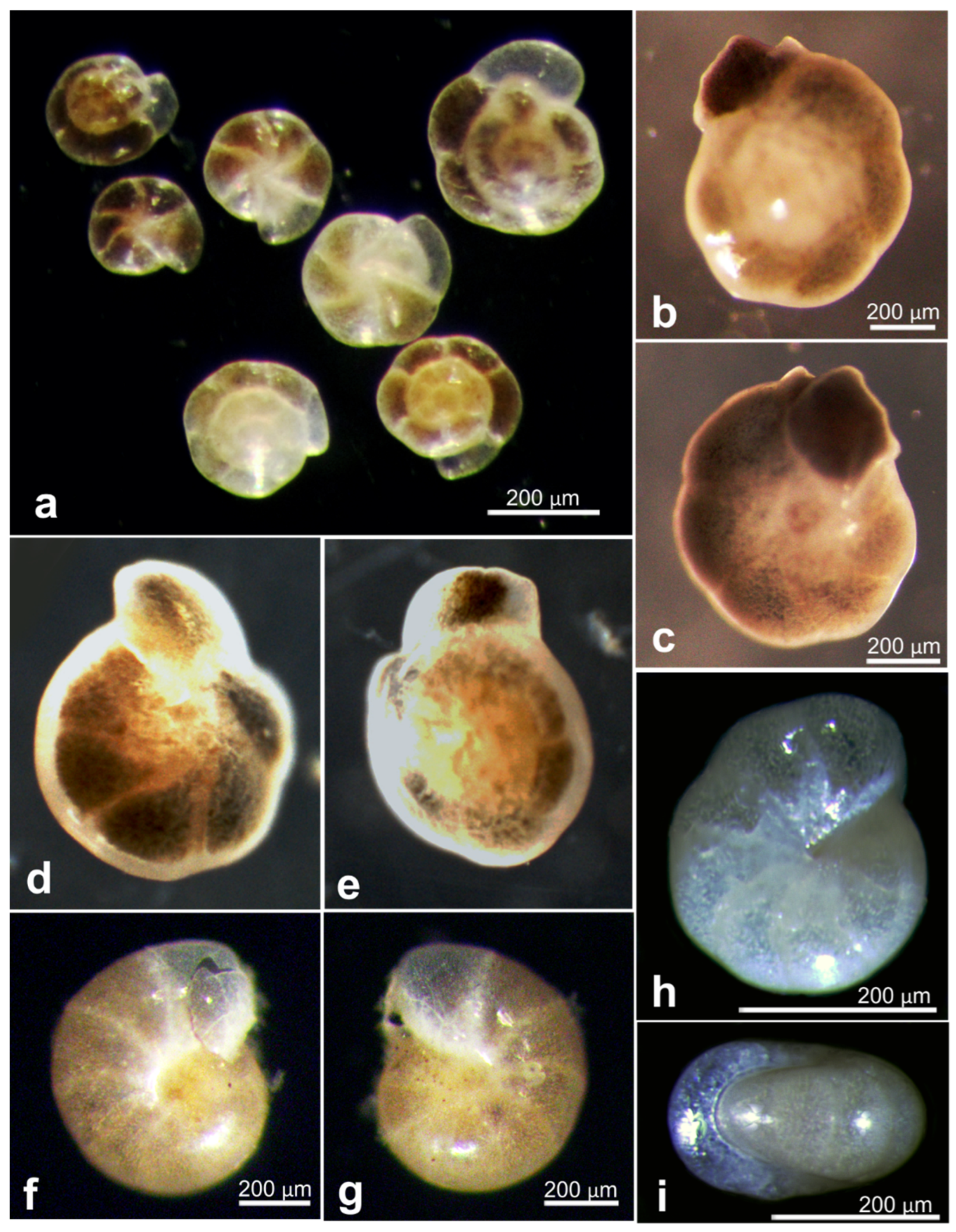
| Cribrostomoides subglobosa | 20977 | OL772050 | RC01 | Depl. BC036, sp. RC1661 | |
| Cribrostomoides subglobosa | 20987 | OL619411 | RC01 | Depl. BC002, sp. RC0136 | |
| Cribrostomoides subglobosa | 21096 | OL619412 | RC01 | Depl. BC015, sp. RC0903 | Figure A7f–h |
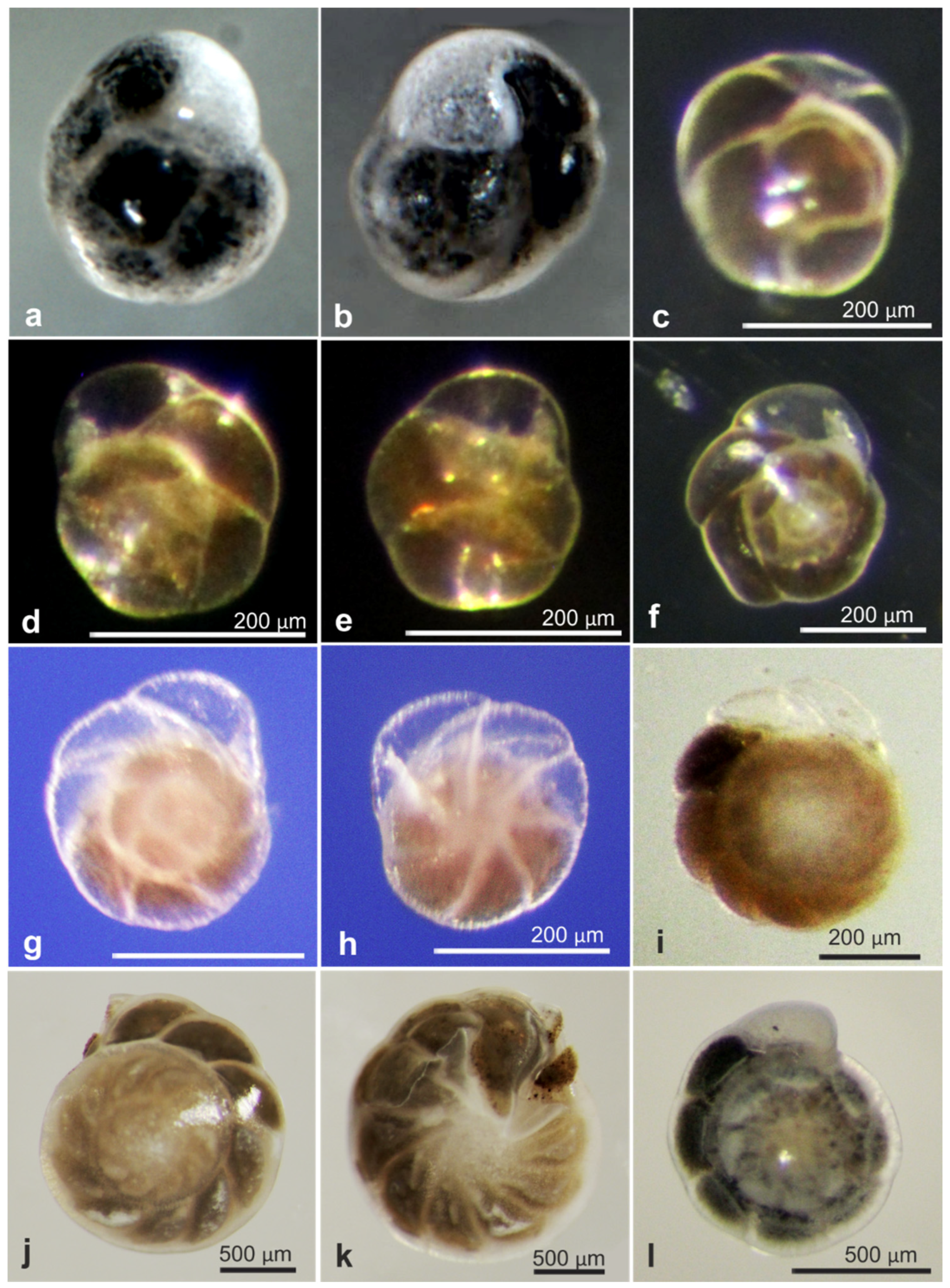
| Epistominella exigua * | 18607 | OL619389, OL619390, OL619391 | AB02 | St. U01, Depl. MC01 | Figure A5d,e |
| Epistominella exigua * | 18608 | OL619392, OL619393, OL619394 | AB02 | St. U04, Depl. MC05 | |
| Epistominella exigua * | 18609 | OL619395, OL619396, OL619397 | AB02 | St. S04, Depl. MC09 | |
| Epistominella exigua * | 18610 | OL619398, OL619399, OL619400 | AB02 | St. S09, Depl. MC12 | |
| Epistominella exigua * | 18611 | OL619401, OL619402 | AB02 | St. S05, Depl. MC11 | Figure A5f |
| Epistominella exigua * | 18619 | OL619403, OL619404 | AB02 | St. S09, Depl. MC19 | |
| Epistominella exigua * | 18621 | OL619405, OL619406 | AB02 | St. S11, Depl. MC23 | |
| Epistominella exigua * | 18622 | OL619407 | AB02 | St. U07, Depl. MC13 | |
| Epistominella vitrea | 8250 | LN873812 | Argentinia, Ushuaia | ||
| Globocassidulina subglobosa | 18631 | MZ262760 | AB02 | St. U10, Depl. MC15 | Figure A5c |
| Globocassidulina subglobosa | 18634 | MZ262761 | AB02 | St. U07, Depl. MC13 | Figure A5a,b |
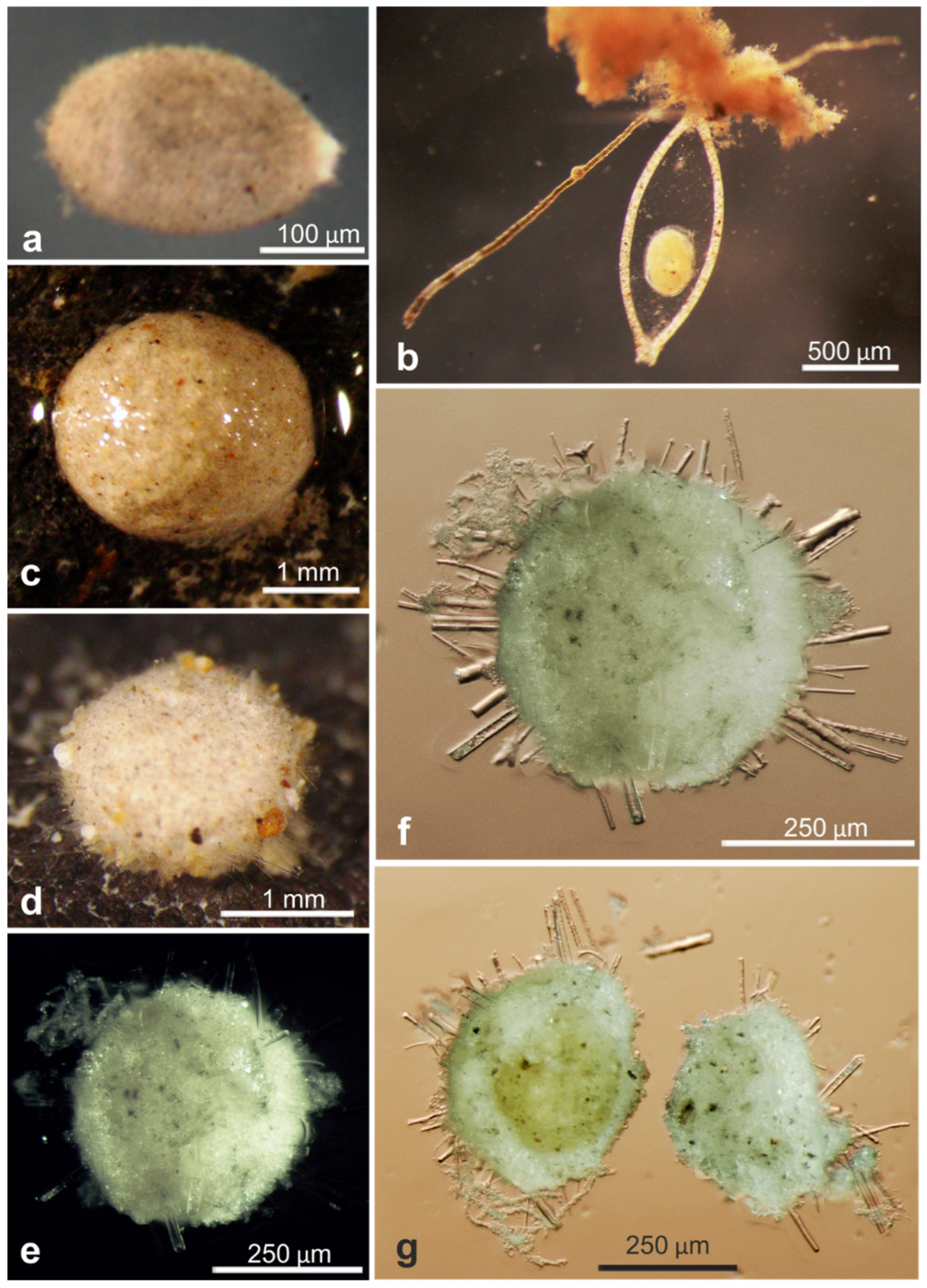
| Hoeglundina elegans * | 17610 | LN873822, OL619408, OL619409 | AB01 | St. C, Depl. EB02 | Figure A5j,k |
| Liebusella goesi | R6 | FR754401 | Norway, Oslo Fjord | ||
| Melonis barleeanus | 19341 | OL639707 | Svalbard | ||
| Melonis pompilioides | 1400 | DQ452697 | Sweden, Tjaerno | ||
| Melonis sp. | 21079 | OL619418 | RC01 | Depl. BC035, sp. RC1542 | Figure A6e,f |
| Nuttallides umbonifer | 10599 | OR381569 | Argentinian basin of Atlantic Ocean, 4605 m | ||
| Nuttallides umbonifer * | 17639 | OL619375, OL619376, OL619377 | AB01 | St. D, Depl. MC04 | Figure A5i |
| Nuttallides umbonifer * | 17644 | OL619378, OL619379, OL619380 | AB01 | St. J, Depl. MC11 | |
| Nuttallides umbonifer * | 18650 | OL619381, OL619382 | AB02 | St. U12, Depl. MC16 | |
| Nuttallides umbonifer * | 18651 | OL619383, OL619384 | AB02 | St. S02, Depl. MC19 | |
| Nuttallides umbonifer | 21055 | OL619420 | RC01 | Depl. BC009, sp. RC0481 | Figure A5g,h |
| Nuttallides umbonifer | 20994 | OL619419 | RC01 | Depl. BC002, sp. RC0137 | |
| Oridorsalis sp. * | 17641 | OL619387, OL619388 | AB01 | St. F, Depl. MC06 | |
| Oridorsalis sp. * | 17661 | OL619385, OL619386 | AB01 | St. C, Depl. EB02 | |
| Oridorsalis sp. * | 18653 | OL619421 | AB02 | St. U09, Depl. MC14 | Figure A6d,e |
| Oridorsalis sp. * | 18654 | OL619422 | AB02 | St. S08, Depl. MC22 | Figure A6a |
| Oridorsalis sp. * | 18655 | OL619423 | AB02 | St. S08, Depl. MC22 | Figure A6a |
| Oridorsalis sp. * | 18656 | OL619424 | AB02 | St. S08, Depl. MC22 | Figure A6a |
| Oridorsalis sp. * | 18658 | OL619425 | AB02 | St. S08, Depl. MC22 | Figure A6a |
| Oridorsalis sp. * | 21027 | OL619426 | RC01 | Depl. BC005, sp. RC0416 | Figure A6b,c |
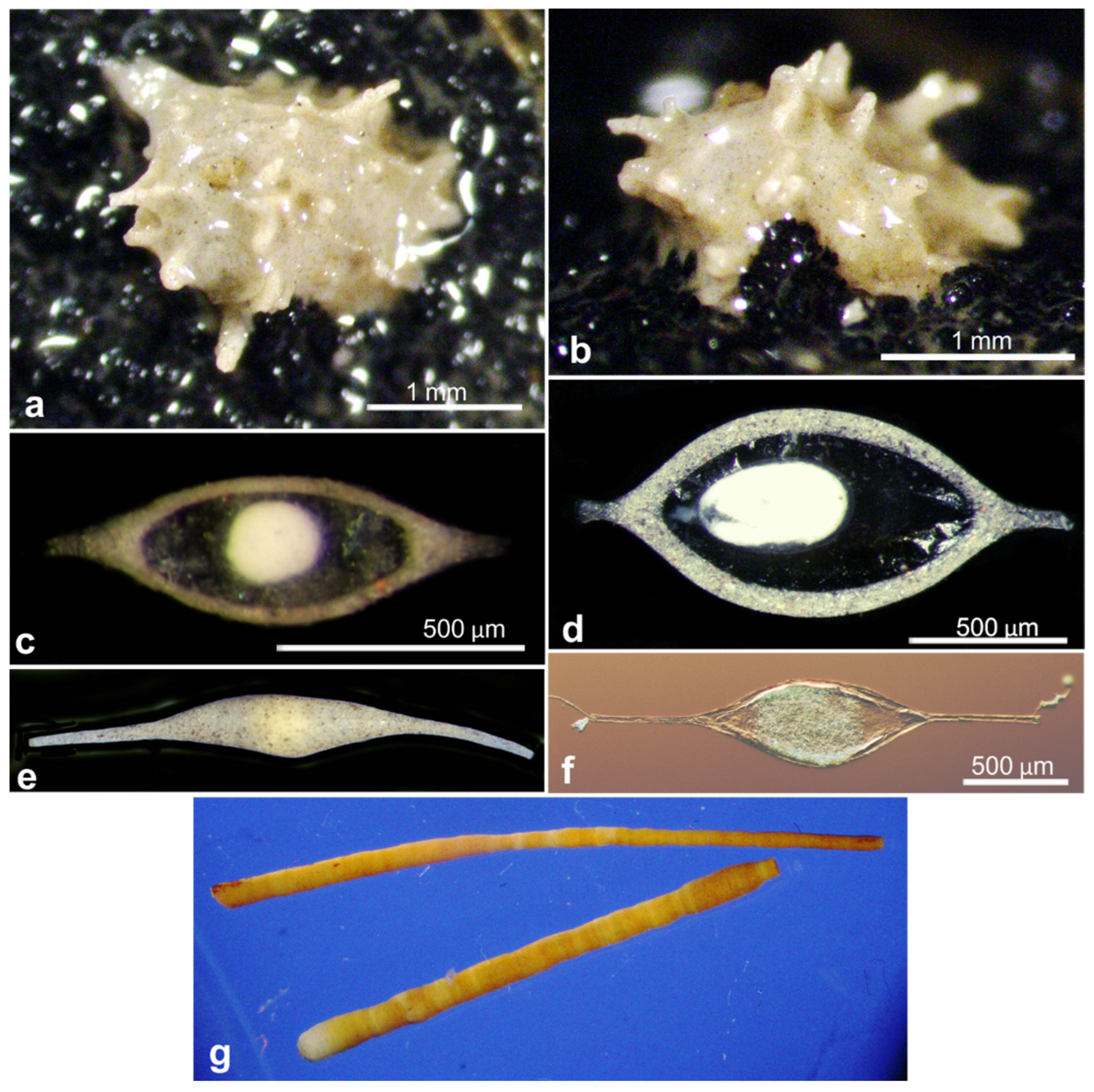
| Reophax aff. helenae | 17621 | OL619417 | AB01 | St. H, Depl. MC09 | Figure A7a similar sp. |
| Reophax bilocularis | 4953 | MK121732 | Antarctica | ||
| Reophax curtus | 9713 | MK121734 | Russia, White Sea | ||
| Reophax pilulifer-arenulata | 8206 | MF770994 | Antarctica, Arctowski | ||
| Reophax sp. | 18820 | OL619416 | BIONOD | St. US10 | Figure A7b |
| Reophax sp. | 20965 | OL619413 | RC01 | Depl. BC001, sp. RC0401 | Figure A7d |
| Reophax sp. | 21004 | OL619414 | RC01 | Depl. BC005, sp. RC0417 | Figure A7c |
| Robertina arctica | 2632 | HE998677 | Svalbard | ||
| Spiroplectammina sp. | 2646 | AJ504689 | Svalbard | ||
| Srinivasania sundarbanensis | EC_4 | MN364400 | India, Sundarbans | ||
| Stainforthia fusiformis | 3965 | AY934744 | Norway, Skagerrak | ||
| Trochammina hadai | 21192 | MZ707232 | West Australia | ||
| indet. Textulariid | 20991 | OL619410 | RC01 | Depl. BC002, sp. RC0136 | Figure A7e |
| Uvigerina peregrina | U26 | AY914569 | Norway, Oslo Fjord |
| Species | Isolate | Seq. Length | GP Content | Cruise | Area | Station | Deployment | Figure |
|---|---|---|---|---|---|---|---|---|
| Hoeglundina elegans * | 17610 | 1020 | 40.1 | AB01 | UK-1 | C | EB02 | Figure A5j,k |
| Reophax aff. helenae | 17621 | 846 | 41.7 | AB01 | UK-1 | H | MC09 | Figure A7a |
| Nuttallides sp. * | 17639 | 1025 | 42.4 | AB01 | UK-1 | D | MC04 | Figure A5i |
| Oridorsalis umbonatus * | 17641 | 958 | 46.3 | AB01 | UK-1 | F | MC06 | |
| Nuttallides sp. * | 17644 | 1033 | 42.5 | AB01 | UK-1 | J | MC11 | |
| Vanhoeffenella squatter | 17657 | 936 | 56.7 | AB01 | UK-1 | C | EB02 | |
| Nemogullmia sp. | 17659 | 958 | 55.7 | AB01 | UK-1 | B-K-E | EB01 | Figure A4i–k |
| Oridorsalis umbonatus * | 17661 | 958 | 46.3 | AB01 | UK-1 | C | EB02 | |
| indet. monothalamid | 17747 | 947 | 48.6 | AB01 | UK-1 | C | EB02 | Figure A2c,d |
| indet. monothalamid | 17751 | 708 | 43.0 | AB01 | UK-1 | C | EB02 | Figure A4e |
| Stortosphaera sp. | 18102 | 753 | 40.3 | AB01 | UK-1 | F | MC06 | Figure A4d |
| Cystammina squatter | 18192 | 817 | 42.6 | AB02 | UK-1 | U06 | MC06 | |
| Xenophyophore squatter | 18534 | 1167 | 49.7 | AB01 | UK-1 | I | MC08 | |
| indet. monothalamid * | 18544 | 924 | 40.3 | AB02 | UK-1 | U02 | MC02 | Figure A1d |
| indet. monothalamid * | 18549 | 997 | 40.5 | AB02 | UK-1 | U05 | MC04 | Figure A1f |
| indet. monothalamid | 18564 | 1013 | 41.0 | AB02 | UK-1 | U05 | MC04 | Figure A1e |
| Hippocrepinella sp. * | 18576 | 756 | 41.8 | AB02 | UK-1 | U12 | MC16 | Figure A3k |
| indet. monothalamid | 18578 | 1163 | 49.7 | AB02 | OMS | S09 | MC19 | Figure A1g |
| indet. monothalamid | 18581 | 1167 | 49.7 | AB02 | OMS | S09 | MC19 | Figure A1c |
| indet. monothalamid * | 18586 | 702 | 45.8 | AB02 | APEI-06 | APEI-06 | BC29 | Figure A4g |
| indet. monothalamid * | 18589 | 831 | 47.5 | AB02 | OMS | S03 | MC08 | Figure A3c,d |
| indet. monothalamid | 18590 | 941 | 57.0 | AB02 | OMS | S02 | MC10 | No photo |
| indet. monothalamid * | 18596 | 1062 | 45.3 | AB02 | UK-1 | U12 | MC16 | Figure A1h |
| indet. monothalamid * | 18602 | 777 | 44.1 | AB02 | OMS | S08 | MC22 | Figure A4a |
| Epistominella exigua * | 18607 | 984 | 44.1 | AB02 | UK-1 | U01 | MC01 | Figure A5d,e |
| Epistominella exigua * | 18608 | 984 | 43.9 | AB02 | UK-1 | U04 | MC05 | |
| Epistominella exigua * | 18609 | 1021 | 44.2 | AB02 | OMS | S04 | MC09 | |
| Epistominella exigua * | 18610 | 1021 | 44.1 | AB02 | OMS | S09 | MC12 | |
| Epistominella exigua * | 18611 | 1021 | 44.2 | AB02 | OMS | S05 | MC11 | Figure A5f |
| Epitominella exigua * | 18619 | 1021 | 44.3 | AB02 | OMS | SO9 | MC19 | |
| Epistominella exigua * | 18621 | 1020 | 44.0 | AB02 | OMS | S11 | MC23 | |
| Epistominella exigua * | 18622 | 1021 | 44.1 | AB02 | OMS | S11 | MC23 | |
| Globocassidulina | 18631 | 1023 | 38.3 | AB02 | UK-1 | U10 | MC15 | Figure A5c |
| Globocassidulina | 18634 | 1022 | 37.9 | AB02 | UK-1 | U07 | MC13 | Figure A5a,b |
| Nuttallides sp. * | 18650 | 1026 | 42.7 | AB02 | UK-1 | U12 | MC16 | |
| Nuttallides sp. * | 18651 | 1062 | 42.9 | AB02 | OMS | S02 | MC19 | |
| Oridorsalis umbonatus | 18653 | 848 | 46.7 | AB02 | UK-1 | U09 | MC14 | Figure A6d,e |
| Oridorsalis umbonatus | 18654 | 849 | 46.9 | AB02 | OMS | S08 | MC22 | Figure A6a |
| Oridorsalis umbonatus | 18655 | 851 | 46.5 | AB02 | OMS | S08 | MC22 | Figure A6a |
| Oridorsalis umbonatus | 18656 | 851 | 46.5 | AB02 | OMS | S08 | MC22 | Figure A6a |
| Oridorsalis umbonatus | 18658 | 851 | 46.4 | AB02 | OMS | S08 | MC22 | Figure A6a |
| indet. monothalamid | 18791 | 976 | 44.9 | AB02 | UK-1 | U04 | MC05 | Figure A1b |
| indet. monothalamid | 18793 | 976 | 43.7 | AB02 | OMS | S03 | MC08 | Figure A1a |
| Reophax sp. | 18820 | 912 | 40.4 | BIO | BGR | 17KG | BC17 | Figure A7b |
| Septuma squatter | 18905 | 716 | 43.3 | BIO | BGR | 24KG | BC24 | |
| Thurammina aff. favosa | 18926 | 722 | 44.7 | BIO | BGR | 56KG | BC56 | Figure A4f |
| indet. monothalamid | 19079 | 765 | 42.5 | AB02 | UK-1 | U03 | MC03 | Figure A1i |
| Ammodiscus squatter | 20913 | 912 | 40.6 | RC01 | OMS | BC004 | ||
| Ammodiscus squatter | 20916 | 735 | 43.9 | RC01 | UK-1 | BC028 | ||
| Storthosphaera squatter | 20930 | 906 | 46.4 | RC01 | UK-1 | BC017 | Figure A3g,h | |
| Reophax squatter | 20931 | 843 | 47.6 | RC01 | UK-1 | BC028 | ||
| Vanhoeffenella squatter | 20952 | 735 | 44.0 | RC01 | UK-1 | BC028 | ||
| Reophax sp. | 20965 | 863 | 43.4 | RC01 | OMS | BC036 | Figure A7d | |
| Reophax squatter | 20966 | 805 | 43.7 | RC01 | OMS | BC036 | ||
| 20977 | 901 | 42.0 | RC01 | OMS | BC036 | |||
| Cribrostomoides squatter | 20986 | 861 | 39.8 | RC01 | OMS | BC002 | ||
| 20987 | 903 | 41.4 | RC01 | OMS | BC002 | |||
| Cribrostomoides squatter | 20988 | 875 | 39.9 | RC01 | OMS | BC002 | ||
| Cribrostomoides squatter | 20990 | 820 | 41.4 | RC01 | OMS | BC002 | ||
| Textulariid | 20991 | 904 | 40.6 | RC01 | OMS | BC002 | Figure A7e | |
| Nuttallides | 20994 | 908 | 42.5 | RC01 | OMS | BC002 | ||
| Trochamminid squatter | 20995 | 856 | 39.7 | RC01 | OMS | BC002 | ||
| indet. Monothalamid | 21000 | 759 | 43.4 | RC01 | OMS | BC007 | Figure A3a,b | |
| Textulariid squatter | 21001 | 914 | 49.1 | RC01 | OMS | BC007 | ||
| Likely komoki squatter | 21002 | 868 | 47.3 | RC01 | OMS | BC007 | Figure A2a,b | |
| Reophax sp. | 21004 | 918 | 40.4 | RC01 | OMS | BC001 | Figure A7c | |
| Reophax squatter | 21011 | 1023 | 43.0 | RC01 | OMS | BC001 | ||
| Oridoralis umbonatus | 21027 | 848 | 45.6 | RC01 | OMS | BC005 | Figure A6b,c | |
| Trochamminid squatter | 21046 | 876 | 39.8 | RC01 | OMS | BC009 | ||
| Nuttallides sp. | 21055 | 909 | 42.6 | RC01 | OMS | BC009 | Figure A5g,h | |
| indet. monothalamid | 21058 | 738 | 43.9 | RC01 | UK-1 | BC020 | Figure A3i,j | |
| Spirillina squatter | 21060 | 836 | 43.0 | RC01 | UK-1 | BC020 | ||
| indet. Monothalamid | 21078 | 1008 | 55.4 | RC01 | UK-1 | BC028 | Figure A4h | |
| Melonis sp. | 21079 | 865 | 43.5 | RC01 | OMS | BC035 | Figure A6e,f | |
| indet. Monothalamid | 21084 | 759 | 42.6 | RC01 | OMS | BC043 | Figure A3e,f | |
| Storthosphaera sp. | 21086 | 791 | 41.0 | RC01 | OMS | BC045 | Figure A4b | |
| Storthosphaera sp. | 21088 | 719 | 40.9 | RC01 | OMS | BC045 | Figure A4c | |
| Textulariid squatter | 21089 | 872 | 39.8 | RC01 | OMS | BC015 | ||
| Textulariid squatter | 21095 | 900 | 40.0 | RC01 | OMS | BC015 | ||
| Cribro. subglobosa | 21096 | 903 | 41.4 | RC01 | OMS | BC015 | Figure A7f–h | |
| Indet monothalamid | 21450 | 948 | 55.7 | RC01 | OMS | BC036 | Figure A2e |
| Species | ASVs | Isolate | Seq. Length | GC Content | Cruise | Area | Station | Deployment | Figures |
|---|---|---|---|---|---|---|---|---|---|
| Oridorsalis umbonatus | 17, 31, 34 | 17641 | 288 | 45.5 | AB01 | UK-1 | F | MC06 | |
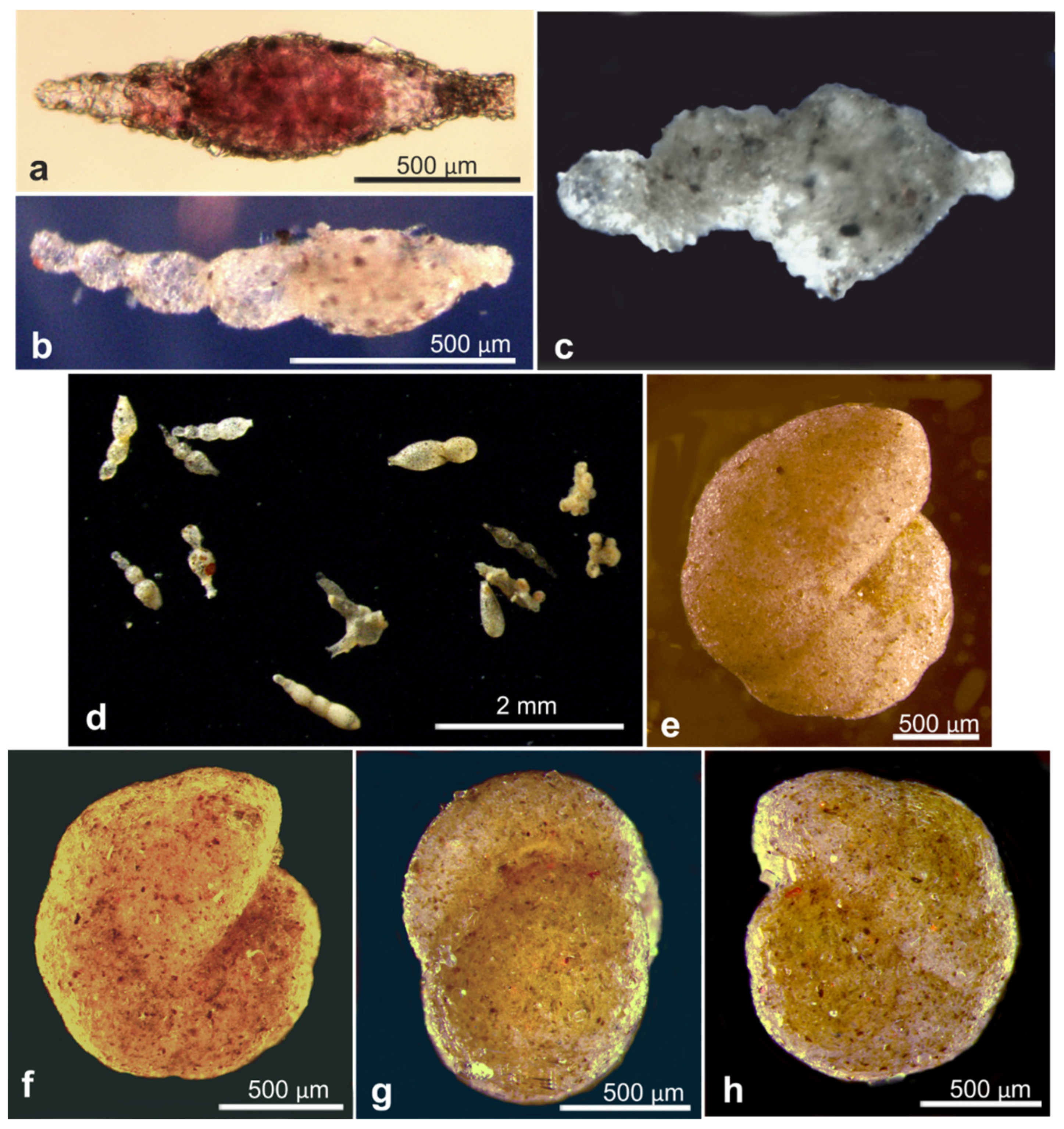
| Vanhoeffenella-like | 8, 32, 35, 66 | 17658 | 260 | 41.3 | AB01 | UK-1 | C | EB02 | Figure A9f |
| Oridorsalis umbonatus | 17, 63 | 17661 | 288 | 45.9 | AB01 | UK-1 | F | MC06 | |
| Crithionina hispida | 9, 58 | 18122 | 249 | 41.1 | AB01 | UK-1 | J | MC22 | Figure A8e–g |
| Horm. distans squatters | 10, 11, 42, 44, 46, 49, 54, 55, 56, 64, 68, 69, 71, 72, 73, 83, 85 | 18127 | 255 | 41.7 | AB01 | UK-1 | K | MC10 | |
| Saccamminid | 51 | 18547 | 367 | 37.8 | AB02 | UK-1 | U03 | MC02 | Figure A8a |
| N. gaussicum squatter | 2, 28 | 18640 | 293 | 40.7 | AB01 | UK-1 | C | EB02 | |
| Oridorsalis umbonatus | 16, 25 | 18653 | 288 | 46.9 | AB02 | UK | Figure A6d,e | ||
| Oridorsalis umbonatus | 1, 6, 37 | 18654 | 287 | 46.3 | AB02 | OMS | Figure A6a | ||
| Oridorsalis umbonatus | 1, 6, 37 | 18655 | 287 | 46.3 | AB02 | OMS | Figure A6a | ||
| Oridorsalis umbonatus | 1, 6, 37 | 18656 | 287 | 46.3 | AB02 | OMS | Figure A6a | ||
| Oridorsalis umbonatus | 1, 6, 21, 37 | 18658 | 282/287 | 46.2 | AB02 | OMS | Figure A6a | ||
| Oridorsalis umbonatus | 1, 6 | 18659 | 287 | 46.4 | AB02 | OMS | |||
| Vanhoeffenella sp. | 3, 59 | 19132 | 361 | 40 | AB02 | OMS | Figure A9c | ||
| Spindle-like | 13 | 20911 | 373 | 40 | RC01 | OMS | Figure A9e | ||
| B. aff. flavidus | 24, 33 | 20926 | 389 | 36.7 | RC01 | OMS | Figure A9g | ||
| Reophax squatter | 22, 70, 77, 93 | 20932 | 255 | 43.9 | RC01 | OMS | |||
| Reophax squatter | 22, 78, 81, 86, 94 | 20934 | 224 | 41.5 | RC01 | UK-1 | |||
| Cornuspira squatter | 22, 48, 84 | 20947 | 245 | 42.3 | RC01 | UK-1 | |||
| Cyclammina squatter | 36, 62 | 20949 | 333 | 38.6 | RC01 | UK-1 | |||
| Vanhoeffenella sp. | 26 | 20963 | 376 | 38.9 | RC01 | UK-1 | Figure A9d | ||
| Vanhoeffenella squatter | 12, 29, 40, 65 | 21019 | 242 | 46.1 | RC01 | OMS | Figure A8b | ||
| Oridorsalis umbonatus | 53, 57, 67, 80, 89, 90, 101 | 21027 | 284 | 44.6 | RC01 | OMS | Figure A6b,c | ||
| V. propinqua | 19, 27 | 21035 | 328 | 38.8 | RC01 | OMS | Figure A10d | ||
| Storthosphaera squatter? | 60 | 21082 | 306 | 44.4 | RC01 | OMS | Figure A9a,b | ||
| Indet. monothalamid | 5 | 21085 | 261 | 44.8 | RC01 | OMS | Figure A8d | ||
| Indet. monothalamid | 4, 95, 96 | 21087 | 238 | 43.2 | RC01 | OMS | Figure A8c | ||
| C. subglobosa squatter | 38, 47 | 21092 | 333 | 37.7 | RC01 | OMS | Figure A10a–c |
| Isolate | Taxon | ASV Numbers |
|---|---|---|
| Clade C | ||
| 20926 | squatter Bathysiphon | 24, 33 |
| 18640 | squatter Nodosinum | 2, 28 |
| 18547 | undet. Monothalamid | 51 |
| Clade G | ||
| 21019 | squatter Vanhoeffenella | 12 |
| Clade M | ||
| 18127 | squatter Hormosinella | 11, 46, 54, 69, 72, 73 |
| 20949 | squatter Cyclammina | 36, 62 |
| 21087 | undet. Monothalamid | 95 |
| Clade F | ||
| 19132 | Vanhoeffenella | 3, 59 |
| 20963 | Vanhoeffenella | 26 |
| 20911 | spindle shaped undet. Monothalamid | 13 |
| Clade Storthosphaera | ||
| 21085 | undet. Monothalamid | 5 |
| 21087 | undet. Monothalamid | 4, 96 |
| 21019 | squatter Vanhoeffenella | 29, 40, 65 |
| 18122 | undet. monothalamid | 9, 58 |
| New Clade | ||
| 20934 | squatter Reophax | 22, 78, 81, 86, 94 |
| 20947 | squatter Cornuspira | 22, 48, 84 |
| 20932 | squatter Reophax | 22, 70, 77, 93 |
| 18127 | squatter Hormosinella | 10, 42, 44, 49, 55, 56, 64, 68, 71, 83, 85 |
| monothalamids branching independently | ||
| 21082 | squatter Storthosphaera | 60 |
| 17658 | undet. monothalamid | 8, 32, 35, 66 |
| Textulariids | ||
| 21092 | squatter? Cribrostomoides | 38, 47 |
| 21035 | undet. textulariid | 19, 27 |
Appendix B
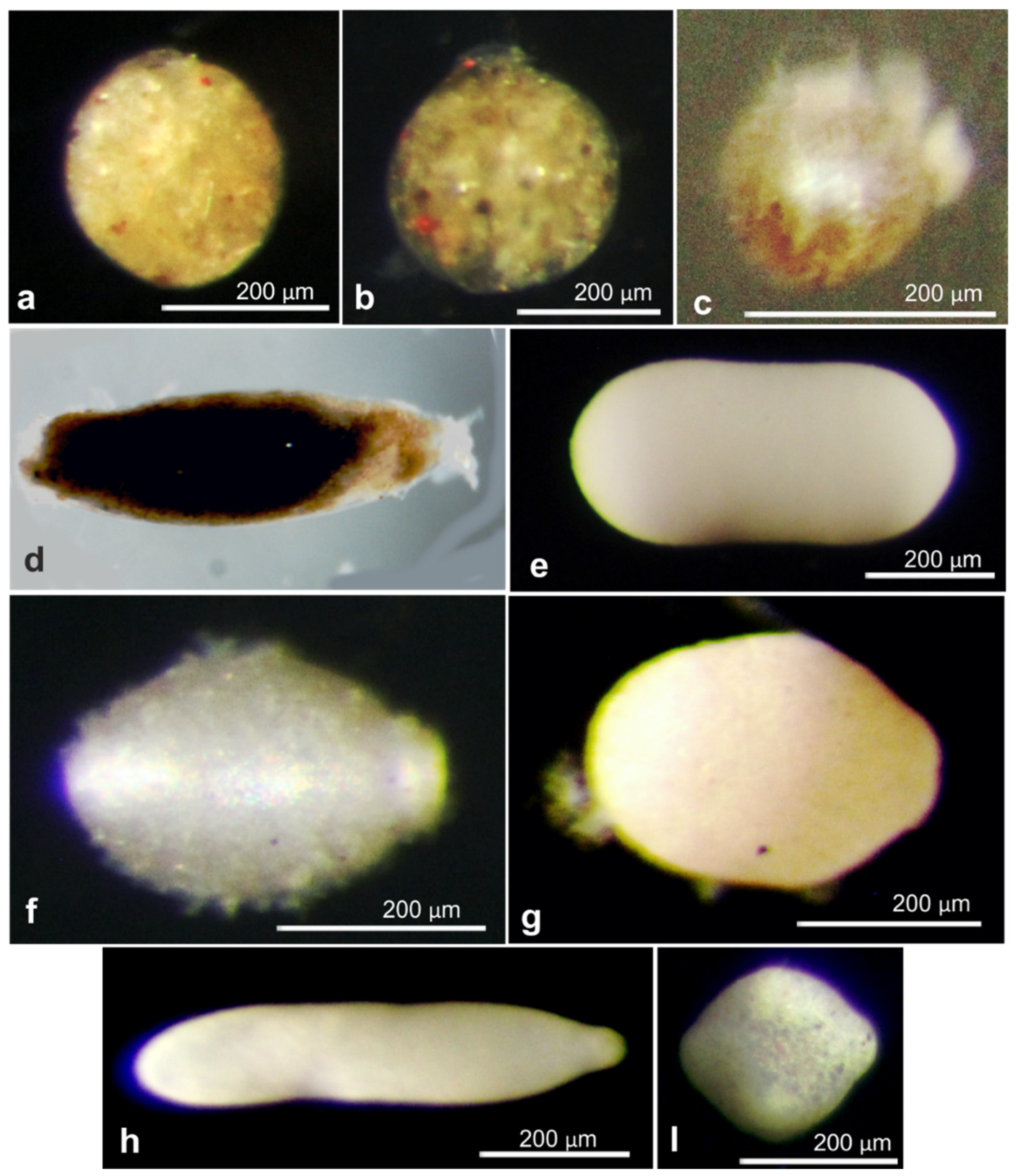
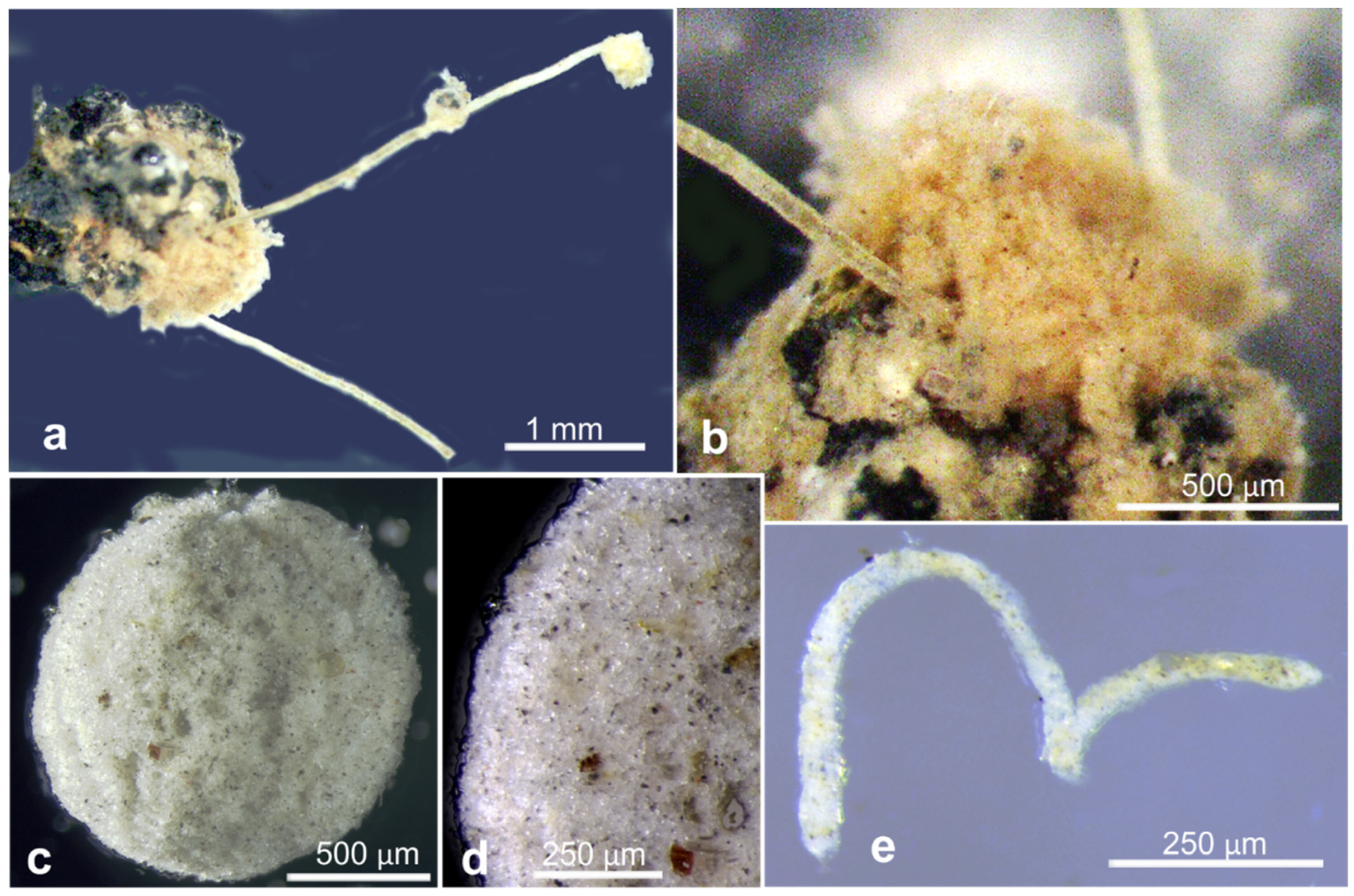


References
- Washburn, T.W.; Jones, D.O.B.; Wei, C.-L.; Smith, C.R. Environmental heterogeneity throughout the Clarion-Clipperton Zone and the potential representativity of the APEI network. Front. Mar. Sci. 2021, 8, 661685. [Google Scholar] [CrossRef]
- Rabone, M.; Wiethase, J.H.; Simon-Lledo, E.; Emery, E.; Jones, D.O.B.; Dahlgren, T.G.; Bribiesca-Contreras, G.; Wiklund, H.; Horton, T.; Glover, A.G. How many metazoan species live in the world’s largest mineral exploration area. Curr. Biol. 2023, 33, 2383–2396. [Google Scholar] [CrossRef]
- Lodge, M.; Johnson, D.; Le Gurun, G.; Wengler, M.; Weaver, P. Seabed mining: International Seabed Authority environmental management plan for the Clarion–Clipperton Zone. A partnership approach. Mar. Policy 2014, 49, 66–72. [Google Scholar] [CrossRef]
- Wedding, L.M.; Friedlander, A.M.; Kittinger, J.N.; Watling, L.; Gaines, S.D.; Bennett, M.; Hardy, S.M.; Smith, C.R. From principles to practice: A spatial approach to systematic planning in the deep sea. Proc. Roy. Soc. B 2013, 280, 20131684. [Google Scholar] [CrossRef]
- Wedding, L.M.; Reiter, S.M.; Smith, C.R.; Gerde, K.M.; Kittinger, J.N.; Friedlander, A.M.; Gaines, S.D.; Clark, M.R.; Thurnherr, A.M.; Hardy, S.M.; et al. Managing mining of the deep seabed. Science 2015, 349, 144–145. [Google Scholar] [CrossRef]
- Peukert, A.; Schoening, T.; Alevizos, E.; Köser, K.; Kwasnitschka, T.; Greinert, J. Understanding Mn-nodule distribution and evaluation of related deep-sea mining impacts using AUV-based hydroacoustic and optical data. Biogeosciences 2018, 15, 2525–2549. [Google Scholar] [CrossRef]
- McQuaid, K.A.; Attrill, M.J.; Clark, M.R.; Cobley, A.; Glover, A.G.; Smith, C.R.; Howell, K.L. Using habitat classification to assess representativity of a protected area network in a large, data-poor area targeted for deep-sea mining. Front. Mar. Sci. 2021, 7, 1066. [Google Scholar] [CrossRef]
- International Seabed Authority (ISA). Deep CCZ Biodiversity Synthesis Workshop Friday Harbor, Washington, USA, 1–4 October 2019. Available online: https://archimer.ifremer.fr/doc/00624/73635/ (accessed on 11 August 2023).
- Smith, C.R.; Clark, M.R.; Goetze, E.; Glover, A.G.; Howell, K.L. Editorial: Biodiversity, connectivity and ecosystem function across the Clarion-Clipperton Zone: A regional synthesis for an area targeted for nodule mining. Front. Mar. Sci. 2021, 8, 797516. [Google Scholar] [CrossRef]
- Gooday, A.J.; Schoenle, A.; Dolan, J.R.; Arndt, H. Protist diversity and function in the dark ocean—Challenging the paradigms of deep-sea ecology with special emphasis on foraminiferans and naked protists. Eur. J. Protistol. 2020, 75, 125721. [Google Scholar] [CrossRef]
- Brady, H.B. Report on the Foraminifera dredged by H.M.S. Challenger during the years 1873–1876: Report of the scientific results of the voyage of H.M.S. Challenger. Zoology 1884, 9, 1–814, pls 1–115. [Google Scholar]
- Cushman, J.A. A monograph of the foraminifera of the North Pacific Ocean. Part 1. Astrorhizidae and Lituolidae. U.S. Natl. Mus. Bull. 1910, 71, 1–134. [Google Scholar]
- Gooday, A.J.; Lejzerowicz, F.J.; Goineau, A.; Holzmann, M.; Kamenskaya, O.; Kitazato, H.; Lim, S.-C.; Pawlowski, J.; Radziejewska, T.; Stachowska, T.; et al. The biodiversity and distribution of abyssal benthic foraminifera and their possible ecological roles: A synthesis across the Clarion-Clipperton Zone. Front. Mar. Sci. 2021, 8, 634726. [Google Scholar] [CrossRef]
- Stachowska-Kaminska, Z.; Gooday, A.J.; Radziejewska, T. Macrofaunal foraminifera from a former benthic impact experiment site (IOM contract area) in the abyssal eastern Clarion-Clipperton Zone. Deep. Sea Res. Part I Oceanogr. Res. Pap. 2022, 188, 103848. [Google Scholar] [CrossRef]
- Gooday, A.J.; Wawrzyniak-Wydrowska, B. Macrofauna-sized foraminifera in epibenthic sedge samples from five areas in the eastern Clarion-Clipperton Zone (equatorial Pacific). Front. Mar. Sci. 2023, 9, 1059616. [Google Scholar] [CrossRef]
- Lejzerowicz, F.; Gooday, A.J.; Barrenechea Angeles, I.; Cordier, T.; Morard, R.; Apothéloz-Perret-Gentil, L.; Lins, L.; Menot, L.; Brandt, A.; Levin, L.A.; et al. Eukaryotic biodiversity and spatial patterns in the Clarion-Clipperton Zone and other abyssal areas: Insights from sediment DNA and RNA metabarcoding. Front. Mar. Sci. 2002, 8, 671033. [Google Scholar] [CrossRef]
- Smith, C.R. Abyssal Baseline Study (ABYSSLINE) Cruise Report. Department Oceanography, University of Hawaii at Manoa: Honolulu, HI, USA, 2013; unpublished work. [Google Scholar]
- Smith, C.R. Abyssal Baseline Study and Geophysical Survey (ABYSSLINE 02). Cruise Report. Department Oceanography, University of Hawaii at Manoa: Honolulu, HI, USA, 2015; unpublished work. [Google Scholar]
- Brenke, N. An epibenthic sledge for operations on marine soft bottom and bedrock. Mar. Technol. Soc. J. 2005, 39, 10–19. [Google Scholar] [CrossRef]
- Pawlowski, J. Introduction to the molecular systematics of foraminifera. Micropaleontology 2000, 46, 1–12. [Google Scholar]
- Pawlowski, J.; Holzmann, M. A plea for DNA barcoding of foraminifera. J. For. Res. 2014, 44, 62–67. [Google Scholar] [CrossRef]
- Dufresne, Y.; Lejzerowicz, F.; Perret-Gentil, L.A.; Pawlowski, J.; Cordier, T. SLIM: A flexible web application for the reproducible processing of environmental DNA metabarcoding data. BMC Bioinform. 2019, 20, 88. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdi, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, A.J. DADA2: High resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef]
- Gouy, M.; Guindon, S.; Gascuel, O. SeaView version 4: A multiplatform graphical user interface for sequence alignment and phylogenetic tree building. Mol. Biol. Evol. 2010, 27, 221–224. [Google Scholar] [CrossRef] [PubMed]
- Guindon, S.; Dufayard, J.F.; Lefort, V.; Anisimova, M.; Hordijk, W.; Gascuel, O. New algorithms and methods to estimate maximum-likelihood phylogenies: Assessing the performance of PhyML 3.0. Syst. Biol. 2010, 59, 307–321. [Google Scholar] [CrossRef] [PubMed]
- Lefort, V.; Longueville, J.E.; Gascuel, O. SMS: Smart model selection in PhyML. Mol. Biol. Evol. 2017, 34, 2422–2424. [Google Scholar] [CrossRef]
- Pawlowski, J.; Holzmann, M.; Tyszka, J. New supraordinal classification of foraminifera: Molecules meet morphology. Mar. Micropaleontol. 2013, 100, 1–10. [Google Scholar] [CrossRef]
- Holzmann, M.; Pawlowski, J. An updated classification of rotaliid foraminifera based on ribosomal DNA phylogeny. Mar. Micropaleontol. 2017, 132, 18–34. [Google Scholar] [CrossRef]
- Pawlowski, J.; Fontaine, D.; Aranda da Silva, A.; Guiard, J. Novel lineages of Southern Ocean deep-sea foraminifera revealed by environmental DNA sequencing. Deep Sea Res. II 2011, 58, 1996–2003. [Google Scholar] [CrossRef]
- Holzmann, M.; Gooday, A.J.; Majewski, W.; Pawlowski, J. Molecular and morphological diversity of monothalamous foraminifera from South Georgia and the Falkland Islands: Description of four new species. Eur. J. Protistol. 2022, 85, 125909. [Google Scholar] [CrossRef]
- Flint, J.M. Recent Foraminifera: A descriptive catalogue of specimens dredged by the U.S. Fish Commission steamer Albatross. Rep. U.S. Natl. Mus. 1897 1899, 249–349. [Google Scholar]
- Goineau, A.; Gooday, A.J. Novel benthic foraminifera are abundant and diverse in an area of the abyssal equatorial Pacific licensed for polymetallic nodule exploration. Sci. Rep. 2017, 7, 45288. [Google Scholar] [CrossRef]
- Cedhagen, T.; Aungtonya, C.; Banchongmanee, S.; Sinniger, F.A.; Pawlowski, J. Gromiids and monothalamous foraminiferans (Rhizaria) from the Andaman Sea, Thailand—Taxonomic notes. Phuket Mar. Biol. Cent. Res. Bull. 2013, 72, 1–17. [Google Scholar]
- Voltski, I.; Gooday, A.J.; Pawlowski, J. Eyes of the deep-sea floor: The integrative taxonomy of the foraminiferal genus Vanhoeffenella. Protist 2017, 169, 235–267. [Google Scholar] [CrossRef] [PubMed]
- de Folin, L. Les Rhizopodes Réticulaires (Suite). Le Nat. n° 10, ser.2 1887, 1, 113–115. [Google Scholar]
- Cushman, J.A. A monograph of the foraminifera of the North Pacific Ocean. Part II. Textulariidae. U.S. Natl. Mus. Bull. 1911, 71, 1–108. [Google Scholar]
- Holzmann, M. Species concept in foraminifera: Ammonia as a case study. Micropaleontology 2000, 46, 21–37. [Google Scholar]
- Ertan, K.T.; Hemleben, V.; Hemleben, C. Molecular phylogeny of selected benthic Foraminifera as inferred from conserved and variable regions of SSU rDNA. Mar. Micropal. 2004, 53, 367–388. [Google Scholar] [CrossRef]
- Schweizer, M.; Pawlowski, J.; Kouwenhoven, T.J.; Guiard, J.; van der Zwaan, G.J. Molecular phylogeny of Rotaliida (Foraminifera) based on complete small subunit rDNA sequences. Mar. Micropaleontol. 2008, 66, 233–246. [Google Scholar] [CrossRef][Green Version]
- Schweizer, M.; Polovodova, I.; Nikulina, A.; Schönfeld, J. Molecular identification of Ammonia and Elphidium species (Foraminifera, Rotaliida) from the Kiel Fjord (SW Baltic Sea) with rDNA sequences. Helgol. Mar. Res. 2011, 65, 1–10. [Google Scholar] [CrossRef]
- Pawlowski, J.; Fahrni, J.F.; Lecroq, B.; Longet, D.; Cornelius, N.; Excoffier, L.; Cedhagen, T.; Gooday, A.J. Bipolar gene flow in deep-sea benthic foraminifera. Mol. Ecol. 2007, 16, 4089–4096. [Google Scholar] [CrossRef]
- Lecroq, B.; Gooday, A.J.; Pawlowski, J. Global genetic homogeneity in deep-sea foraminiferan Epistominella exigua. Zootaxa 2009, 2096, 23–32. [Google Scholar] [CrossRef]
- Gooday, A.J.; Jorissen, F.J. Benthic foraminiferal biogeography: Controls on global distribution patterns in deep-water settings. Ann. Rev. Mar. Sci. 2012, 4, 237–262. [Google Scholar] [CrossRef]
- Holbourn, A.; Henderson, A.; McLeod, N. Atlas of Benthic Foraminifera; Wiley-Blackwell: Chichester, UK, 2013; pp. 1–642. [Google Scholar] [CrossRef]
- Gooday, A.J. A response by benthic Foraminifera to the deposition of phytodetritus in the deep-sea. Nature 1988, 332, 70–73. [Google Scholar] [CrossRef]
- Gooday, A.J. Deep-sea benthic foraminiferal species which exploit phytodetritus: Characteristic features and controls on distribution. Mar. Micropaleontol. 1993, 22, 187–205. [Google Scholar] [CrossRef]
- Cushman, J.A. A monograph of the foraminifera of the North Pacific Ocean. Part V. Rotaliidae. U.S. Natl. Mus. Bull. 1915, 71, 1–81. [Google Scholar]
- Smith, P.B. Foraminifera of the North Pacific Ocean. U.S. Geol. Surv. Prof. Pap. 1973, 766, 1–17. [Google Scholar]
- Ciardo, D.E.; Schar, G.; Bottger, E.C.; Altwegg, M.; Bosshard, P.P. Internal transcribed spacer sequencing versus biochemical profiling for identification of medically important yeasts. J. Clin. Microbiol. 2006, 44, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Caron, D.A. Past president’s address: Protistan biogeography: Why all the fuss? J. Eukaryot. Microbiol. 2009, 56, 105–112. [Google Scholar] [CrossRef]
- Weber, A.A.-T.; Pawlowski, J. Wide occurrence of SSU rDNA intragenomic polymorphism in Foraminifera and its implications for molecular species identification. Protist 2014, 165, 645–661. [Google Scholar] [CrossRef]
- Hayward, B.; Grenfell, H.R.; Sabaa, A.T.; Neil, H.L.; Buzas, M.A. Recent New Zealand deep-water benthic foraminifera: Taxonomy, ecological distribution, biogeography, and use in paleoenvironmental assessment. GNS Sci. Monogr. 2010, 26, 1–363. [Google Scholar]
- Kawagata, S.; Kamihashi, T. Middle Pleistocene to Holocene upper bathyal benthic foraminifera from IODP Hole U1352B in Canterbury Basin, New Zealand. Paleontol. Res. 2016, 20, 1–85. [Google Scholar] [CrossRef]
- Lohmann, G.P. Abyssal benthonic foraminifera as hydrographic indicators in the western South Atlantic Ocean. J. Foram. Res. 1978, 8, 6–34. [Google Scholar] [CrossRef]
- Schulze, R.E. Zoologische Ergebnisse der Nord-seefahrt, vom 21 Juli bis 9 September, 1872. I, Rhizopoden. II, Jahresberichte Kommission zur Untersuchung der Deutschen Meer in Kiel für die Jahr 1872, 1873, 1875. pp. 99–114. Available online: https://www.zobodat.at/pdf/MON-ALLGEMEIN_0299_0001-0447.pdf (accessed on 11 August 2023).
- Jones, R.W.; Bender, H.; Charnock, M.A.; Kaminski, M.A.; Whittaker, J.E. Emendation of the foraminiferal genus Cribrostomoides Cushman, 1910, and its taxonomic implications. J. Micropalaeontol. 1993, 12, 181–193. [Google Scholar] [CrossRef][Green Version]
- Saidova, K.M. Benthonic Foraminifera of the Pacific Ocean; Academy of Sciences of the USSR. P.P. Shirshov Institute of Oceanology: Moscow, Russia, 1975; pp. 1–875. pls 1–116. (In Russian). [Google Scholar]
- Rhumbler, L. Beiträge zur Kenntnis der Rhizopoden. II. Saccammina sphaerica M. Sars. Zweiter Theil. Z. Wiss. Zool. 1894, 57, 587–617. [Google Scholar]
- Christiansen, B.O. Notes on the biology of foraminifera. Troisième Symposium Européen de Biologie Marine. Vie Milieu 1971, 2, 465–478. [Google Scholar]
- Moodley, L. “Squatter” behaviour in soft-shelled foraminifera. Mar. Micropaleontol. 1990, 16, 149–153. [Google Scholar] [CrossRef]
- Hughes, J.A.; Gooday, A.J. The influence of dead Syringammina fragilissima (Xenophyophorea) tests on the distribution of benthic foraminifera in the Darwin Mounds region (NE Atlantic). Deep-Sea Res. I 2004, 51, 1741–1758. [Google Scholar] [CrossRef]
- Gooday, A.J. Meiofaunal foraminiferans from the bathyal Porcupine Seabight (northeast Atlantic): Size structure, standing stock, taxonomic composition, species diversity and vertical distribution in the sediment. Deep-Sea Res. 1986, 33, 1345–1373. [Google Scholar] [CrossRef]
- Gooday, A.J.; Rothe, N.; Pearce, R.B. New and poorly known benthic foraminifera (Protista, Rhizaria) inhabiting the shells of planktonic foraminifera on the bathyal Mid-Atlantic Ridge. Mar. Biol. Res. 2013, 9, 447–461. [Google Scholar] [CrossRef]
- Goineau, A.; Gooday, A.J. Radiolarian tests as microhabitats for novel benthic foraminifera: Observations from the abyssal eastern equatorial Pacific (Clarion–Clipperton Fracture Zone). Deep-Sea Res. I 2015, 103, 73–85. [Google Scholar] [CrossRef][Green Version]
- Lecoq, B.; Gooday, A.J.; Cedhagen, T.; Sabbatini, A.; Pawlowski, J. Molecular analyses reveal high levels of eukaryotic richness associated with enigmatic deep-sea protists (Komokiacea). Mar. Biodiv. 2009, 39, 45–55. [Google Scholar] [CrossRef]
- Gooday, A.J.; Goineau, A. The contribution of fine sieve fractions (63–150 µm) to foraminiferal abundance and diversity in an area of the eastern Pacific Ocean licensed for polymetallic nodule exploration. Front. Mar. Sci. 2019, 6, 114. [Google Scholar] [CrossRef]
- Goineau, A.; Gooday, A.J. Diversity and spatial patterns of foraminiferal assemblages in the eastern Clarion–Clipperton zone (abyssal eastern equatorial Pacific). Deep Sea Res. Part I Oceanogr. Res. Pap. 2019, 149, 103036. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Himmighofen, O.E.; Holzmann, M.; Barrenechea-Angeles, I.; Pawlowski, J.; Gooday, A.J. An Integrative Taxonomic Survey of Benthic Foraminiferal Species (Protista, Rhizaria) from the Eastern Clarion-Clipperton Zone. J. Mar. Sci. Eng. 2023, 11, 2038. https://doi.org/10.3390/jmse11112038
Himmighofen OE, Holzmann M, Barrenechea-Angeles I, Pawlowski J, Gooday AJ. An Integrative Taxonomic Survey of Benthic Foraminiferal Species (Protista, Rhizaria) from the Eastern Clarion-Clipperton Zone. Journal of Marine Science and Engineering. 2023; 11(11):2038. https://doi.org/10.3390/jmse11112038
Chicago/Turabian StyleHimmighofen, Oceanne E., Maria Holzmann, Inés Barrenechea-Angeles, Jan Pawlowski, and Andrew J. Gooday. 2023. "An Integrative Taxonomic Survey of Benthic Foraminiferal Species (Protista, Rhizaria) from the Eastern Clarion-Clipperton Zone" Journal of Marine Science and Engineering 11, no. 11: 2038. https://doi.org/10.3390/jmse11112038
APA StyleHimmighofen, O. E., Holzmann, M., Barrenechea-Angeles, I., Pawlowski, J., & Gooday, A. J. (2023). An Integrative Taxonomic Survey of Benthic Foraminiferal Species (Protista, Rhizaria) from the Eastern Clarion-Clipperton Zone. Journal of Marine Science and Engineering, 11(11), 2038. https://doi.org/10.3390/jmse11112038






