High-Frequency Responses of the Blue Mussel (Mytilus edulis) Feeding and Ingestion Rates to Natural Diets
Abstract
1. Introduction
2. Material and Methods
2.1. Experimental Design
2.2. Water Quality Measurements
2.3. Mussel Physiology
2.4. Statistical Analyses
3. Results
3.1. Environmental Conditions
3.2. Pumping Rate
3.3. Ingestion Rate
3.4. Functional Responses to Food Availability
4. Discussion
4.1. Feeding Activity in Response to Natural Fluctuations in Diet
4.2. Intra- and Interindividual Variability in Feeding Activity
4.3. Energy Acquisition
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Prins, T.C.; Smaal, A.C.; Dame, R.F. A review of the feedbacks between bivalve grazing and ecosystem processes. Aquat. Ecol. 1998, 31, 349–359. [Google Scholar] [CrossRef]
- Newell, R. Ecosystem influences of natural and cultivated populations of suspension-feeding bivalve molluscs: A review. J. Shellfish Res. 2004, 23, 51–61. [Google Scholar]
- Trottet, A.; Roy, S.; Tamigneaux, E.; Lovejoy, C.; Tremblay, R. Impact of suspended mussels (Mytilus edulis L.) on plankton communities in a Magdalen Islands lagoon (Québec, Canada): A mesocosm approach. J. Exp. Mar. Biol. Ecol. 2008, 365, 103–115. [Google Scholar] [CrossRef]
- Smaal, A.C.; Verbagen, J.H.G.; Coosen, J.; Haas, H.A. Interaction between seston quantity and quality and benthic suspension feeders in the Oosterschelde, the Netherlands. Ophelia 1986, 26, 385–399. [Google Scholar] [CrossRef]
- Smaal, A.C.; Schellekens, T.; van Stralen, M.R.; Kromkamp, J.C. Decrease of the carrying capacity of the Oosterschelde estuary (SW Delta, NL) for bivalve filter feeders due to overgrazing? Aquaculture 2013, 404–405, 28–34. [Google Scholar] [CrossRef]
- Bratbak, G.; Jacquet, S.; Larsen, A.; Pettersson, L.H.; Sazhin, A.F.; Thyrhaug, R. The plankton community in Norwegian coastal waters—abundance, composition, spatial distribution and diel variation. Cont. Shelf Res. 2011, 31, 1500–1514. [Google Scholar] [CrossRef]
- Foster-Smith, R.L. The effect of concentration of suspension on the filtration rates and pseudofaecal production for Mytilus edulis L., Cerastoderma edule (L.) and Venerupis pullastra (Montagu). J. Exp. Mar. Biol. Ecol. 1975, 17, 1–22. [Google Scholar] [CrossRef]
- Shumway, S.E.; Cucci, T.L.; Newell, R.C.; Yentsch, C.M. Particle selection, ingestion, and absorption in filter-feeding bivalves. J. Exp. Mar. Biol. Ecol. 1985, 91, 77–92. [Google Scholar] [CrossRef]
- Velasco, L.; Navarro, J. Feeding physiology of infaunal (Mulinia edulis) and epifaunal (Mytilus chilensis) bivalves under a wide range of concentrations and qualities of seston. Mar. Ecol. Prog. Ser. 2002, 240, 143–155. [Google Scholar] [CrossRef]
- Bayne, B.L.; Iglesias, J.I.P.; Hawkins, A.J.S.; Navarro, E.; Heral, M.; Deslous-Paoli, J.M. Feeding behaviour of the mussel, Mytilus edulis: Responses to variations in quantity and organic content of the seston. J. Mar. Biol. Assoc. UK 1993, 73, 813–829. [Google Scholar] [CrossRef]
- Smaal, A.C.; Vonck, A.P.M.A.; Bakker, M. Seasonal Variation in Physiological Energetics of Mytilus edulis and Cerastoderma edule of Different Size Classes. J. Mar. Biol. Assoc. UK 1997, 77, 817–838. [Google Scholar] [CrossRef]
- Strohmeier, T.; Strand, Ø.; Cranford, P. Clearance rates of the great scallop (Pecten maximus) and blue mussel (Mytilus edulis) at low natural seston concentrations. Mar. Biol. 2009, 156, 1781–1795. [Google Scholar] [CrossRef]
- Riisgard, H.U. Filtration rate and growth in the blue mussel, Mytilus edulis Linneaus, 1758: Dependence on algal concentration. J. Shellfish Res. 1991, 10, 29–35. [Google Scholar]
- Clausen, I.; Riisgård, H. Growth, filtration and respiration in the mussel Mytilus edulis: No evidence for physiological regulation of the filter-pump to nutritional needs. Mar. Ecol. Prog. Ser. 1996, 141, 37–45. [Google Scholar] [CrossRef]
- Hawkins, A.; Smith, R.; Bayne, B.; Héral, M. Novel observations underlying the fast growth of suspension-feeding shellfish in turbid environments: Mytilus edulis. Mar. Ecol. Prog. Ser. 1996, 131, 179–190. [Google Scholar] [CrossRef]
- Navarro, E.; Iglesias, J.I.P.; Ortega, M.M. Natural sediment as a food source for the cockle Cerastoderma edule (L.): Effect of variable particle concentration on feeding, digestion and the scope for growth. J. Exp. Mar. Biol. Ecol. 1992, 156, 69–87. [Google Scholar] [CrossRef]
- Velasco, L.; Navarro, J. Feeding physiology of two bivalves under laboratory and field conditions in response to variable food concentrations. Mar. Ecol. Prog. Ser. 2005, 291, 115–124. [Google Scholar] [CrossRef]
- Willows Optimal digestive investment: A model for filter feeders experiencing variable diets. Am. Soc. Limnol. Oceanogr. 1992, 37, 829–847. [CrossRef]
- Rueda, J.L.; Smaal, A.C. Physiological response of Spisula subtruncata (da Costa, 1778) to different seston quantity and quality. In Nutrients and Eutrophication in Estuaries and Coastal Waters; Orive, E., Elliott, M., de Jonge, V.N., Eds.; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2002; pp. 505–511. [Google Scholar]
- Widdows, J.; Fieth, P.; Worrall, C.M. Relationships between seston, available food and feeding activity in the common mussel Mytilus edulis. Mar. Biol. 1979, 50, 195–207. [Google Scholar] [CrossRef]
- Jøsrgensen, C.B. Bivalve filter feeding revisited. Mar. Ecol. Prog. Ser. 1996, 142, 297–302. [Google Scholar]
- Riisgård, H.U.; Egede, P.P.; Barreiro Saavedra, I. Feeding Behaviour of the Mussel, Mytilus edulis: New Observations, with a Minireview of Current Knowledge. J. Mar. Biol. 2011, 2011, 312459. [Google Scholar] [CrossRef]
- Winter, J. A critical review on some aspects of filter-feeding in lamellibranchiate bivalves. Haliotis 1976, 7, 1–87. [Google Scholar]
- Steeves, L.; Strohmeier, T.; Filgueira, R.; Strand, Ø. Exploring feeding physiology of Mytilus edulis across geographic and fjord gradients in low-seston environments. Mar. Ecol. Prog. Ser. 2020, 651, 71–84. [Google Scholar] [CrossRef]
- Navarro, J.M.; Winter, J.E. Ingestion rate, assimilation efficiency and energy balance in Mytilus chilensis in relation to body size and different algal concentrations. Mar. Biol. 1982, 67, 255–266. [Google Scholar] [CrossRef]
- Navarro, J.; Widdows, J. Feeding physiology of Cerastoderma edule in response to a wide range of seston concentrations. Mar. Ecol. Prog. Ser. 1997, 152, 175–186. [Google Scholar] [CrossRef]
- Holling, C.S. The Functional Response of Invertebrate Predators to Prey Density. Mem. Entomol. Soc. Can. 1966, 98, 5–86. [Google Scholar] [CrossRef]
- Picoche, C.; Le Gendre, R.; Flye-Sainte-Marie, J.; Françoise, S.; Maheux, F.; Simon, B.; Gangnery, A. Towards the Determination of Mytilus edulis Food Preferences Using the Dynamic Energy Budget (DEB) Theory. PLoS ONE 2014, 9, e109796. [Google Scholar] [CrossRef]
- Montalto, V.; Martinez, M.; Rinaldi, A.; Sarà, G.; Mirto, S. The effect of the quality of diet on the functional response of Mytilus galloprovincialis (Lamarck, 1819): Implications for integrated multitrophic aquaculture (IMTA) and marine spatial planning. Aquaculture 2017, 468, 371–377. [Google Scholar] [CrossRef]
- Figueiras, F.; Labarta, U.; Reiriz, M. Coastal upwelling, primary production and mussel growth in the Rías Baixas of Galicia. In Sustainable Increase of Marine Harvesting: Fundamental Mechanisms and New Concepts; Springer: Berlin/Heidelberg, Germany, 2002; pp. 121–131. [Google Scholar]
- Erga, S.R. Ecological studies on the phytoplankton of Boknafjorden, western Norway. II. Environmental control of photosynthesis. J. Plankton Res. 1989, 11, 785–812. [Google Scholar] [CrossRef]
- Frette, Ø.; Rune Erga, S.; Hamre, B.; Aure, J.; Stamnes, J.J. Seasonal variability in inherent optical properties in a western Norwegian fjord. Sarsia 2004, 89, 276–291. [Google Scholar] [CrossRef]
- Cranford, P.J.; Grant, J. Particle clearance and absorption of phytoplankton and detritus by the sea scallop Placopecten magellanicus (Gmelin). J. Exp. Mar. Biol. Ecol. 1990, 137, 105–121. [Google Scholar] [CrossRef]
- Vajedsamiei, J.; Melzner, F.; Raatz, M.; Kiko, R.; Khosravi, M.; Pansch, C. Simultaneous recording of filtration and respiration in marine organisms in response to short-term environmental variability. Limnol. Oceanogr. Methods 2021, 19, 196–209. [Google Scholar] [CrossRef]
- Palmer, R.E.; Williams, L.G. Effect of particle concentration on filtration efficiency of the bay scallop Argopecten irradians and the oyster Crassostrea virginica. Ophelia 1980, 19, 163–174. [Google Scholar] [CrossRef]
- Filgueira, R.; Labarta, U.; Fernandez-Reiriz, M.J. Flow-through chamber method for clearance rate measurements in bivalves: Design and validation of individual chambers and mesocosm: CR protocol for validation. Limnol. Oceanogr. Methods 2006, 4, 284–292. [Google Scholar] [CrossRef]
- Strohmeier, T.; Strand, ø.; Alunno-Bruscia, M.; Duinker, A.; Rosland, R.; Aure, J.; Erga, S.; Naustvoll, L.; Jansen, H.; Cranford, P. Response of Mytilus edulis to enhanced phytoplankton availability by controlled upwelling in an oligotrophic fjord. Mar. Ecol. Prog. Ser. 2015, 518, 139–152. [Google Scholar] [CrossRef][Green Version]
- Steeves, L.; Vimond, C.; Strohmeier, T.; Casas, S.; Strand, Ø.; Comeau, L.; Filgueira, R. Relationship between pumping rate and particle capture efficiency in three species of bivalves. Mar. Ecol. Prog. Ser. 2022, 691, 55–68. [Google Scholar] [CrossRef]
- Sunde, B.K. Gill and Labial Palp Areas in Blue Mussels (Mytilus edulis) at Sites with Different Food Quantity; University of Bergen: Bergen, Norway, 2013. [Google Scholar]
- Kooijman, S. Dynamic Energy Budget Theory for Metabolic Organisation; Cambridge University Press: Cambridge, UK, 2010; ISBN 0-521-13191-X. [Google Scholar]
- Cleveland, W.S.; Devlin, S.J. Locally Weighted Regression: An Approach to Regression Analysis by Local Fitting. J. Am. Stat. Assoc. 1988, 83, 596–610. [Google Scholar] [CrossRef]
- Cranford, P.; Strohmeier, T.; Filgueira, R.; Strand, Ø. Potential methodological influences on the determination of particle retention efficiency by suspension feeders: Mytilus edulis and Ciona intestinalis. Aquat. Biol. 2016, 25, 61–73. [Google Scholar] [CrossRef]
- Filgueira, R.; Fernandez-Reiriz, M.J.; Labarta, U. Clearance rate of the mussel Mytilus galloprovincialis. I. Response to extreme chlorophyll ranges. Cienc. Mar. 2009, 35, 405–417. [Google Scholar] [CrossRef]
- Pascoe, P.; Parry, H.; Hawkins, A. Observations on the measurement and interpretation of clearance rate variations in suspension-feeding bivalve shellfish. Aquat. Biol. 2009, 6, 181–190. [Google Scholar] [CrossRef]
- Hawkins, A.J.S.; Bayne, B.L.; Bougrier, S.; Heral, M.; Iglesias, J.I.P.; Navarro, E.; Smith, R.F.M.; Urrutia, M.B. Some general relationships in comparing the feeding physiology of suspension-feeding bivalve molluscs. J. Exp. Mar. Biol. Ecol. 1998, 17, 87–103. [Google Scholar] [CrossRef]
- Pouvreau, S.; Bodoy, A.; Buestel, D. In situ suspension feeding behaviour of the pearl oyster, Pinctada margaritifera: Combined effects of body size and weather-related seston composition. Aquaculture 2000, 181, 91–113. [Google Scholar] [CrossRef]
- Pouvreau, S.; Jonquières, G.; Buestel, D. Filtration by the pearl oyster, Pinctada margaritifera, under conditions of low seston load and small particle size in a tropical lagoon habitat. Aquaculture 1999, 176, 295–314. [Google Scholar] [CrossRef]
- Cranford, P.J.; Ward, J.E.; Shumway, S.E. Bivalve Filter Feeding: Variability and Limits of the Aquaculture Biofilter. In Shellfish Aquaculture and the Environment; Shumway, S.E., Ed.; Wiley-Blackwell: Oxford, UK, 2011; pp. 81–124. ISBN 978-047-096-096-7. [Google Scholar]
- Filgueira, R. Clearance rate of the mussel Mytilus galloprovincialis. II. Response to uncorrelated seston variables (quantity, quality, and chlorophyll content). Cienc. Mar. 2010, 36, 15–28. [Google Scholar] [CrossRef]
- Hawkins, A.; James, M.; Hickman, R.; Hatton, S.; Weatherhead, M. Modelling of suspension-feeding and growth in the green-lipped mussel Perna canaliculus exposed to natural and experimental variations of seston availability in the Marlborough Sounds, New Zealand. Mar. Ecol. Prog. Ser. 1999, 191, 217–232. [Google Scholar] [CrossRef]
- Iglesias, J.I.P.; Navarro, E.; Alvarez Jorna, P.; Armentina, I. Feeding, particle selection and absorption in cockles Cerastoderma edule (L.) exposed to variable conditions of food concentration and quality. J. Exp. Mar. Biol. Ecol. 1992, 162, 177–198. [Google Scholar] [CrossRef]
- Bayne, B.; Hawkins, A.; Navarro, E.; Iglesias, I. Effects of seston concentration on feeding, digestion and growth in the mussel Mytilus edulis. Mar. Ecol. Prog. Ser. 1989, 55, 47–54. [Google Scholar] [CrossRef]
- Hawkins, A.J.S.; Bayne, B.L. Seasonal variation in the balance between physiological mechanisms of feeding and digestion in Mytilus edulis (Bivalvia: Mollusca). Mar. Biol. 1984, 82, 233–240. [Google Scholar] [CrossRef]
- Bayne, B.L.; Hawkins, A.J.S.; Navarro, E. Feeding and Digestion by the Mussel Mytilus edulis L. (Bivalvia: Mollusca) in Mixtures of Silt and Algal Cells at Low Concentrations. J. Exp. Mar. Biol. Ecol. 1987, 111, e0205981. [Google Scholar] [CrossRef]
- Cranford, P.J.; Emerson, C.W.; Hargrave, B.T.; Milligan, T.G. In Situ Feeding and Absorption Responses of Sea Scallops Placopecten Magellanicus (Gmelin) to Storm-Induced Changes in the Quantity and Composition of the Seston. J. Exp. Mar. Biol. Ecol. 1998, 219, 45–70. [Google Scholar] [CrossRef]
- Frechette, M.; Bourget, E. Significance of Small-Scale Spatio-Temporal Heterogeneity in Phytoplankton Abundance for Energy Flow in Mytilus Edulis. Mar. Biol. 1987, 94, 231–240. [Google Scholar] [CrossRef]
- Bayne, B.L.; Svensson, S.; Nell, J.A. The Physiological Basis for Faster Growth in the Sydney Rock Oyster, Saccostrea Commercialis. Biol. Bull. 1999, 197, 377–387. [Google Scholar] [CrossRef] [PubMed]
- Fuentes-Santos, I.; Labarta, U.; Fernández-Reiriz, M.J. Characterizing Individual Variability in Mussel (Mytilus galloprovincialis) Growth and Testing Its Physiological Drivers Using Functional Data Analysis. PLoS ONE 2018, 13, e0205981. [Google Scholar] [CrossRef] [PubMed]
- Tamayo, D.; Ibarrola, I.; Urrutia, M.B.; Navarro, E. The Physiological Basis for Inter-Individual Growth Variability in the Spat of Clams (Ruditapes philippinarum). Aquaculture 2011, 321, 113–120. [Google Scholar] [CrossRef]
- Hawkins, A.J.S.; Magoulas, A.; Héral, M.; Bougrier, S.; Naciri-Graven, Y.; Day, A.J.; Kotoulas, G. Separate Effects of Triploidy, Parentage and Genomic Diversity upon Feeding Behaviour, Metabolic Efficiency and Net Energy Balance in the Pacific Oyster Crassostrea gigas. Genet. Res. 2000, 76, 273–284. [Google Scholar] [CrossRef]
- Fernández-Reiriz, M.; Garrido, J.; Irisarri, J. Fatty Acid Composition in Mytilus Galloprovincialis Organs: Trophic Interactions, Sexual Differences and Differential Anatomical Distribution. Mar. Ecol. Prog. Ser. 2015, 528, 221–234. [Google Scholar] [CrossRef]
- Griffith, A.W.; Gobler, C.J. Transgenerational Exposure of North Atlantic Bivalves to Ocean Acidification Renders Offspring More Vulnerable to Low PH and Additional Stressors. Sci. Rep. 2017, 7, 11394. [Google Scholar] [CrossRef]
- Zhang, F.; Hu, B.; Fu, H.; Jiao, Z.; Li, Q.; Liu, S. Comparative Transcriptome Analysis Reveals Molecular Basis Underlying Fast Growth of the Selectively Bred Pacific Oyster, Crassostrea Gigas. Front. Genet. 2019, 10, 610. [Google Scholar] [CrossRef]
- de Villemereuil, P.; Gaggiotti, O.; Mouterde, M.; Till-Bottraud, I. Common Garden Experiments in the Genomic Era: New Perspectives and Opportunities. Heredity 2016, 116, 249–254. [Google Scholar] [CrossRef]
- Jørgensen, C.; Larsen, P.; Riisgård, H. Effects of Temperature on the Mussel Pump. Mar. Ecol. Prog. Ser. 1990, 64, 89–97. [Google Scholar] [CrossRef]
- Rosa, M.; Ward, J.E.; Shumway, S.E. Selective Capture and Ingestion of Particles by Suspension-Feeding Bivalve Molluscs: A Review. J. Shellfish Res. 2018, 37, 727–746. [Google Scholar] [CrossRef]
- Coughlan, J. The Estimation of Filtering Rate from the Clearance of Suspensions. Mar. Biol. 1969, 2, 356–358. [Google Scholar] [CrossRef]
- Møhlenberg, F.; Riisgård, H.U. Efficiency of Particle Retention in 13 Species of Suspension Feeding Bivalves. Ophelia 1978, 17, 239–246. [Google Scholar] [CrossRef]
- Rosa, M.; Ward, J.E.; Holohan, B.A.; Shumway, S.E.; Wikfors, G.H. Physicochemical Surface Properties of Microalgae and Their Combined Effects on Particle Selection by Suspension-Feeding Bivalve Molluscs. J. Exp. Mar. Biol. Ecol. 2017, 486, 59–68. [Google Scholar] [CrossRef]
- Pales Espinosa, E.; Perrigault, M.; Ward, J.E.; Shumway, S.E.; Allam, B. Lectins Associated With the Feeding Organs of the Oyster Crassostrea Virginica Can Mediate Particle Selection. Biol. Bull. 2009, 217, 130–141. [Google Scholar] [CrossRef] [PubMed]
- Yahel, G.; Marie, D.; Beninger, P.; Eckstein, S.; Genin, A. In Situ Evidence for Pre-Capture Qualitative Selection in the Tropical Bivalve Lithophaga Simplex. Aquat. Biol. 2009, 6, 235–246. [Google Scholar] [CrossRef]
- Strohmeier, T.; Strand, Ø.; Alunno-Bruscia, M.; Duinker, A.; Cranford, P.J. Variability in Particle Retention Efficiency by the Mussel Mytilus Edulis. J. Exp. Mar. Biol. Ecol. 2012, 412, 96–102. [Google Scholar] [CrossRef]
- Bayne, B.L.; Hawkins, A.J.S.; Navarro, E. Feeding and Digestion in Suspension-Feeding Bivalve Molluscs: The Relevance of Physiological Compensations. Am Zool 1988, 28, 147–159. [Google Scholar] [CrossRef]
- Ibarrola, I.; Larretxea, X.; Iglesias, J.I.P.; Urrutia, M.B.; Navarro, E. Seasonal Variation of Digestive Enzyme Activities in the Digestive Gland and the Crystalline Style of the Common Cockle Cerastoderma Edule. Comp. Biochem. Physiol. 1998, 25–34. [Google Scholar] [CrossRef]
- Navarro, E.; Iglesias, J.I.P.; Ortega, M.M.; Larretxea, X. The Basis for a Functional Response to Variable Food Quantity and Quality in Cockles Cerastoderma Edule (Bivalvia, Cardiidae). Physiol. Zool. 1994, 67, 468–496. [Google Scholar] [CrossRef]
- Ibarrola, I.; Navarro, E.; Iglesias, J.I.P. Short-Term Adaptation of Digestive Processes in the Cockle Cerastoderma Edule Exposed to Different Food Quantity and Quality. J. Comp. Physiol. B Biochem. Syst. Environ. Physiol. 1998, 168, 32–40. [Google Scholar] [CrossRef]
- Scholten, H.; Smaal, A.C. Responses of Mytilus Edulis L. to Varying Food Concentrations: Testing EMMY, an Ecophysiological Model. J. Exp. Mar. Biol. Ecol. 1998, 219, 217–239. [Google Scholar] [CrossRef]
- Scholten, H.; Smaal, A.C. The Ecophysiological Response of Mussels (Mytilus Edulis) in Mesocosms to a Range of Inorganic Nutrient Loads: Simulations with the Model EMMY. Aquat. Ecol. 1999, 33, 83–100. [Google Scholar] [CrossRef]
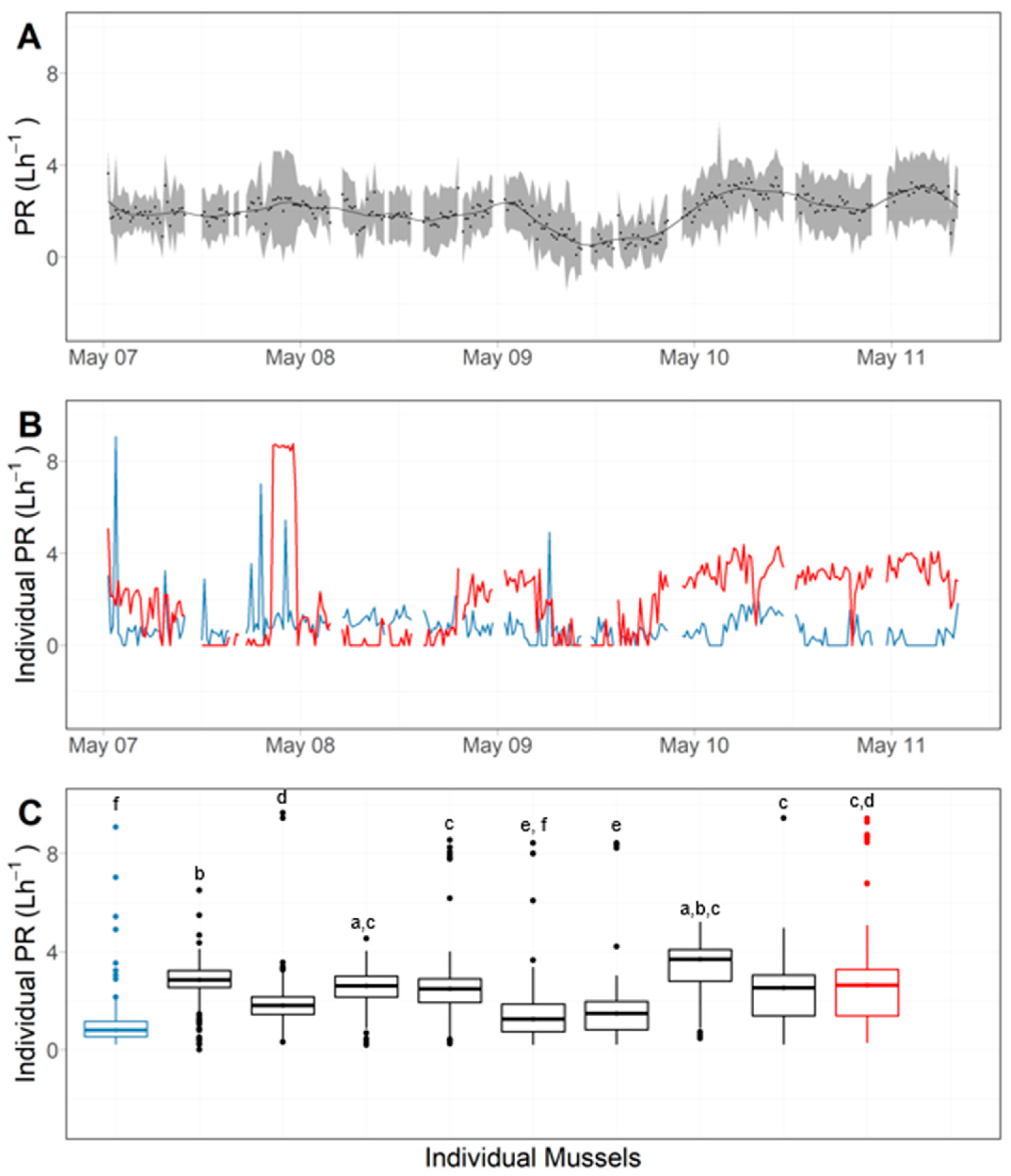


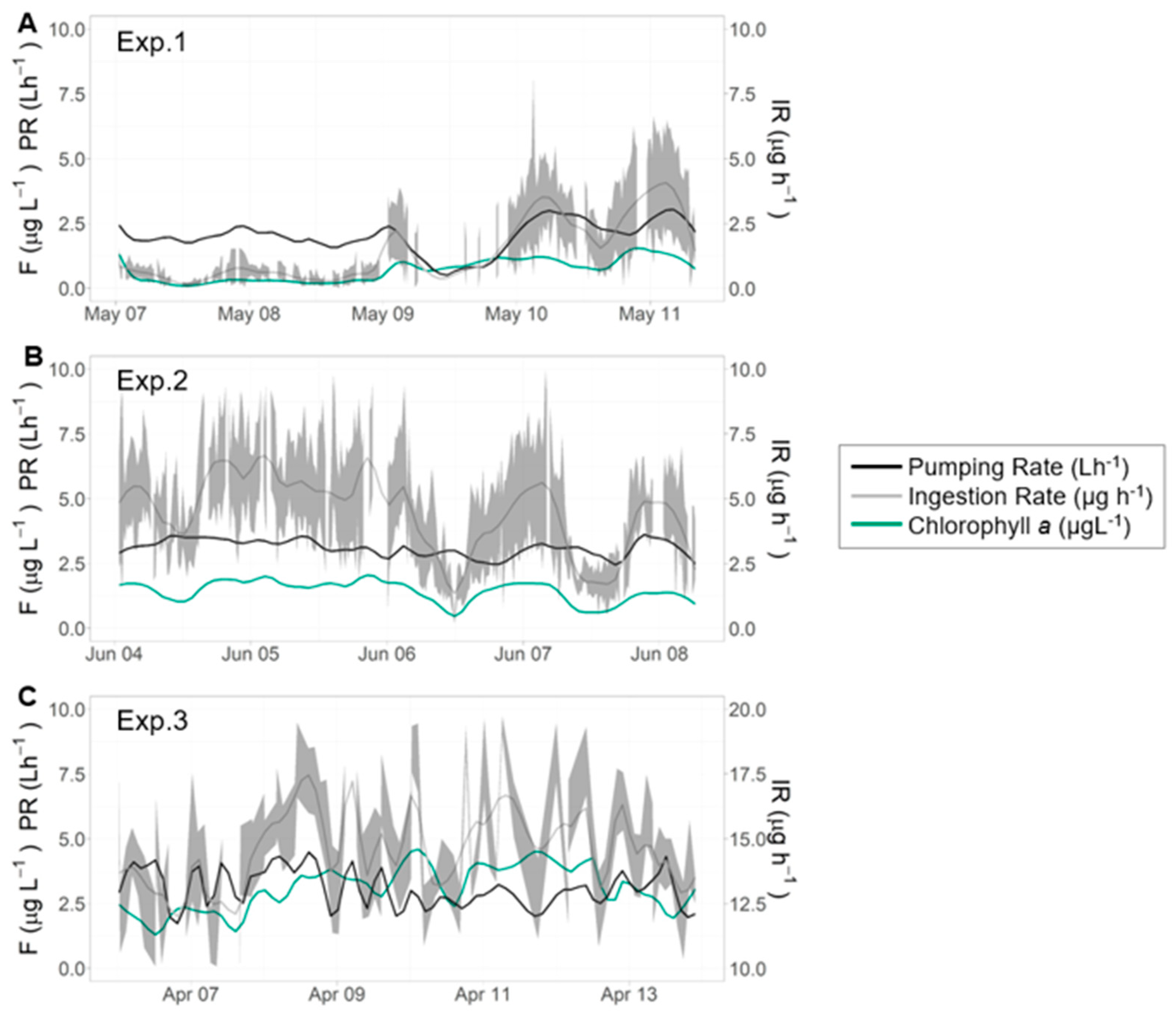
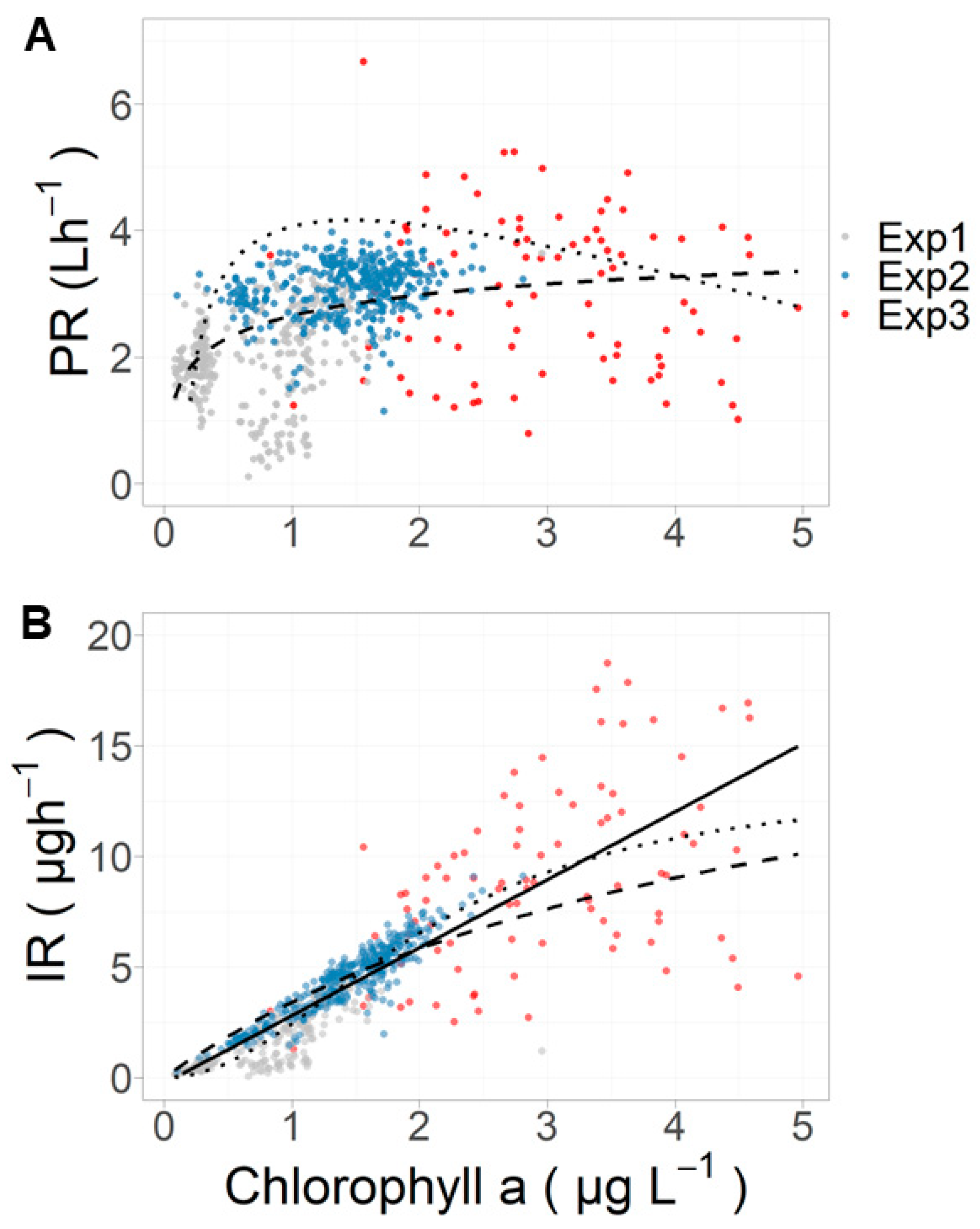
| Exp. 1 | Exp. 2 | Exp. 3 | |
|---|---|---|---|
| Dates | May 07–11 | June 04–08 | April 06–13 |
| Temperature (°C) | 8.31 ± 0.16 (2) | 10.51 ± 0.63 (6) | 6.85 ± 0.14 (2) |
| Fluorescence (µg L−1) | 0.67 ± 0.44 (66) | 1.47 ± 0.47 (32) | 2. 99 ± 0.89 (30) |
| Suspended particulate matter (mg L−1) | 1.68 ± 0.31 (18) | 2.64 ± 0.52 (20) | 1.92 ± 0.57 (30) |
| Energy (J L−1) | 5.83 ± 1.74 (30) | 11 ± 2.83 (26) | 9.00 ± 1.87 (21) |
| Shell length (mm) | 55.9 ± 1.6 (3) | 59.5 ± 1.4 (2) | 35.0 ± 2.5 (7) |
| Median pumping rate (L h−1) | 2.0 ± 0.7 (35) | 3.2 ± 0.4 (13) | 3.1 ± 1.1 (35) |
| Median ingestion rate (µg h−1) | 0.8 ± 1.2 (150) | 4.4 ± 2.3 (52) | 8.9 ± 4.1 (46) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Steeves, L.; Agüera, A.; Filgueira, R.; Strand, Ø.; Strohmeier, T. High-Frequency Responses of the Blue Mussel (Mytilus edulis) Feeding and Ingestion Rates to Natural Diets. J. Mar. Sci. Eng. 2022, 10, 1290. https://doi.org/10.3390/jmse10091290
Steeves L, Agüera A, Filgueira R, Strand Ø, Strohmeier T. High-Frequency Responses of the Blue Mussel (Mytilus edulis) Feeding and Ingestion Rates to Natural Diets. Journal of Marine Science and Engineering. 2022; 10(9):1290. https://doi.org/10.3390/jmse10091290
Chicago/Turabian StyleSteeves, Laura, Antonio Agüera, Ramón Filgueira, Øivind Strand, and Tore Strohmeier. 2022. "High-Frequency Responses of the Blue Mussel (Mytilus edulis) Feeding and Ingestion Rates to Natural Diets" Journal of Marine Science and Engineering 10, no. 9: 1290. https://doi.org/10.3390/jmse10091290
APA StyleSteeves, L., Agüera, A., Filgueira, R., Strand, Ø., & Strohmeier, T. (2022). High-Frequency Responses of the Blue Mussel (Mytilus edulis) Feeding and Ingestion Rates to Natural Diets. Journal of Marine Science and Engineering, 10(9), 1290. https://doi.org/10.3390/jmse10091290






