Geographic Differentiation of Morphological Characteristics in the Brown Seaweed Sargassum thunbergii along the Korean Coast: A Response to Local Environmental Conditions
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Environmental Parameters
2.3. Sampling Design and Morphological Measurements
2.4. Data Analysis
3. Results
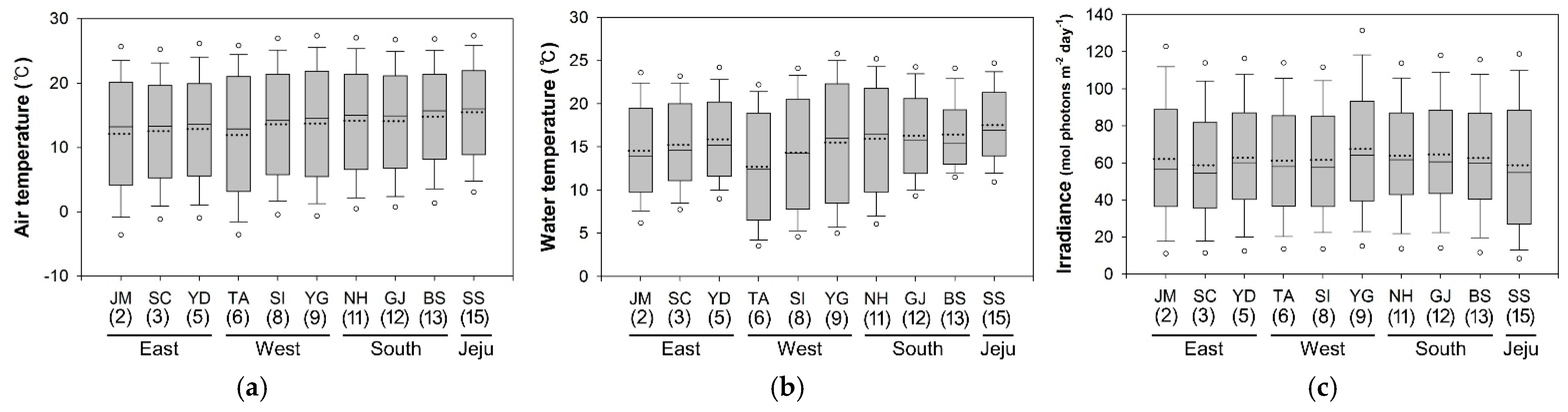
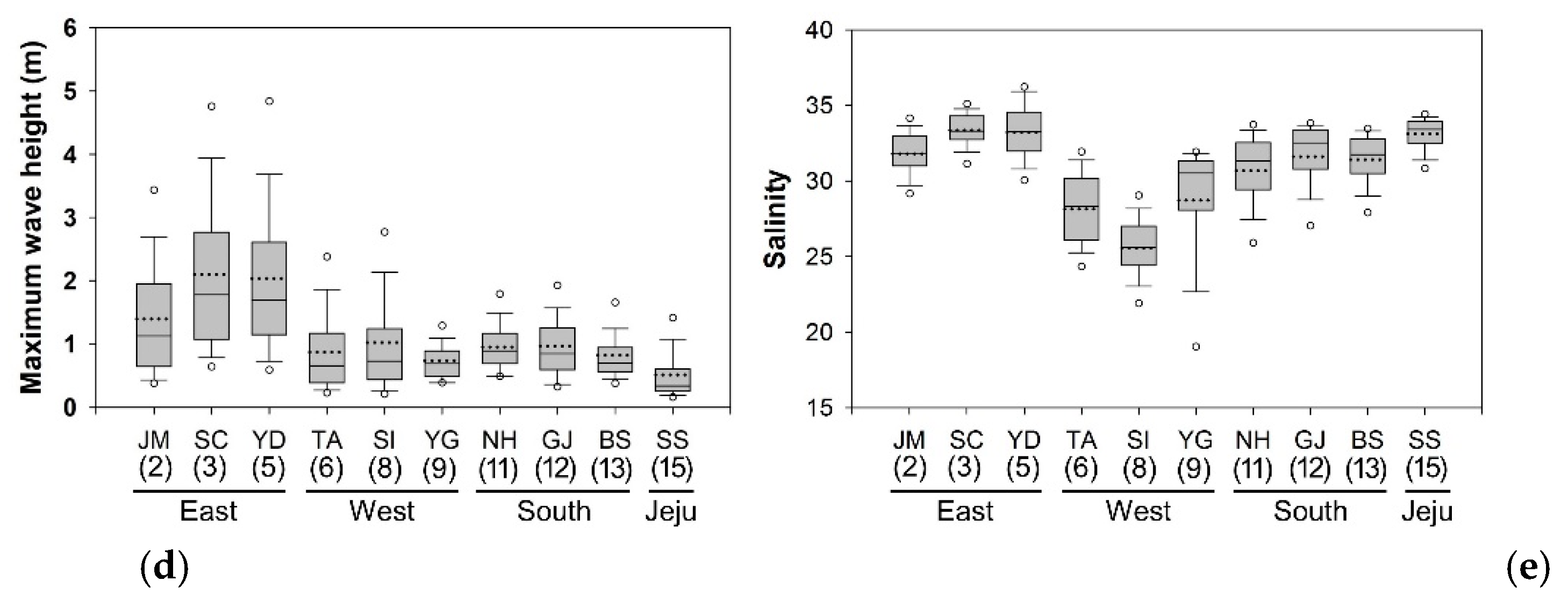
3.1. Environmental Parameters
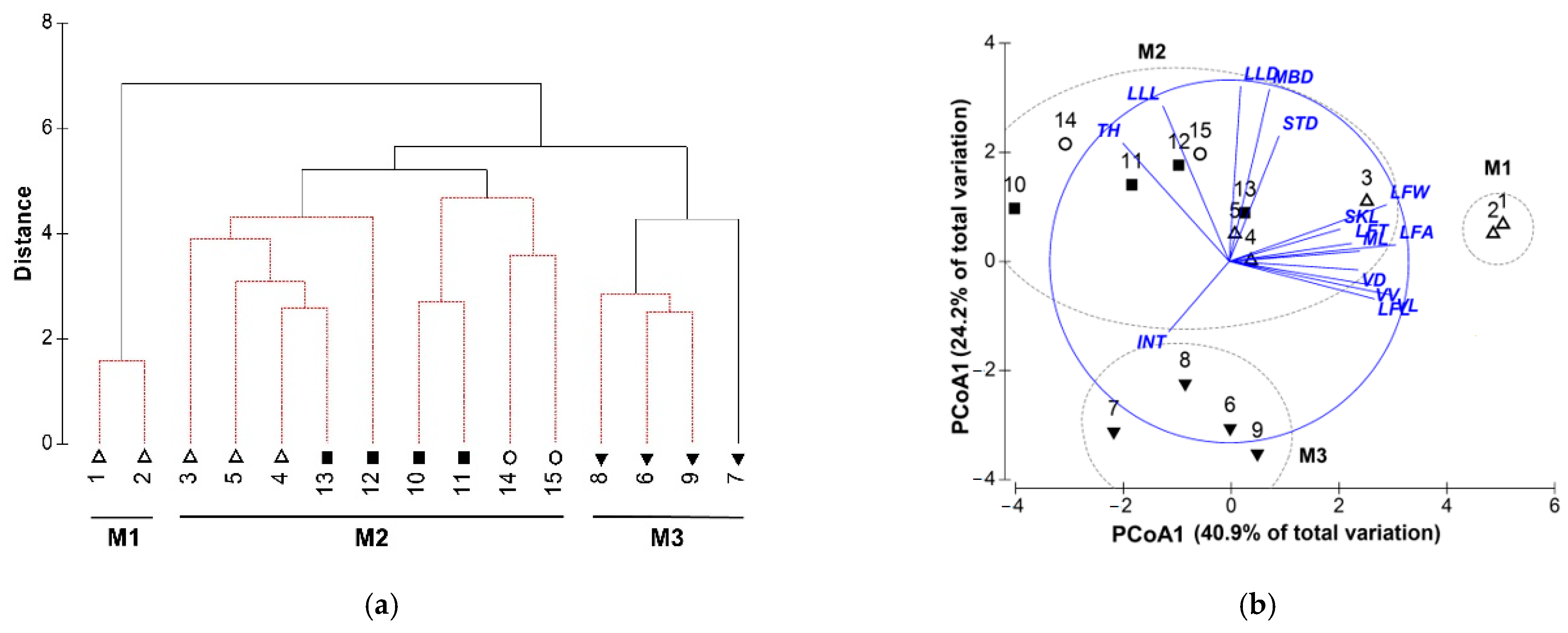
3.2. Morphological Variations
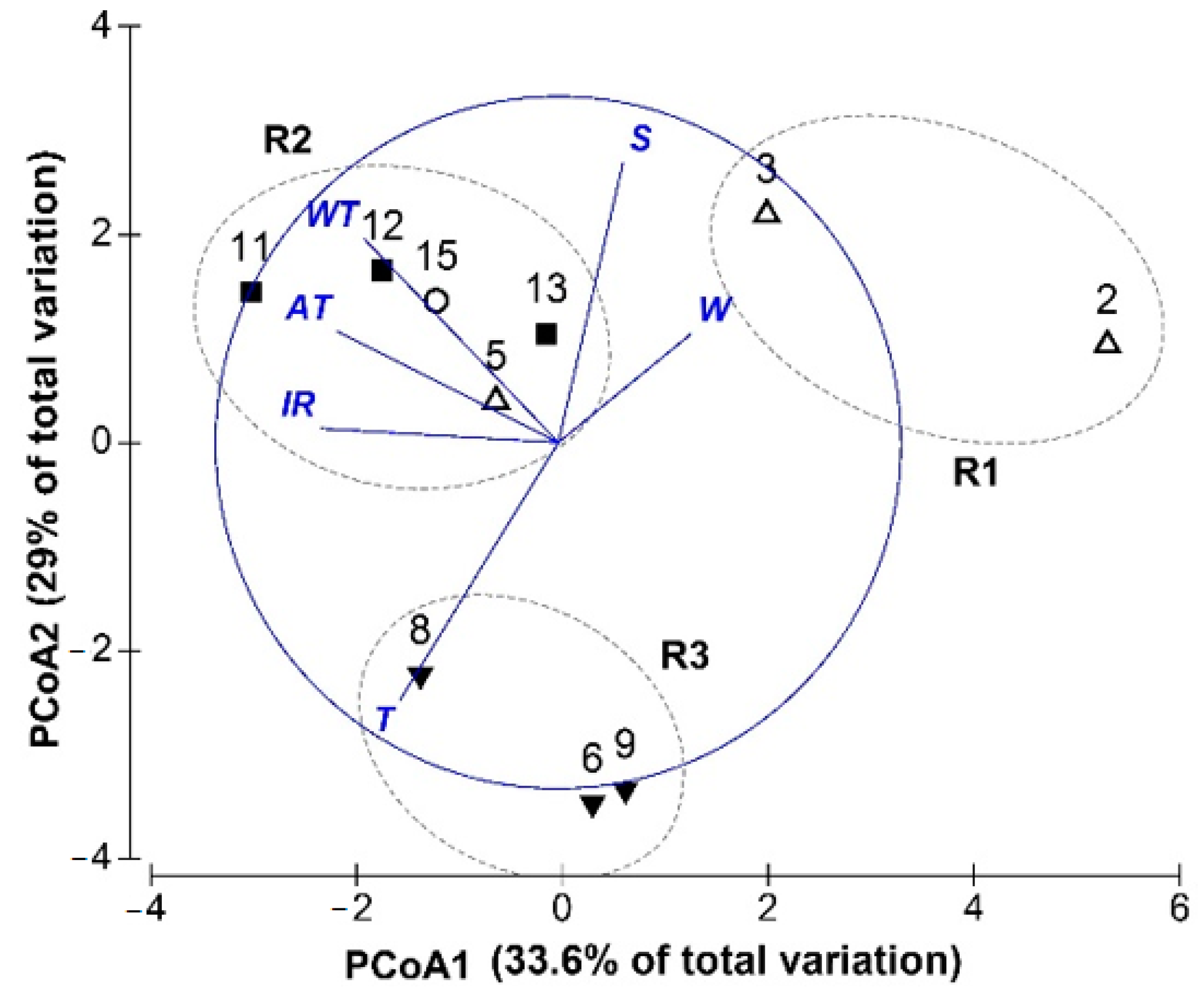
3.3. Relationships between Morphological Variations and Environments
4. Discussion
4.1. Relationships between Morphological Variation and Environments
4.2. Environmental Factors in the Intertidal Zone
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A

References
- Slatkin, M. Gene flow and the geographic structure of natural populations. Science 1987, 236, 787–792. [Google Scholar] [CrossRef] [PubMed]
- Blanchette, C.A.; Miner, B.G.; Gaines, S.D. Geographic variability in form, size and survival of Egregia menziesii around Point Conception, California. Mar. Ecol. Prog. Ser. 2002, 239, 69–82. [Google Scholar] [CrossRef]
- Fowler-Walker, M.J.; Gillanders, B.M.; Connell, S.D.; Irving, A.D. Patterns of association between canopy-morphology and understorey assemblages across temperate Australia. Estuar. Coast. Shelf. Sci. 2005, 63, 133–141. [Google Scholar] [CrossRef]
- Cacabelos, E.; Olabarria, C.; Incera, M.; Troncoso, J.S. Effects of habitat structure and tidal height on epifaunal assemblages associated with macroalgae. Estuar. Coast. Shelf. Sci. 2010, 89, 43–52. [Google Scholar] [CrossRef]
- Wernberg, T.; Vanderklift, M.A. Contribution of temporal and spatial components to morphological variations in the kelp Ecklonia (Laminariales). J. Phycol. 2010, 46, 153–161. [Google Scholar] [CrossRef]
- Littler, M.M.; Littler, D.S. The evolution of thallus form and survival strategies in benthic marine macroalgae: Field and laboratory tests of a functional form model. Am. Nat. 1980, 116, 25–44. [Google Scholar] [CrossRef]
- Duggins, D.O.; Eckman, J.E.; Siddon, C.E.; Klinger, T. Population, morphometric and biomechanical studies of three understory kelps along a hydrodynamic gradient. Mar. Ecol. Prog. Ser. 2003, 265, 57–76. [Google Scholar] [CrossRef][Green Version]
- Roberson, M.L.; Coyer, J.A. Variation in blade morphology of the kelp Eisenia arborea: Incipient speciation due to local water motion? Mar. Ecol. Prog. Ser. 2004, 282, 115–128. [Google Scholar] [CrossRef]
- Benedetti-Cecchi, L.; Bertocci, I.; Vaselli, S.; Maggi, E. Morphological plasticity and variable spatial patterns in different populations of the red alga Rissoella verrucosa. Mar. Ecol. Prog. Ser. 2006, 315, 87–98. [Google Scholar] [CrossRef][Green Version]
- Charrier, B.; Le Bail, A.; de Reviers, B. Plant Proteus: Brown algal morphological plasticity and underlying developmental mechanisms. Trends Plant. Sci. 2012, 17, 468–477. [Google Scholar] [CrossRef]
- Gerard, V.A.; Mann, K.H. Growth and production of Laminaria longicruris (Phaeophyta) populations exposed to different intensities of water movement. J. Phycol. 1979, 15, 33–41. [Google Scholar] [CrossRef]
- Falace, A.; Bressan, G. Seasonal variations of Cystoseira barbata (Stackhouse) C. Agardh frond architecture. Hydrobiologia 2006, 555, 193–206. [Google Scholar] [CrossRef]
- Engelen, A.H.; Åberg, P.; Olsen, J.L.; Stam, W.T.; Breeman, A.M. Effects of wave exposure and depth on biomass, density and fertility of the fucoid seaweed Sargassum polyceratium (Phaeophyta, Sargassaceae). Eur. J. Phycol. 2005, 40, 149–158. [Google Scholar] [CrossRef]
- Stewart, H. Morphological variation and phenotypic plasticity of buoyancy in the macroalga Turbinaria ornata across a barrier reef. Mar. Biol. 2006, 149, 721–730. [Google Scholar] [CrossRef]
- Malta, E.-J.; Ferreira, D.G.; Vergara, J.J.; Pérez-Lloréns, L. Nitrogen load and irradiance affect morphology, photosynthesis and growth of Caulerpa prolifera (Bryopsidales: Chlorophyta). Mar. Ecol. Prog. Ser. 2005, 298, 101–114. [Google Scholar] [CrossRef]
- D’Amours, O.; Scheibling, R.E. Effect of wave exposure on morphology, attachment strength and survival of the invasive green alga Codium fragile ssp. tomentosoides. J. Exp. Mar. Biol. Ecol. 2007, 351, 129–142. [Google Scholar] [CrossRef]
- Toth, G.B.; Harrysson, H.; Wahlström, N.; Olsson, J.; Oerbekke, A.; Steinhagen, S.; Kinnby, A.; White, J.; Albers, E.; Edlund, U.; et al. Effects of irradiance, temperature, nutrients, and pCO2 on the growth and biochemical composition of cultivated Ulva fenestrata. J. Appl. Phycol. 2020, 32, 3243–3254. [Google Scholar] [CrossRef]
- Monro, K.; Poore, A.G.B.; Brooks, R. Multivariate selection shapes environment-dependent variation in the clonal morphology of a red seaweed. Evol. Ecol. 2007, 21, 765–782. [Google Scholar] [CrossRef]
- Vettori, D.; Nikora, V.; Biggs, H. Implications of hyposaline stress for seaweed morphology and biomechanics. Aquat. Bot. 2020, 162, 103188. [Google Scholar] [CrossRef]
- Kalvas, A.; Kautsky, L. Morphological variation in Fucus vesiculosus populations along temperature and salinity gradients in Iceland. J. Mar. Biol. Assoc. U. K. 1998, 78, 985–1001. [Google Scholar] [CrossRef]
- Kübler, J.E.; Dudgeon, S.R. Temperature dependent change in the complexity of form of Chondrus crispus fronds. J. Exp. Mar. Biol. Ecol. 1996, 207, 15–24. [Google Scholar] [CrossRef]
- Blanchette, C.A. Size and survival of intertidal plants in response to wave action: A case study with Fucus gardneri. Ecology 1997, 78, 1563–1578. [Google Scholar] [CrossRef]
- Ruuskanen, A.; Bäck, S.; Reitalu, T. A comparison of two cartographic exposure methods using Fucus vesiculosus as an indicator. Mar. Biol. 1999, 134, 139–145. [Google Scholar] [CrossRef]
- Fowler-Walker, M.J.; Wernberg, T.; Connell, S.D. Differences in kelp morphology between wave sheltered and exposed localities: Morphologically plastic or fixed traits? Mar. Biol. 2006, 148, 755–767. [Google Scholar] [CrossRef]
- Kilar, J.A.; McLachlan, J. Branching morphology as an indicator of environmental disturbance: Testing the vegetative fragmentation of Acanthophora spicifera and the turf morphology of Laurencia papillosa. Aquat. Bot. 1986, 24, 115–130. [Google Scholar] [CrossRef]
- Yñiguez, A.T.; McManus, J.W.; Collado-Vides, L. Capturing the dynamics in benthic structures: Environmental effects on morphology in the macroalgal genera Halimeda and Dictyota. Mar. Ecol. Prog. Ser. 2010, 411, 17–32. [Google Scholar] [CrossRef]
- Miner, B.G.; Sultan, S.E.; Morgan, S.G.; Padilla, D.K.; Relyea, R.A. Ecological consequences of phenotypic plasticity. Trends Ecol. Evol. 2005, 20, 685–692. [Google Scholar] [CrossRef]
- Balata, D.; Piazzi, L.; Rindi, F. Testing a new classification of morphological functional groups of marine macroalgae for the detection of responses to stress. Mar. Biol. 2011, 158, 2459–2469. [Google Scholar] [CrossRef]
- Hurd, C.L. Water motion, marine macroalgal physiology, and production. J. Phycol. 2000, 36, 453–472. [Google Scholar] [CrossRef]
- Hay, M.E. The functional morphology of turf-forming seaweeds: Persistence in stressful marine habitats. Ecology 1981, 62, 739–750. [Google Scholar] [CrossRef]
- Gylle, A.M.; Nygård, C.A.; Ekelund, N.G.A. Desiccation and salinity effects on marine and brackish Fucus vesiculosus L. (Phaeophyceae). Phycologia 2009, 48, 156–164. [Google Scholar] [CrossRef]
- Wernberg, T.; Thomsen, M.S. The effect of wave exposure on the morphology of Ecklonia radiata. Aquat. Bot. 2005, 83, 61–70. [Google Scholar] [CrossRef]
- Boundouresque, C.F. Taxonomy and Phylogeny of Unicellular Eukaryotes. In Environmental Microbiology: Fundamentals and Applications; Bertrand, J.C., Caumette, P., Lebaron, P., Matheron, R., Normand, P., Sime-Ngando, T., Eds.; Springer: Dordrecht, The Netherlands, 2015; pp. 191–257. [Google Scholar] [CrossRef]
- Liu, F.-L.; Li, J.-J.; Liang, Z.-R.; Zhang, Q.-S.; Zhao, F.-J.; Jueterbock, A.; Critchley, A.T.; Morrell, S.L.; Assis, J.; Tang, Y.-Z.; et al. A concise review of the brown seaweed Sargassum thunbergia—A knowledge base to inform large-scale cultivation efforts. J. Appl. Phycol. 2021, 33, 3469–3482. [Google Scholar] [CrossRef]
- Umezaki, I. Ecological studies of Sargassum thunbergii (Mertens) O. Kuntze in Maizuru Bay, Japan Sea. Bot. Mag. Tokyo 1974, 87, 285–292. [Google Scholar] [CrossRef]
- Zhang, Q.S.; Li, W.; Liu, S.; Pan, J.H. Size-dependence of reproductive allocation of Sargassum thunbergii (Sargassaceae, Phaeophyta) in Bohai Bay, China. Aquat. Bot. 2009, 91, 194–198. [Google Scholar] [CrossRef]
- Cho, S.M.; Lee, S.M.; Ko, Y.D.; Mattio, L.; Boo, S.M. Molecular systematic reassessment of Sargassum (Fucales, Phaeophyceae) in Korea using four gene regions. Bot. Mar. 2012, 55, 473–484. [Google Scholar] [CrossRef]
- Yendo, K. The Fucaceae of Japan. J. Coll. Sci. Tokyo Imp. Univ. 1907, 21, 1–174. [Google Scholar]
- Okamura, K. Icones of Japanese Algae; Kazamashobo: Tokyo, Japan, 1923; Volume V, pp. 6–8. [Google Scholar]
- Oak, J.H.; Lee, I.K. Taxonomy of the genus Sargassum (Fucales, Phaeophyceae) from Korea I. Subgenus Bactrophycus Section Teretia. Algae 2005, 20, 77–90. [Google Scholar] [CrossRef]
- Chu, S.H.; Zhang, Q.S.; Liu, S.K.; Tang, Y.Z.; Zhang, S.B.; Lu, Z.C.; Yu, Y.Q. Tolerance of Sargassum Thunbergii germlings to thermal, osmotic and desiccation stress. Aquat. Bot. 2012, 96, 1–6. [Google Scholar] [CrossRef]
- Kang, J.Y.; Khan, M.N.A.; Park, N.H.; Cho, J.Y.; Lee, M.C.; Fujii, H.; Hong, Y.K. Antipyretic, analgesic, and anti-inflammatory activities of the seaweed Sargassum fulvellum and Sargassum thunbergii in mice. J. Ethnopharmacol. 2008, 116, 187–190. [Google Scholar] [CrossRef]
- Kim, J.-A.; Karadeniz, F.; Ahn, B.-N.; Kwon, M.S.; Mun, O.-J.; Bae, M.J.; Seo, Y.; Kim, M.; Lee, S.-H.; Kim, Y.Y.; et al. Bioactive quinone derivatives from the marine brown alga Sargassum thunbergii induce anti-adipogenic and pro-osteoblastogenic activities. J. Sci. Food Agric. 2015, 96, 783–790. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Wernberg, T.; de Bettignies, T.; Holmer, M.; Li, K.; Wu, J.; Lin, F.; Yu, Y.; Xu, J.; Zhou, C.; et al. Screening of seaweeds in the East China Sea as potential bio-monitors of heavy metals. Environ. Sci. Pollut. Res. 2018, 25, 16640–16651. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.Q.; Zhang, Q.S.; Tang, Y.Z.; Zhang, S.B.; Lu, Z.C.; Chu, S.H.; Tang, X.X. Establishment of intertidal seaweed beds of Sargassum thunbergii through habitat creation and germling seeding. Ecol. Eng. 2012, 44, 10–17. [Google Scholar] [CrossRef]
- Li, X.M.; Zhang, Q.S.; Tang, Y.Z.; Yu, Y.Q.; Liu, H.L.; Li, L.X. Highly efficient photoprotective responses to high light stress in Sargassum thunbergii germlings, a representative brown macroalga of intertidal zone. J. Sea Res. 2014, 85, 491–498. [Google Scholar] [CrossRef]
- Liang, Z.; Wang, F.; Sun, X.; Wang, W.; Liu, F. Reproductive biology of Sargassum thunbergii (Fucales, Phaeophyceae). Am. J. Plant. Sci. 2014, 5, 2574–2581. [Google Scholar] [CrossRef]
- Stiger, V.; Horiguchi, T.; Yoshida, T.; Coleman, A.W.; Masuda, M. Phylogenetic relationships within the genus Sargassum (Fucales, Phaeophyceae), inferred from ITS-2 nrDNA, with an emphasis on the taxonomic subdivision of the genus. Phycol. Res. 2003, 51, 1–10. [Google Scholar] [CrossRef]
- Zhao, F.; Wang, X.; Liu, J.; Duan, D. Population genetic structure of Sargassum thunbergii (Fucales, Phaeophyta) detected by RAPD and ISSR markers. J. Appl. Phycol. 2007, 19, 409–416. [Google Scholar] [CrossRef]
- Li, J.-J.; Hu, Z.-M.; Gao, X.; Sun, Z.-M.; Choi, H.-G.; Duan, D.-L.; Endo, H. Oceanic currents drove population genetic connectivity of the brown alga Sargassum thunbergii in the north-west Pacific. J. Biogeogr. 2017, 44, 230–242. [Google Scholar] [CrossRef]
- Gong, Y.; Suh, Y.-S.; Seong, K.-T.; Han, I.-S. Climate Change and Marine Ecosystem; Academy Book: Seoul, Korea, 2010; pp. 39–64. [Google Scholar]
- Carruthers, T.J.B.; Longstaff, B.J.; Dennison, W.C.; Abal, E.G.; Aioi, K. Measurement of Light Penetration in Relation to Seagrass. In Global Seagrass Research Motheds; Short, F.T., Coles, R.G., Short, C.A., Eds.; Elsevier Science: Amsterdam, The Netherlands, 2001; pp. 369–392. [Google Scholar]
- Clarke, K.R.; Gorley, R.N. PRIMER Version 6: User Manual/Tutorial; Primer-E Ltd.: Plymouth, UK, 2006. [Google Scholar]
- Umanzor, S.; Ladah, L.; Calderon-Aguilera, L.E.; Zertuche-González, J.A. Testing the relative importance of intertidal seaweeds as ecosystem engineers across tidal heights. J. Exp. Mar. Biol. Ecol. 2019, 511, 100–107. [Google Scholar] [CrossRef]
- Anderson, M.J.; Gorley, R.N.; Clarke, K.R. PERMANOVA+ for PRIMER: Guide to Software and Statistical Methods; Primer-E Ltd.: Plymouth, UK, 2008. [Google Scholar]
- Taylor, R.B.; Sotka, E.; Hay, M.E. Tissue-specific induction of herbivore resistance: Seaweed response to amphipod grazing. Oecologia 2002, 132, 68–76. [Google Scholar] [CrossRef]
- Diaz-Pulido, G.; Villamil, L.; Almanza, V. Herbivory effects on the morphology of the brown alga Padina boergesenii (Phaeophyta). Phycologia 2007, 46, 131–136. [Google Scholar] [CrossRef]
- Mueller, R.; Fischer, A.M.; Bolch, C.J.; Wright, J.T. Environmental correlates of phenotypic variation: Do variable tidal regimes influence morphology in intertidal seaweeds? J. Phycol. 2015, 51, 859–871. [Google Scholar] [CrossRef] [PubMed]
- Pearson, G.A.; Davison, I.R. Freezing rate and duration determine the physiological response of intertidal fucoids to freezing. Mar. Biol. 1993, 115, 353–362. [Google Scholar] [CrossRef]
- Dudgeon, S.R.; Kübler, J.E.; Vadas, R.L.; Davison, I.R. Physiological responses to environmental variation in intertidal red algae: Does thallus morphology matter? Mar. Ecol. Prog. Ser. 1995, 117, 193–206. [Google Scholar] [CrossRef]
- Liu, F.; Pang, S.J. Stress tolerance and antioxidant enzymatic activities in the metabolisms of the reactive oxygen species in two intertidal red algae Grateloupia turuturu and Palmaria palmata. J. Exp. Mar. Biol. Ecol. 2010, 382, 82–87. [Google Scholar] [CrossRef]
- Norton, T.A.; Mathieson, A.C.; Neushul, M. Morphology and Environment. In The Biology of Seaweeds; Lobban, C.S., Wynne, M.J., Eds.; University of California Press: Oakland, CA, USA, 1981; pp. 421–451. [Google Scholar]
- Sjøtun, K.; Fredriksen, S.; Rueness, J. Effect of canopy biomass and wave exposure on growth in Laminaria hyperborea (Laminariaceae: Phaeophyta). Eur. J. Phycol. 1998, 33, 337–343. [Google Scholar] [CrossRef]
- De Paula, E.J.; de Oliveira F°, E.C. Wave exposure and ecotypical differentiation in Sargassum cymosum (Phaeophyta-Fucales). Phycologia 1982, 21, 145–153. [Google Scholar] [CrossRef]
- Viejo, R.M.; Arrontes, J.; Andrew, N.L. An experimental evaluation of the effect of wave action on the distribution of Sargassum muticum in northern Spain. Bot. Marina 1995, 38, 437–442. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, J.H.; Li, J.Q.; Zhang, Y.T.; Sui, H.D.; Wu, W.G.; Niu, Y.L.; Gao, Z.K. The growth characteristics of long-line cultured seaweed Sargassum thunbergii in the Sanggou Bay. Prog. Fish Sci. 2016, 37, 120–126. [Google Scholar]
- Sideman, E.J.; Mathieson, A.C. Morphological variation within and between natural populations of non-tide pool Fucus distichus (Phaeophyta) in New England. J. Phycol. 1985, 21, 250–257. [Google Scholar] [CrossRef]
- Stengel, D.B.; Dring, M.J. Morphology and in situ growth rates of plants of Ascophyllum nodosum (Phaeophyta) from different shore levels and responses of plants to vertical transplantation. Eur. J. Phycol. 1997, 35, 193–202. [Google Scholar] [CrossRef][Green Version]

| Morphological Characteristics | Code | Group M1 | Group M2 | Group M3 |
|---|---|---|---|---|
| Thallus | ||||
| Thallus height (cm) *** | TH | 27.8 ± 1.1 c | 68.3 ± 2.7 a | 35.2 ± 1.3 b |
| Main branch diameter (mm) *** | MBD | 2.0 ± 0.1 a | 2.0 ± 0.1 a | 1.2 ± 0.1 b |
| Stipe length (mm) NS | STL | 4.1 ± 0.2 a | 3.7 ± 0.2 a | 3.5 ± 0.2 a |
| Stipe diameter (mm) *** | STD | 6.6 ± 0.3 a | 4.9 ± 0.2 b | 3.2 ± 0.1 c |
| Longest lateral branch length (cm) *** | LLL | 4.4 ± 0.4 b | 9.8 ± 0.5 a | 3.2 ± 0.1 c |
| Longest lateral branch diameter (mm) *** | LLD | 1.1 ± 0.0 b | 1.2 ± 0.0 a | 0.4 ± 0.0 c |
| Internode interval (mm) *** | INT | 2.0 ± 0.0 c | 4.2 ± 0.2 b | 4.9 ± 0.2 a |
| Leaf | ||||
| Leaf length (mm) *** | LFL | 9.2 ± 0.2 a | 6.0 ± 0.2 c | 6.7 ± 0.2 b |
| Leaf width (mm) *** | LFW | 0.8 ± 0.0 a | 0.5 ± 0.0 b | 0.4 ± 0.0 c |
| Leaf thickness (mm) ** | LFT | 0.41 ± 0.0 a | 0.36 ± 0.0 b | 0.34 ± 0.0 b |
| Leaf area (mm2) *** | LFA | 7.2 ± 0.2 a | 3.1 ± 0.2 b | 2.9 ± 0.1 b |
| Vesicle | ||||
| Air-vesicle length (mm) *** | VL | 2.9 ± 0.1 a | 2.2 ± 0.0 b | 2.4 ± 0.1 b |
| Air-vesicle diameter (mm) * | VD | 1.0 ± 0.0 a | 0.9 ± 0.0 b | 0.9 ± 0.0 b |
| Air-vesicle volume (mm3) *** | VV | 6.7 ± 0.4 a | 4.1 ± 0.2 b | 4.6 ± 0.4 b |
| Stalk length (mm) *** | SKL | 3.1 ± 0.1 a | 2.4 ± 0.1 b | 2.2 ± 0.1 b |
| Mucro length (mm) *** | ML | 1.3 ± 0.1 a | 0.9 ± 0.0 b | 1.0 ± 0.1 b |
| Number of Variables | Best Combinations of Environmental Variables |
|---|---|
| 1 | T (0.342), IR (0.339), AT (0.233), WT (0.182), S (0.099), W (0.002) |
| 2 | IR+T (0.464) 5, AT+T (0.397), AT+IR (0.393), WT+T (0.390) |
| 3 | AT+IR+T (0.519) 2, WT+IR+T (0.519) 2, AT+WT+T (0.440) |
| 4 | AT+WT+IR+T (0.556) 1, AT+S+IR+T (0.446), WT+S+IR+T (0.443) |
| 5 | AT+WT+S+IR+T (0.475) 4, AT+WT+IR+W+T (0.354) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.; Choi, S.K.; Van, S.; Kim, S.T.; Kang, Y.H.; Park, S.R. Geographic Differentiation of Morphological Characteristics in the Brown Seaweed Sargassum thunbergii along the Korean Coast: A Response to Local Environmental Conditions. J. Mar. Sci. Eng. 2022, 10, 549. https://doi.org/10.3390/jmse10040549
Kim S, Choi SK, Van S, Kim ST, Kang YH, Park SR. Geographic Differentiation of Morphological Characteristics in the Brown Seaweed Sargassum thunbergii along the Korean Coast: A Response to Local Environmental Conditions. Journal of Marine Science and Engineering. 2022; 10(4):549. https://doi.org/10.3390/jmse10040549
Chicago/Turabian StyleKim, Sangil, Sun Kyeong Choi, Seohyeon Van, Seong Taek Kim, Yun Hee Kang, and Sang Rul Park. 2022. "Geographic Differentiation of Morphological Characteristics in the Brown Seaweed Sargassum thunbergii along the Korean Coast: A Response to Local Environmental Conditions" Journal of Marine Science and Engineering 10, no. 4: 549. https://doi.org/10.3390/jmse10040549
APA StyleKim, S., Choi, S. K., Van, S., Kim, S. T., Kang, Y. H., & Park, S. R. (2022). Geographic Differentiation of Morphological Characteristics in the Brown Seaweed Sargassum thunbergii along the Korean Coast: A Response to Local Environmental Conditions. Journal of Marine Science and Engineering, 10(4), 549. https://doi.org/10.3390/jmse10040549






