A Study of Arca noae (Linnaeus, 1758) in Elounda Bay, Crete, Eastern Mediterranean
Abstract
:1. Introduction
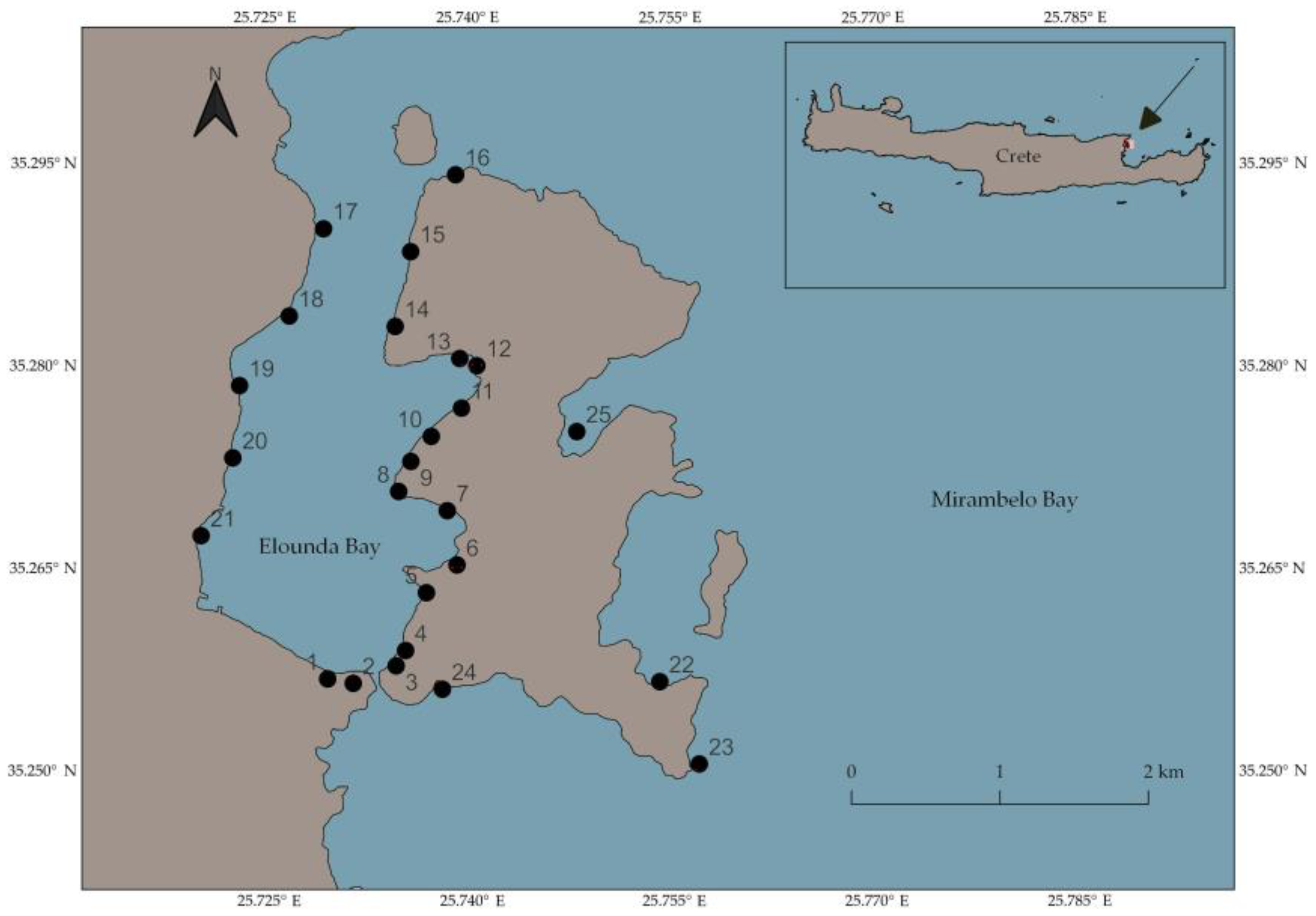
2. Materials and Methods
2.1. Population Structure
2.2. Sex Ratio and Reproductive Biology
2.3. Environmental Parameters
3. Results
3.1. Population Structure
3.1.1. Population Densities
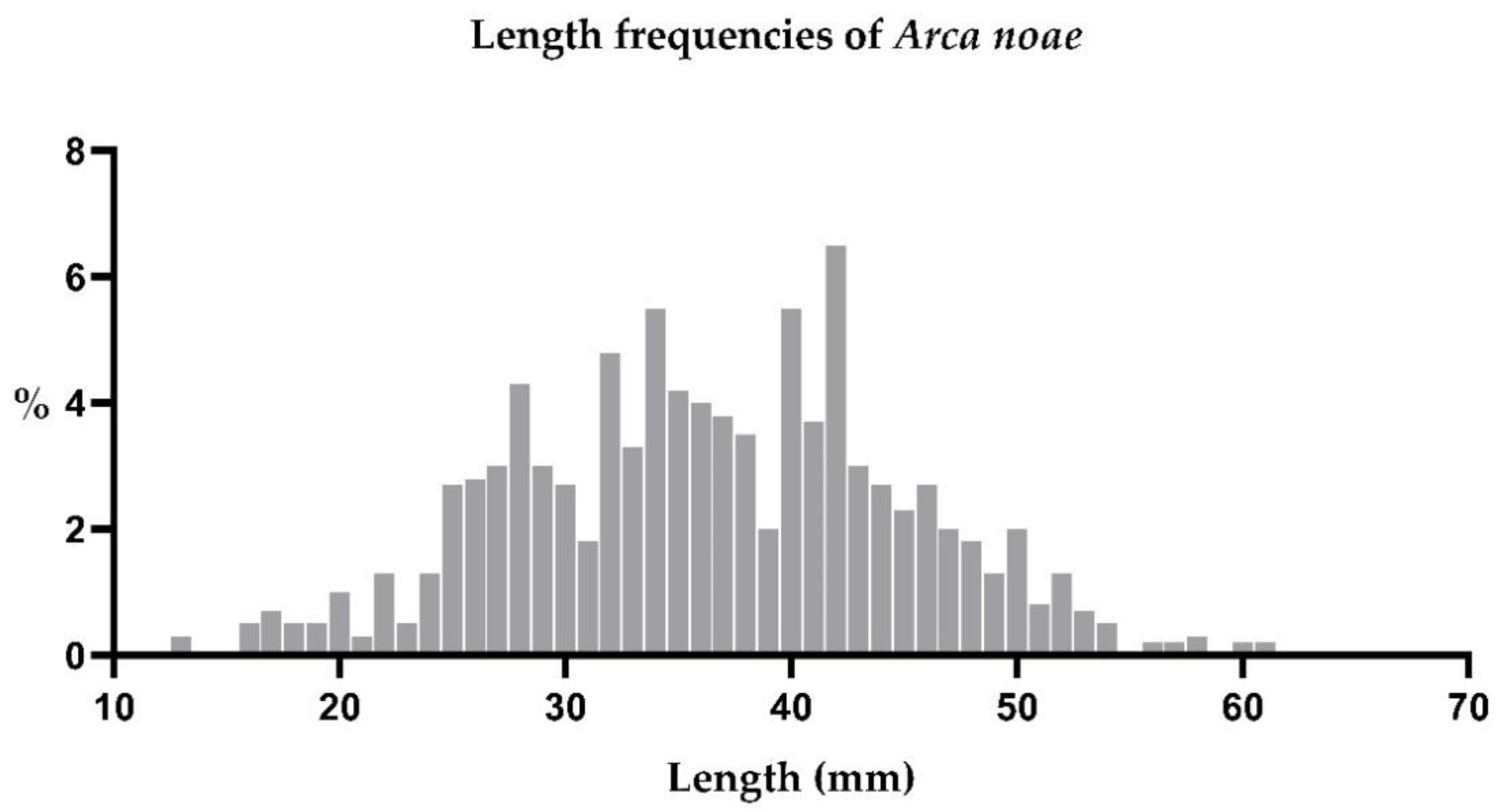
3.1.2. Shell Lengths
3.2. Reproduction
3.2.1. Sex Ratio
3.2.2. Reproductive Cycle
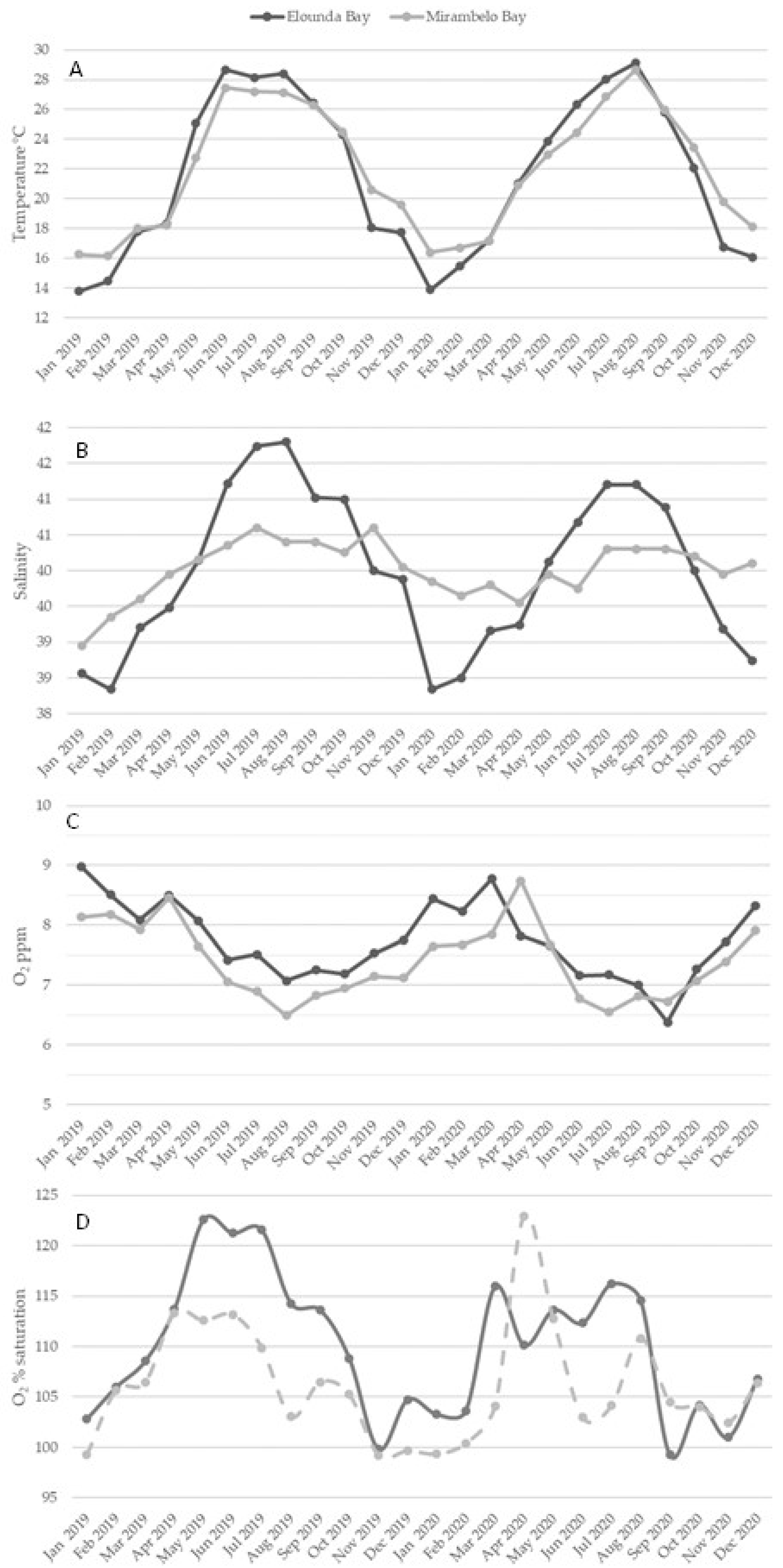
3.3. Environmental Paramaters
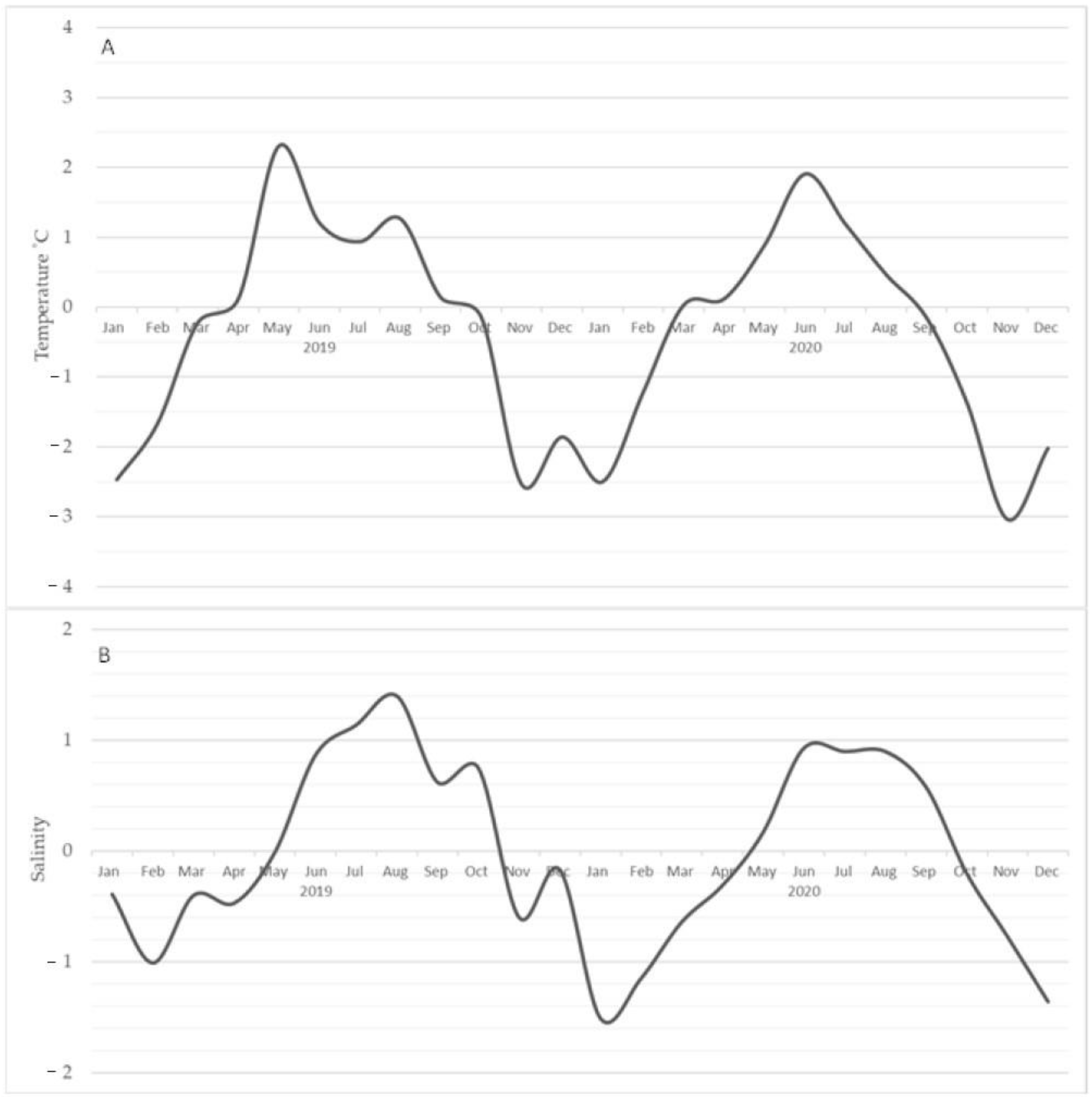
3.3.1. Temperature
3.3.2. Salinity
3.3.3. Dissolved Oxygen
3.3.4. pH
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Smaal, A.C.; Ferreira, J.G.; Grant, J.; Petersen, J.K.; Strand, Ø. Goods and Services of Marine Bivalves; Springer Nature: Berlin, Germany, 2018; ISBN 9783319967769. [Google Scholar]
- Shumway, S.E.; Davis, C.; Downey, R.; Karney, R.; Kraeuter, J.; Parsons, J.; Rheault, R.; Wikfors, G. Shellfish aquaculture—In praise of sustainable economies and environments. World Aquac. 2003, 34, 15–17. [Google Scholar]
- Alonso, A.A.; Álvarez-Salgado, X.A.; Antelo, L.T. Assessing the impact of bivalve aquaculture on the carbon circular economy. J. Clean. Prod. 2021, 279. [Google Scholar] [CrossRef]
- Morton, B.; Peharda, M. The biology and functional morphology of Arca noae (Bivalvia: Arcidae) from the Adriatic Sea, Croatia, with a discussion on the evolution of the bivalve mantle margin. Acta Zool. 2008, 89, 19–28. [Google Scholar] [CrossRef]
- Peharda, M.; Richardson, C.A.; Onofri, V.; Bratoš, A.; Crnčević, M. Age and growth of the bivalve Arca noae L. in the Croatian Adriatic Sea. J. Molluscan Stud. 2002, 68, 307–310. [Google Scholar] [CrossRef] [Green Version]
- Ghribi, F.; Bejaoui, S.; Rabeh, I.; Aouini, F.; Chetoui, I.; El Cafsi, M. Effects of Culinary Methods on Nutritional Characteristics of the Edible Shellfish Noah’s Ark (Arca noae L., 1758) from Tunisian Coasts. J. Aquat. Food Prod. Technol. 2017, 26, 1324–1336. [Google Scholar] [CrossRef]
- Puljas, S.; Peharda, M.; Župan, I.; Bukša, F. Maximum recorded life span of Arca noae Linnaeus, 1758 in the marine protected area Tela šč ica, Adriatic Sea Maximum recorded life span of Arca noae Linnaeus, 1758 in the marine protected area Telaščica, Adriatic Sea. Cah. Biol. Mar. 2015, 56, 163–168. [Google Scholar] [CrossRef]
- Puljas, S.; Lukić, J. Oocyte analysis of species Arca noae Linnaeus, 1758 (Mollusca: Bivalvia) from Adriatic Sea. Eur. Zool. J. 2021, 88, 925–932. [Google Scholar] [CrossRef]
- Bello, G.; Paparella, P.; Corriero, A.; Santamaria, N. Protandric hermaphroditism in the bivalve Arca noae (Mollusca: Arcidae). Mediterr. Mar. Sci. 2013, 14, 86–91. [Google Scholar] [CrossRef]
- Peharda, M.; Mladineo, I.; Bolotin, J.; Kekez, L.; Skaramuca, B. The reproductive cycle and potential protandric development of the Noah’s Ark shell, Arca noae L.: Implications for aquaculture. Aquaculture 2006, 252, 317–327. [Google Scholar] [CrossRef]
- Ghribi, F.; Bello, G.; Zupa, R.; Passantino, L.; Santamaria, N.; El Cafsi, M.; Corriero, A. Reproductive and tissue plasticity in Arca noae (Bivalvia: Arcidae). Eur. Zool. J. 2017, 84, 473–487. [Google Scholar] [CrossRef]
- Župan, I.; Peharda, M.; Dolenec, T.; Dolenec, M.; Rožič, P.Ž.; Lojen, S.; Ezgeta-Balić, D.; Arapov, J. Aquaculture assessment of Noah’s Ark (Arca noae Linnaeus, 1758) in the Central Adriatic Sea (Croatia). J. Shellfish Res. 2014, 33, 433–441. [Google Scholar] [CrossRef] [Green Version]
- Peharda, M.; Ezgeta-Balić, D.; Davenport, J.; Vrgoč, N. The potential for aquaculture of the bearded horse mussel (Modiolus barbatus) and Noah’s Ark shell (Arca noae) in southern Croatia. Aquac. Int. 2013, 21, 639–653. [Google Scholar] [CrossRef]
- Maidanou, M.; Koulouri, P.; Arvanitidis, C.; Koutsoubas, D.; Dounas, C. Macrobenthic assemblage structure associated with a Caulerpa prolifera meadow in the eastern Mediterranean Sea (Elounda Bay, Crete Island). Reg. Stud. Mar. Sci. 2017, 14, 1–14. [Google Scholar] [CrossRef]
- Koulouri, P.; Kalogirou, S.; Maidanou, M.; Koutsoubas, D.; Dounas, C. Fish and cephalopod assemblage structure of green alga Caulerpa prolifera (Chlorophyta) meadow in the eastern Mediterranean Sea (Elounda Bay, Crete Island). Reg. Stud. Mar. Sci. 2016, 3, 33–41. [Google Scholar] [CrossRef]
- McDowell, E.M.; Trump, B.F. Histologic fixatives suitable for diagnostic light and electron microscopy. Arch. Pathol. Lab. Med. 1976, 100, 405–414. [Google Scholar] [PubMed]
- Bennett, H.S.; Wyrick, A.D.; Lee, S.W.; Mcneil, J.H. Science and art in preparing tissues embedded in plastic for light microscopy, with special reference to glycol methacrylate, glass knives and simple stains. Stain Technol. 1976, 51, 71–97. [Google Scholar] [CrossRef] [PubMed]
- Peharda, M.; Stagličić, N.; Ezgeta, D. Distribution and population structure of Arca noae in the Pašman channel. Croat. J. Fish. Ribar. 2009, 124, 3–10. [Google Scholar]
- Knott, N.A.; Underwood, A.J.; Chapman, M.G.; Glasby, T.M. Epibiota on vertical and on horizontal surfaces on natural reefs and on artificial structures. J. Mar. Biol. Assoc. UK 2004, 84, 1117–1130. [Google Scholar] [CrossRef]
- Connell, S.D. Effects of surface orientation on the cover of epibiota. Biofouling 1999, 14, 219–226. [Google Scholar] [CrossRef]
- Ushiama, S.; Smith, J.A.; Suthers, I.M.; Lowry, M.; Johnston, E.L. The effects of substratum material and surface orientation on the developing epibenthic community on a designed artificial reef. Biofouling 2016, 32, 1049–1060. [Google Scholar] [CrossRef]
- Glasby, T.M.; Connell, S.D. Orientation and position of substrata have large effects on epibiotic assemblages. Mar. Ecol. Prog. Ser. 2001, 214, 127–135. [Google Scholar] [CrossRef]
- Hrs-brenko, M. Preliminary survey of populations of the bivalve Noah’s ark (Arca noae, Linne) in the Northern Adriatic Sea. Aquaculture 1980, 21, 357–363. [Google Scholar] [CrossRef]
- Marin, A.; Belluga, M.D.L. Sponge coating decreases predation on the bivalve Arca noae. J. Molluscan Stud. 2005, 71, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Reitner, J.; Keupp, H. Fossil and Recent Sponges; Springer: Berlin, Germany; Heidelberg, Germany, 1991. [Google Scholar] [CrossRef]
- Peharda, M.; Bolotin, J.; Vrgoč, N.; Jasprica, N.; Bratoš, A.; Skaramuca, B. A study of the Noah’s Ark shell (Arca noae Linnaeus 1758) in Mali Ston Bay, Adriatic Sea. J. Shellfish Res. 2003, 22, 705–709. [Google Scholar]
- Maidanou, M.; Koulouri, P.; Karachle, P.K.; Arvanitidis, C.; Koutsoubas, D.; Dounas, C. Trophic diversity of a fish community associated with a caulerpa prolifera (Forsskål) meadow in a shallow semi-enclosed embayment. J. Mar. Sci. Eng. 2021, 9, 165. [Google Scholar] [CrossRef]




| Female | Male | |
|---|---|---|
| Early & late developing | Initially dense oogonia present in acini, followed by previtellogenic oocytes smaller than 60 μm. | Acini initially filled with germinative cells and spermatogonia, followed by the development of small spermatozoa. |
| Ripe | Acini filled with large mature oocytes (>60 μm). | Acini filled with spermatozoa. Central part of acini intensively pinkish. |
| Partially spawned | Acini almost empty with a few mature oocytes remaining. | No spermatogonia present and spermatozoa mainly evacuated. |
| Spent/Inactive | Empty acini with occasional scarce degenerative oocytes. | Empty acini with occasional small numbers of remaining spermatozoa. |
| Adapted from Peharda et al. (2006) [10] | ||
| Stage | Early and Late Developing | Ripe | Partially Spawned | Spent/Inactive |
|---|---|---|---|---|
| Month | ||||
| June | 2 | 1 | 1 | 6 |
| August | 8 | 0 | 0 | 0 |
| September | 5 | 0 | 1 | 3 |
| November | 5 | 0 | 0 | 5 |
| May | 5 | 3 | 1 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Skouradakis, G.; Dounas, C.; Androulakis, D.N.; Papadaki, M.; Koulouri, P.; Pavlidis, M. A Study of Arca noae (Linnaeus, 1758) in Elounda Bay, Crete, Eastern Mediterranean. J. Mar. Sci. Eng. 2022, 10, 673. https://doi.org/10.3390/jmse10050673
Skouradakis G, Dounas C, Androulakis DN, Papadaki M, Koulouri P, Pavlidis M. A Study of Arca noae (Linnaeus, 1758) in Elounda Bay, Crete, Eastern Mediterranean. Journal of Marine Science and Engineering. 2022; 10(5):673. https://doi.org/10.3390/jmse10050673
Chicago/Turabian StyleSkouradakis, Grigorios, Costas Dounas, Dimitrios N. Androulakis, Maria Papadaki, Panayota Koulouri, and Michail Pavlidis. 2022. "A Study of Arca noae (Linnaeus, 1758) in Elounda Bay, Crete, Eastern Mediterranean" Journal of Marine Science and Engineering 10, no. 5: 673. https://doi.org/10.3390/jmse10050673
APA StyleSkouradakis, G., Dounas, C., Androulakis, D. N., Papadaki, M., Koulouri, P., & Pavlidis, M. (2022). A Study of Arca noae (Linnaeus, 1758) in Elounda Bay, Crete, Eastern Mediterranean. Journal of Marine Science and Engineering, 10(5), 673. https://doi.org/10.3390/jmse10050673








