Oxidative Stress in Far Eastern Mussel Mytilus trossulus (Gould, 1850) Exposed to Combined Polystyrene Microspheres (µPSs) and CuO-Nanoparticles (CuO-NPs)
Abstract
:1. Introduction
2. Material and Methods
2.1. Description of the Experiment
2.2. Preparation of Working Solutions
2.3. Comet Assay
2.4. Lysosomal Membrane Stability
2.5. Integral Antioxidant Activity
2.6. MDA Concentration
2.7. Carbonyl Concentration
2.8. Statistical Analysis
2.9. Quality Assurance and Quality Control Assessment
3. Results
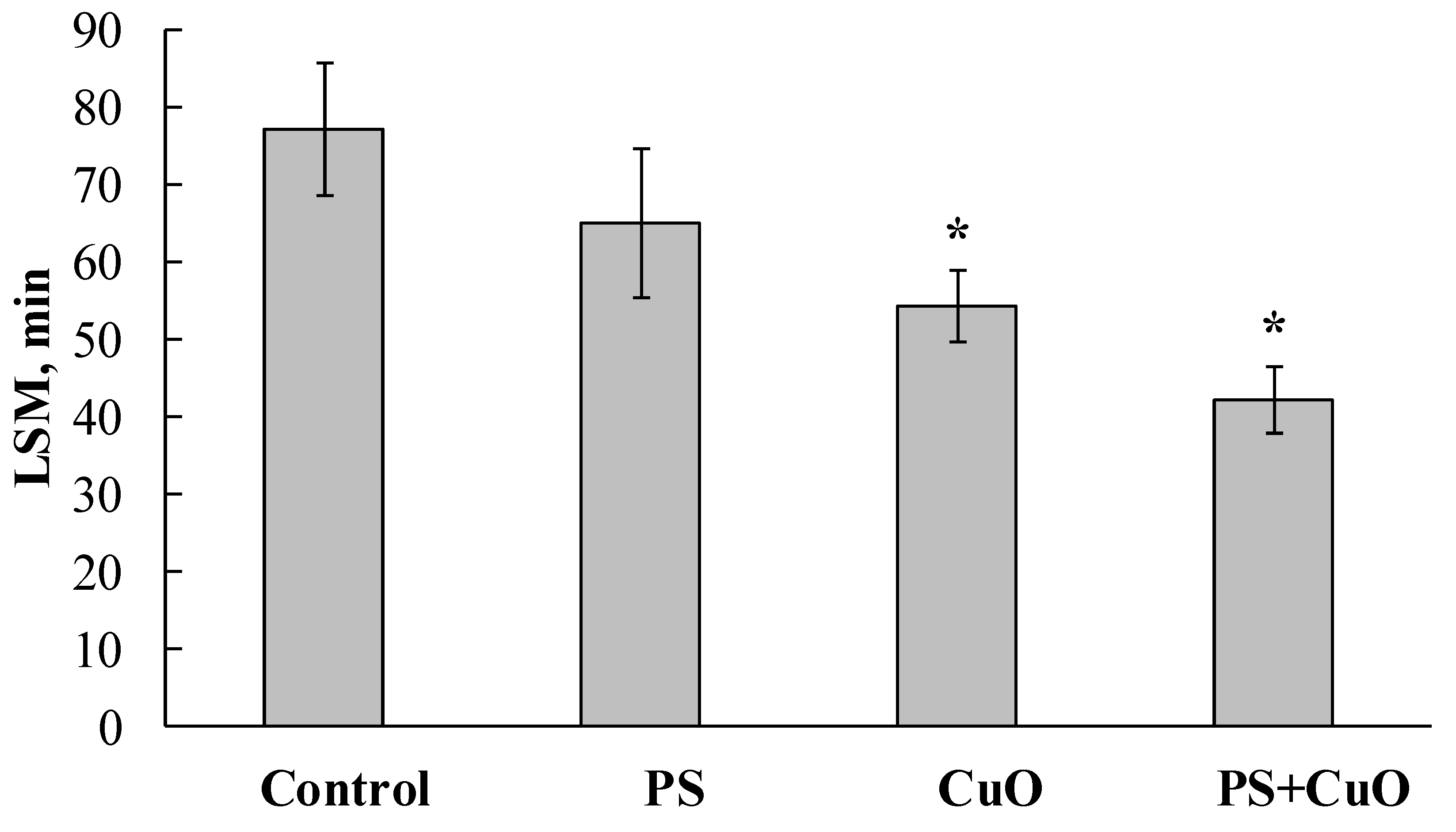
3.1. Lysosomal Membrane Stability
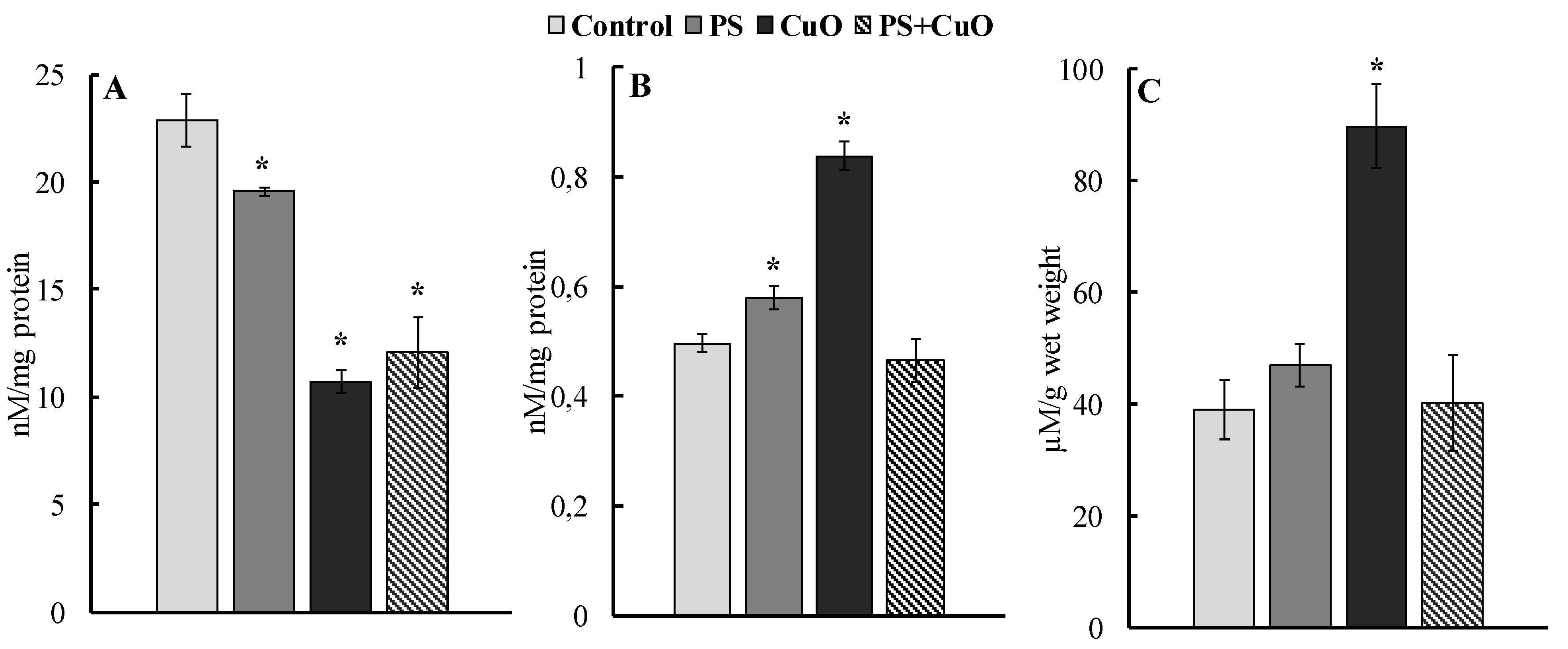
3.2. Integral Antiradical Activity
3.3. Carbonyl Concentration
3.4. MDA
3.5. Comet Assay
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bundschuh, M.; Filser, J.; Luderwald, S.; McKee, M.S.; Metreveli, G.; Schaumann, G.E.; Schulz, R.; Wagner, S. Nanoparticles in the environment: Where do we come from, where do we go to? Environ. Sci. Eur. 2018, 30, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, T.Y.; Bornhöft, N.A.; Hungerbühler, K.; Nowack, B. Dynamic probabilistic modeling of environmental emissions of engineered nanomaterials. Environ. Sci Technol. 2016, 50, 4701–4711. [Google Scholar] [CrossRef] [PubMed]
- Von Moos, N.; Burkhardt-Holm, P.; Köhler, A. Uptake and effects of microplastics on cells and tissue of the blue mussel Mytilus edulis L. after an experimental exposure. Environ. Sci. Technol. 2012, 46, 11327–11335. [Google Scholar] [CrossRef] [PubMed]
- Avio, C.G.; Gorbi, S.; Milan, M.; Benedetti, M.; Fattorini, D.; D’Errico, G.; Pauletto, M.; Bargelloni, L.; Regoli, F. Pollutants bioavailability and toxicological risk from microplastics to marine mussels. Environ. Pollut. 2015, 198, 211–222. [Google Scholar] [CrossRef]
- Buffet, P.E.; Tankoua, O.F.; Pan, J.F.; Berhanu, D.; Herrenknecht, C.; Poirier, L.; Amiard-Triquet, C.; Amiard, J.C.; Bérard, J.B.; Risso, C.; et al. Behavioural and biochemical responses of two marine invertebrates Scrobicularia plana and Hediste diversicolor to copper oxide nanoparticles. Chemosphere 2011, 84, 166–174. [Google Scholar] [CrossRef] [Green Version]
- Gambardella, C.; Morgana, S.; Bari, G.D.; Ramoino, P.; Bramini, M.; Diaspro, A.; Falugi, C.; Faimali, M. Multidisciplinary screening of toxicity induced by silica nanoparticles during sea urchin development. Chemosphere 2015, 139, 486–495. [Google Scholar] [CrossRef]
- Madhav, M.R.; David, S.E.M.; Kumar, R.S.S.; Swathy, J.S.; Bhuvaneshwari, M.; Mukherjee, A.; Chandrasekaran, N. Toxicity and accumulation of copper oxide (CuO) nanoparticles in different life stages of Artemia salina. Environ. Toxicol. Pharmacol. 2017, 52, 227–238. [Google Scholar] [CrossRef]
- Chelomin, V.P.; Slobodskova, V.V.; Zakhartsev, M.K.; Kukla, S.P. Genotoxic potential of copper oxide nanoparticles in the bivalve mollusk Mytilus trossulus. J. Ocean Univ. China 2017, 16, 339–345. [Google Scholar] [CrossRef]
- Oliviero, M.; Schiavo, S.; Dumontet, S.; Manzo, S. DNA damages and offspring quality in sea urchin Paracentrotus lividus sperms exposed to ZnO nanoparticles. Sci. Total Environ. 2019, 651, 756–765. [Google Scholar] [CrossRef]
- Kukla, S.; Slobodskova, V.; Mazur, A.; Chelomin, V.; Kamenev, Y. Genotoxic testing of titanium dioxide nanoparticles in Far Eastern mussels, Mytilus trossulus. Pollution 2021, 7, 129–140. [Google Scholar] [CrossRef]
- Mazur, A.A.; Zhuravel, E.V.; Slobodskova, V.V.; Mazur, M.A.; Kukla, S.P.; Chelomin, V.P. Waterborne exposure of adult sand dollar, Scaphechinus mirabilis (Agassiz, 1864), to zinc ions and zinc oxide nanoparticles affects early development of its offspring. Water Air Soil Pollut. 2020, 231, 115. [Google Scholar] [CrossRef]
- Mazur, A.A.; Chelomin, V.P.; Zhuravel, E.V.; Kukla, S.P.; Slobodskova, V.V.; Dovzhenko, N.V. Genotoxicity of polystyrene (PS) microspheres in short-term exposure to gametes of the sand dollar Scaphechinus mirabilis (Agassiz, 1864) (Echinodermata, Echinoidea). J. Mar. Sci. Eng. 2021, 9, 1088. [Google Scholar] [CrossRef]
- Chelomin, V.P.; Mazur, A.A.; Slobodskova, V.V.; Kukla, S.P.; Dovzhenko, N.V. Genotoxic properties of polystyrene (PS) microspheres in the filter-feeder mollusk Mytilus trossulus (Gould, 1850). J. Mar. Sci. Eng. 2022, 10, 273. [Google Scholar] [CrossRef]
- Regoli, F.; Giuliani, M.E. Oxidative pathways of chemical toxicity and oxidative stress biomarkers in marine organisms. Mar. Environ. Res. 2014, 93, 106–117. [Google Scholar] [CrossRef] [PubMed]
- Détrée, C.; Gallardo-Escárate, C. Single and repetitive microplastics exposures induce immune system modulation and homeostasis alteration in the edible mussel Mytilus galloprovincialis. Fish Shellfish Immunol. 2018, 83, 52–60. [Google Scholar] [CrossRef]
- Paul-Pont, I.; Lacroix, C.; Fernández, C.G.; Hégaret, H.; Lambert, C.; Le Goïc, N.; Frère, L.; Cassone, A.-L.; Sussarellu, R.; Fabioux, C. Exposure of marine mussels Mytilus spp. to polystyrene microplastics: Toxicity and influence on fluoranthene bioaccumulation. Environ. Pollut. 2016, 216, 724–737. [Google Scholar] [CrossRef] [Green Version]
- Santos, D.; Félix, L.; Luzio, A.; Parra, S.; Bellas, J.; Monteiro, S.M. Single and combined acute and subchronic toxic effects of MPs and copper in zebrafish (Danio rerio) early life stages. Chemosphere 2021, 277, 130262. [Google Scholar] [CrossRef]
- Banaee, M.; Soltanian, S.; Sureda, A.; Gholamhosseini, A.; Haghi, B.N.; Akhlaghi, M.; Derikvandy, A. Evaluation of single and combined effects of cadmium and micro-plastic particles on biochemical and immunological parameters of common carp (Cyprinus carpio). Chemosphere 2019, 236, 124335. [Google Scholar] [CrossRef]
- Gonzalez-Soto, N.; Hatfield, J.; Katsumiti, A.; Duroudier, N.; Lacave, J.M.; Bilbao, E.; Orbea, A.; Navarro, E.; Cajaraville, M.P. Impacts of dietary exposure to different sized polystyrene microplastics alone and with sorbed benzo[a]pyrene on biomarkers and whole organism responses in mussels Mytilus galloprovincialis. Sci. Total Environ. 2019, 684, 548–566. [Google Scholar] [CrossRef]
- Faggio, C.; Tsarpali, V.; Dailianis, S. Mussel digestive gland as a model tissue for assessing xenobiotics: An overview. Sci. Total Environ. 2018, 636, 220–229. [Google Scholar] [CrossRef]
- Martinez-Gomez, C.; Bignell, J.; Lowe, D. Lysosoma membrane stability in mussels. ICES Tech. Mar. Environ. Sci. 2015, 56, 41. [Google Scholar] [CrossRef]
- Belcheva, N.N.; Istomina, A.A.; Dovzhenko, N.V.; Lishavskaya, T.; Chelomin, V.P. Using heavy metal content and lipid peroxidation indicators in the tissues of the mussel Crenomytilus grayanus for pollution assessment after marine environmental remediation. Bull. Environ. Contam. Toxicol. 2015, 95, 481–487. [Google Scholar] [CrossRef] [PubMed]
- Buege, J.A.; Aust, S.D. Microsomal lipid peroxidation. Methods. Enzymol. 1978, 52, 302–310. [Google Scholar] [CrossRef] [PubMed]
- Mesquita, C.S.; Oliveira, R.; Bento, F.; Geraldo, D.; Rodrigues, J.V.; Marcos, J.C. Simplified 2,4-dinitrophenylhydrazine spectrophotometric assay for quantification of carbonyls in oxidized proteins. Anal. Biochem. 2014, 458, 69–71. [Google Scholar] [CrossRef]
- de Ruijter, V.N.; Redondo-Hasselerharm, P.E.; Gouin, T.; Koelmans, A.A. Quality criteria for microplastic effect studies in the context of risk assessment: Acritical review. Environ. Sci Technol. 2020, 54, 11692–11705. [Google Scholar] [CrossRef]
- Markwell, M.A.; Haas, S.M.; Bieber, L.L.; Tolbert, N.E. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal. Biochem. 1978, 87, 206–210. [Google Scholar] [CrossRef]
- Rainieri, S.; Conlledo, N.; Larsen, B.K.; Granby, K.; Barranco, A. Combined effects of microplastics and chemical contaminants on the organ toxicity of zebrafish (Danio rerio). Environ. Res. 2018, 162, 135–143. [Google Scholar] [CrossRef]
- Luís, L.G.; Ferreira, P.; Fonte, E.; Oliveira, M.; Guilhermino, L. Does the presence of microplastics influence the acute toxicity of chromium(VI) to early juveniles of the common goby (Pomatoschistus microps)? A study with juveniles from two wild estuarine populations. Aquat. Toxicol. 2015, 164, 163–174. [Google Scholar] [CrossRef]
- Ferreira, P.; Fonte, E.; Soares, M.E.; Carvalho, F.; Guilhermino, L. Effects of multi-stressors on juveniles of the marine fish Pomatoschistus microps: Gold nanoparticles, microplastics and temperature. Aquat. Toxicol. 2016, 170, 89–103. [Google Scholar] [CrossRef]
- Xia, B.; Zhang, J.; Zhao, X.; Feng, J.; Teng, Y.; Chen, B.; Sun, X.; Zhu, L.; Sun, X.; Qu, K. Polystyrene microplastics increase uptake, elimination and cytotoxicity of decabromodiphenyl ether (BDE-209) in the marine scallop Chlamys farreri. Environ. Pollut. 2020, 258, 113657. [Google Scholar] [CrossRef]
- Li, Z.; Yi, X.; Zhou, H.; Chi, T.; Li, W.; Yang, K. Combined effect of polystyrene microplastics and dibutyl phthalate on the microalgae Chlorella pyrenoidosa. Environ. Pollut. 2020, 257, 113604. [Google Scholar] [CrossRef] [PubMed]
- Yi, X.; Chi, T.; Li, Z.; Wang, J.; Yu, M.; Wu, M.; Zhou, H. Combined effect of polystyrene plastics and triphenyltin chloride on the green algae Chlorella pyrenoidosa. Environ. Sci. Pollut. Res. Int. 2019, 26, 15011–15018. [Google Scholar] [CrossRef] [PubMed]
- Yi, X.; Wang, J.; Li, Z.; Zhang, Z.; Chi, T.; Guo, M.; Li, W.; Zhou, H. The effect of polystyrene plastics on the toxicity of triphenyltin to the marine diatom Skeletonema costatum-influence of plastic particle size. Environ. Sci. Pollut. Res. Int. 2019, 26, 25445–25451. [Google Scholar] [CrossRef] [PubMed]
- Davarpanah, E.; Guilhermino, L. Are gold nanoparticles and microplastics mixtures more toxic to the marine microalgae Tetraselmis chuii than the substances individually? Ecotoxicol. Environ. Saf. 2019, 181, 60–68. [Google Scholar] [CrossRef]
- Thiagarajan, V.; Iswarya, V.P.A.J.; Seenivasan, R.; Chandrasekaran, N.; Mukherjee, A. Influence of differently functionalized polystyrene microplastics on the toxic effects of P25 TiO2 NPs towards marine algae Chlorella sp. Aquat. Toxicol. 2019, 207, 208–216. [Google Scholar] [CrossRef]
- Zhu, X.; Zhao, W.; Chen, X.; Zhao, T.; Tan, L.; Wang, J. Growth inhibition of the microalgae Skeletonema costatum under copper nanoparticles with microplastic exposure. Mar. Environ. Res. 2020, 158, 105005. [Google Scholar] [CrossRef]
- Dong, S.; Qu, M.; Rui, Q.; Wang, D. Combinational effect of titanium dioxide nanoparticles and nanopolystyrene particles at environmentally relevant concentrations on nematode Caenorhabditis elegans. Ecotoxicol. Environ. Saf. 2018, 161, 444–450. [Google Scholar] [CrossRef]
- Browne, M.A.; Dissanayake, A.; Galloway, T.S.; Lowe, D.M.; Thompson, R.C. Ingested microscopic plastic translocates to the circulatory system of the mussel, Mytilus edulis (L.). Environ. Sci. Technol. 2008, 42, 5026–5031. [Google Scholar] [CrossRef]
- Kukla, S.P.; Slobodskova, V.V.; Chelomin, V.P. The genotoxicity of copper oxide nanoparticles to marine organisms based on the example of the Pacific mussel Mytilus trossulus Gould, 1850 (Bivalvia: Mytilidae). J. Mar. Bio. 2017, 43, 171–175. [Google Scholar] [CrossRef]
- Studer, A.M.; Limbach, L.K.; Van Duc, L.; Krumeich, F.; Athanassiou, E.K.; Gerber, L.C.; Moch, H.; Stark, W.J. Nanoparticle cytotoxicity depends on intracellular solubility: Comparison of stabilized copper metal and degradable copper oxide nanoparticles. Toxicol. Lett. 2010, 197, 169–174. [Google Scholar] [CrossRef]
- Bhattacharya, P.; Lin, S.; Turner, J.P.; Ke, P.C. Physical adsorption of charged plastic nanoparticles affects algal photosynthesis. J. Phys. Chem. 2010, 114, 16556–16561. [Google Scholar] [CrossRef]
- Barboza, L.G.A.; Vieira, L.R.; Branco, V.; Figueiredo, N.; Carvalho, F.; Carvalho, C.; Guilhermino, L. Microplastics cause neurotoxicity, oxidative damage and energy-related changes and interact with the bioaccumulation of mercury in the European seabass, Dicentrarchus labrax (Linnaeus, 1758). Aquat. Toxicol. 2018, 195, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Revel, M.; Lagarde, F.; Perrein-Ettajani, H.; Bruneau, M.; Akcha, F.; Sussarellu, R.; Rouxel, J.; Costil, K.; Decottignies, P.; Cognie, B. Tissue-specific biomarker responses in the blue mussel Mytilus spp. exposed to a mixture of microplastics at environmentally relevant concentrations. Front. Environ. Sci. 2019, 7, 33. [Google Scholar] [CrossRef]
- Bondarenko, O.; Juganson, K.; Ivask, A.; Kasemets, K.; Mortimer, M.; Kahru, A. Toxicity of Ag, CuO and ZnO nanoparticles to selected environmentally relevant test organisms and mammalian cells in vitro: A critical review. Arch. Toxicol. 2013, 87, 1181–1200. [Google Scholar] [CrossRef] [Green Version]
- Sun, T.; Zhan, J.; Li, F.; Ji, C.; Wu, H. Evidence-based meta-analysis of the genotoxicity induced by microplastics in aquatic organisms at environmentally relevant concentrations. Sci. Total Environ. 2021, 783, 147076. [Google Scholar] [CrossRef]
- Berber, A.A. Genotoxic evaluation of polystyrene microplastic. Sak. Univ. J. Sci. 2019, 23, 358–367. [Google Scholar] [CrossRef] [Green Version]
- Ribeiro, F.; Garcia, A.R.; Pereira, B.P.; Fonseca, M.; Mestre, N.C.; Fonseca, T.G.; Ilharco, L.M.; Bebianno, M.J. Microplastics effects in Scrobicularia plana. Mar. Pollut. Bull. 2017, 122, 379–391. [Google Scholar] [CrossRef]
- Pittura, L.; Avio, C.G.; Giuliani, M.E.; d’Errico, G.; Keiter, S.H.; Cormier, B.; Gorbi, S.; Regoli, F. Microplastics as vehicles of environmental PAHs to marine organisms: Combined chemical and physical hazards to the Mediterranean mussels, Mytilus galloprovincialis. Front. Mar. Sci. 2018, 5, 103. [Google Scholar] [CrossRef] [Green Version]
- Santana, M.F.; Moreira, F.T.; Pereira, C.D.; Abessa, D.M.; Turra, A. Continuous exposure to microplastics does not cause physiological effects in the cultivated mussel Perna perna. Arch. Environ. Contam. Toxicol. 2018, 74, 594–604. [Google Scholar] [CrossRef] [Green Version]
- Sıkdokur, E.; Belivermi¸s, M.; Sezer, N.; Pekmez, M.; Bulan, Ö.K.; Kılıç, Ö. Effects of microplastics and mercury on manila clam Ruditapes philippinarum: Feeding rate, immunomodulation, histopathology and oxidative stress. Environ. Pollut. 2020, 262, 114247. [Google Scholar] [CrossRef]
- Wang, S.; Hu, M.; Zheng, J.; Huang, W.; Shang, Y.; Fang, J.K.-H.; Shi, H.; Wang, Y. Ingestion of nano/micro plastic particles by the mussel Mytilus coruscus is size dependent. Chemosphere 2021, 263, 127957. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, P.; Barboza, L.G.A.; Branco, V.; Figueiredo, N.; Carvalho, C.; Guilhermino, L. Effects of microplastics and mercury in the freshwater bivalve Corbicula fluminea (Müller, 1774): Filtration rate, biochemical biomarkers and mercury bioconcentration. Ecotoxicol. Environ. Saf. 2018, 164, 155–163. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dovzhenko, N.V.; Chelomin, V.P.; Mazur, A.A.; Kukla, S.P.; Slobodskova, V.V.; Istomina, A.A.; Zhukovskaya, A.F. Oxidative Stress in Far Eastern Mussel Mytilus trossulus (Gould, 1850) Exposed to Combined Polystyrene Microspheres (µPSs) and CuO-Nanoparticles (CuO-NPs). J. Mar. Sci. Eng. 2022, 10, 707. https://doi.org/10.3390/jmse10050707
Dovzhenko NV, Chelomin VP, Mazur AA, Kukla SP, Slobodskova VV, Istomina AA, Zhukovskaya AF. Oxidative Stress in Far Eastern Mussel Mytilus trossulus (Gould, 1850) Exposed to Combined Polystyrene Microspheres (µPSs) and CuO-Nanoparticles (CuO-NPs). Journal of Marine Science and Engineering. 2022; 10(5):707. https://doi.org/10.3390/jmse10050707
Chicago/Turabian StyleDovzhenko, Nadezda Vladimirovna, Victor Pavlovich Chelomin, Andrey Alexandrovich Mazur, Sergey Petrovich Kukla, Valentina Vladimirovna Slobodskova, Aleksandra Anatolievna Istomina, and Avianna Fayazovna Zhukovskaya. 2022. "Oxidative Stress in Far Eastern Mussel Mytilus trossulus (Gould, 1850) Exposed to Combined Polystyrene Microspheres (µPSs) and CuO-Nanoparticles (CuO-NPs)" Journal of Marine Science and Engineering 10, no. 5: 707. https://doi.org/10.3390/jmse10050707
APA StyleDovzhenko, N. V., Chelomin, V. P., Mazur, A. A., Kukla, S. P., Slobodskova, V. V., Istomina, A. A., & Zhukovskaya, A. F. (2022). Oxidative Stress in Far Eastern Mussel Mytilus trossulus (Gould, 1850) Exposed to Combined Polystyrene Microspheres (µPSs) and CuO-Nanoparticles (CuO-NPs). Journal of Marine Science and Engineering, 10(5), 707. https://doi.org/10.3390/jmse10050707





