Abstract
Paracentrotus lividus gonads, often referred to as “roe” or “uni” for gastronomical purposes, are among the most appreciated seafood delicacies in the Mediterranean area and worldwide. However, the increasing demand for human consumption has caused a growing pressure on its wild stocks, prompting the need to develop efficient aquaculture systems for its production. The set-up of effective feeds for various life stages and optimal procedures for breeding post-larvae and adult sea urchins still need to be improved. Here, for the first time, we aim at developing artificial feeds for the post-larvae of P. lividus because a critical step to improve our productive skills is post-larval growth. We tested various natural and prepared components to speed up the growth and enhance the survival rates of post-larvae, while taking into account the abiotic and biotic factors influencing the culture conditions in three replicate sets of tanks, characterized by different volumes. We tested formulated feeds and compared them with the effect of fresh foods in the frame of canonical culture practices. Our results indicated the efficiency of a feed composed of spirulina and Ulva rigida. Statistical analyses demonstrated the positive impact of this diet on the growth of post-larvae, behavior and survival rates. In addition, we demonstrated the efficacy of prepared feeds in the sea urchin aquaculture system, because they facilitated manipulation and control of the culture procedures for the satisfactory growth of P. lividus post-larvae.
1. Introduction
The purple sea urchin Paracentrotus lividus (Lamarck, 1816) is diffused throughout the Mediterranean Sea and along the northeastern Atlantic coast, from Scotland and Ireland to the Southern Morocco and the Canary Islands [1]. P. lividus is considered a key species for several coastal communities associated with vegetated ecosystems, due to its essential role in their food webs [2]. In addition, it is often the dominant herbivore of shallow reefs, and its removal can impact the associated communities [3]. The feeding preferences of purple sea urchins vary according to their size and the diversity and abundance of the plant cover [4]. The edible part of the sea urchin, named “roe”, is represented by gonads, which are made of somatic cells (nutritive phagocytes) and germinal cells (oogonia and spermatogonia). Sea urchin roe is a highly valued food and is considered a delicacy in the international marketplace [5,6]. The demand for roes has grown in the last decades, hence the sea urchin natural stocks have been heavily overfished in various coastal areas [7,8]. Consequently, the interest in the production of sea urchins through aquaculture has grown, as a necessary alternative to fishery [9,10], not only to meet the demand for human consumption but also to reduce the harvesting pressure on wild populations. The identification of suitable diets and rearing methods enables the production of high-quality gonads, available on the market throughout the year [11]. Additionally, the production of a large number of juveniles and their subsequent introduction into the wild could be a solution for the conservation of natural populations [3]. Sea urchins, as most marine invertebrates, exhibit external fertilization and further spawning in the water column: female and male gametes are released simultaneously by gonopores. Gametes can be emitted following slight modification of the environmental conditions (e.g., seasonal changes of temperature or even mechanical disturbances) [12,13].
Most adult sea urchins are opportunistic plant feeders. P. lividus mainly feeds on algae and seagrasses, ingesting some animal items along with plant biomass [2]. For this reason, the set-up of a diet for adults is relatively simple, taking into account that, in aquaculture practice, it should promote gonadal growth and quality to provide marketable roes. Sea urchin aquaculture is a promising field, but it may be challenging. The major bottleneck limiting the aquaculture production of various marine species is represented by high mortality rates during larval and post-larval stages [14]. For P. lividus, post-settlement mortality may achieve rates higher than 90%, in some cases within the first weeks of benthic life [15].
Several attempts to improve larval survival and to develop suitable rearing methods for post-larvae [16], as well as diets and feed ratios [17,18] were made. In addition, it is essential to improve larval diets and feed ratios, because they also affect the survival and growth of post-larvae [19,20]. After fertilization, larvae usually take 18–25 days to reach the competent stage for settlement, shifting to the benthic life [21]. At this stage, suitable environmental cues, such as the presence of macroalgae or compounds emitted by adult conspecifics, may be important triggers to stimulate settlement [11]. While the larval morphology changes, the benthic juvenile stage may start only after the complete development of the mouth and anus, which become functional, along with the completion of the digestive system. During larval growth, sea urchins modify their morphology and feed on microorganisms suspended in the seawater. According to Lawrence et al. [22], many species of cultured algae support the growth and development of larvae up to metamorphosis. Rodhomonas lens, Dunaliella tertiolecta, Phaeodactylum tricornutum, Isochrysis galbana and Cricosphera elongata are the most effective to promote the larval growth in aquaculture plants. However, they show significant differences in terms of effectiveness at promoting development and survival [22]. Larvae are exotrophic, yet after about 48 h post-fertilization, they filter microorganisms in the water column, but post-larvae start to graze algal biofilms immediately after the settlement [11].
When individuals reach a test diameter of 3–4 mm, a biofilm diet should be supplied, containing macroalgae species such as Enteromorpha spp., Ulva spp., Saccharina spp. and Laminaria spp. [11]. While rearing and feeding techniques for the larval stages are relatively well developed, studies aimed at improving the quality of feeds applied to post-larval productions have received little attention. Indeed, only a few studies focused on the post-larval growth and survival of various species of sea urchins, and these investigations have been carried out just within the first 10 days post-settlement [18,19]. Moreover,Taylor et al. [23] suggested that juveniles as small as 3–4 mm can use formulated feeds for growth. In fact, formulated feeds for juvenile sea urchins are theoretically feasible and more practical than live, natural diets for supporting and maintaining growth in a culture, which is important for developing a sea urchin full-cycle aquaculture industry and in line with the aims of our research.
Despite the previous and current scientific production, the definition of the “perfect” diet for both post-larvae and adult P. lividus is still to be obtained, and investigations adding information to this puzzle can be useful to reach well-performing aquaculture procedures [24]. Recently, there has been an increase in research efforts focused on the use of microalgae, such as spirulina, for aquaculture purposes. These studies were mainly aimed at investigating the use of spirulina, with the urge to find alternatives to fish meal and fish oil in compound feeds, due to their rapidly rising prices and the demand for sustainable fisheries [25]. In general, microalgae have emerged as a natural nutritional source for novel bioactive compounds [26], and spirulina (namely, Arthrospira platensis, a cyanobacterium) is the most common cultured microorganism easily produced on a commercial scale [27]. The mass culture of spirulina could be a potential solution to the world food crisis and the global protein shortage and thus, serve as an excellent source of proteins to replace animal-derived diets. The aim of this research is to investigate future possibilities of developing effective formulated feeds for the post-larvae of purple sea urchin. We performed tests on post-larval feeds formulated ad-hoc to identify the most effective ones in terms of the survival and development of post-larvae. To this end, we monitored the abiotic and biotic factors affecting the survival and the development of post-larvae of P. lividus subjected to a range of formulated diets.
2. Materials and Methods
2.1. Gamete Collection and Larval Development
Adult sea urchins were collected by scuba divers around the island of Procida (Bay of Naples, Italy) in February 2021 at 10 m depth. The collected specimens were immediately transported in a thermally insulated box to the laboratory and maintained in tanks with circulating sea water for ten days until testing. Gamete emission was stimulated by gently injecting some specimens with 1 mL of 0.5 M KCl through the peribuccal membrane. Females (identified according to Brundu et al. [28]) were positioned over beakers filled with sterilized seawater to collect eggs. Dry sperm was collected and used for in vitro fertilization, activated by adding sterilized seawater and counted in a cell-counting chamber in order to mix adequate proportions of eggs and spermatozoans, to obtain successful fertilization. We opted for a sperm-to-egg ratio of 100:1 [29]. The beakers were kept at 20 °C in a controlled temperature chamber on a 12/12 h light/dark cycle. Forty-eight hours post-fertilization, the embryos reached the pluteus stage and were distributed in equal concentrations (1.5 plutei mL−1) in 15 L plastic tanks containing 3 L of sterilized seawater (Figure 1), because these conditions were demonstrated to assure good survival rates and settlement capability [30]. According to the culture conditions suggested in the literature [30,31], larvae were fed on Dunaliella tertiolecta (5000 cells mL−1) along with a composed algal food (SHG Snow Reef, Super High Group, Ovada, Italy; www.superhigroup.com, accessed on 30 October 2022) containing yeast, fish oils, Chlorella and various HUFAs; they mimic the composition of natural marine snow in a suspension of particles sized 5–10 μm [31]. Larval rearing lasted 30 days. Water renewals were assured every one or two days and continuous aeration was assured until the complete settlement of all survivorships. The young sea urchins, just settled, were used to test experimental post-larval feeds.
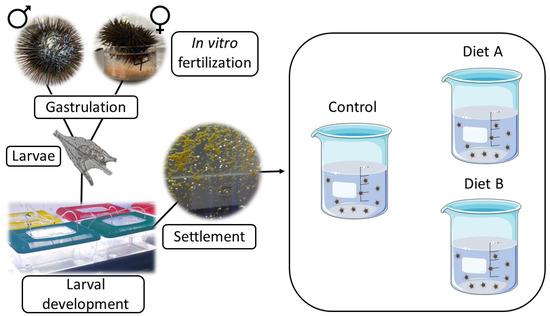
Figure 1.
Experimental scheme of the feeding trial with control diet and diets A and B, starting from the in vitro fertilization.
2.2. Feeding Experiments
Feeding tests on post-larvae were conducted in 2 L glass beakers containing 1.5 L of sterilized seawater (Figure 1) equipped with an aeration system. One main trial was performed, using three different diets containing various proportions of basic ingredients to progressively improve their efficiency. All diets were offered after incorporation into an agarose matrix, identical for all the formulations and for the controls, to stabilize the content and avoid the excessive leaching of the dietetic components between the next two distributions of feed, performed every three days. Water renewals were performed every two to three days, and the control of the culture system conditions was consistently achieved by means of abiotic and biotic measurements. The base agarose matrix preparation consisted of the addition of 3 g of agarose (dry powder Agarose LE, Roche, USA) to 100 mL of seawater (3% agarose in weight) heated to 70 °C. All ingredients (Table 1) were added prior to reaching the ambient temperature, when the agarose gel was still liquid. Finally, the agarose mixtures were poured into Petri dishes, and, after complete gelification at 5 °C, each plate was cut into small pieces of 1 g each and kept in a refrigerator at 5 °C. The experiment lasted 80 days.

Table 1.
The ingredient composition (weight expressed in percentage) of two diets tested on P. lividus post-larvae, offered in an agarose matrix. Diets indicated with the letters A and B were consistently compared to a control diet containing only the common alga Ulva rigida often used as a convenient feeding substrate for sea urchins.
As is evident, the experiment contained U. rigida as a natural dietetic item in order to provide a control contained in the same agarose matrix as the other diets. U. rigida also represented an attractant for some formulated diets. Samples of U. rigida were hand-collected in shallow waters off the bay of Naples and transferred to the laboratory in tanks containing seawater from the collection site. In the laboratory, samples were carefully checked and washed in order to discard undesired material and fragments of other algal species. U. rigida supplies were renewed weekly to provide fresh feeds.
The two diets used for these experiments both contained SHG® “microperle”, a supplementary food contained in convenient disposable capsules and usually used for marine invertebrates. In addition, they contained Biomarin Algamac Enrich®, a dry mix usually adopted for pelagic marine larvae; spirulina is usually also used in the preparation of freshwater and marine fish species feeds (Table 2).

Table 2.
Food elemental composition of spirulina (A), SHG® Microperle (B) and Biomarin Algamac Enrich® (C).
2.3. Biotic and Abiotic Measurements
Abiotic and biotic measurements were recorded regularly during the experiment to follow the development of post-larvae and the effect of each feed. Physical and chemical characteristics of the seawater from each tank were measured every two to three days. Water samples were taken in 50 mL beakers from the tanks to be analyzed with the filter photometer “AL450” by Aqualytic. Chemical analyses were conducted to evaluate the concentrations of nitrites, nitrates, ammonium and phosphates. Temperature, dissolved O2 and salinity were measured using a precision mercury thermometer, an oxygen portable meter ProfiLine oxi 41 3310 (WTW, Germany) and a refractometer model Bioeropeak RFT-PA Series, respectively. A multi-parameter set XS PC 70 allowed the measurement of pH. Each water parameter was compared with the values characterizing the local marine environment, measured in the seawater collected at the same site as the collection of the sea urchins. In particular, post-larval stages are very sensitive to chemical and physical stressors, and the highest mortalities usually occur in this phase compared to the juvenile or the adult stages. Fluctuations of temperature were avoided by performing all the experiments in a thermostatic chamber constantly kept at 18 °C (Table S1 in the Supplementary Material file).
Biotic data considered to test the conditions of post-larvae were the number of post-larvae (counted under an optical microscope) and their size (test diameter estimated using millimetric paper). In addition, movement patterns of cultured specimens were controlled every two days in order to relate their behavior to a normal development. The movement patterns were evaluated using an arbitrary scale ranging from one to three, wherein: 1: low movements; 2: moderate movements and 3: fast movements.
Growth increments of the post-larvae during the experiment were evaluated by taking pictures of all the individuals at the start of the feeding experiment (t0), at an intermediate time of the experiment (tf/2 = 40th day) and at the end of the experiment (tf = 82nd day) Twenty post-larvae submitted to these measures were randomly chosen and analyzed at these three times. Size measures were obtained using a millimetric paper positioned under the Petri dish containing the post-larvae and observed under a stereomicroscope. The sizes of individuals were further classified into three classes corresponding to: “<1 mm”, “1–2 mm” and “>2 mm”.
2.4. Statistical Analyses
Data to evaluate both the numerical variation of P. lividus post-larvae and their growth increments were tested for normality and the homogeneity of variances by the D’Agostino and Pearson’s normality test. Further, the significance of differences among replicates was evaluated using ordinary one-way ANOVA, and individual relationships were assessed by means of the Dunnett’s multiple comparisons test. The size of post-larvae was assessed at three time-intervals for each of three diets (two experimental diets and a control), and results were reported as size-frequency histograms. The significance of differences among diets and time intervals was evaluated by means of two-way ANOVA. Non parametric one-way ANOVA was used to check the significance of differences in the movement pattern score obtained from the post-larvae between treatments. A value of p < 0.05 was chosen as a threshold for significance. Correlation matrix analysis was also performed to evaluate the correlations between larger data sets in the same experiment, and Dunnett’s multiple comparisons test was used to evaluate all pairwise treatment comparisons (Tables S2 and S3). All data were analyzed by means of GraphPad Prism 7.0 (GraphPad Software, San Diego, CA, USA, www.graphpad.com, accessed 30 October 2022).
3. Results
The density of post-larvae decreased progressively along the experiment for all diet types; however, the mortality rates were significantly different. In the replicates of the post-larvae feeding on the control diet (U. rigida), the number of specimens dropped from 20 to 14 individuals (data recorded on the 57th day) after ten days. A higher mortality was recorded on the 67th day of the experiment, and the number of individuals kept decreasing up to the end of the experiment (88% mortality). The mortality of post-larvae feeding on diet B (U. rigida, SHG microperle and Algamac Enrich) was almost constant during the whole experiment, with the only exception of the period between the 7th and 20th day. In all replicates, a survival of 30% was recorded at the end of the experiment. Lower mortality rates were recorded in post-larvae fed on diet B (U. rigida and spirulina). The number of larvae was constant until the 30th day of the experiment (18 post-larvae); at this point, mortality rates increased by 18%, yet the average number of post-larvae remained almost constant until the end of the experiment (Figure 2).
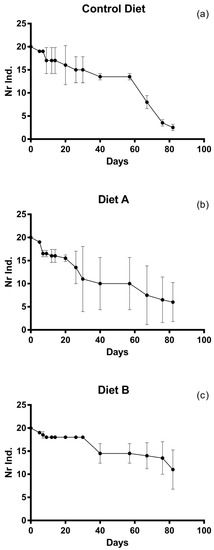
Figure 2.
Mortality rates of post-larvae during the experimental period. Dots indicate the average number of individuals for each diet: (a) control diet (U. rigida); (b) diet A (U. rigida, SHG® microperle, Algamac Enrich); (c) diet B (U. rigida, 2% spirulina SHG®).
The significance of differences among replicates was evaluated using ordinary one-way ANOVA, and individual relationships were assessed by means of the Dunnett’s multiple comparisons test performed on the average number in the replicates feeding on the two diets (Table 3). Data were tested for normality by the D’Agostino and Pearson normality test (Table S2), respectively. Results showed a significant difference between the control diet (U. rigida) and diet B (U. rigida + 2% Spirulina), whereas the abundances of post-larvae feeding on the control diet (U. rigida) and diet A (U. rigida + microperle + Algamac) were not significantly different at the end of the experiment.

Table 3.
Dunnett’s multiple comparisons test between post-larvae of P. lividus feeding on control diet (U. rigida), diet A (U. rigida, SHG® microperle, Algamac Enrich) and diet B (U. rigida, 2% spirulina SHG®) of the trial. Threshold p < 0.05. (* = statistically significant; ns = not statistically significant).
Post-larvae feeding on U. rigida + 2% spirulina (Diet B) were the most active, exhibiting higher movement scores compared to diet A (see Table S7 in the Supplementary Material). In fact, the latter showed stable behavior compared to the post-larvae fed on (U. rigida+ Microperle + Algamac), which were less responsive all along the experiment. Differently, individuals fed on the control diet (U. rigida) were more responsive at the start of the experiment, but their movement patterns completely turned off at the end of the experiment.
Dunnett’s multiple comparisons were also performed to define statistically significant differences in the movement scores of P. lividus post-larvae fed on different diets. Intriguingly, a significant difference was found between the control diet (U. rigida) and diet B (U. rigida+ 2% spirulina), whereas no significant difference was found between individuals feeding on the control diet and diet A (Table 4). Statistical differences in the movement scores of post-larvae fed on different diets were tested by non-parametric one-way ANOVA. The analysis indicated that diet A and diet B promoted significant differences in animal activity (Table 5).

Table 4.
Dunnett’s multiple comparisons test between movement scores of P.lividus post-larvae feeding on control diet (U. rigida), diet A (U. rigida, SHG® microperle, Algamac Enrich) and diet B (U. rigida, 2% spirulina SHG®) AB. Threshold p < 0.05 (** = statistically significant; ns = not statistically significant).

Table 5.
Non parametric one way Anova among movement scores of P. lividus post-larvae feeding on Control Diet, diet A (U. rigida, SHG® microperle, Algamac Enrich) and diet B (U. rigida, 2% spirulina SHG®). Threshold p < 0.05 (* = statistically significant).
The test diameter of post-larvae feeding on the Control diet (U. rigida) was evaluated at the start of the third phase of the experiment (t0) when most post-larvae (62%) had a size between 1 and 2 mm. By the end of the experiment, post-larval size ranging from 1 to 2 mm increased by 23.7%, and individuals larger than 2 mm accounted for 14.20% (Figure 3a; see also Figure 4). Similar patterns were noticed on post-larvae fed on the second diet (U. rigida + Microperle + Algamac). Their size was between 1–2 mm in most individuals (72%). By the end of the experiment, about 20% of post-larvae were larger than 2 mm, whereas most of them (80%) ranged between 1 and 2 mm (Figure 3b). At the start of the experiment with the third diet (U. rigida + spirulina), more than half post-larvae (63%) were between 1 and 2 mm, and only 35% were smaller than 1 mm. Interestingly, at tf, post-larvae wider than 2 mm accounted for 11% of the total, while there was an increase of roughly 22% of individuals in the range of 1–2 mm (Figure 3c).
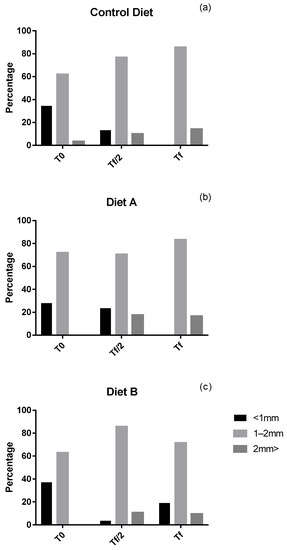
Figure 3.
Size-frequency histograms of P. lividus post-larvae feeding of (a) control diet (U. rigida), (b) diet A (U. rigida, SHG® microperle, Algamac Enrich), (c) and diet B (U. rigida, spirulina SHG®) at three experimental times (T0 = start of the experiment; Tf/2 = 40th day; Tf = 82nd day).
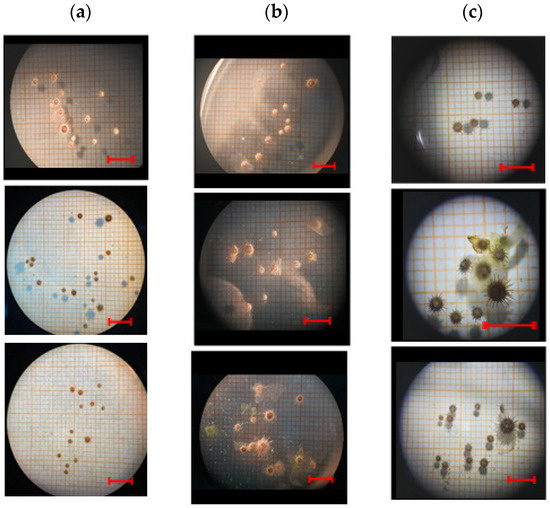
Figure 4.
P. lividus post-larvae size captures at t0, tf/2 and tf in the replicates of (a) control diet, (b) diet A and (c) diet B. The red scale bar measures 10 mm, according to the magnification under optical microscopy.
The results of two-way ANOVA performed on the size frequency distributions of post-larvae indicated no significant differences among treatments at all the time intervals. The unique significant factor (at p < 0.001) was time, indicating that all treatments produced growth during the experimental time.
4. Discussion
This study was conducted aiming at determining the physiological responses (in terms of growth, survival and physiologic performances) of P. lividus according to three feed formulations, varying in quality (plant or animal source ingredients) and biochemical composition, following the changes in environmental conditions (both physical and chemical). The results reported above demonstrated that artificial diets have a potential to be integrated and used for the feeding of post-larvae.
Previous research focused primarily on composed feeds developed for the adult stage [32]. However, a sustainable aquaculture practice for any marine species requires the culture of all life stages. Our findings confirmed the suggestion by George et al. [33], indicating that artificial feeds may reduce the need for culturing phytoplankton for the post settlement stage and provide consistency of results. In this view, artificial feeds are likely to be more practical to use and economically efficient. It was shown that the preparation of artificial feeds through incorporation in agarose permits a longer conservation of the feeds and a higher stability in the seawater, as well as an easy manipulation of the doses to be offered, and it guarantees the continuous availability of post-larval feeds, while requiring minimum maintenance. Our findings are in line with Jangoux [34], demonstrating that the quality of the culture environment for juveniles of P. lividus is improved by the use of a proper “water-resistant” diet and that it is crucial for intensified echinoculture.
The growth model established with the data obtained from the results of this investigation, comparing the performances of individuals fed with two different feeds, indicated that the growth of the sea urchins can be maximized by an appropriate formulated feed. As a matter of fact, the preparation of feeds and the determination of ratios was made easy by the possibility of manipulation and conservation in the refrigerator, which was quite more complex when using microalgae such as Ulvella lens or live diatoms, requiring initial laboratory isolation and culture with the risk of collapse, due to external environmental factors or accidental events. In contrast, a formulated feed for post-larvae is efficient in terms of growth and survival rates, while facilitating an easy manipulation and a consistency of the dietetic formulation.
In parallel, our study confirmed the outcomes of the research conducted by Solari et al. [35], indicating the diet efficacy of spirulina integrated within a formulated food. In fact, we demonstrated that, notwithstanding that spirulina cannot be considered as a “natural” food item for post-larvae of Mediterranean invertebrates, P. lividus exhibited a clear attraction towards feeds containing spirulina by showing clear chemotactic activity, recently demonstrated by Luis and Gago [36]. During the trial of post-larval feeding experiments, the combination of spirulina and U. rigida in equal proportions yielded interesting results in terms of growth, movement, survival rates and water quality (low turbidity influence) compared to the other diet. In addition, biotic measurements indicated that, combining the size increase and the survival rates, spirulina and Ulva mixed in equal proportions represented the most efficient diet, resulting in the highest number of post-larvae and the fastest size increases.
The abiotic descriptors of water also demonstrated that the physical and chemical quality of the medium was not negatively impacted by the addition of spirulina. In contrast, other diets tested (e.g., diet A “U. rigida + Microperle + Algamac”) caused water turbidity. Nonetheless, growth measurements showed a positive influence of diet A “U. rigida+ Microperle + Algamac” on the size increments of post-larvae, in agreement with the conclusions of Pearce et al. [37] on the efficiency of highly proteic feeds for Strongylocentrotus droebachiensis. However, the latter study also demonstrated that feeds that easily break down may sink to the bottom and promote conditions of anoxia, consequently leading to increased mortalities. Assuming that frequent water changes must be assured to preserve water quality, it is important to also take into consideration other factors, such as temperature, whose increase directly affects the post-larvae survival [38]. However, despite temperature variations, the diet B also showed the best results in terms of water turbidity and the presence of pollutants.
An important finding to be underlined concerned the greater movement activity of post-larvae fed on U. rigida + 2% spirulina with respect to those fed on “U. rigida + Microperle + Algamac”. Considering the food elemental composition, spirulina is characterized by a higher protein content with respect to the other components used in the two diets. No evidence was reported in literature about the possible relationship between the food composition and the post-larvae reactivity. Data are instead present for P. lividus adults, demonstrating that their responsiveness was closely dependent on its nutritional state [36]. In particular, clear ability of this sea urchins was evidenced to positively discriminate proteins, and incitants/stimulants for them were only found among proteins. It could be the same for the post-larvae, for which the high protein content of a food could represent an exciting stimulus for faster movement.
Our results are important for productive aquaculture purposes because this type of feeds fulfills two major concerns of aquaculture facilities, i.e., the productive yields and the costs of production, as indicated by [39]. In fact, the main advantages of formulated feeds are represented by their consistent quality, besides the reduced work effort and their easy storage, which reduce the possible seasonal concerns about feed availability. Most studies that used formulated feeds for sea urchin aquaculture explored their effects on mature gonadal growth, the ingestion rates and the somatic growth of adults [31,40]. In various species of sea urchins [41] the gonadal growth was faster with formulated feeds than with fresh algal foods [42]. A recent study by Zupo et al. [31] investigated the gonadosomatic indices, the histological features of gonads and the quality of gametes and larvae produced by individuals reared under a range of formulated feeds. The study also compared the effects of plant items naturally found in sea urchin typical habitats, such as the macroalga U. rigida and the seagrass Posidonia oceanica [31,43], aiming at evaluating the effects of five formulated diets on growth and gonadosomatic indices. This study confirmed that formulated pellets are efficient at enhancing the production of large gonads [44] and favored higher gonadosomatic indices as compared to the other tested diets and a natural meal based on P. oceanica leaves [31]. However, other factors, such as gamete quality and individual variability, may impact the fitness of plutei and consequently the survival of post-larvae [30].
Based on the results reported above, we conclude that an artificially formulated feed containing equal proportions of spirulina and algae is adequate food for sea urchins in an aquaculture environment. Such conclusions are in line with those of previous research, demonstrating that a feasible diet for adult sea urchins should contain both animal and plant items [32], and it produces maximum growth especially when it contains a smaller animal-protein component (around 10%) and a larger amount of plant components (over 90%) [45]. In the case of the post-larvae herein considered, the “animal” component was successfully replaced by spirulina, a cyanobacterium characterized by a high protein content.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jmse11010154/s1, Table S1. Abiotic descriptors of water quality in beckers during the experiment trial; Table S2. Correlation matrix for abiotic and biotic measures from replicates of control diet. Table S3. Correlation matrix for abiotic and biotic measures from repiclates of Diet A. Table S4. Correlation matrix for different abiotic and biotic measures from replicates of diet B; Table S5. D’Agostino & Pearson normality test on number of P. lividus post-larvae feeding on Control diet (U. rigida), and diet A (U. rigida, SHG® microperle, Algamac enrich) and diet B (U. rigida, 2% Spirulina SHG®). Table S6. Average values of biotic data corresponding to the different replicates of post-larvae feeding on Control Diet, Diet A, and Diet B. Table S7. D’Agostino & Pearson normality test on post-larvae of P. lividus movement scores on Control diet (U. rigida), and diet A (U. rigida, SHG® microperle, Algamac enrich) and diet B (U. rigida, 2% Spirulina SHG®).
Author Contributions
M.G. performed the experimental work, the statistical analyses and produced the first draft of the manuscript; F.G. performed and supervised the experimental work in Procida. S.F. and B.P. contributed to the experimental work in Procida. A.D.C. planned the research and supervised the production of the manuscript. V.Z. planned the research, revised the manuscript, supervised the experimental work in Procida and contributed to the production of the first draft and its final revision; M.C. helped in planning the research, contributing the first draft, revising the manuscript and the supervision of students. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We acknowledge the private firm Echinoidea at Procida Island (Gulf of Naples), managed by Chiara and Michele Trapanese, where feeding experiments and in vitro fertilization of the sea urchin Paracentrotus lividus were performed, including post-larvae culture. Thanks to the technical help of Mario Loffredo e Domenico Mattera. Francesca Glaviano was supported by a PhD fellowship (PhD in Biology, University of Naples Federico II) at the Stazione Zoologica Anton Dohrn.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Boudouresque, C.F.; Verlaque, M. Ecology of Paracentrotus lividus. In Edible Sea Urchin: Biology and Ecology; Lawrence, J.M., Ed.; Elsevier Science B.V: Amsterdam, The Netherlands, 2007; pp. 243–285. [Google Scholar]
- Zupi, V.; Fresi, E. A study on the food web of the Posidonia oceanica ecosystem: Analysis of the gut contents of echinoderms. In International Workshop on Posidonia oceanica Beds 1; GIS Posidonie Publishing: Marseille, France, 1984; pp. 373–379. [Google Scholar]
- Andrew, N.L.; Agatsuma, Y.; Ballesteros, E.; Bazhin, A.G.; Creaser, E.P.; Barnes, D.K.A.; Xiaoqi, Z. Status and management of world sea urchin fisheries. Oceanogr. Mar. Biol. 2002, 40, 343–425. [Google Scholar]
- Boudouresque, C.F.; Verlaque, M. Paracentrotus lividus. In Developments in Aquaculture and Fisheries Science; Elsevier: Amsterdam, The Netherlands, 2013; pp. 297–327. [Google Scholar]
- McBride, S.C. Sea urchin aquaculture. Am. Fish. Soc. Symp. 2005, 46, 179. [Google Scholar]
- Unuma, T. Gonadal growth,and its relationship to aquaculture in sea urchins. In The Sea Urchin: From Basic Biology to Aquaculture; Swets & Zeitlinger: Lisse, The Netherlands, 2002; pp. 115–127. [Google Scholar]
- Addis, P.; Secci, M.; Manunza, A.; Corrias, S.; Niffoi, A.; Cau, A. A geostatistical approach for the stock assessment of the edible sea urchin, Paracentrotus lividus, in four coastal zones of Southern and West Sardinia (SW Italy, Mediterranean Sea). Fish. Res. 2009, 100, 215–221. [Google Scholar] [CrossRef]
- Pais, A.; Chessa, L.A.; Serra, S.; Ruiu, A.; Meloni, G.; Donno, Y. The impact of commercial and recreational harvesting for Paracentrotus lividus on shallow rocky reef sea urchin communities in North-western Sardinia, Italy. Estuarine. Coast. Shelf Sci. 2007, 73, 589–597. [Google Scholar] [CrossRef]
- Pearce, C. Sea urchin aquaculture. Bull. Aquac. Assoc. Can. 2010, 108, 1–2. [Google Scholar]
- Grosjean, P. Growth Model of the Reared Sea Urchin Paracentrotus lividus (Lamarck, 1816). Ph.D. Thesis, Universite Libre De Bruxelles, Brussels, Belgium, 2001. [Google Scholar]
- Carboni, S.; Hughes, A.D.; Atack, T.; Tocher, D.R.; Migaud, H. Influence of broodstock diet on somatic growth, fecundity, gonad carotenoids and larval survival of sea urchin. Aquac. Res. 2013, 46, 969–976. [Google Scholar] [CrossRef]
- Spirlet, C. Biologie de L’oursin Comestible (Paracentrotus lividus): Contrôle du cycle reproducteur et optimalisation de la phase de remplissage gonadique. Ph.D. Thesis, Universite Libre De Bruxelles, Brussels, Belgium, 1999. [Google Scholar]
- Spirlet, C.; Grosjean, P.; Jangoux, M. Reproductive cycle of the echinoid Paracentrotus lividus: Analysis by means of maturity index. Invertebr. Reprod. Dev. 1998, 1, 69–81. [Google Scholar] [CrossRef]
- Dhert, P.; Rombaut, G.; Suantika, G.; Sorgeloos, P. Advancement of rotifer culture and manipulation techniques in Europe. Aquaculture 2001, 200, 129–146. [Google Scholar] [CrossRef]
- Buitrago, E.; Lodeiros, C.; Lunar, K.; Alvarado, D.; Indorf, F.; Frontado, K.; Moreno, P.; Vasquez, Z. Mass production of competent larvae of the sea urchin Lytechinus variegatus (Echinodermata: Echinoidea). Aquac. Int. 2005, 4, 359–367. [Google Scholar] [CrossRef]
- Carboni, S.; Kelly, M.S.; Hughes, A.D.; Vignier, J.; Atack, T.; Migaud, H. Evaluation of flow through culture technique for commercial production of sea urchin (Paracentrotus lividus) larvae. Aquac. Res. 2014, 45, 768–772. [Google Scholar] [CrossRef]
- Carboni, S.; Vignier, J.; Chiantore, M.; Tocher, D.R.; Migaud, H. Effects of dietary microalgae on growth, survival and fatty acid composition of sea urchin Paracentrotus lividus throughout larval development. Aquaculture 2012, 324, 250–258. [Google Scholar] [CrossRef]
- Kelly, M.S.; Hunter, A.J.; Scholfield, C.L.; McKenzie, J.D. Morphology and survivorship of larval Psammechinus miliaris (Gmelin) (Echinodermata: Echinoidea) in response to varying food quantity and quality. Aquaculture 2000, 183, 223–240. [Google Scholar] [CrossRef]
- Liu, H.; Kelly, M.S.; Cook, E.J.; Black, K.; Orr, H.; Zhu, J.X.; Dong, S.L. The effect of diet type on growth and fatty-acid composition of sea urchin larvae, I. Paracentrotus lividus (Lamarck, 1816) (Echinodermata). Aquaculture 2007, 264, 247–262. [Google Scholar] [CrossRef]
- Jimmy, R.A.; Kelly, M.S.; Beaumont, A.R. The effect of diet type and quantity on the development of common sea urchin larvae Echinus esculentus. Aquaculture 2003, 220, 261–275. [Google Scholar] [CrossRef]
- Falugi, C.; Angelini, C.; Yokota, Y.; Matranga, V.; Smolenicka, Z. Sea urchin development from the egg to metamorphosis: An integrated model for cell-to-cell and environment interaction. In The Sea Urchin: From Basic Biology to Aquaculture; Swets & Zeitlinger: Lisse, The Netherlands, 2002; pp. 73–93. [Google Scholar]
- Lawrence, J.M.; Olave, S.; Otaiza, R.; Lawrence, A.L.; Bustos, E. Enhancement of gonad production in the sea urchin Loxechinus albus in Chile fed extruded feeds. J. World Aquac. Soc. 1977, 28, 91–96. [Google Scholar] [CrossRef]
- Taylor, A.M.; Powell, M.L.; Watts, S.A.; Lawrence, A.L. Formulated feed supports weight gain and survivorship in juvenile sea urchins Lytechinus variegatus. J. World Aquac. Soc. 2009, 40, 780–787. [Google Scholar] [CrossRef]
- Fernandez, C. Croissance et nutrition de Paracentrotus lividus dans le cadre d’un projet aquacole avec alimentation artificielle. Ph.D. Thesis, Ocean University Sri Lanka, Colombo, Sri Lanka, 1996; p. 279. [Google Scholar]
- Zhang, F.; Man, Y.B.; Mo, W.Y.; Wong, M.H. Application of Spirulina in aquaculture: A review on wastewater treatment and fish growth. Rev. Aquac. 2020, 12, 582–599. [Google Scholar] [CrossRef]
- Christaki, E.; Florou-Paneri, P.; Bonos, E. Microalgae: A novel ingredient in nutrition. Int. J. Food Sci. Nutr. 2011, 62, 794–799. [Google Scholar] [CrossRef]
- Priyadarshani, I.; Rath, B. Commercial and industrial applications of micro algae—A review. J. Algal Biomass Util. 2012, 3, 89–100. [Google Scholar]
- Brundu, G.; Cannavacciuolo, A.; Nannini, M.; Somma, E.; Munari, M.; Zupo, V.; Farina, S. Development of an efficient, noninvasive method for identifying gender year-round in the sea urchin Paracentrotus lividus. Aquaculture 2022, 564, 739082. [Google Scholar] [CrossRef]
- Varrella, S.; Romano, G.; Ianora, A.; Bentley, M.G.; Ruocco, N.; Costantini, M. Molecular response to toxic diatom-derived aldehydes in the sea urchin Paracentrotus lividus. Mar. Drugs 2014, 12, 2089–2113. [Google Scholar] [CrossRef] [PubMed]
- Federico, S.; Glaviano, F.; Esposito, R.; Pinto, B.; Gharbi, M.; Di Cosmo, A.; Costantini, M.; Zupo, V. Gene expression detects the factors influencing the reproductive success and the survival rates of Paracentrotus lividus offspring. Int. J. Mol. Sci. 2022, 23, 12790. [Google Scholar] [CrossRef] [PubMed]
- Zupo, V.; Glaviano, F.; Caramiello, D.; Mutalipassi, M. Effect of five benthic diatoms on the survival and development of Paracentrotus lividus post-larvae in the laboratory. Aquaculture 2018, 495, 13–20. [Google Scholar] [CrossRef]
- Fernandez, C.; Boudouresque, C.F. Evaluating artificial diets for small Paracentrotus lividus (Echinodermata: Echinoidea). Echinoderms San Fr. 1998, 651–657. [Google Scholar]
- George, S.B.; Lawrence, J.M.; Lawrence, A.L. Complete larval development of the sea urchin Lytechinus variegatus fed an artificial feed. Aquaculture 2004, 242, 217–228. [Google Scholar] [CrossRef]
- Jangoux, M. Land-based, closed-cycle echiniculture of Paracentrotus lividus (Lamarck) (Echinoidea: Echinodermata): A long-term experiment at a pilot scale. J. Shellfish. Res. 1998, 17, 152–153. [Google Scholar]
- Solari, P.; Pasquini, V.; Secci, M.; Giglioli, A.; Crnjar, R.; Addis, P. Chemosensitivity in the sea urchin Paracentrotus lividus (Echinodermata: Echinoidea) to food-related compounds: An innovative behavioral bioassay. Front. Ecol. Evol. 2021, 9, 723. [Google Scholar] [CrossRef]
- Luis, O.J.; Gago, J.M. Perception of Dissolved Food-Related Compounds by the Sea Urchin Paracentrotus lividus (Echinodermata: Echinoidea). Front. Mar. Sci. 2021, 8, 719670. [Google Scholar] [CrossRef]
- Pearce, C.M.; Daggett, T.L.; Robinson, S.M. Effect of protein source ratio and protein concentration in prepared diets on gonad yield and quality of the green sea urchin, Strongylocentrotus droebachiensis. Aquaculture 2002, 214, 307–332. [Google Scholar] [CrossRef]
- Mos, B.; Cowden, K.L.; Nielsen, S.J.; Dworjanyn, S.A. Do Cues Matter? Highly inductive settlement cues don’t ensure high post-settlement survival in sea urchin aquaculture. PLoS ONE 2011, 6, e28054. [Google Scholar] [CrossRef]
- Keesing, J.K.; Hall, K.C. Review of harvests and status of world sea urchin fisheries points to opportunities for aquaculture. J. Shellfish. Res. 1998, 17, 1597–1604. [Google Scholar]
- Fernandez, C.; Pergent, G. Effect of different formulated diets and rearing conditions on growth parameters in the sea urchin Paracentrotus lividus. J. Shellfish. Res. 1998, 17, 1571–1581. [Google Scholar]
- Woods, C.; James, P.J.; Moss, G.A.; Wright, J.; Siikavuopio, S. A comparison of the effect of urchin size and diet on gonad yield and quality in the sea urchin Evechinus chloroticus Valenciennes. Aquac. Int. 2008, 16, 49–68. [Google Scholar] [CrossRef]
- Lawrence, J.M. Sea urchin roe cuisine. In Developments in Aquaculture and Fisheries Science; Elsevier: Amsterdam, The Netherlands, 2007; Volume 37, pp. 521–523. [Google Scholar]
- Zupo, V. The use of feeding indices for the study of food webs: An application to a “Posidonia oceanica” ecosystem. Coenoses 1993, 8, 85–95. [Google Scholar]
- Sartori, D.; Gaion, A. Can sea urchins benefit from an artificial diet? Physiological and histological assessment for echinoculture feasibility evaluation. Aquac. Nutr. 2016, 22, 1214–1221. [Google Scholar] [CrossRef]
- Tomsic, S.; Conides, A.J.; Anicic, I. Growth and Gonad Changes in Stony Sea Urchin, Paracentrotus lividus (Lamark, 1816) Fed Artificially Formulated Feed and Benthic Macrophyte Diet. Nase More 2015, 62, 85–90. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).