1. Introduction
With the increasingly serious energy crisis and environmental problems, the awareness of environmental protection from all walks of life at home and abroad has been continuously enhanced. As the main source of marine air pollution, ship engine exhaust has attracted more and more attention from governments and regions, and the requirements for controlling the emission of polluting gases have become increasingly strong [
1]. In order to protect the marine environment and effectively control the emission of harmful gases, the International Maritime Organization (IMO) and the governments of various countries have issued regulations to limit pollutants and set up emission control areas. Among these, the International Convention for the Prevention of Pollution from Ships issued by the IMO, “Regulations for Prevention of Air Pollution from Ships”, clearly stipulated the emission limits of exhaust pollutants from ships’ engines and clarified the standards and time limits for the reduction of ship exhaust emissions [
2].”Clean, low-carbon, safe, and efficient” energy reform has become the general trend [
3].
In order to reduce environmental pollution and harmful substances emissions, diesel and natural gas dual-fuel engines are the subjects of a development trend in internal combustion engines. However, both diesel and natural gas are nonrenewable energy sources, and excessive exploitation of these leads to resource depletion and vegetation destruction. At the same time, the supply and demand of oil and gas are greatly affected by the international situation, and price fluctuations can intensify, which is not conducive to economic benefits. In addition, diesel–natural gas dual-fuel engines will not meet the IMO’s ambitious goal of reducing carbon dioxide emissions from shipping to 50% of the 2008 levels by 2050.
Biodiesel is a kind of fatty acid ester that can be produced from waste oil. Therefore, it is carbon-neutral, renewable, and easy to degrade. Most importantly, it does not contain sulfur and is nontoxic, so it is an ideal alternative fuel for diesel. It has been proven that the use of biodiesel on ships can effectively reduce the emissions of harmful substances, such as sulfur oxides (SO
x), carbon monoxide (CO), particulate matter (PM), and hydrocarbons (HC) [
4]. Methyl decanoate (MD) is an important manifestation of biodiesel [
5,
6,
7].Therefore, the research conducted on biodiesel by domestic and international scholars has focused mainly on MD. Herbinet et al. [
8] studied the low- and high-temperature oxidation performance of MD, developed the chemical reaction mechanism, and constructed a detailed combustion kinetics model, which was effectively verified in ignition delay and JSR experiments, laying a foundation for the combustion kinetics study of MD. Szybist et al. [
9] studied the self-ignition characteristics and heat release laws of MD and other fuels on the engine bench. The study showed that MD produced a large amount of CO
2 during the low-temperature heat release process, which was the result of ester decarboxylation and not oxidation. Wang et al. [
10] experimentally measured the oxidation behavior of MD in laminar premixed and nonpremixed flames in a countercurrent structure under atmospheric pressure and compared the experimental data with the experimental data of an n-alkane flame with a similar carbon number to evaluate the influence of saturation, the carbon chain length, and the existence of the ester group on the flame. In addition, Wang Shiye et al. [
11] studied the emission performance of a mixed fuel of MD and butanol at high temperature and pressure and concluded that the NO
x emission was reduced. However, under the current background of decarbonization, it is difficult to break through the barrier of carbon reduction simply for the development and research of organic carbon-based fuels.
Ammonia (NH
3), as a zero-carbon fuel, is attracting the attention of scholars. Ammonia combustion is associated with carbon-related air pollutants; black carbon or soot, unburned hydrocarbons (HC), and methane and carbon monoxide (CO) emissions are basically eliminated, and only nitrogen and nitrous compounds are the products of ammonia’s complete combustion. Pfahl et al. [
12] and Takizawa et al. [
13] successively investigated the outward propagation speed of a spherical flame when an ammonia/air mixture was burned at 0.1 MPa. The results showed that the laminar combustion rate of NH
3 was generally lower than that of hydrocarbon fuels. Wang et al. [
14] used laser ignition technology to study the laminar combustion characteristics of a premixed ammonia/hydrogen/air mixture and reasonably predicted the accuracy of various mechanism models. Many studies have shown that the poor combustion performance of NH
3 is the main reason for its limited application.
Based on the above, it is believed that MD and NH
3 are highly complementary, Therefore, in this paper, the author propose to mix these two fuels, which could not only improve the quality and performance of their combustion but effectively respond to the energy crisis and climate change. Chemical properties are crucial. At present, there have been relatively few studies on the mixed combustion of these two fuels, and most actual internal combustion engines are in the medium- and low-temperature regions. In this study, referring to the research methods of Liu [
15] and Pei [
16] and starting from the medium- and low-temperature reaction path of MD, the mixed combustion was studied. The effect of ammonia on the mechanism of the MD ignition delay time (IDT) provides a reference for exploring biodiesel–ammonia dual-fuel engines.
3. Results and Discussion
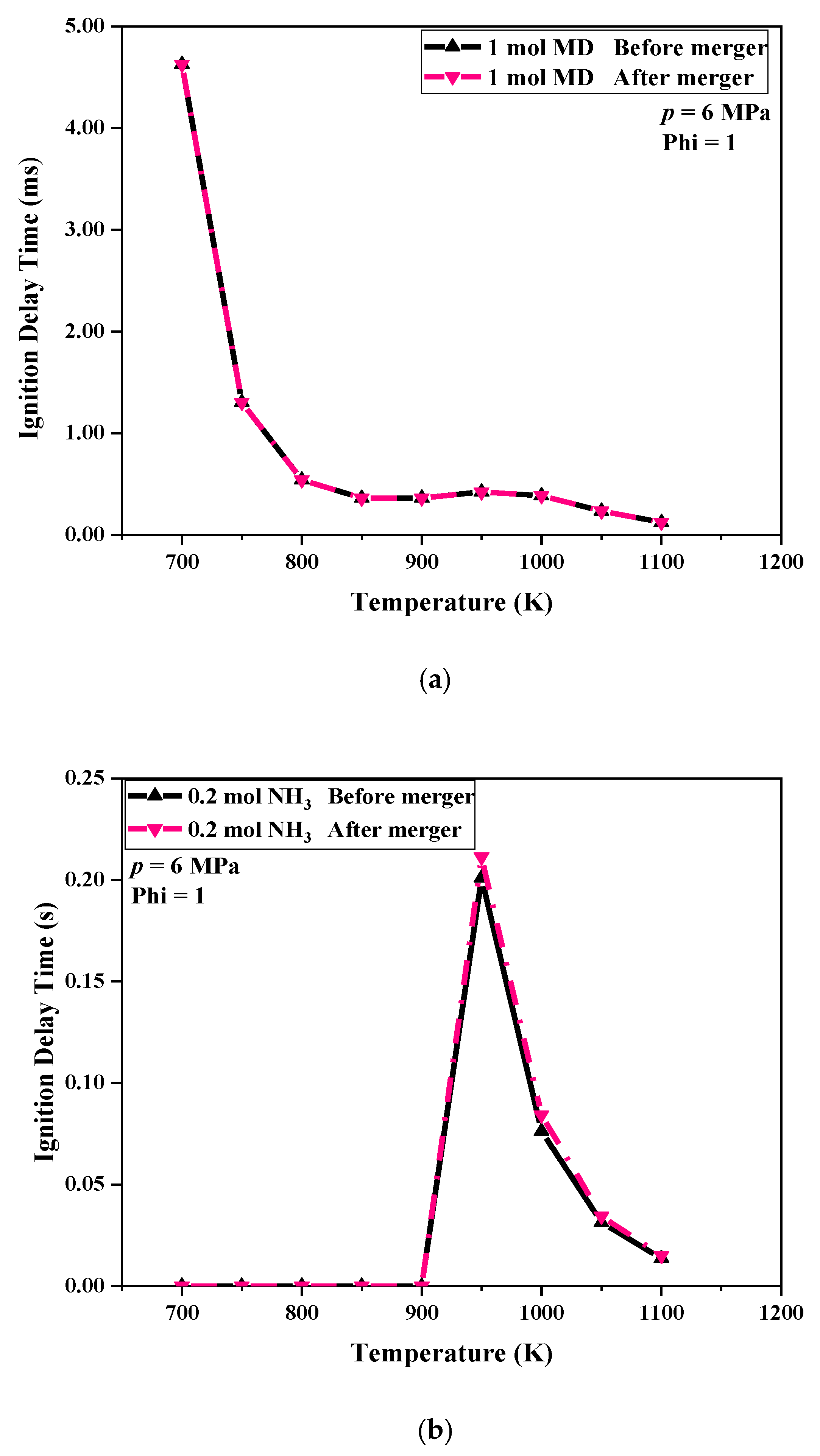
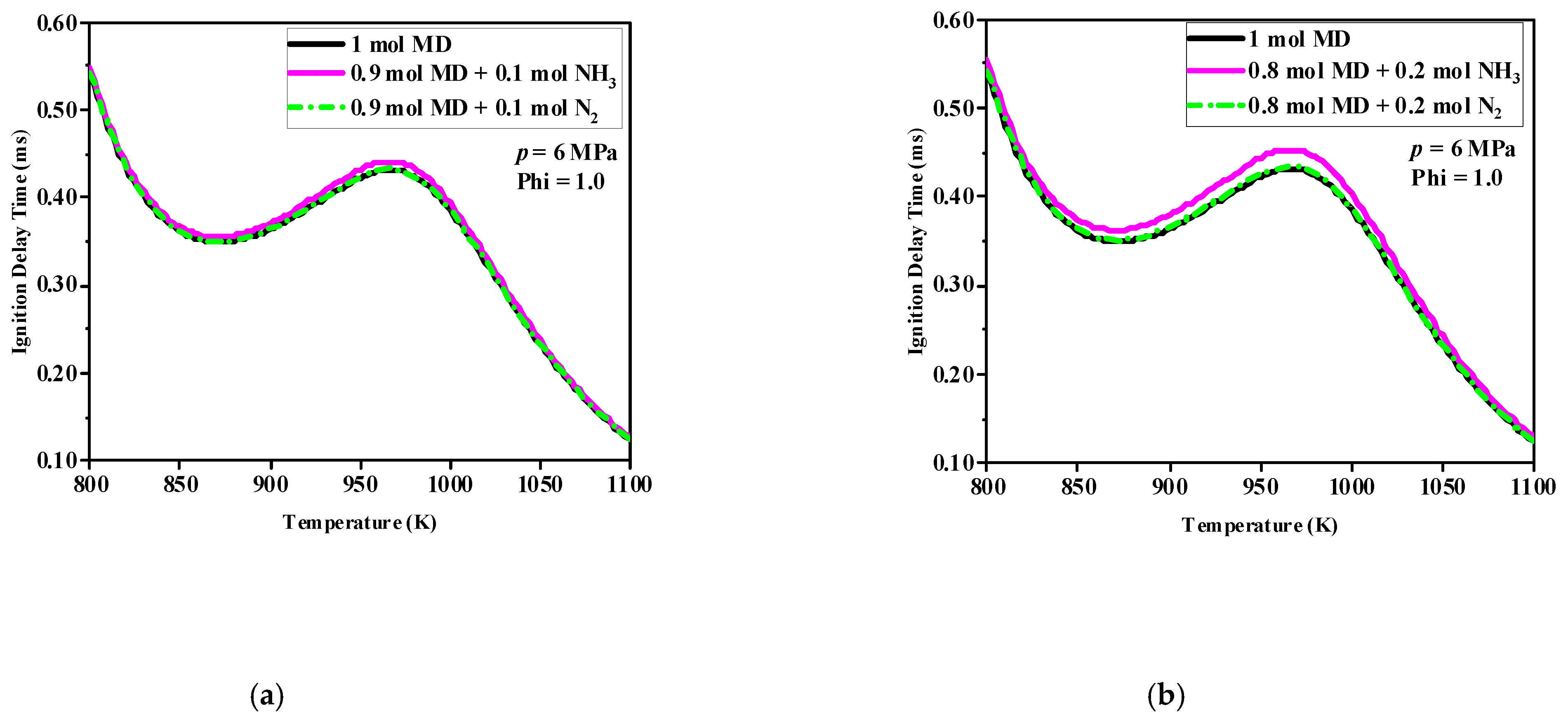
3.1. Analysis of Ignition Delay Time
In order to better compare the effect of NH
3 on the IDT of MD, the change curves of the IDT at the initial temperature are shown in
Figure 2a–d. It is generally believed that the IDT of a fuel decreases with increasing temperature, but this was not the case for the MD. The IDT of the MD first decreased as the initial temperature increased but then increased and then decreases after 900 K. This was the negative temperature coefficient (NTC) behavior exhibited by MD [
19]. It is obvious that the NTC phenomenon of MD appeared between 750 K and 950 K. This was due to the fact that MD with long carbon chains exhibits the properties of alkane groups [
20,
21].
Figure 2 shows that under the same conditions, the addition of N
2 had almost no effect on the ignition of MD, that is, the black and green lines of each figure almost overlapped, which also shows that NH
3 was the only material variable that really affected the ignition of MD. As the NH
3 increased, the distance between the purple curve and the other two lines increased, indicating that after MD was mixed with NH
3, the IDT was prolonged, and it increased as the amount of NH
3 increased, especially in the NTC region, this was because NH
3 absorbed part of the heat and slowed down the oxidation decomposition rate of MD.
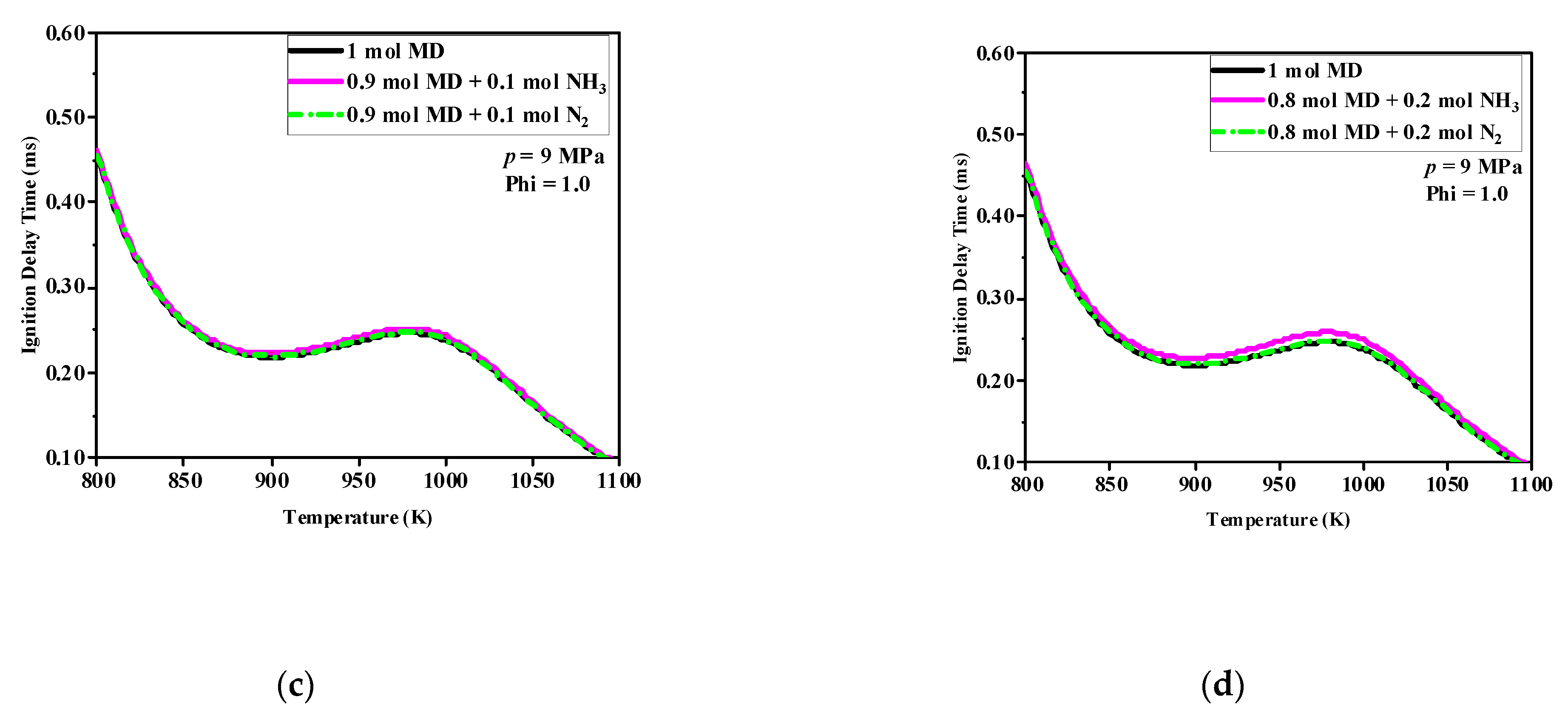
Comparing
Figure 2a–d shows that when the initial pressure increased from 6 to 9 MPa, the IDT of the MD significantly decreased over the whole temperature range. With the same number of moles of NH
3, the pressure increased, and the purple curve approached the black and green curves. The reason for this was that the inhibitory effect of the NH
3 on MD ignition was reduced. In addition, the effect of NH
3 addition on the IDT of the MD was mainly concentrated in the temperature region corresponding to the occurrence of the NTC phenomenon in MD.
3.2. Reaction Path and Sensitivity Analysis
3.2.1. The Main Reaction Pathways
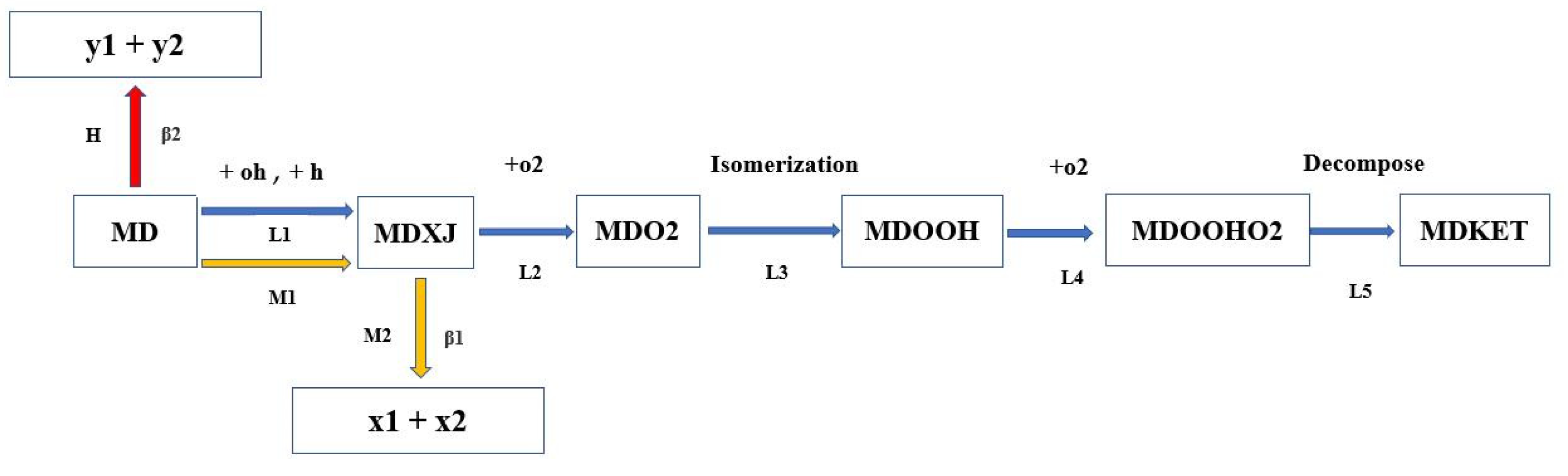
The reaction pathway of MD is very similar to that of long-chain hydrocarbons. In
Figure 3, the MD low-temperature (L1–L5), medium-temperature (M1–M2), and high-temperature (H) reactions are marked with blue, yellow, and red colors, respectively, and each step represents the same type of reaction. Under low-temperature conditions, the MD was dehydrogenated by the free radicals OH and H to form 10 isomers of methyl decanoate, MDXJ (X = 1, 2, … 9, 10), among which the MD and dehydrogenation reaction of the radical OH dominated. The generated MDXJ then oxidized to generate a peroxide group (MDO2), which was then isomerized to form a hydroperoxy group (MDOOH). MDOOH continued to be oxygenated to form a double peroxy hydrogen group (MDOOHO2), and MDOOHO2 decomposed into a free radical OH and ketoester (MDKET). Finally, the ketoester C-C bond was broken and decomposed into small molecular compounds. As the temperature increased, the middle–high temperature reactions were enhanced and competed with the low-temperature reaction, resulting in the backward shift of the MD reaction pathway coupled with the enhancement of the β1 cleavage reaction. Then, the MD decomposed into the relatively smaller molecules x1 and x2, inhibiting the low-temperature reactivity, interfering with the overall reaction order of the NTC system, and resulting in a prolonged IDT. In the high-temperature region, MD directly sheared β2 into the relatively smaller molecules y1 and y2, which promoted the depletion and decomposition of MD, and the IDT began to decrease again.
3.2.2. Sensitivity Analysis of Ignition Delay Time
Regarding the above discussion, in order to further illustrate the effect of NH
3 on the IDT of MD, a representative reaction was selected, as shown in
Table 3, and an IDT mechanism sensitivity analysis was carried out on the mixing of NH
3 into MD.
Two cases of 1 mol MD and 0.8 mol MD + 0.2 mol NH
3 were selected to study the sensitivity of elementary reactions promoting and inhibiting reactivity at initial temperatures of 800 K, 850 K, 900 K, 950 K, 1000 K, and 1050 K. This range included the low-, medium-, and high-temperature regions and the NTC region. The IDT sensitivity coefficient Si was defined per Equation (1), that is, the rate of change in IDT after the reaction constant is doubled:
where
τ is the ignition delay period of the mixed fuel, and
is the specific rate coefficient of the ith reaction. This means that a positive sensitivity coefficient indicated that the elementary reaction inhibited the ignition of fuel, and a negative value indicated that the reaction promoted ignition.The greater the absolute value of the sensitivity coefficient was, the greater the influence of the reaction on fuel ignition was.
Figure 4a–f is a comparison of the sensitivity changes of the main elements in
Table 3 under the conditions of 6 MPa and equivalence ratio Phi = 1. The promotion or inhibition of all reactions in the low–medium temperature range (800–950 K) increased with the temperature, and the R1404 elementary reaction was the most active, indicating that the consumption path of MD in this temperature range was R1404-dominated. However, the activity began to decrease at 950 K. In the NTC behavior region (about 870–970 K), the activity of R1451 increased with the initial temperature, indicating that the inhibitory effect of this reaction on MD ignition was enhanced and reached its maximum at 950 K, which means that starting from 950 K, the consumption of MD with the low-temperature reaction was inhibited, the β1 cleavage reaction was enhanced, and its reaction channel moved backward to β1.
In the medium–high temperature range (950–1050 K), the elementary reactions in the low-temperature reaction were as follows: The activities of R1404, R4117, R4770, R5933, and R6257 decreased as the initial temperature increased and had little effect on the MD at 1050 K. Even at the initial temperature of 1050K, the sensitivity coefficient of R1404 was positive, which inhibited the reaction activity. In addition, the inhibitory effect of the R1451 reaction with β1 cleavage on the MD weakened as the initial temperature increased, while the activity of the R1337 reaction with β2 cleavage was extremely active in this temperature range. This means that in the medium–high temperature range, as the initial temperature increased, the β1 cleavage reaction of the MD was weakened, the β2 cleavage reaction was enhanced, and the channel continued to move back to β2, which released the inhibitory factor of the MD response, and the IDT was greatly reduced.
As the number of moles of NH
3 increased from 0 to 0.2 mol, the absolute values of the sensitivity coefficients of various elementary reactions at different initial temperatures decreased to varying degrees. Some of the promoting/inhibiting effects were counteracted.
Figure 4 also shows that the temperature range where NH
3 weakened the elementary reaction of the MD the most was near the NTC region. This was because, in the NTC region, the MD transformed from a low-temperature chain reaction to a medium- to high-temperature β-cleavage reaction, and the addition of the NH
3 was involved. Therefore, the addition of NH
3 snatched parts of the OH and inhibited the low-temperature chain reaction of MD, resulting in the transfer of a small part of the MD to the β cleavage reaction. At the same time, the medium and high temperatures were not enough to completely crack the MD, which greatly increased the IDT of the MD.
3.3. Rate of Production Analysis
The rate of production (ROP) is an important reference for the study of fuel generation and consumption. It constitutes the main basis for the study of fuel reaction paths. Different external conditions have different reaction paths as well as different reaction rates and products. Regarding
Figure 3, the key points of low-, medium-, and high-temperature transformation were selected for analysis, and the reaction steps and primitives are shown in
Table 4.
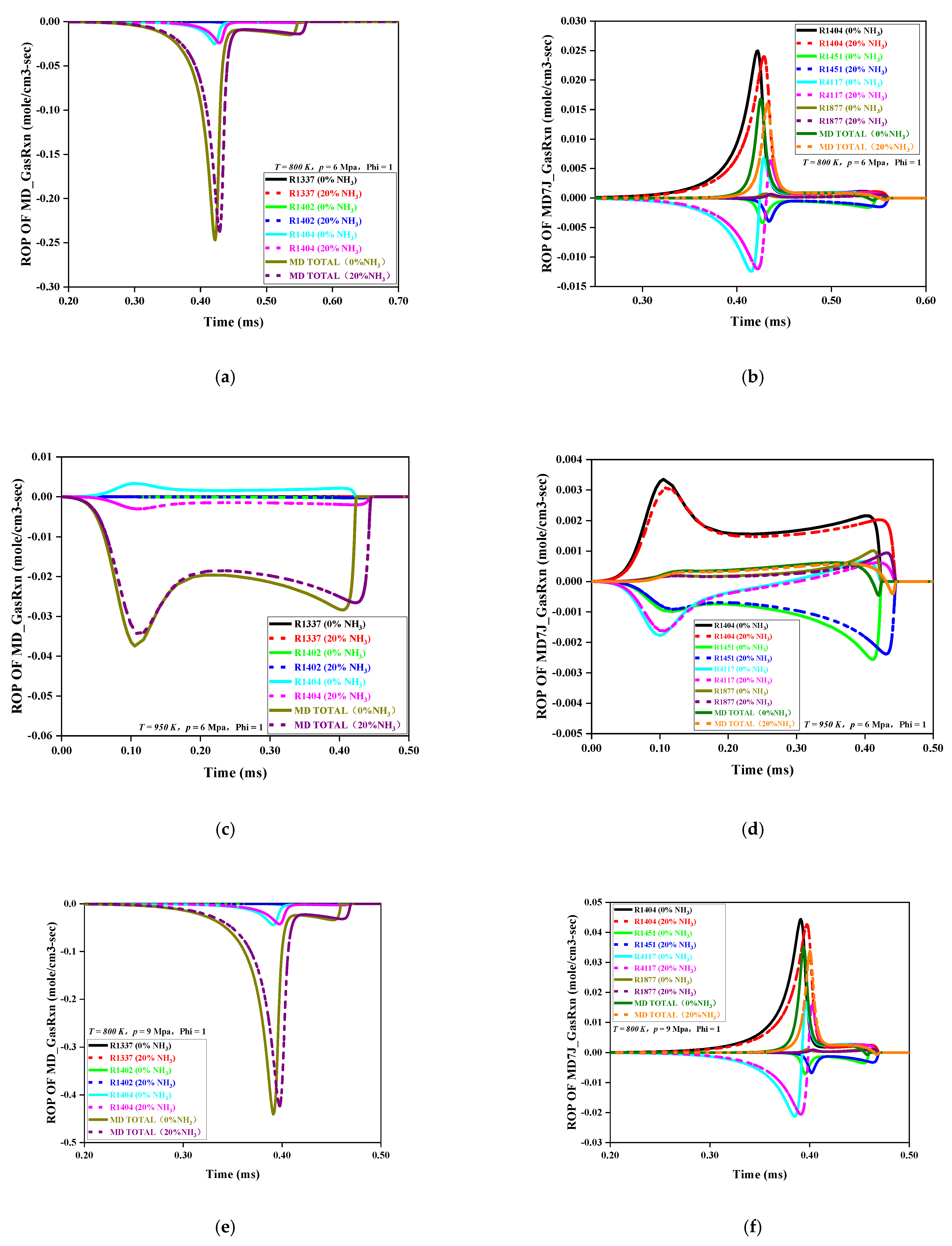
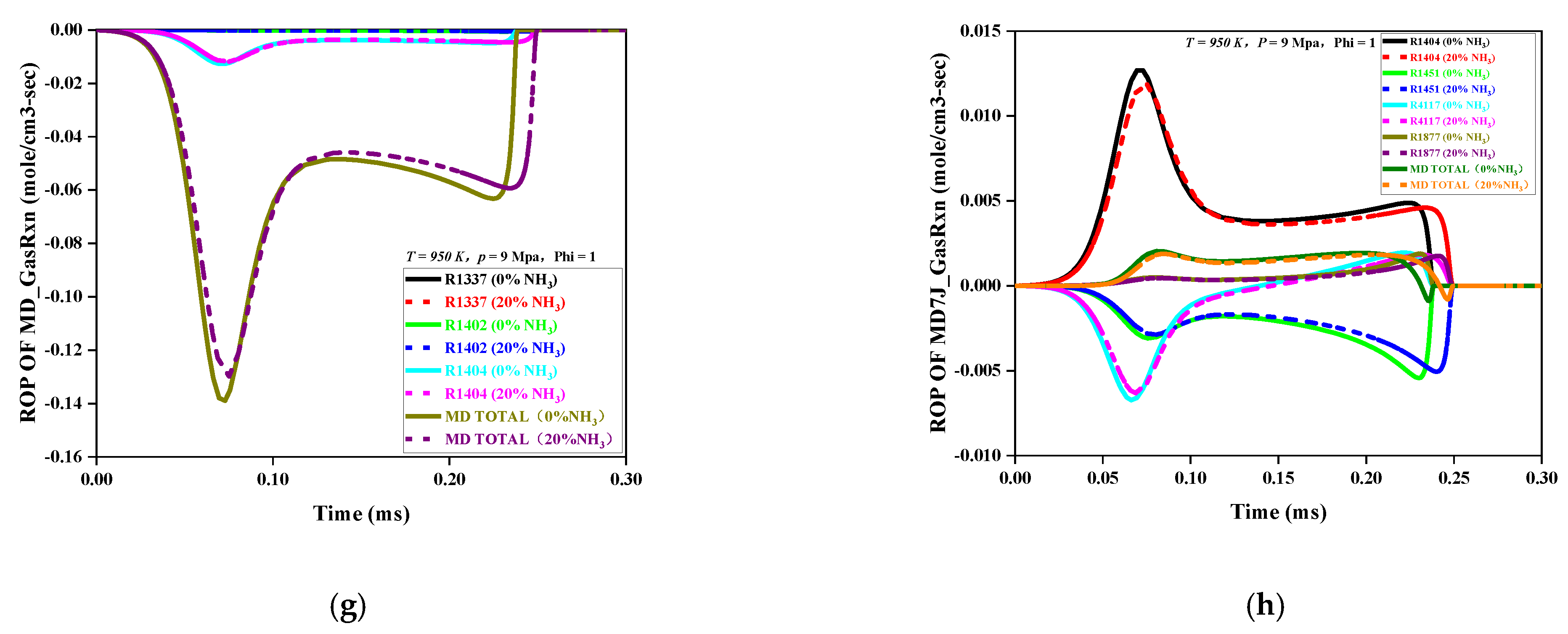
Figure 5a–h is an ROP analysis diagram of MD and MD7J under the initial pressures of 6 MPa and 9 MPa and the initial temperatures of 800 K and 950 K. The curve in the figure shows a negative value. This indicates that the corresponding ROP was a consumption reaction; a positive value would indicate production reaction.
Because of the effect of the initial pressure on the ROP, the MD and MD7J had the same change trend in ROP under different initial pressures. As the initial pressure increased, the absolute value of the ROP was obviously enhanced, indicating that the reaction rate of each primitive accelerated as the initial pressure increased and that the time required for the reaction to reach equilibrium was relatively reduced, that is, the IDT decreased.
Because of the effect of the initial temperature on the ROP, the ROP variation trends of the MD and MD7J were different at different initial temperatures. At the initial low temperature of 800 K, the overall reaction rate trends for the MD and MD7J were consistent with the change trends for the low-temperature reaction channel. The MD was mainly consumed by the dehydrogenation reaction with H and OH to generate the MD7J, and its main elemental reactions were R1402 and R1404, respectively. The dehydrogenation reaction through the OH radical played a dominant role, while the pyrolysis reaction of MD did not exist, that is, the R1337 curve was 0. Correspondingly, the main source of MD7J was R1404, and the MD7J was consumed through oxygenation, pyrolysis, isomerization, and other reactions. The main elemental reactions were R4117, R1451, and R1877, among which the oxidation reaction of R4117 was dominant.
The temperature of 950 K fell inside the range of medium-temperature reaction and was in the NTC region. Compared with those at low temperature, the reactions of MD and MD7J at this initial temperature were relatively complicated. At this initial temperature, the reaction rates of low-temperature primitives, such as R1402, R1404, R1877, R4117, and R4770, dropped sharply or even became zero, and both the MD and MD7J underwent β-cleavage (R1337 and R1451), but the β-cleavage of the MD7J was more significant than that of MD. This indicates that the low-temperature chain reaction and β-cleavage of the MD and MD7J coexisted at 950 K and that there was a competitive relationship.
When the NH3 mole ratio increased from 0 to 20%, the consumption rate and production rate of MD and its intermediates decreased at the same initial pressure and temperature, and the time required for the reaction to reach equilibrium was relatively prolonged, that is, the IDT increased. At 950 K in the NTC region, it was obvious that the addition of NH3 had the most sensitive effect on the reaction rate of the MD. The consumption and average production rate of all the elements, including intermediates, decreased the most, and the delay of reaction toward equilibrium reached the maximum, that is, the IDT reached the maximum. This indicates that in the NTC system, the addition of NH3 had a significant inhibition effect on the MD consumption rate. In practical applications of dual-fuel engine, the fuel injection temperature should be avoided in the NTC region to ensure good maneuverability of a power device.