Effects of Early Thermal Environment on Growth, Age at Maturity, and Sexual Size Dimorphism in Arctic Charr
Abstract
1. Introduction
2. Materials and Methods
2.1. General Methods
2.2. Experimental Design
2.2.1. Experimental Phase 1
2.2.2. Experimental Phase 2
2.3. Data Analysis and Statistical Methods
3. Results
3.1. Mortality
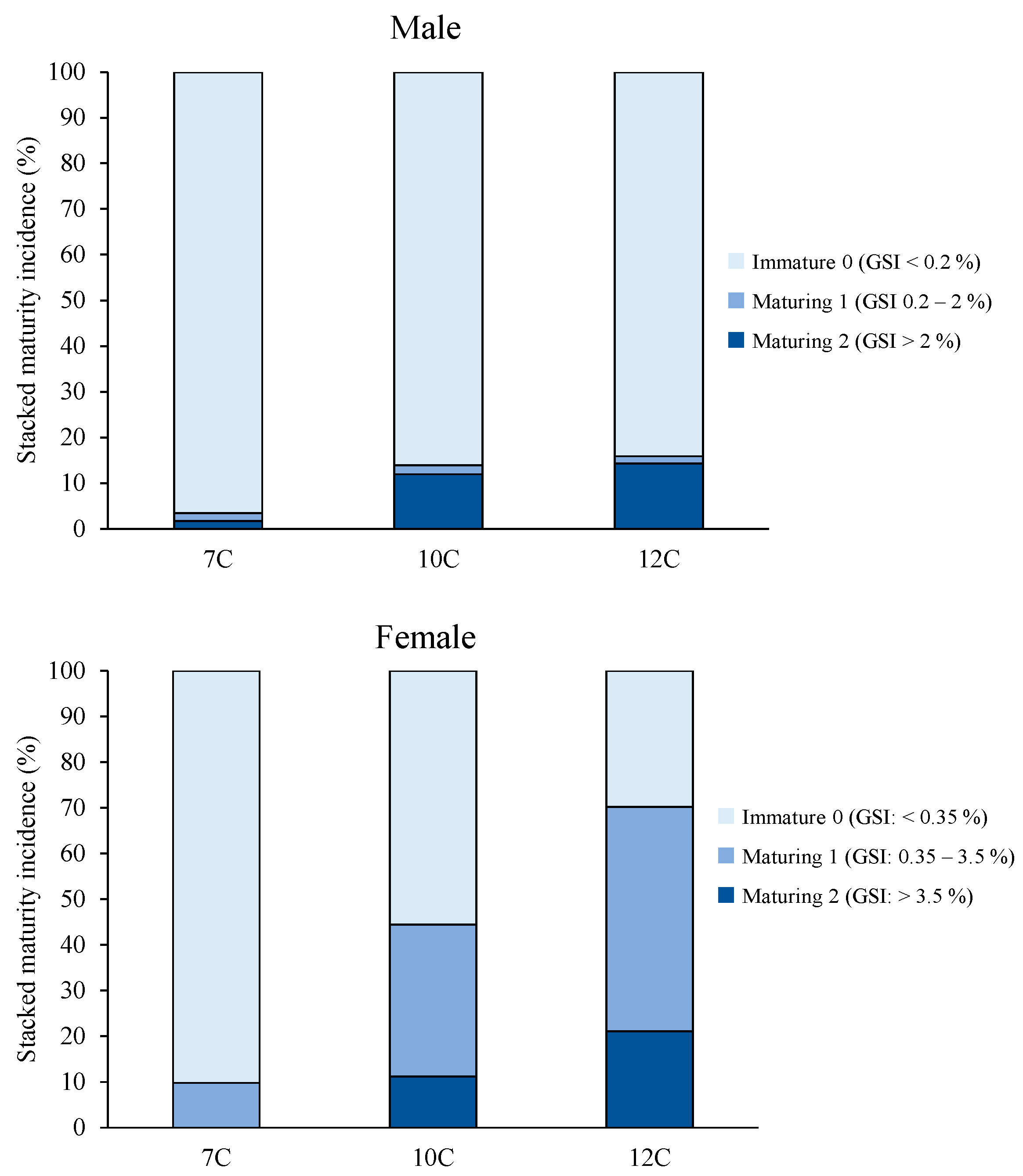
3.2. Growth, Puberty, and Sexual Size Dimorphism (SSD)
4. Discussion
4.1. Sexual Size Dimorphism (SSD)
4.2. Implications of the Experimental Set-Up
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brett, J.R. Environmental factors and growth. In Fish Physiology. Bioenergetics and Growth; Hoar, W.S., Randall, D.J., Brett, J.R., Eds.; Academic Press: New York, NY, USA, 1979; Volume 8, pp. 599–675. [Google Scholar]
- Little, A.G.; Seebacher, F. Physiological performance curves: When are they useful? Front. Physiol. 2021, 12, 805102. [Google Scholar] [CrossRef] [PubMed]
- Pavlov, D.A.; Moksness, E. Development of the axial skeleton in wolffish, Anarhichas lupus (Pisces, Anarhichadidae), at different temperatures. Environ. Biol. Fishes 1997, 49, 401–416. [Google Scholar] [CrossRef]
- Johnston, I.A.; Cole, N.J.; Abercromby, M.; Vieira, V.L.A. Embryonic temperature modulates muscle growth characteristics in larval and juvenile herring. J. Exp. Biol. 1998, 201, 623–646. [Google Scholar] [CrossRef] [PubMed]
- Kuparinen, A.; Cano, J.M.; Loehr, J.; Herczeg, G.; Gonda, A.; Merilä, J. Fish age at maturation is influenced by temperature independently of growth. Oceologia 2011, 167, 435–443. [Google Scholar] [CrossRef]
- Hooker, O.; Adams, C.; Chavarie, L. Arctic charr phenotypic responses to abrupt temperature change: An insight into how cold water fish could respond to extreme climatic events. bioRxiv 2020. [Google Scholar] [CrossRef]
- Pittman, K.; Yúfera, M.; Pavlidis, M.; Geffen, A.J.; Koven, W.; Ribeiro, L.; Zambonino-Infante, J.L.; Tandler, A. Fantastically plastic: Fish larvae equipped for a new world. Rev. Aquac. 2013, 5, 224–267. [Google Scholar] [CrossRef]
- Jonsson, B.; Jonsson, N. Phenotypic plasticity and epigenetics of fish: Embryo temperature affects later-developing life-history traits. Aquat. Biol. 2019, 28, 21–32. [Google Scholar] [CrossRef]
- Gavery, M.R.; Roberts, S.B. Epigenetic considerations in aquaculture. PeerJ 2017, 5, e4147. [Google Scholar] [CrossRef]
- Klemetsen, A. The charr problem revisited: Exceptional phenotypic plasticity promotes ecological speciation in postglacial lakes. Freshw. Rev. 2010, 3, 49–74. [Google Scholar] [CrossRef]
- Dempson, J.B.; Kristofferson, A.H. Spatial and temporal aspects of the ocean migration of anadromous Arctic char. Am. Fish. Soc. Symp. 1987, 1, 340–357. [Google Scholar]
- Jonsson, B.; Jonsson, N. Polymorphism and speciation in Arctic charr. J. Fish Biol. 2001, 58, 605–638. [Google Scholar] [CrossRef]
- Sigurjónsdóttir, H.; Gunnarsson, K. Alternative mating tactics of Arctic charr, Salvelinus alpinus, in Thingvallavatn, Iceland. Environ. Biol. Fishes 1989, 26, 159–176. [Google Scholar] [CrossRef]
- Brattli, M.B.; Egeland, T.B.; Nordeide, J.T.; Folstad, I. Spawning behavior of Arctic charr (Salvelinus alpinus): Spawning synchrony, vibrational communication, and mate guarding. Ecol. Evol. 2018, 8, 8076–8087. [Google Scholar] [CrossRef]
- Árnason, T.; Gunnarsson, S.; Imsland, A.K.; Thorarensen, H.; Smáradóttir, H.; Steinarsson, A.; Gústavsson, A.; Johanssson, M.; Björnsson, B.T.H. Long-term rearing of Arctic charr Salvelinus alpinus under different salinity regimes at constant temperature. J. Fish Biol. 2014, 85, 1145–1162. [Google Scholar] [CrossRef]
- Gunnarsson, S.; Johansson, M.; Gústavsson, A.; Árnason, T.; Árnason, J.; Smáradóttir, H.; Björnsson, B.T.H.; Thorarensen, H.; Imsland, A.K. Effects of short-day treatment on long-term growth performance and maturation of farmed Arctic charr Salvelinus alpinus reared in brackish water. J. Fish Biol. 2014, 85, 1211–1226. [Google Scholar] [CrossRef]
- Nilsson, J.; Brännäs, E.; Eriksson, L.-O. The Swedish Arctic charr breeding programme. Hydrobiologia 2010, 650, 275–282. [Google Scholar] [CrossRef]
- Yossa, R.; Bardon-Albaret, A.; Chiasson, M.A.; Liu, Q.; Duston, J.; Manning, T.; Benfey, T.J. Controlling preharvest maturity in farmed Arctic char: A review from the Canadian perspective. J. World Aquac. Soc. 2019, 50, 894–907. [Google Scholar] [CrossRef]
- Fairbairn, D.J. Allometry for sexual size dimorphism: Testing two hypotheses for Rench’s rule in the water strider Aquarius remigis. Am. Nat. 2005, 166, 69–84. [Google Scholar] [CrossRef]
- Stillwell, R.C.; Fox, C.W. Environmental effects on sexual size dimorphism of a seed-feeding beetle. Oceologia 2007, 153, 273–280. [Google Scholar] [CrossRef]
- Starostová, Z.; Kubicka, L.; Kratochvíl, L. Macroevolutionary pattern of sexual size dimorphism in geckos corresponds to intraspecific temperature-induced variation. J. Evol. Biol. 2010, 23, 670–677. [Google Scholar] [CrossRef]
- Swift, D.R. The effect of temperature and oxygen on the growth rate of the Windermere charr (Salvelinus alpinus Willughbii). Comp. Biochem. Physiol. 1964, 12, 179–183. [Google Scholar] [CrossRef]
- Lyytikäinen, T.; Koskela, J.; Rissanen, I. The influence of temperature on growth and proximate body composition of under yearling lake Inari Arctic char (Salvelinus alpinus (L.)). J. Appl. Ichtyol. 1997, 13, 191–194. [Google Scholar] [CrossRef]
- Larsson, S.; Berglund, I. Growth and food consumption of 0+ Arctic charr fed pelleted or natural food at six different temperatures. J. Fish Biol. 1998, 52, 230–242. [Google Scholar] [CrossRef]
- Larsson, S.; Berglund, I. The effect of temperature on the energetic growth efficiency of Arctic charr (Salvelinus alpinus L.) from four Swedish populations. J. Therm. Biol. 2005, 30, 29–36. [Google Scholar] [CrossRef]
- Larsson, S.; Forseth, T.; Berglund, I.; Jensen, A.J.; Näslund, I.; Elliott, J.M.; Jonsson, B. Thermal adaptation of Arctic charr: Experimental studies of growth in eleven charr populations from Sweden, Norway and Britain. Freshw. Biol. 2005, 50, 353–368. [Google Scholar] [CrossRef]
- Beuvard, C.; Imsland, A.K.D.; Thorarensen, H. The effect of temperature on growth performance and aerobic metabolic scope in Arctic charr, Salvelinus alpinus (L.). J. Therm. Biol. 2021, 103117. [Google Scholar] [CrossRef]
- Pétursdóttir, Þ.; Eyþórsdóttir, E. Áhrif mismunandi hitastigs á vöxt og kynþroska bleikju. Eldisfréttir 1993, 9, 41–44. (In Icelandic) [Google Scholar]
- Gunnarsson, S.; Imsland, A.K.; Árnason, J.; Gústavsson, A.; Arnarson, I.; Jónsson, J.K.; Foss, A.; Stefansson, S.; Thorarensen, H. Effects of rearing temperatures on the growth and maturation of Arctic charr (Salvelinus alpinus) during juvenile and on-growing periods. Aquac. Res. 2011, 42, 221–229. [Google Scholar] [CrossRef][Green Version]
- Svavarsson, E. Árangur í kynbótum á bleikju og næstu skref. Fræðaþing landbúnaðarins 2007, 4, 121–125. (In Icelandic) [Google Scholar]
- Fjelldal, P.G.; Hansen, T.J.; Wargelius, A.; Ayllon, F.; Glover, K.A.; Schulz, R.W.; Fraser, T.W.K. Development of supermale and all-male Atlantic salmon to research the vgll3 allele–puberty link. BMC Genet. 2020, 21, 123. [Google Scholar] [CrossRef]
- Taranger, G.L.; Carrillo, M.; Schulz, R.W.; Fontaine, P.; Zanuy, S.; Felip, A.; Weltzien, F.-A.; Dufour, S.; Karlsen, Ø.; Norberg, B.; et al. Control of puberty in farmed fish. Gen. Comp. Endocrinol. 2010, 165, 483–515. [Google Scholar] [CrossRef]
- Jonsson, B.; Jonsson, N. Early environment influences later performance in fishes. J. Fish Biol. 2014, 85, 151–188. [Google Scholar] [CrossRef]
- Jonsson, B.; Jonsson, N.; Finstad, A.G. Linking embryonic temperature with adult reproductive investment in Atlantic salmon Salmo salar. Mar. Ecol. Prog. Ser. 2014, 515, 217–226. [Google Scholar] [CrossRef]
- Scott, G.R.; Johnston, I.A. Temperature during embryonic development has persistent effects on thermal acclimation capacity in zebrafish. Proc. Natl. Acad. Sci. USA 2012, 109, 14247–14252. [Google Scholar] [CrossRef]
- Schnurr, M.E.; Yin, Y.; Scott, G.R. Temperature during embryonic development has persistent effects on metabolic enzymes in the muscle of zebrafish. J. Exp. Biol. 2014, 217, 1370–1380. [Google Scholar] [CrossRef]
- Johnston, I.A.; Manthri, S.; Alderson, R.; Smart, A.; Campbell, P.; Nickell, D.; Robertson, B.; Paxton, C.G.M.; Burt, L. Freshwater environment affects growth rate and muscle fibre recruitment in seawater stages of Atlantic salmon (Salmo salar L.). J. Exp. Biol. 2003, 206, 1337–1351. [Google Scholar] [CrossRef]
- Macqueen, D.J.; Robb, D.H.F.; Olsen, T.; Melstveit, L.; Paxton, C.G.M.; Johnston, I.A. Temperature until the ‘eyed stage’ of embryogenesis programmes the growth trajectory and muscle phenotype of adult Atlantic salmon. Biol. Lett. 2008, 4, 294–298. [Google Scholar] [CrossRef]
- Burgerhout, E.; Mommens, M.; Johnsen, H.; Aunsmo, A.; Santi, N.; Andersen, Ø. Genetic background and embryonic temperature affect DNA methylation and expression of myogenin and muscle development in Atlantic salmon (Salmo salar). PLoS ONE 2017, 12, e0179918. [Google Scholar] [CrossRef]
- Craig, J.K.; Foote, C.J.; Wood, C.C. Evidence for temperature-dependent sex determination in sockeye salmon (Oncorhynchus nerka). Can. J. Fish. Aquat. Sci. 1996, 53, 141–147. [Google Scholar] [CrossRef]
- Imsland, A.K.D.; Gunnarsson, S.; Thorarensen, H. Impact of environmental factors on the growth and maturation of farmed Arctic charr. Rev. Aquac. 2020, 12, 1689–1707. [Google Scholar] [CrossRef]
- Schulte, P.M.; Healy, T.M.; Fangue, N.A. Thermal performance curves, phenotypic plasticity, and the time scales of temperature exposure. Integr. Comp. Biol. 2011, 51, 691–702. [Google Scholar] [CrossRef] [PubMed]
- Schulte, P.M. What is environmental stress? Insights from fish living in variable environment. J. Exp. Biol. 2014, 217, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Little, A.G.; Loughland, I.; Seebacher, F. What do warming waters mean for fish physiology and fisheries? J. Fish Biol. 2020, 97, 328–340. [Google Scholar] [CrossRef]
- Tveiten, H.; Mayer, I.; Johnsen, H.K.; Jobling, M. Sex steroids, growth and condition of Arctic charr broodstock during an annual cycle. J. Fish Biol. 1998, 53, 714–727. [Google Scholar] [CrossRef]
- Adams, C.E.; Huntingford, A. Growth, maturation and reproductive investment in Arctic charr. J. Fish Biol. 1997, 51, 750–759. [Google Scholar] [CrossRef]
- Tveiten, H.; Johnsen, H.K.; Jobling, M. Influence of maturity status on the annual cycles of feeding and growth in Arctic charr reared at constant temperature. J. Fish Biol. 1996, 48, 910–924. [Google Scholar] [CrossRef]
- Damsgård, B.; Arnesen, A.M.; Jobling, M. Seasonal patterns of feed intake and growth of Hammerfest and Svalbard Arctic charr maturing at different ages. Aquaculture 1999, 171, 149–160. [Google Scholar] [CrossRef]
- Fraser, T.W.K.; Hansen, T.J.; Remø, S.C.; Fjelldal, P.G. Triploidy effects growth, life history strategies, and bone health in Arctic char (Salvelinus alpinus), but does not impact cataract incidence. Aquaculture 2022, 547, 737465. [Google Scholar] [CrossRef]
- Jobling, M.; Baardvik, B.M. Patterns of growth of maturing and immature Arctic charr, Salvelinus alpinus, in a hatchery population. Aquaculture 1991, 94, 343–354. [Google Scholar] [CrossRef]
- Sæther, B.S.; Johnsen, H.K.; Jobling, M. Seasonal changes in food consumption and growth of arctic charr exposed to either simulated natural or a 12:12 LD photoperiod at constant water temperature. J. Fish Biol. 1996, 48, 1113–1122. [Google Scholar] [CrossRef]
- Liu, Q.; Duston, J. Long photoperiod in winter is more effective than food deprivation in stopping unwanted sexual maturation in Arctic charr. Aquaculture 2019, 501, 213–218. [Google Scholar] [CrossRef]
- Liu, Q.; Duston, J. Preventing sexual maturation in Arctic charr by 24h light overwinter and suppressing somatic growth. Aquaculture 2016, 464, 537–544. [Google Scholar] [CrossRef]
- Küttner, E.; Moghadam, H.K.; Skúlason, S.; Danzmann, R.G.; Ferguson, M.M. Genetic architecture of body weight, condition factor and age of sexual maturation in Icelandic Arctic charr (Salvelinus alpinus). Mol. Genet. Genom. 2011, 286, 67–79. [Google Scholar] [CrossRef]
- Rowe, D.K.; Thorpe, J.E. Differences in growth between maturing and non-maturing male Atlantic salmon, Salmo salar L., parr. J. Fish Biol. 1990, 36, 643–658. [Google Scholar] [CrossRef]
- Silverstein, J.T.; Shearer, K.D.; Dickhoff, W.W.; Plisetskaya, E.M. Effects of growth and fatness on sexual development of chinook salmon (Oncorhynchus tshawytscha) parr. Can. J. Aquat. Sci. 1998, 55, 2376–2382. [Google Scholar] [CrossRef]
- Sloat, M.R.; Reeves, G.H. Individual condition, standard metabolic rate, and rearing temperature influence steelhead and rainbow trout (Oncorhynchus mykiss) life histories. Can. J. Fish. Aquat. Sci. 2014, 71, 491–501. [Google Scholar] [CrossRef]
- Rice, C.D. Restricted Feeding, Spermatogenesis and Growth in Arctic Charr, Salvelinus alpinus: The Identification of Two Possible Gematogenic Control Points. Master’s Thesis, Memorial University of Newfoundland, St. John’s, NL, Canada, 1999. [Google Scholar]
- Duston, J.; Astaikie, T.; MacIsaac, P.F. Long-to-short photoperiod in winter halves the incidence of sexual maturity among Arctic charr. Aquaculture 2003, 221, 567–580. [Google Scholar] [CrossRef]
- Atse, C.B.; Audet, C.; de la Noüe, J. Effects of temperature and salinity on the reproductive success of Arctic charr, Salvelinus alpinus (L.): Egg composition, milt characteristics and fry survival. Aquac. Res. 2002, 33, 299–309. [Google Scholar] [CrossRef]
- Schreck, C.B. Stress and fish reproduction: The roles of allostasis and hormesis. Gen. Comp. Endocrinol. 2010, 165, 549–556. [Google Scholar] [CrossRef]
- Beverton, R.J.H.; Hylen, A.; Østvedt, O.J. Growth, maturation, and longevity of maturation cohorts of Northeast Arctic cod. ICES Mar. Sci. Symp. 1994, 198, 482–501. [Google Scholar]
- Beverton, R.J.H.; Hylen, A.; Østvedt, O.J.; Alsvaag, J.; Iles, T.C. Growth, maturation, and longevity of maturation cohorts of Norwegian spring-spawning herring. ICES J. Mar. Sci. 2004, 61, 165–175. [Google Scholar] [CrossRef]
- Atkinson, D. Temperature and organism size—A biological law for ectotherms? Adv. Ecol. Res. 1994, 25, 1–58. [Google Scholar] [CrossRef]
- Forster, J.; Hirst, A.G.; Atkinson, D. Warming-induced reductions in body size are greater in aquatic than terrestrial species. Proc. Natl. Acad. Sci. USA 2012, 109, 19310–19314. [Google Scholar] [CrossRef] [PubMed]
- Baudron, A.R.; Needle, C.L.; Rijnsdorp, A.D.; Marshall, C.T. Warming temperatures and smaller body sizes: Synchronous changes in growth of North Sea fishes. Glob. Change Biol. 2014, 20, 1023–1031. [Google Scholar] [CrossRef]
- Stamps, J.; Krishnan, V.V. Sexual bimaturation and sexual size dimorphism in animals with asymptotic growth after maturity. Ecol. Evol. 1997, 11, 21–39. [Google Scholar] [CrossRef]
- Zohar, Y. Fish reproductive biology—Reflecting on five decades of fundamental and translational research. Gen. Comp. Endocrinol. 2021, 300, 113544. [Google Scholar] [CrossRef]
- Tamate, T.; Maekawa, K. Latitudinal variation in sexual size dimorphism of sea-run Masu salmon, Oncorhynchus masaou. Evolution 2006, 60, 196–201. [Google Scholar] [CrossRef]
- Valiente, A.G.; Juanes, F.; Garcia-Vazquez, E. Reproductive strategies explain genetic diversity in Atlantic salmon, Salmo salar. Environ. Biol. Fishes 2005, 74, 323–334. [Google Scholar] [CrossRef]
- Estlander, S.; Kahilainen, K.K.; Horppila, J.; Olin, M.; Rask, M.; Kubecka, J.; Peterka, J.; Ríha, M.; Huuskonen, H. Latitudinal variation in sexual dimorphism in life-history traits of a freshwater fish. Ecol. Evol. 2017, 7, 665–673. [Google Scholar] [CrossRef]
- Morbey, Y.E. Female-biased dimorphism in size and age at maturity is reduced at higher latitudes in lake whitefish Coregonus clupeaformis. J. Fish Biol. 2008, 93, 40–46. [Google Scholar] [CrossRef]
- Roitberg, E.S.; Orlova, V.F.; Bulakhova, N.A.; Kuranova, V.N.; Eplanova, G.V.; Zinenko, O.I.; Arribas, O.; Kratochvíl, L.; Ljubisavljević, K.; Starikov, V.P.; et al. Variation in body size and sexual size dimorphism in the most widely ranging lizard: Testing the effects of reproductive mode and climate. Ecol. Evol. 2020, 10, 4531–4561. [Google Scholar] [CrossRef]
- Stillwell, R.C.; Fox, C.W. Geographic variation in body size, sexual size dimorphism and fitness components of a seed beetle: Local adaption versus phenotypic plasticity. Oikos 2009, 118, 703–712. [Google Scholar] [CrossRef]
- Stillwell, R.C.; Blanckenhorn, W.U.; Teder, T.; Davidowitz, G.; Fox, C.W. Sex differences in phenotypic plasticity affect variation in sexual size dimorphism in insects: From physiology to evolution. Annu. Rev. Entomol. 2010, 55, 227–245. [Google Scholar] [CrossRef]
- Roff, D. An allocation model of growth and reproduction in fish. Can. J. Fish. Aquat. Sci. 1983, 40, 1395–1404. [Google Scholar] [CrossRef]
- Mandiki, S.N.M.; Babiak, I.; Bopopi, J.M.; Leprieur, F.; Kestemont, P. Effects of sex steroids and their inhibitors on endocrine parameters and gender growth differences in Eurasian perch (Perca fluviatilis) juveniles. Steroids 2005, 70, 85–94. [Google Scholar] [CrossRef]
- Mizzau, T.W.; Garner, S.R.; Marklevitz, S.A.C.; Thompson, G.J.; Morbey, Y.E. A genetic test of sexual size dimorphism in pre-emergent chinook salmon. PLoS ONE 2013, 8, e78421. [Google Scholar] [CrossRef]





| Group | Maturation Stage | GSI Range | GSI (%) | n | W5 (g) | W10 (g) |
|---|---|---|---|---|---|---|
| Male | ||||||
| 7C | 0 | <0.2 | 0.10 ± 0.003 | 112 | 9.1 ± 0.31 | 1483 ± 39 |
| 7C | 1 | 0.2–2 | 0.44 ± 0.14 | 2 | 6.4 ± 1.0 | 1465 ± 145 |
| 7C | 2 | >2 | 7.6 ± 1.60 | 2 | 11.3 ± 2.9 | 600 ± 78 |
| 7C | Overall | 0.01–8.2 | 0.23 ± 0.20 | 116 | 9.17 ± 0.3 | 1478 ± 36 |
| 10C | 0 | <0.2 | 0.09 ± 0.002 | 223 | 11.5 ± 0.3 | 1564 ± 26 |
| 10C | 1 | 0.2–2 | 0.43 ± 0.07 | 5 | 12.0 ± 1.5 | 1254 ± 208 |
| 10C | 2 | >2 | 5.8 ± 0.34 | 31 | 12.0 ± 0.6 | 812 ± 61 |
| 10C | Overall | 0.01–8.1 | 0.77 ± 0.12 | 262 | 11.5 ± 0.3 | 1449 ± 28 |
| 12C | 0 | <0.2 | 0.09 ± 0.003 | 106 | 14.1 ± 0.4 | 1411 ± 34 |
| 12C | 1 | 0.2–2 | 0.37 ± 0.009 | 2 | 13.4 ± 1.0 | 1831 ± 39 |
| 12C | 2 | >2 | 6.3 ± 0.289 | 18 | 14.4 ± 1.0 | 810 ± 65 |
| 12C | Overall | 0.01–7.7 | 1.02 ± 0.20 | 126 | 14.0 ± 0.4 | 1326 ± 35 |
| Female | ||||||
| 7C | 0 | <0.35 | 0.24 ± 0.01 | 109 | 9.2 ± 0.3 | 1345 ± 33 |
| 7C | 1 | 0.35–3.5 | 0.45 ± 0.04 | 12 | 9.4 ± 1.1 | 950 ± 107 |
| 7C | 2 | >3.5 | - | 0 | - | - |
| 7C | Overall | 0.06–0.9 | 0.26 ± 0.001 | 121 | 9.1 ± 0.3 | 1306 ± 32 |
| 10C | 0 | <0.35 | 0.27 ± 0.002 | 144 | 11.2 ± 0.4 | 1372 ± 32 |
| 10C | 1 | 0.35–3.5 | 0.43 ± 0.007 | 86 | 11.8 ± 0.5 | 1107 ± 38 |
| 10C | 2 | >3.5 | 9.1 ± 0.62 | 29 | 12.8 ± 0.8 | 857 ± 68 |
| 10C | Overall | 0.12–14.4 | 1.31 ± 0.19 | 259 | 11.7 ± 0.3 | 1226 ± 24.4 |
| 12C | 0 | <0.35 | 0.29 ± 0.01 | 34 | 12.5 ± 0.6 | 1365 ± 55 |
| 12C | 1 | 0.35–3.5 | 0.43 ± 0.01 | 56 | 13.4 ± 0.5 | 1185 ± 48 |
| 12C | 2 | >3.5 | 10.2 ± 0.59 | 24 | 12.2 ± 0.7 | 626 ± 45 |
| 12C | Overall | 0.14–14.5 | 2.46 ± 0.40 | 114 | 12.8 ± 0.3 | 1113 ± 38 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Árnason, T.; Smáradóttir, H.; Thorarensen, H.; Steinarsson, A. Effects of Early Thermal Environment on Growth, Age at Maturity, and Sexual Size Dimorphism in Arctic Charr. J. Mar. Sci. Eng. 2022, 10, 167. https://doi.org/10.3390/jmse10020167
Árnason T, Smáradóttir H, Thorarensen H, Steinarsson A. Effects of Early Thermal Environment on Growth, Age at Maturity, and Sexual Size Dimorphism in Arctic Charr. Journal of Marine Science and Engineering. 2022; 10(2):167. https://doi.org/10.3390/jmse10020167
Chicago/Turabian StyleÁrnason, Tómas, Heiðdís Smáradóttir, Helgi Thorarensen, and Agnar Steinarsson. 2022. "Effects of Early Thermal Environment on Growth, Age at Maturity, and Sexual Size Dimorphism in Arctic Charr" Journal of Marine Science and Engineering 10, no. 2: 167. https://doi.org/10.3390/jmse10020167
APA StyleÁrnason, T., Smáradóttir, H., Thorarensen, H., & Steinarsson, A. (2022). Effects of Early Thermal Environment on Growth, Age at Maturity, and Sexual Size Dimorphism in Arctic Charr. Journal of Marine Science and Engineering, 10(2), 167. https://doi.org/10.3390/jmse10020167






