The Effects of Anthropogenic Electromagnetic Fields (EMF) on the Early Development of Two Commercially Important Crustaceans, European Lobster, Homarus gammarus (L.) and Edible Crab, Cancer pagurus (L.)
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Collection and Husbandry
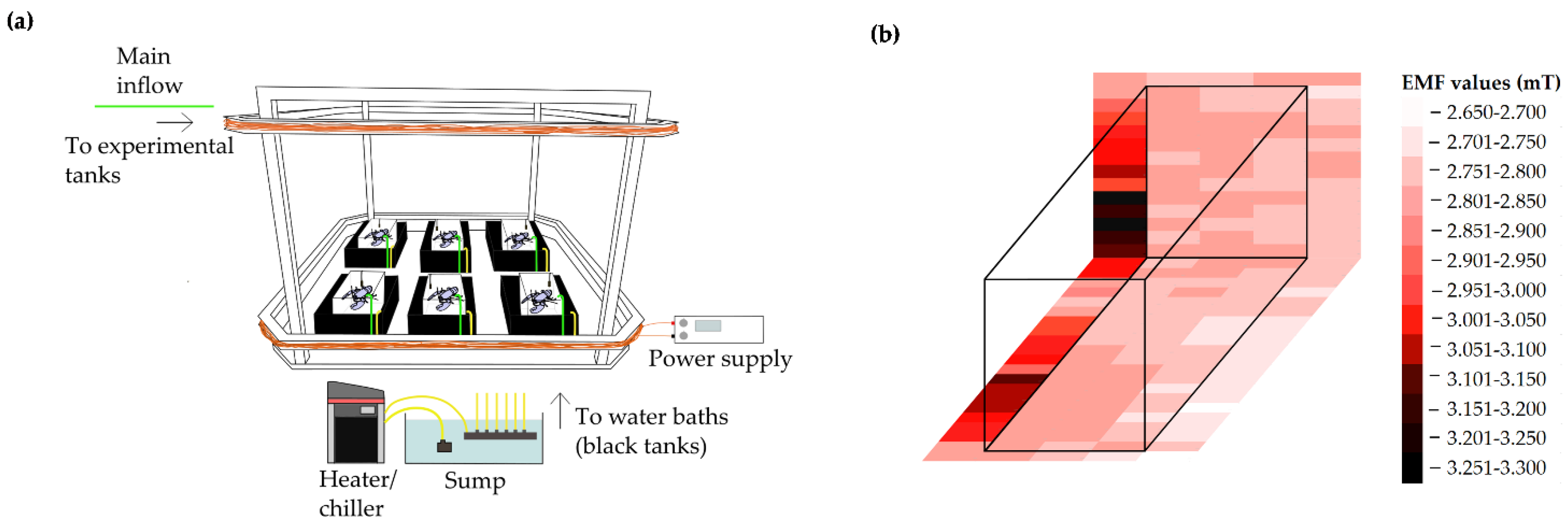
2.2. Experimental Setup
2.3. Measured Parameters
2.3.1. Embryonic Development
2.3.2. Hatching
2.3.3. Larval Assessment
2.3.4. Vertical Swimming Speed
2.4. Statistical Analysis
3. Results
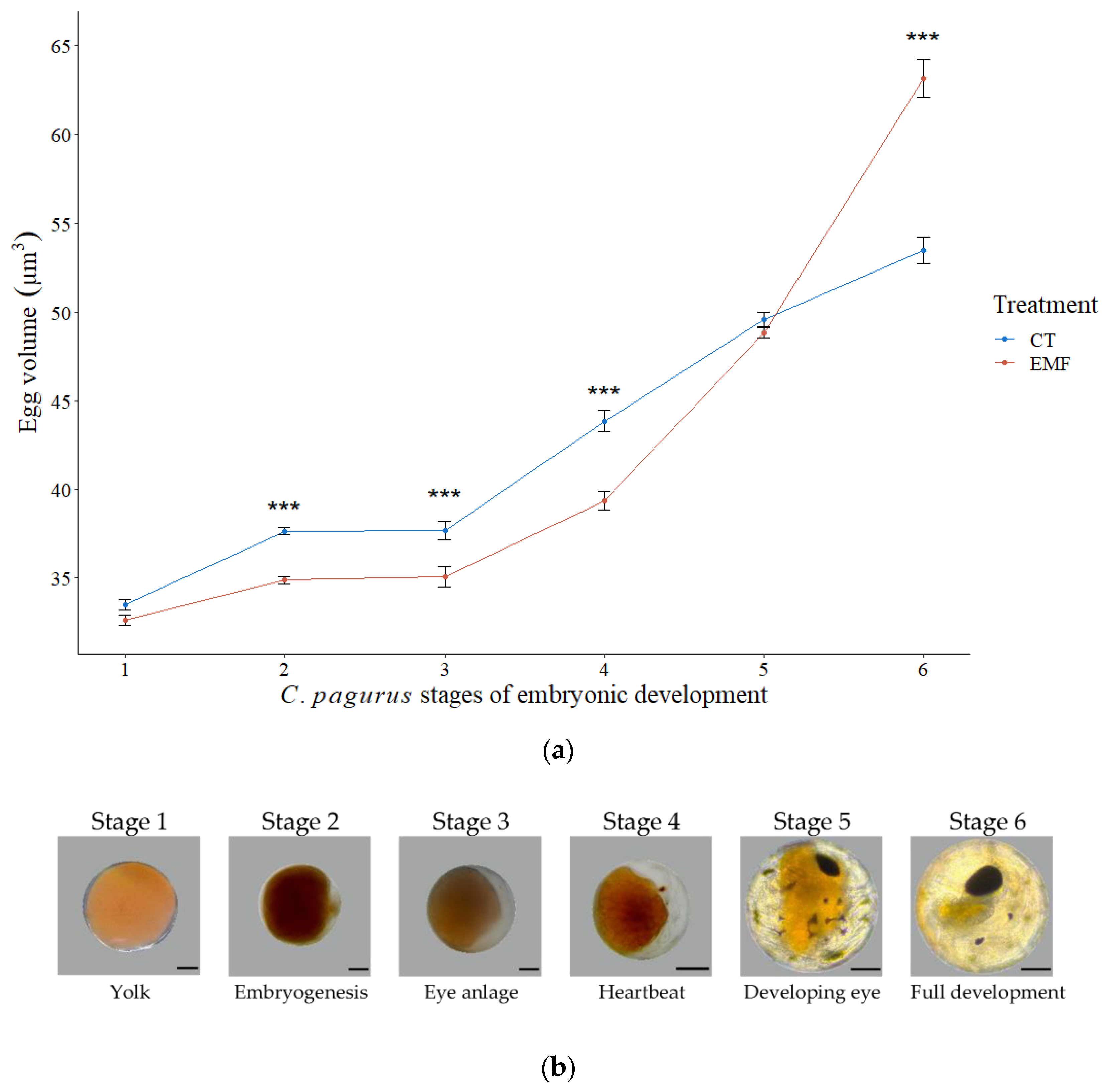
3.1. Embryonic Development
3.1.1. Homarus Gammarus
3.1.2. Cancer Pagurus
3.2. Hatching
3.2.1. Homarus Gammarus
3.2.2. Cancer Pagurus
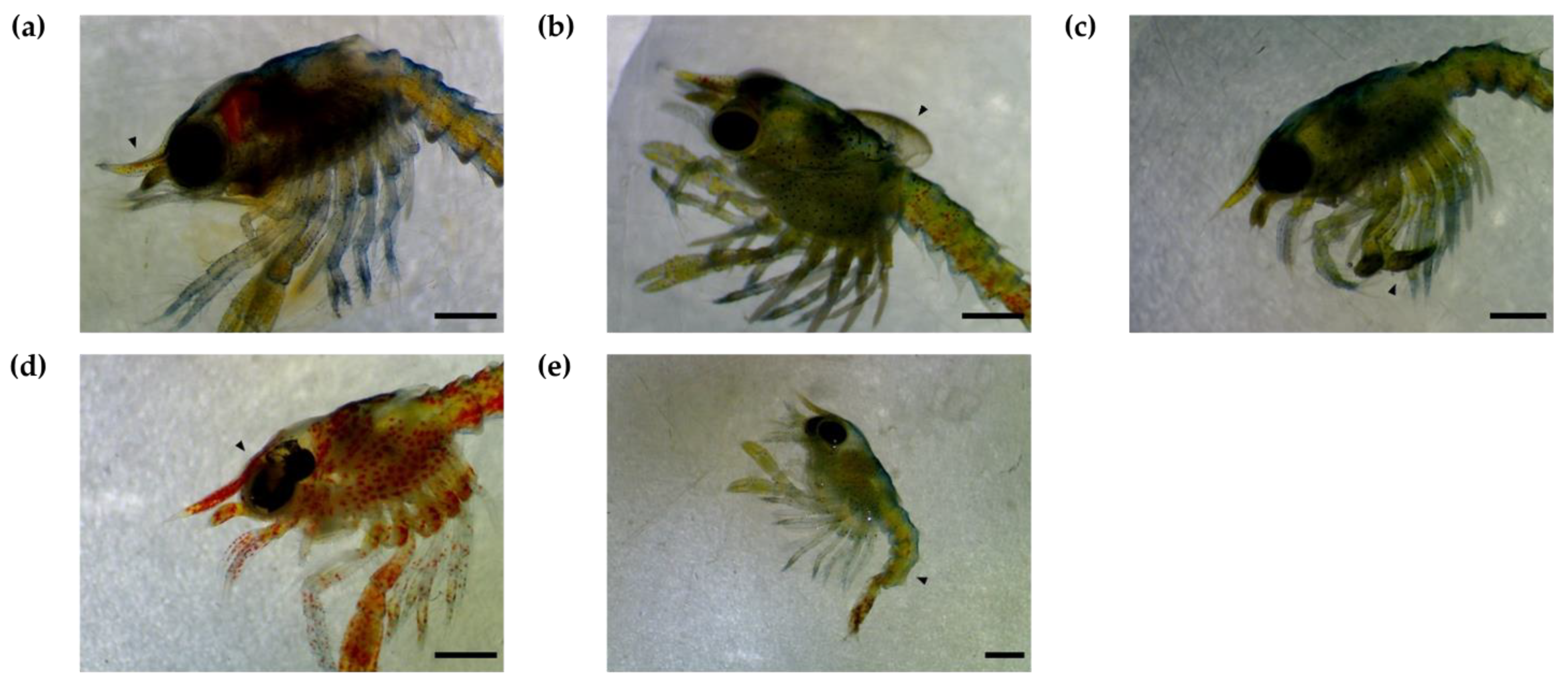
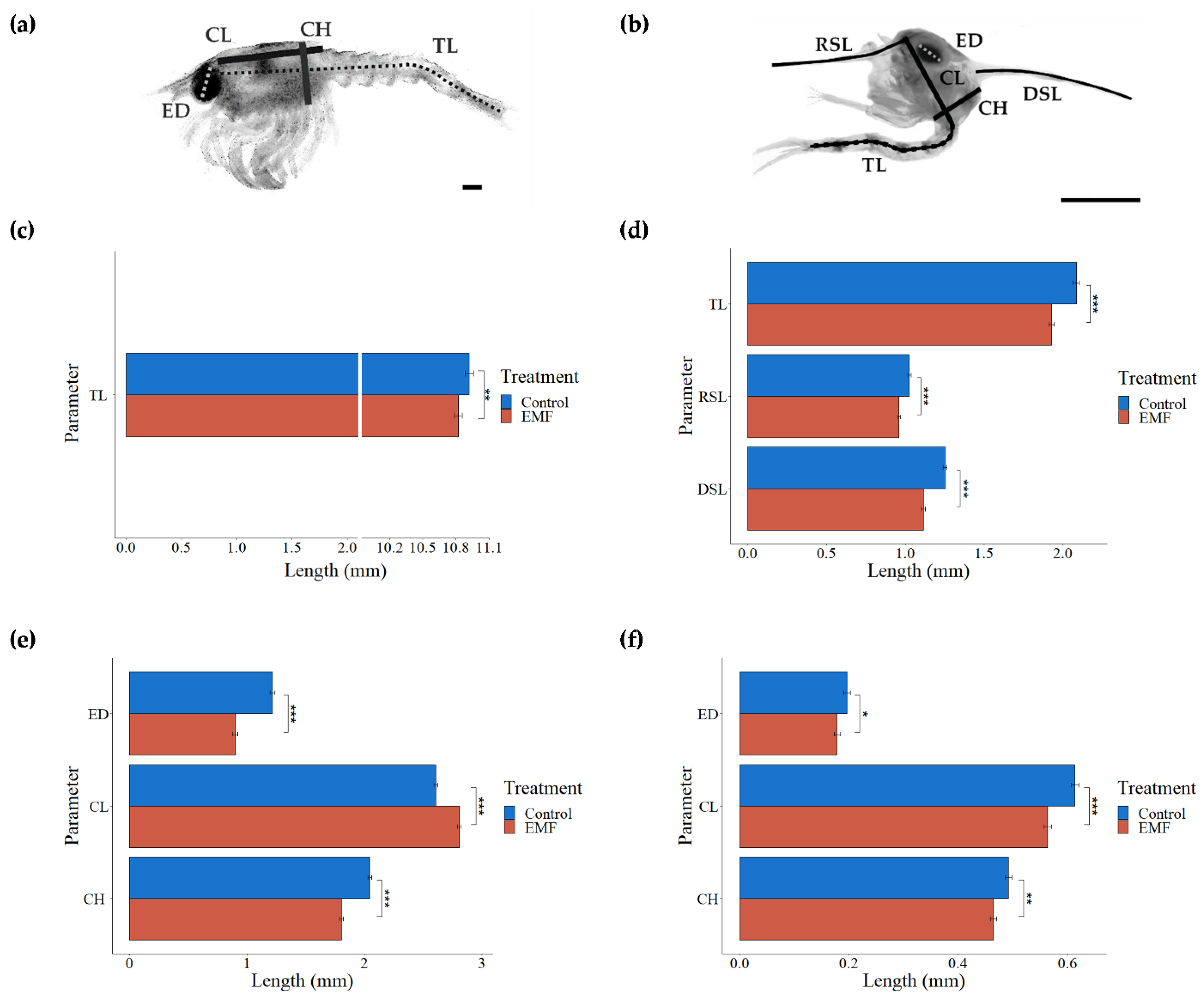
3.3. Larval Assessment
3.3.1. Homarus Gammarus
3.3.2. Cancer Pagurus
3.4. Vertical Swimming Speed
3.4.1. Homarus Gammarus
3.4.2. Cancer Pagurus
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kirschvink, J.L. Homing in on vertebrates. Nature 1997, 390, 339–340. [Google Scholar] [CrossRef]
- Gill, A.B. Offshore renewable energy: Ecological implications of generating electricity in the coastal zone. J. Appl. Ecol. 2005, 42, 605–615. [Google Scholar] [CrossRef]
- Bochert, R.; Zettler, M.L. Long-term exposure of several marine benthic animals to static magnetic fields. Bioelectromagnetics 2004, 25, 498–502. [Google Scholar] [CrossRef] [PubMed]
- Tricas, T.; Gill, A.B. Effects of EMFs from Undersea Power Cables on Elasmobranchs and Other Marine Species; OCS Study BOEMRE 2011-09; U.S. Department of the Interior: Washington, DC, USA, 2011. [Google Scholar]
- Taormina, B.; Bald, J.; Want, A.; Thouzeau, G.; Lejart, M.; Desroy, N.; Carlier, A. A review of potential impacts of submarine power cables on the marine environment: Knowledge gaps, recommendations and future directions. Renew. Sustain. Energy Rev. 2018, 96, 380–391. [Google Scholar] [CrossRef]
- Centre for Marine and Coastal Studies. A Baseline Assessment of Electromagnetic Fields Generated by Offshore Windarm Cables; University of Liverpool: Birkenhead, UK, 2003; p. 71. [Google Scholar]
- Bochert, R.; Zettler, M.L. Effect of Electromagnetic Fields on Marine Organisms. In Offshore Wind Energy: Research on Environmental Impacts; Köller, J., Köppel, J., Peters, W., Eds.; Springer: Berlin/Heidelberg, Germany, 2006; pp. 223–234. [Google Scholar]
- Hutchison, Z.L.; Gill, A.B.; Sigray, P.; He, H.; King, J.W. Anthropogenic electromagnetic fields (EMF) influence the behaviour of bottom-dwelling marine species. Sci. Rep. 2020, 10, 4219. [Google Scholar] [CrossRef]
- Kavet, R.; Wyman, M.T.; Klimley, A.P. Modeling Magnetic Fields from a DC Power Cable Buried Beneath San Francisco Bay Based on Empirical Measurements. PLoS ONE 2016, 11, e0148543. [Google Scholar] [CrossRef][Green Version]
- Otremba, Z.; Jakubowska, M.; Urban-Malinga, B.; Andrulewicz, E. Potential effects of electrical energy transmission–the case study from the Polish Marine Areas (southern Baltic Sea). Oceanol. Hydrobiol. Stud. 2019, 48, 196–208. [Google Scholar] [CrossRef]
- Taormina, B.; Di Poi, C.; Agnalt, A.-L.; Carlier, A.; Desroy, N.; Escobar-Lux, R.H.; D’eu, J.-F.; Freytet, F.; Durif, C.M.F. Impact of magnetic fields generated by AC/DC submarine power cables on the behavior of juvenile European lobster (Homarus gammarus). Aquat. Toxicol. 2020, 220, 105401. [Google Scholar] [CrossRef]
- Formicki, K.; Sadowski, M.; Tański, A.; Korzelecka-Orkisz, A.; Winnicki, A. Behaviour of trout (Salmo trutta L.) larvae and fry in a constant magnetic field. J. Appl. Ichthyol. 2004, 20, 290–294. [Google Scholar] [CrossRef]
- Cada, G.F.; Bevelhimer, M.S.; Riemer, K.P.; Turner, J.W. Effects on Freshwater Organisms of Magnetic Fields Associated with Hydrokinetic Turbines; Technical Report; U.S. Department of Energy: Washington, DC, USA, 2011. [Google Scholar]
- Woodruff, D.L.; Schultz, I.R.; Marshall, K.E.; Ward, J.A.; Cullinan, V.I. Effects of Electromagnetic Fields on Fish and Invertebrates; Pacific Northwest National Laboratory: Richland, WA, USA, 2012. [Google Scholar]
- Scott, K.; Harsanyi, P.; Lyndon, A.R. Understanding the effects of electromagnetic field emissions from Marine Renewable Energy Devices (MREDs) on the commercially important edible crab, Cancer pagurus (L.). Mar. Pollut. Bull. 2018, 131, 580–588. [Google Scholar] [CrossRef]
- Panagopoulos, D.J.; Johansson, O.; Carlo, G.L. Polarization: A Key Difference between Man-made and Natural Electromagnetic Fields, in regard to Biological Activity. Sci. Rep. 2015, 5, 14914. [Google Scholar] [CrossRef] [PubMed]
- Panagopoulos, D.J. Electromagnetic interaction between environmental fields and living systems determines health and well-being. In Electromagnetic Fields: Principles, Engineering Applications and Biophysical Effects; Kwang, M.-H., Yoon, S.-O., Eds.; Nova Science Publishers: New York, NY, USA, 2013; Volume 13. [Google Scholar]
- Pecoraro, R.; Pavone, S.C.; Scalisi, E.M.; Sica, C.; Ignoto, S.; Contino, M.; Salvaggio, A.; Sorbello, G.; Di Donato, L.; Brundo, M.V. Biological Effects of Non-ionizing Electromagnetic Fields to 27 GHz on Sperm Quality of Mytilus galloprovincialis. J. Mar. Sci. Eng. 2022, 10, 521. [Google Scholar] [CrossRef]
- Levin, M.; Ernst, S.G. Applied DC magnetic fields cause alterations in the time of cell divisions and developmental abnormalities in early sea urchin embryos. Bioelectromagnetics 1997, 18, 255–263. [Google Scholar] [CrossRef]
- Denegre James, M.; Valles James, M.; Lin, K.; Jordan, W.B.; Mowry Kimberly, L. Cleavage planes in frog eggs are altered by strong magnetic fields. Proc. Natl. Acad. Sci. USA 1998, 95, 14729–14732. [Google Scholar] [CrossRef]
- Sadowski, M.; Winnicki, A.; Formicki, K.; Sobocinski, A.; Tanski, A. The effect of magnetic field on permeability of egg shells of salmonid fishes. Acta Ichthyol. Piscat. 2007, 37, 129–135. [Google Scholar] [CrossRef]
- Krylov, V.V. Effects of electromagnetic fields on parthenogenic eggs of Daphnia magna Straus. Ecotoxicol. Environ. Saf. 2010, 73, 62–66. [Google Scholar] [CrossRef]
- Skauli, K.S.; Reitan, J.B.; Walther, B.T. Hatching in zebrafish (Danio rerio) embryos exposed to a 50 Hz magnetic field. Bioelectromagnetics 2000, 21, 407–410. [Google Scholar] [CrossRef]
- Ernst, D.A.; Lohmann, K.J. Effect of magnetic pulses on Caribbean spiny lobsters: Implications for magnetoreception. J. Exp. Biol. 2016, 219, 1827–1832. [Google Scholar] [CrossRef]
- Tański, A.; Formicki, K.; ŚMietana, P.; Sadowski, M.; Winnicki, A. Sheltering behaviour of Spinycheek crayfish (Orconectes limosus) in the presence of an artifical magnetic field. Bull. Fr. Pêche Piscic. 2005, 376–377, 787–793. [Google Scholar] [CrossRef]
- Hutchison, Z.; Sigray, P.; He, H.; Gill, A.; King, J.; Gibson, C. Electromagnetic Field (EMF) impacts on elasmobranch (shark, rays, and skates) and American lobster movement and migration from direct current cables. BOEM 2018, 3, 2018. [Google Scholar]
- Scott, K. Understanding the Biology of Two Commercially Important Crustaceans in Relation to Fisheries and Anthropogenic Impacts. Ph.D. Thesis, Heriot-Watt University, Edinburgh, UK, 2019. [Google Scholar]
- Scott, K.; Harsanyi, P.; Easton, B.A.A.; Piper, A.J.R.; Rochas, C.M.V.; Lyndon, A.R. Exposure to Electromagnetic Fields (EMF) from Submarine Power Cables Can Trigger Strength-Dependent Behavioural and Physiological Responses in Edible Crab, Cancer pagurus (L.). J. Mar. Sci. Eng. 2021, 9, 776. [Google Scholar] [CrossRef]
- Holthuis, L.B. Marine Lobsters of the World; Food and Agriculture Organization of the United Nations: Rome, Italy, 1991; Volume 13. [Google Scholar]
- Scott, K.; Harsanyi, P.; Lyndon, A.R. Baseline measurements of physiological and behavioural stress markers in the commercially important decapod Cancer pagurus (L.). J. Exp. Mar. Biol. Ecol. 2018, 507, 1–7. [Google Scholar] [CrossRef]
- Bridges, T. Crab and Lobster Stock Assessment. 2017. Available online: https://www.gov.uk/government/publications/crab-and-lobster-stock-assessment-2017 (accessed on 17 March 2022).
- Lizárraga-Cubedo, H.A.; Tuck, I.; Bailey, N.; Pierce, G.J.; Kinnear, J.A.M. Comparisons of size at maturity and fecundity of two Scottish populations of the European lobster, Homarus gammarus. Fish. Res. 2003, 65, 137–152. [Google Scholar] [CrossRef]
- Branford, J.R. Incubation period for the lobster Homarus gammarus at various temperatures. Mar. Biol. 1978, 47, 363–368. [Google Scholar] [CrossRef]
- Williamson, H.C. Contributions to the Life-Histories of the Edible Crab (Cancer Pagurus) and of Other Decapod Crustacea; Edinburgh, UK, 1904. [Google Scholar]
- Ungfors, A. Sexual maturity of the edible crab (Cancer pagurus) in the Skagerrak and the Kattegat, based on reproductive and morphometric characters. ICES J. Mar. Sci. 2007, 64, 318–327. [Google Scholar] [CrossRef][Green Version]
- Hunter, E.; Eaton, D.; Stewart, C.; Lawler, A.; Smith, M.T. Edible Crabs “Go West”: Migrations and Incubation Cycle of Cancer pagurus Revealed by Electronic Tags. PLoS ONE 2013, 8, e63991. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Edwards, E. The Edible Crab and Its Fishery in British Waters; Farnham (UK) Fishing News Books: Farnham, UK, 1979. [Google Scholar]
- Howard, A.E. The distribution and behaviour of ovigerous edible crabs (Cancer pagurus), and consequent sampling bias. ICES J. Mar. Sci. 1982, 40, 259–261. [Google Scholar] [CrossRef]
- Naylor, J.K.; Taylor, E.W.; Bennett, D.B. The oxygen uptake of ovigerous edible crabs (Cancer pagurus) (L.) and their eggs. Mar. Freshw. Behav. Physiol. 1997, 30, 29–44. [Google Scholar] [CrossRef]
- Langhamer, O.; Wilhelmsson, D. Colonisation of fish and crabs of wave energy foundations and the effects of manufactured holes—A field experiment. Mar. Environ. Res. 2009, 68, 151–157. [Google Scholar] [CrossRef]
- Hunter, W.R.; Sayer, M.D.J. The comparative effects of habitat complexity on faunal assemblages of northern temperate artificial and natural reefs. ICES J. Mar. Sci. 2009, 66, 691–698. [Google Scholar] [CrossRef]
- Hiscock, K.; Sharrock, S.; Highfield, J.; Snelling, D. Colonization of an artificial reef in south-west England—ex-HMS ‘Scylla’. J. Mar. Biol. Assoc. UK 2010, 90, 69–94. [Google Scholar] [CrossRef]
- Helluy, S.M.; Beltz, B.S. Embryonic Development of the American Lobster (Homarus americanus): Quantitative Staging and Characterization of an Embryonic Molt Cycle. Biol. Bull. 1991, 180, 355–371. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Lei, S.; Zhang, X.; Zhang, P.; Ikeda, Y. Biochemical composition of cuttlefish (Sepia esculenta) eggs during embryonic development. Molluscan Res. 2014, 34, 1–9. [Google Scholar] [CrossRef]
- Chung, J.S.; Webster, S.G. Expression and release patterns of neuropeptides during embryonic development and hatching of the green shore crab, Carcinus maenas. Development 2004, 131, 4751–4761. [Google Scholar] [CrossRef]
- Hadley, P.B. The behavior of the larval and adolescent stages of the American lobster (Homarus americanus). J. Comp. Neurol. Psychol. 1908, 18, 199–301. [Google Scholar] [CrossRef]
- Ingle, R. The larval and post-larval development of the edible crab, Cancer pagurus Linnaeus (Decapoda: Brachyura). Bull. Br. Mus. (Nat. Hist.) Zool. 1981, 40, 211–236. [Google Scholar]
- Ennis, G.P. Endogenous rhythmicity associated with larval hatching in the lobster Homarus gammarus. J. Mar. Biol. Assoc. United Kingd. 1973, 53, 531–538. [Google Scholar] [CrossRef]
- Agnalt, A.L.; Grefsrud, E.S.; Farestveit, E.; Larsen, M.; Keulder, F. Deformities in larvae and juvenile European lobster (Homarus gammarus) exposed to lower pH at two different temperatures. Biogeosciences 2013, 10, 7883–7895. [Google Scholar] [CrossRef]
- Botero, L.; Atema, J. Behavior and Substrate Selection During Larval Settling in the Lobster Homarus Americanus. J. Crustacean Biol. 1982, 2, 59–69. [Google Scholar] [CrossRef]
- Webley, J.A.C.; Connolly, R.M. Vertical movement of mud crab megalopae (Scylla serrata) in response to light: Doing it differently down under. J. Exp. Mar. Biol. Ecol. 2007, 341, 196–203. [Google Scholar] [CrossRef]
- Schmalenbach, I.; Buchholz, F. Vertical positioning and swimming performance of lobster larvae (Homarus gammarus) in an artificial water column at Helgoland, North Sea. Mar. Biol. Res. 2010, 6, 89–99. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing 4.0.3; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- Yu, G.; Xu, S.; Chen, M.; Feng, T.; Zhan, L.; Zhou, L. Use ggbreak to effectively utilize plotting space to deal with large datasets and outliers. Front. Genet. 2021, 12, 2122. [Google Scholar] [CrossRef]
- Wear, R.G. Incubation in British Decapod Crustacea, and the Effects of Temperature on the Rate and Success of Embryonic Development. J. Mar. Biol. Assoc. UK 1974, 54, 745–762. [Google Scholar] [CrossRef]
- Charmantier, G.; Mounet-Guillaume, R. Temperature-specific rates of embryonic development of the European lobster Homarus gammarus (L.). J. Exp. Mar. Biol. Ecol. 1992, 160, 61–66. [Google Scholar] [CrossRef]
- Hjort, J. Fluctuations in the great fisheries of northern Europe viewed in the light of biological research. In Proceedings of the RAPPORTS ET PROCRS-VERBAUX, Copenhagen, Denmark, May 1914. [Google Scholar]
- Fey, D.P.; Jakubowska, M.; Greszkiewicz, M.; Andrulewicz, E.; Otremba, Z.; Urban-Malinga, B. Are magnetic and electromagnetic fields of anthropogenic origin potential threats to early life stages of fish? Aquat. Toxicol. 2019, 209, 150–158. [Google Scholar] [CrossRef]
- Zimmerman, S.; Zimmerman, A.M.; Winters, W.D.; Cameron, I.L. Influence of 60-Hz magnetic fields on sea urchin development. Bioelectromagnetics 1990, 11, 37–45. [Google Scholar] [CrossRef]
- Cameron, I.L.; Hunter, K.E.; Winters, W.D. Retardation of embryogenesis by extremely low frequency 60 Hz electromagnetic fields. Physiol. Chem. Phys. Med. NMR 1985, 17, 135–138. [Google Scholar]
- Fey, D.P.; Greszkiewicz, M.; Otremba, Z.; Andrulewicz, E. Effect of static magnetic field on the hatching success, growth, mortality, and yolk-sac absorption of larval Northern pike Esox lucius. Sci. Total Environ. 2019, 647, 1239–1244. [Google Scholar] [CrossRef]
- Petrov, E.; Martinac, B. Modulation of channel activity and gadolinium block of MscL by static magnetic fields. Eur. Biophys. J. 2006, 36, 95. [Google Scholar] [CrossRef]
- Davis, C.C. Mechanisms of hatching in aquatic invertebrate eggs. In Oceanography and Marine Biology: An Annual Review; Taylor & Francis: Aberdeen, Scotland, 1968; Volume 6, pp. 325–376. [Google Scholar]
- Pandian, T.J. Ecophysiological studies on the developing eggs and embryos of the European lobster Homarus gammarus. Mar. Biol. 1970, 5, 154–167. [Google Scholar] [CrossRef]
- Fonds, M. Laboratory Observations on the Influence of Temperature and Salinity on Development of the Eggs and Growth of the Larvae of Solea solea (Pisces). Mar. Ecol. Prog. Ser. 1979, 1, 91–99. [Google Scholar] [CrossRef]
- Gardner, C.; Maguire, G.B. Effect of photoperiod and light intensity on survival, development and cannibalism of larvae of the Australian giant crab Pseudocarcinus gigas (Lamarck). Aquaculture 1998, 165, 51–63. [Google Scholar] [CrossRef]
- Keppel, E.A.; Scrosati, R.A.; Courtenay, S.C. Ocean acidification decreases growth and development in American lobster (Homarus americanus) larvae. J. Northwest Atl. Fish. Sci. 2012, 44, 61–66. [Google Scholar] [CrossRef]
- Zahedi, Y.; Zaun, G.; Maderwald, S.; Orzada, S.; Pütter, C.; Scherag, A.; Winterhager, E.; Ladd, M.E.; Grümmer, R. Impact of repetitive exposure to strong static magnetic fields on pregnancy and embryonic development of mice. J. Magn. Reson. Imaging 2014, 39, 691–699. [Google Scholar] [CrossRef] [PubMed]
- Rooze, M.; Hinsenkamp, M. In vivo modifications induced by electromagnetic stimulation of chicken embryos. Reconstr. Surg. Traumatol. 1985, 19, 87–92. [Google Scholar]
- Fernie, K.J.; Bird, D.M.; Dawson, R.D.; Laguë, P.C. Effects of electromagnetic fields on the reproductive success of American kestrels. Physiol Biochem. Zool 2000, 73, 60–65. [Google Scholar] [CrossRef]
- Kurihara, H. Effects of CO2-driven ocean acidification on the early developmental stages of invertebrates. Mar. Ecol. Prog. Ser. 2008, 373, 275–284. [Google Scholar] [CrossRef]
- Kawaguchi, S.; Kurihara, H.; King, R.; Hale, L.; Berli, T.; Robinson, J.P.; Ishida, A.; Wakita, M.; Virtue, P.; Nicol, S. Will krill fare well under Southern Ocean acidification? Biol. Lett. 2011, 7, 288–291. [Google Scholar] [CrossRef]
- Byrne, M. Impact of ocean warming and ocean acidification on marine invertebrate life history stages: Vulnerabilities and potential for persistence in a changing ocean. In Oceanography and Marine Biology: Annual Review; Gibson, R.N., Atkinson, R.J.A., Gordon, J.D.M., Eds.; Taylor & Francis: London, UK, 2011; Volume 49, p. 42. [Google Scholar]
- Giorgi, G.; Guerra, D.; Pezzoli, C.; Cavicchi, S.; Bersani, F. Genetic effects of static magnetic fields. Body size increase and lethal mutations induced in populations of Drosophila melanogaster after chronic exposure. Genet. Sel. Evol. 1992, 24, 393–413. [Google Scholar] [CrossRef]
- Ueno, S.; Harada, K.; Shiokawa, K. The embryonic development of frogs under strong DC magnetic fields. IEEE Trans. Magn. 1984, 20, 1663–1665. [Google Scholar] [CrossRef]
- Nichols, J.H.; Thompson, B.M.; Cryer, M. Production, drift and mortality of the planktonic larvae of the edible crab (cancer pagurus) off the North-East coast of England. Neth. J. Sea Res. 1982, 16, 173–184. [Google Scholar] [CrossRef]
- Ennis, G. Swimming ability of larval American lobsters, Homarus americanus, in flowing water. Can. J. Fish. Aquat. Sci. 1986, 43, 2177–2183. [Google Scholar] [CrossRef]
- Scarratt, D.J. Abundance and Distribution of Lobster Larvae (Homarus americanus) in Northumberland Strait. J. Fish. Res. Board Can. 1964, 21, 661–680. [Google Scholar] [CrossRef]
- Ward, B.K.; Tan, G.X.; Roberts, D.C.; Della Santina, C.C.; Zee, D.S.; Carey, J.P. Strong static magnetic fields elicit swimming behaviors consistent with direct vestibular stimulation in adult zebrafish. PLoS ONE 2014, 9, e92109. [Google Scholar] [CrossRef]
- Fedele, G.; Green, E.W.; Rosato, E.; Kyriacou, C.P. An electromagnetic field disrupts negative geotaxis in Drosophila via a CRY-dependent pathway. Nat. Commun. 2014, 5, 4391. [Google Scholar] [CrossRef]
- Ugolini, A. Equatorial sandhoppers use body scans to detect the earth’s magnetic field. J. Comp. Physiol. A 2006, 192, 45–49. [Google Scholar] [CrossRef]
- Love, M.S.; Nishimoto, M.M.; Clark, S.; McCrea, M.; Bull, A.S. Assessing potential impacts of energized submarine power cables on crab harvests. Cont. Shelf Res. 2017, 151, 23–29. [Google Scholar] [CrossRef]
- Milton, S.L.; Mary, M.N.; Scott, C.; Ann Scarborough, B. Identical Response of Caged Rock Crabs (Genera Metacarcinus and Cancer) to Energized and Unenergized Undersea Power Cables in Southern California, USA. Bull. South. Calif. Acad. Sci. 2015, 114, 33–41. [Google Scholar] [CrossRef]
- Campbell, A.; Stasko, A.B. Movements of lobsters (Homarus americanus) tagged in the Bay of Fundy, Canada. Mar. Biol. 1986, 92, 393–404. [Google Scholar] [CrossRef]


| H. gammarus | C. pagurus | |||
|---|---|---|---|---|
| Females | Control | EMF | Control | EMF |
| 1 | 63 | 80 | N/A | 113 |
| 2 | N/A | 61 | 112 | 113 |
| 3 | 55 | 57 | 106 | 102 |
| 4 | 96 | 68 | 133 | 133 |
| 5 | 99 | 100 | 106 | 145 |
| 6 | 101 | 52 | 107 | 137 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Harsanyi, P.; Scott, K.; Easton, B.A.A.; de la Cruz Ortiz, G.; Chapman, E.C.N.; Piper, A.J.R.; Rochas, C.M.V.; Lyndon, A.R. The Effects of Anthropogenic Electromagnetic Fields (EMF) on the Early Development of Two Commercially Important Crustaceans, European Lobster, Homarus gammarus (L.) and Edible Crab, Cancer pagurus (L.). J. Mar. Sci. Eng. 2022, 10, 564. https://doi.org/10.3390/jmse10050564
Harsanyi P, Scott K, Easton BAA, de la Cruz Ortiz G, Chapman ECN, Piper AJR, Rochas CMV, Lyndon AR. The Effects of Anthropogenic Electromagnetic Fields (EMF) on the Early Development of Two Commercially Important Crustaceans, European Lobster, Homarus gammarus (L.) and Edible Crab, Cancer pagurus (L.). Journal of Marine Science and Engineering. 2022; 10(5):564. https://doi.org/10.3390/jmse10050564
Chicago/Turabian StyleHarsanyi, Petra, Kevin Scott, Blair A. A. Easton, Guadalupe de la Cruz Ortiz, Erica C. N. Chapman, Althea J. R. Piper, Corentine M. V. Rochas, and Alastair R. Lyndon. 2022. "The Effects of Anthropogenic Electromagnetic Fields (EMF) on the Early Development of Two Commercially Important Crustaceans, European Lobster, Homarus gammarus (L.) and Edible Crab, Cancer pagurus (L.)" Journal of Marine Science and Engineering 10, no. 5: 564. https://doi.org/10.3390/jmse10050564
APA StyleHarsanyi, P., Scott, K., Easton, B. A. A., de la Cruz Ortiz, G., Chapman, E. C. N., Piper, A. J. R., Rochas, C. M. V., & Lyndon, A. R. (2022). The Effects of Anthropogenic Electromagnetic Fields (EMF) on the Early Development of Two Commercially Important Crustaceans, European Lobster, Homarus gammarus (L.) and Edible Crab, Cancer pagurus (L.). Journal of Marine Science and Engineering, 10(5), 564. https://doi.org/10.3390/jmse10050564








