Morphometric Analyses of Phenotypic Plasticity in Habitat Use in Two Caspian Sea Mullets
Abstract
:1. Introduction
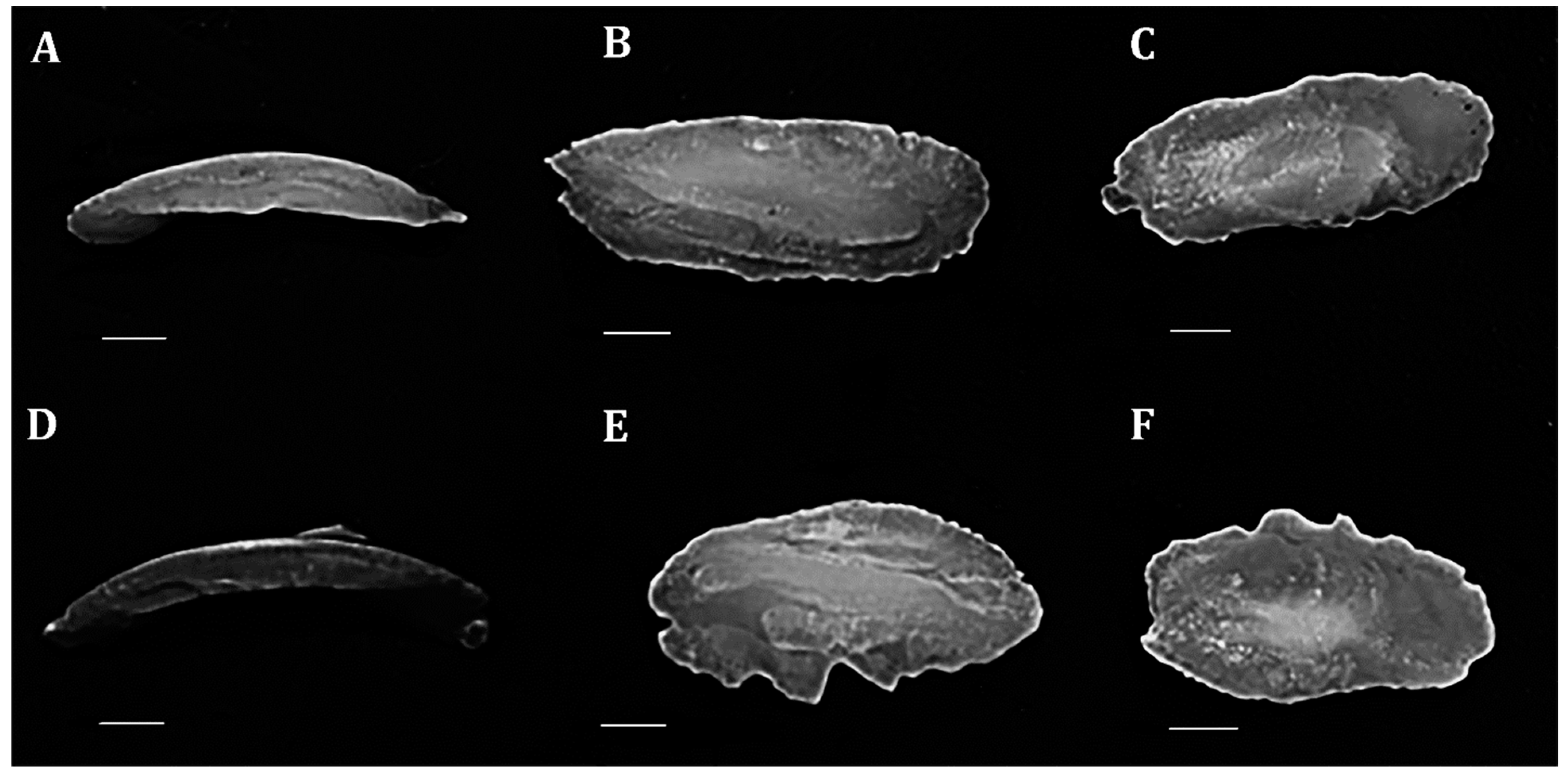
2. Materials and Methods
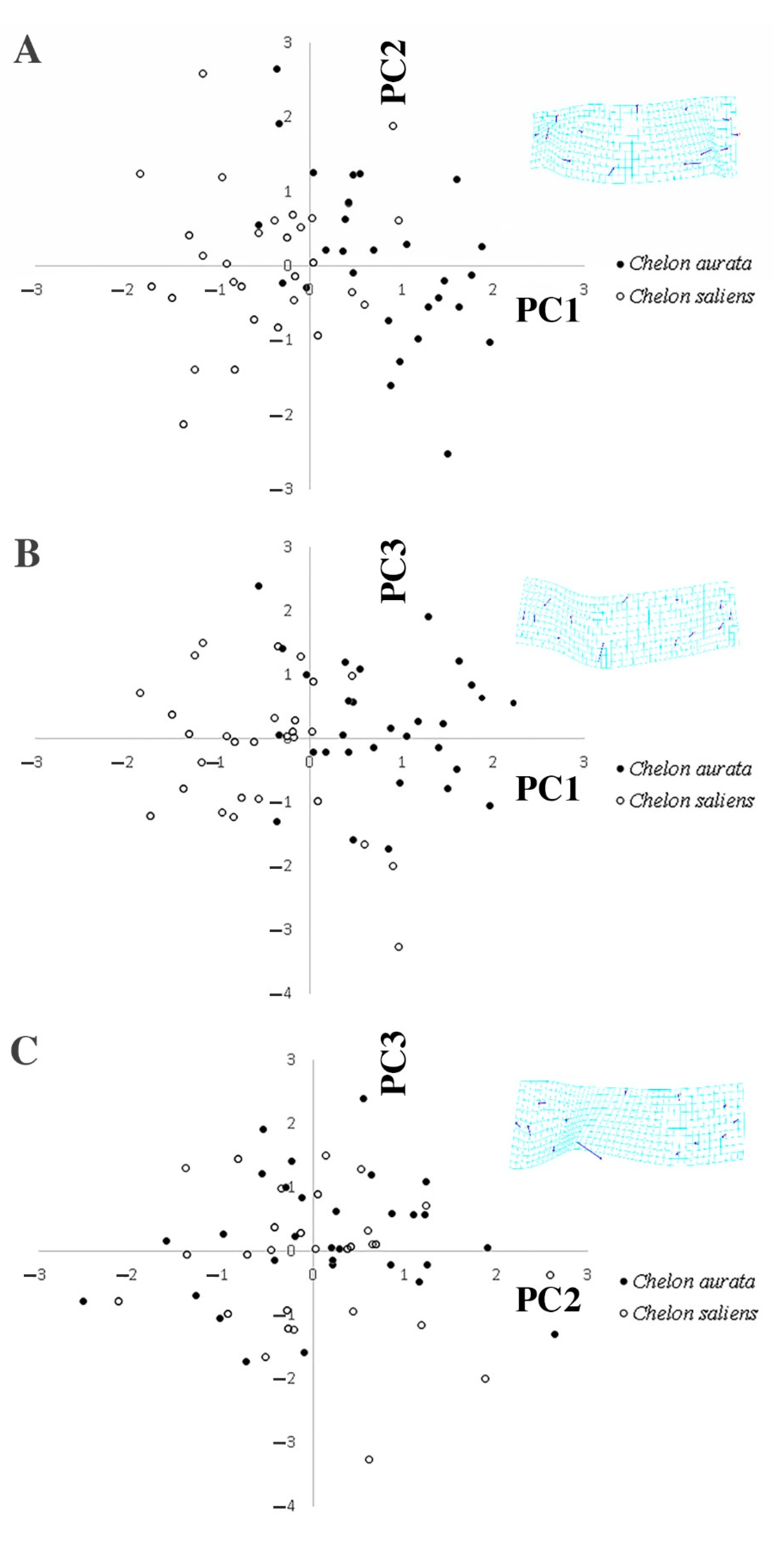
3. Results
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- D’iglio, C.; Natale, S.; Albano, M.; Savoca, S.; Famulari, S.; Gervasi, C.; Lanteri, G.; Panarello, G.; Spanò, N.; Capillo, G. Otolith Analyses Highlight Morpho-Functional Differences of Three Species of Mullet (Mugilidae) from Transitional Water. Sustainability 2022, 14, 398. [Google Scholar] [CrossRef]
- Elgueta, A.; Thoms, M.C.; Górski, K.; Díaz, G.; Habit, E.M. Functional process zones and their fish communities in temperate Andean river networks. River Res. Appl. 2019, 35, 1702–1711. [Google Scholar] [CrossRef]
- Kelley, J.L.; Grierson, P.F.; Collin, S.P.; Davies, P.M. Habitat disruption and the identification and management of functional trait changes. Fish Fish. 2018, 19, 716–728. [Google Scholar] [CrossRef]
- Weiskopf, S.R.; Rubenstein, M.A.; Crozier, L.G.; Gaichas, S.; Griffis, R.; Halofsky, J.E.; Hyde, K.J.W.; Morelli, T.L.; Morisette, J.T.; Muñoz, R.C.; et al. Climate change effects on biodiversity, ecosystems, ecosystem services, and natural resource management in the United States. Sci. Total Environ. 2020, 733, 137782. [Google Scholar] [CrossRef]
- Mattone, C.; Bradley, M.; Barnett, A.; Konovalov, D.A.; Sheaves, M. Environmental conditions constrain nursery habitat value in Australian sub-tropical estuaries. Mar. Environ. Res. 2022, 175, 105568. [Google Scholar] [CrossRef]
- Michaud, B.C.; Kilborn, J.P.; MacDonald, T.C.; Peebles, E.B. A description of Florida estuarine gradient complexes and the implications of habitat factor covariation for community habitat analysis. Estuar. Coast. Shelf Sci. 2022, 264, 107669. [Google Scholar] [CrossRef]
- Arai, T. Migration ecology in the freshwater eels of the genus Anguilla Schrank, 1798. Trop. Ecol. 2022, 63, 155–170. [Google Scholar] [CrossRef]
- Bakhshalizadeh, S.; Bani, A. Morphological analysis of pectoral fin spine for identifying ecophenotypic variation of Persian Sturgeon Acipenser persicus. Mar. Ecol. 2018, 39, e12516. [Google Scholar] [CrossRef]
- DeWitt, T.J.; Troendle, N.J.; Mateos, M.; Mauricio, R. Population genetics and independently replicated evolution of predator-associated burst speed ecophenotypy in mosquitofish. Heredity 2022, 128, 45–55. [Google Scholar] [CrossRef]
- Proćków, M.; Proćków, J.; Błażej, P.; Mackiewicz, P. The influence of habitat preferences on shell morphology in ecophenotypes of Trochulus hispidus complex. Sci. Total Environ. 2018, 630, 1036–1043. [Google Scholar] [CrossRef]
- Smith, S.R.; Amish, S.J.; Bernatchez, L.; le Luyer, J.; Wilson, C.; Boeberitz, O.; Luikart, G.; Scribner, K.T. Mapping of Adaptive Traits Enabled by a High-Density Linkage Map for Lake Trout. Genes Genomes Genet. 2020, 10, 1929–1947. [Google Scholar] [CrossRef]
- Cerda, J.M.; Palacios-Fuentes, P.; Díaz-Santana-Iturrios, M.; Ojeda, F.P. Description and discrimination of sagittae otoliths of two sympatric labrisomid blennies Auchenionchus crinitus and Auchenionchus microcirrhis using morphometric analyses. J. Sea Res. 2021, 173, 102063. [Google Scholar] [CrossRef]
- Coad, B.W. Review of the freshwater mullets of Iran (family mugilidae). Iran. J. Ichthyol. 2017, 4, 75–130. [Google Scholar] [CrossRef]
- Gallien, L.; Carboni, M. The community ecology of invasive species: Where are we and what’s next? Ecography 2017, 40, 335–352. [Google Scholar] [CrossRef]
- Latorre Espeso, D. Effects of Environmental Conditions on Phenotypic Plasticity of Fishes in Iberian Waters: Life-History, Physiological and Morphological Traits. Ph.D. Thesis, Universitat de Girona, Girona, Spain, 2019. [Google Scholar]
- Ren, P.; He, H.; Song, Y.; Cheng, F.; Xie, S. The spatial pattern of larval fish assemblages in the lower reach of the Yangtze River: Potential influences of river–lake connectivity and tidal intrusion. Hydrobiologia 2016, 766, 365–379. [Google Scholar] [CrossRef]
- Bakhshalizadeh, S.; Abbasi, K.; Rostamzade Liafuie, A.; Nezamdoost Darestani, R. A Guideline on the Identification of Economical Fish of the South Caspian Sea; Jahad Daneshgahi University: Teheran, Iran, 2021. [Google Scholar]
- Barati, A. Diet and growth of chicks of the Great Cormorant, Phalacrocorax carbo, at Ramsar, northern Iran. (Aves: Phalacrocoracidae). Zool. Middle East 2009, 46, 29–36. [Google Scholar] [CrossRef]
- Fazli, H.; Ghaninejad, D.; Janbaz, A.A.; Daryanabard, R. Population ecology parameters and biomass of golden grey mullet (Liza aurata) in Iranian waters of the Caspian Sea. Fish. Res. 2008, 93, 222–228. [Google Scholar] [CrossRef]
- Pourfaraj, V.; Karami, M.; Nezami, S.; Rafiee, G.; Khara, H. Morphological variation of Golden mullet, Liza aurata, of southern coasts of the Caspian Sea. Iran. Sci. Fish. J. 2008, 17, 35–48. [Google Scholar]
- Cardona, L. Selección del hábitat por los mugílidos (Osteichthyes: Mugilidae) en los estuarios mediterráneos: El papel de la salinidad. Sci. Mar. 2007, 70, 443–455. [Google Scholar] [CrossRef]
- Froese, R.; Pauly, D. FishBase. 2022. Available online: www.fishbase.org. (accessed on 13 September 2022).
- Kesiktaş, M.; Yemişken, E.; Yildiz, T.; Eryilmaz, L. Age, growth and reproduction of the golden grey mullet, Chelon auratus (Risso, 1810) in the Golden Horn Estuary, Istanbul. J. Mar. Biol. Assoc. UK 2020, 100, 989–995. [Google Scholar] [CrossRef]
- Katselis, G.; Hotos, G.; Minos, G.; Vidalis, V. Phenotypic affinities on fry of four Mediterranean grey mullet species. Turk. J. Fish. Aquat. Sci. 2006, 6, 49–55. [Google Scholar]
- Quattrocchi, F.; D’Anna, G.; Fiorentino, F.; Titone, A.; Zenone, A.; Garofalo, G. Phenotypic variation across populations of red mullet (Mullus barbatus) in different environments of the central Mediterranean. Mar. Freshw. Res. 2020, 71, 1313–1326. [Google Scholar] [CrossRef]
- Cadrin, S.X. Advances in morphometric identification of fishery stocks. Rev. Fish Biol. Fish. 2000, 10, 91–112. [Google Scholar] [CrossRef]
- Pazzaglia, J.; Reusch, T.B.H.; Terlizzi, A.; Marín-Guirao, L.; Procaccini, G. Phenotypic plasticity under rapid global changes: The intrinsic force for future seagrasses survival. Evol. Appl. 2021, 14, 1181–1201. [Google Scholar] [CrossRef]
- Xue, B.K.; Leibler, S. Benefits of phenotypic plasticity for population growth in varying environments. Proc. Natl. Acad. Sci. USA 2018, 115, 12745–12750. [Google Scholar] [CrossRef]
- Azzurro, E.; Tuset, V.M.; Lombarte, A.; Maynou, F.; Simberloff, D.; Rodríguez-Pérez, A.; Solé, R.V. External morphology explains the success of biological invasions. Ecol. Lett. 2014, 17, 1455–1463. [Google Scholar] [CrossRef]
- Smith, S.M.; Fox, R.J.; Donelson, J.M.; Head, M.L.; Booth, D.J. Predicting range-shift success potential for tropical marine fishes using external morphology. Biol. Lett. 2016, 12, 3–7. [Google Scholar] [CrossRef]
- Ugrin, N.; Škeljo, F.; Ferri, J.; Krstulović Šifner, S. Use of otolith morphology and morphometry for species discrimination of megrims Lepidorhombus spp. in the Central Eastern Adriatic Sea. J. Mar. Biol. Assoc. UK 2021, 101, 735–741. [Google Scholar] [CrossRef]
- Helland, I.P.; Vøllestad, L.A.; Freyhof, J.; Mehner, T. Morphological differences between two ecologically similar sympatric fishes. J. Fish Biol. 2006, 75, 2756–2767. [Google Scholar] [CrossRef]
- Valentin, A.E.; Penin, X.; Chanut, J.-P.; Sévigny, J.-M.; Rohlf, F.J. Arching effect on fish body shape in geometric morphometric studies. J. Fish Biol. 2008, 73, 623–638. [Google Scholar] [CrossRef]
- Zelditch, M.L.; Swiderski, D.L.; Sheets, H.D. Introduction. Geom. Morphometrics Biol. 2012, 95, 1–20. [Google Scholar] [CrossRef]
- Franssen, N.R. Anthropogenic habitat alteration induces rapid morphological divergence in a native stream fish. Evol. Appl. 2011, 4, 791–804. [Google Scholar] [CrossRef]
- Khan, M.A.; Nazir, A. Stock delineation of the long-whiskered catfish, Sperata aor (Hamilton 1822), from River Ganga by using morphometrics. Mar. Freshw. Res. 2018, 70, 107–113. [Google Scholar] [CrossRef]
- Tuset, V.M.; Lombarte, A.; Assis, C.A. Otolith atlas for the western Mediterranean, north and central eastern Atlantic. Sci. Mar. 2008, 72, 7–198. [Google Scholar] [CrossRef]
- Bani, A.; Poursaeid, S.; Tuset, V.M. Comparative morphology of the sagittal otolith in three species of south Caspian gobies. J. Fish Biol. 2013, 82, 1321–1332. [Google Scholar] [CrossRef]
- Lombarte, A.; Gordoa, A.; Whitfield, A.K.; James, N.C.; Tuset, V.M. Ecomorphological analysis as a complementary tool to detect changes in fish communities following major perturbations in two South African estuarine systems. Environ. Biol. Fishes 2012, 94, 601–614. [Google Scholar] [CrossRef]
- Lombarte, A.; Lleonart, J. Otolith size changes related with body growth, habitat depth and temperature. Environ. Biol. Fishes 1993, 37, 297–306. [Google Scholar] [CrossRef]
- Quinn, G.; Keough, M. Experimental Design and Data Analysis for Biologists; Cambridge University Press: Cambridge, UK, 2002. [Google Scholar] [CrossRef]
- Chenuil, A.; Cahill, A.E.; Délémontey, N.; Salliant du Luc, E.D.; Fanton, H. Problems and questions posed by cryptic species. A framework to guide future studies. In From Assessing to Conserving Biodiversity; Springer: Cham, Switzerland, 2019; pp. 77–106. [Google Scholar]
- Fi, C.; Robinson, C.T.; Malard, F. Cryptic species as a window into the paradigm shift of the species concept. Mol. Ecol. 2018, 27, 613–635. [Google Scholar] [CrossRef]
- Naciri, Y.; Linder, H.P. Species delimitation and relationships: The dance of the seven veils. Taxon 2015, 64, 3–16. [Google Scholar] [CrossRef]
- Assis, C.A. The utricular otoliths, lapilli, of teleosts: Their morphology and relevance for species identification and systematics studies. Sci. Mar. 2005, 69, 259–273. [Google Scholar] [CrossRef]
- Assis, I.O.; da Silva, V.E.L.; Souto-Vieira, D.; Lozano, A.P.; Volpedo, A.V.; Fabré, N.N. Ecomorphological patterns in otoliths of tropical fishes: Assessing trophic groups and depth strata preference by shape. Environ. Biol. Fishes 2020, 103, 349–361. [Google Scholar] [CrossRef]
- Callicó Fortunato, R.; Benedito Durà, V.; Volpedo, A. The morphology of saccular otoliths as a tool to identify different mugilid species from the Northeastern Atlantic and Mediterranean Sea. Estuar. Coast. Shelf Sci. 2014, 146, 95–101. [Google Scholar] [CrossRef]
- He, T.; Chen, C.J.; Qin, J.G.; Li, Y.; Wu, R.H.; Gao, T.X. The use of otolith shape to identify stocks of redlip mullet, Liza haematocheilus. Pak. J. Zool. 2020, 52, 2265–2273. [Google Scholar] [CrossRef]
- Nazir, A.; Khan, M.A. Using otoliths for fish stock discrimination: Status and challenges. Acta Ichthyol. Piscat. 2021, 51, 199–218. [Google Scholar] [CrossRef]
- Radhakrishnan, K.V.; Li, Y.; Jayalakshmy, K.V.; Liu, M.; Murphy, B.R.; Xie, S. Application of otolith shape analysis in identifying different ecotypes of Coilia ectenes in the Yangtze Basin, China. Fish. Res. 2012, 125, 156–160. [Google Scholar] [CrossRef]
- SriHari, M.; Bhushan, S.; Nayak, B.B.; Pavan-Kumar, A.; Abidi, Z.J. Spatial Variations in the Stocks of Randall’s Threadfin Bream, Nemipterus randalli Russell 1986 Along the Indian Coast Inferred Using Body and Otolith Shape Analysis. Thalassas 2021, 37, 883–890. [Google Scholar] [CrossRef]
- Gagliano, M.; Mccormick, M.I. Feeding history influences otolith shape in tropical fish. Mar. Ecol. Prog. Ser. 2004, 278, 291–296. [Google Scholar] [CrossRef]
- Mille, T.; Mahé, K.; Cachera, M.; Villanueva, M.C.; de Pontual, H.; Ernande, B. Diet is correlated with otolith shape in marine fish. Mar. Ecol. Prog. Ser. 2016, 555, 167–184. [Google Scholar] [CrossRef]
- Volpedo, A.; Echeverr, D.D. Ecomorphological patterns of the sagitta in fish on the continental shelf off Argentine. Fish. Res. 2003, 60, 551–560. [Google Scholar] [CrossRef]
- Gauldie, R.W.; Crampton, J.S. An eco-morphological explanation of individual variability in the shape of the fish otolith: Comparison of the otolith of Hoplostethus atlanticus with other species by depth. J. Fish Biol. 2002, 60, 1204–1221. [Google Scholar] [CrossRef]
- Libungan, L.A.; Óskarsson, G.J.; Slotte, A.; Jacobsen, J.A.; Pálsson, S. Otolith shape: A population marker for Atlantic herring Clupea harengus. J. Fish Biol. 2015, 86, 1377–1395. [Google Scholar] [CrossRef]
- Callicó Fortunato, R.; González-Castro, M.; Reguera Galán, A.; García Alonso, I.; Kunert, C.; Benedito Durà, V.; Volpedo, A. Identification of potential fish stocks and lifetime movement patterns of Mugil liza Valenciennes 1836 in the Southwestern Atlantic Ocean. Fish. Res. 2017, 193, 164–172. [Google Scholar] [CrossRef]
- Conith, A.J.; Kidd, M.R.; Kocher, T.D.; Albertson, R.C. Ecomorphological divergence and habitat lability in the context of robust patterns of modularity in the cichlid feeding apparatus. BMC Evol. Biol. 2020, 20, 95. [Google Scholar] [CrossRef] [PubMed]
- Costeur, L.; Grohé, C.; Aguirre-Fernández, G.; Ekdale, E.; Schulz, G.; Müller, B.; Mennecart, B. The bony labyrinth of toothed whales reflects both phylogeny and habitat preferences. Sci. Rep. 2018, 8, 8–13. [Google Scholar] [CrossRef]
- Pasisingi, N.; Olii, A.H.; Habibie, S.A. Morphology and growth pattern of Nike fish (amphidromous goby larvae) in Gorontalo Waters, Indonesia. Tomini. J. Aquat. Sci. 2020, 1, 1–7. [Google Scholar] [CrossRef]
- Schrandt, M.N.; Switzer, T.S.; Stafford, C.J.; Flaherty-Walia, K.E.; Paperno, R.; Matheson, R.E. Similar habitats, different communities: Fish and large invertebrate assemblages in eastern Gulf of Mexico polyhaline seagrasses relate more to estuary morphology than latitude. Estuar. Coast. Shelf Sci. 2018, 213, 217–229. [Google Scholar] [CrossRef]
- Torres-Dowdall, J.; Handelsman, C.A.; Reznick, D.N.; Ghalambor, C.K. Local Adaptation and the Evolution of Phenotypic Plasticity in Trinidadian Guppies (Poecilia reticulata). Evolution 2012, 66, 3432–3443. [Google Scholar] [CrossRef]
- Wegscheider, B.; Linnansaari, T.; Curry, R.A. Mesohabitat modelling in fish ecology: A global synthesis. Fish Fish. 2020, 21, 927–939. [Google Scholar] [CrossRef]
- Yedier, S.; Bostanci, D. Aberrant otoliths in four marine fishes from the Aegean Sea, Black Sea, and Sea of Marmara (Turkey). Reg. Stud. Mar. Sci. 2020, 34, 101011. [Google Scholar] [CrossRef]
- Clark, F.J.K.; da Silva Lima, C.S.; Pessanha, A.L.M. Otolith shape analysis of the Brazilian silverside in two northeastern Brazilian estuaries with distinct salinity ranges. Fish. Res. 2021, 243, 106094. [Google Scholar] [CrossRef]
- D’Iglio, C.; Albano, M.; Famulari, S.; Savoca, S.; Panarello, G.; di Paola, D.; Perdichizzi, A.; Rinelli, P.; Lanteri, G.; Spanò, N.; et al. Intra- and interspecific variability among congeneric Pagellus otoliths. Sci. Rep. 2021, 11, 16315. [Google Scholar] [CrossRef]
- Khayyami, H.; Movahedinia, A.; Zolgharnein, H.; Salamat, N. Morphological variability of Liza aurata (Risso, 1810), along the southern Caspian Sea. J. Basic Appl. Zool. 2014, 67, 100–107. [Google Scholar] [CrossRef]
- Whitfield, A.K.; Panfili, J.; Durand, J.-D. A global review of the cosmopolitan flathead mullet Mugil cephalus Linnaeus 1758 (Teleostei: Mugilidae), with emphasis on the biology, genetics, ecology and fisheries aspects of this apparent species complex. Rev. Fish Biol. Fish. 2012, 22, 641–681. [Google Scholar] [CrossRef]
- Ghaninejad, D. Maturity Stages, Gonado-Somatic Index (GSI) and Fecundity of Leaping Grey Mullet, Liza saliens (Risso, 1810) in the Western Part of Iranian Waters of the Caspian Sea (Guilan Province, Iran). Asian Fish. Sci. 2011, 24, 115–124. [Google Scholar] [CrossRef]
- Ghaninejad, D.; Parafkandeh Haghighi, F.; Abdolmaleki, S.; Nahrevar, R.; Khedmati, K.; Rastin, R.; Nikpour, M. Morphometric and Meristic Characteristics of Liza aurata Risso 1810 in the South of Caspian Sea. New Technol. Aquac. Dev. J. Fish. 2012, 6, 31–42. [Google Scholar]
- Qiao, J.; Zhu, R.; Chen, K.; Zhang, D.; Yan, Y.; He, D. Comparative Otolith Morphology of Two Morphs of Schizopygopsis thermalis Herzenstein 1891 (Pisces, Cyprinidae) in a Headwater Lake on the Qinghai-Tibet Plateau. Fishes 2022, 7, 99. [Google Scholar] [CrossRef]
- Bakhshalizadeh, S.; Bani, A. Uso de morfometría geométrica para la identificación de variaciones ecofenotípicas en juveniles de esturión persa acipenser persicus. Sci. Mar. 2017, 81, 187–193. [Google Scholar] [CrossRef]
- Bostanci, D.; Yedier, S. Discrimination of invasive fish Atherina boyeri (Pisces: Atherinidae) populations by evaluating the performance of otolith morphometrics in several lentic habitats. Fresenius Environ. Bull. 2018, 27, 4493–4501. [Google Scholar]
- Terada, C.; Saitoh, T. Phenotypic and genetic divergence among island populations of sika deer (Cervus nippon) in southern Japan: A test of the local adaptation hypothesis. Popul. Ecol. 2018, 60, 211–221. [Google Scholar] [CrossRef]

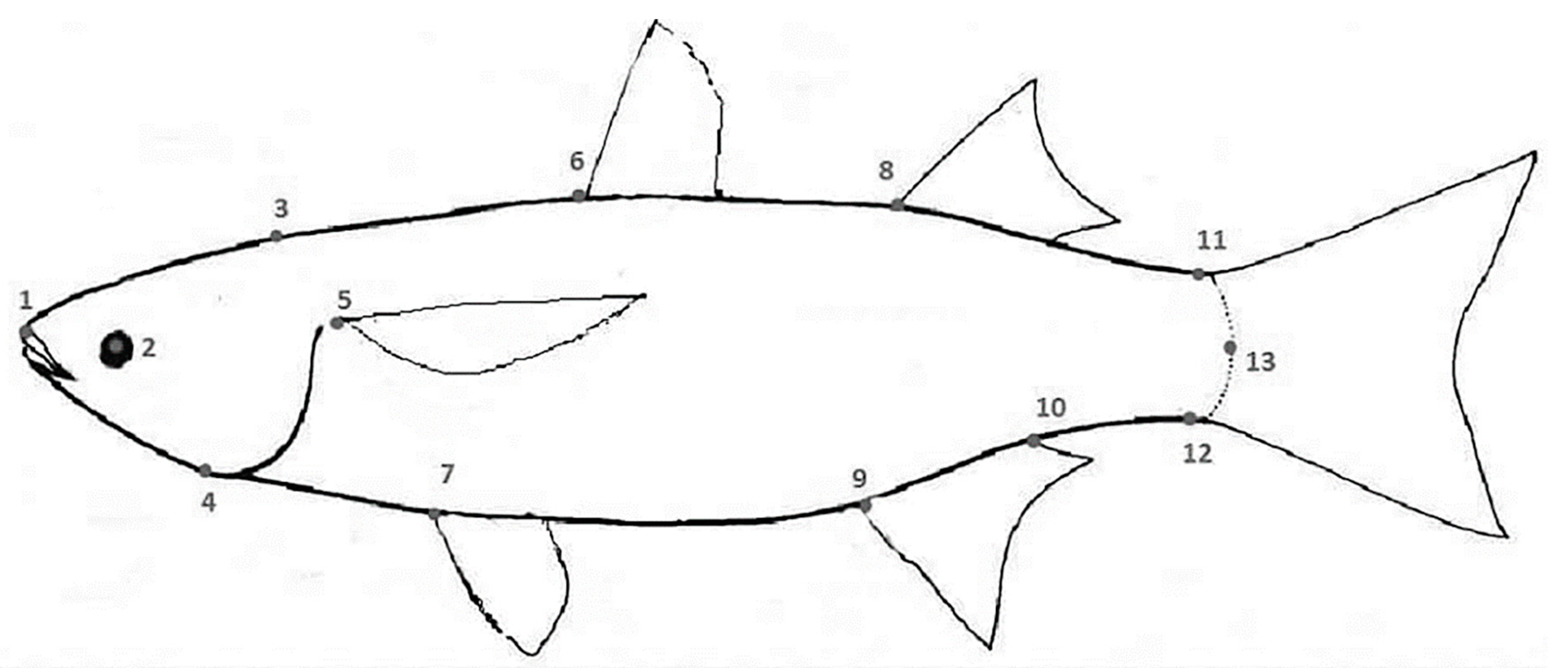

| Morphometric Measurements | F Value | p Value | Morphometric Measurements | F Value | p Value | Morphometric Measurements | F Value | p Value |
|---|---|---|---|---|---|---|---|---|
| 1-2 | 14.92 | 0.00 | 3-7 | 1.01 | 0.32 | 6-9 | 20.73 | 0.00 |
| 1-3 | 23.39 | 0.00 | 3-8 | 2.66 | 0.11 | 6-10 | 4.89 | 0.03 |
| 1-4 | 16.19 | 0.00 | 3-9 | 45.38 | 0.00 | 6-11 | 1.69 | 0.20 |
| 1-5 | 18.50 | 0.00 | 3-10 | 19.78 | 0.00 | 6-12 | 4.12 | 0.05 |
| 1-6 | 7.84 | 0.01 | 3-11 | 0.70 | 0.40 | 6-13 | 2.77 | 0.10 |
| 1-7 | 1.62 | 0.21 | 3-12 | 4.16 | 0.05 | 7-8 | 6.01 | 0.02 |
| 1-8 | 4.71 | 0.03 | 3-13 | 2.46 | 0.12 | 7-9 | 23.13 | 0.00 |
| 1-9 | 16.67 | 0.00 | 4-5 | 0.20 | 0.66 | 7-10 | 8.65 | 0.00 |
| 1-10 | 1.20 | 0.28 | 4-6 | 0.82 | 0.06 | 7-11 | 0.00 | 0.97 |
| 1-11 | 37.66 | 0.00 | 4-7 | 3.30 | 0.07 | 7-12 | 1.81 | 0.18 |
| 1-12 | 56.88 | 0.00 | 4-8 | 11.00 | 0.00 | 7-13 | 0.85 | 0.36 |
| 1-13 | 55.90 | 0.00 | 4-9 | 40.82 | 0.00 | 8-9 | 2.12 | 0.15 |
| 2-3 | 41.68 | 0.00 | 4-10 | 18.30 | 0.00 | 8-10 | 2.16 | 0.15 |
| 2-4 | 1.21 | 0.27 | 4-11 | 0.92 | 0.34 | 8-11 | 5.12 | 0.03 |
| 2-5 | 19.55 | 0.00 | 4-12 | 0.00 | 0.95 | 8-12 | 2.33 | 0.13 |
| 2-6 | 6.81 | 0.01 | 4-13 | 0.04 | 0.84 | 8-13 | 5.51 | 0.02 |
| 2-7 | 0.26 | 0.61 | 5-6 | 0.28 | 0.60 | 9-10 | 15.74 | 0.00 |
| 2-8 | 1.64 | 0.20 | 5-7 | 7.73 | 0.01 | 9-11 | 50.81 | 0.00 |
| 2-9 | 47.42 | 0.00 | 5-8 | 6.29 | 0.01 | 9-12 | 56.42 | 0.00 |
| 2-10 | 9.29 | 0.00 | 5-9 | 50.50 | 0.00 | 9-13 | 62.21 | 0.00 |
| 2-11 | 19.33 | 0.00 | 5-10 | 19.58 | 0.00 | 10-11 | 19.02 | 0.00 |
| 2-12 | 22.34 | 0.00 | 5-11 | 0.00 | 1.00 | 10-12 | 24.35 | 0.00 |
| 2-13 | 29.79 | 0.00 | 5-12 | 0.81 | 0.37 | 10-13 | 27.38 | 0.00 |
| 3-4 | 13.75 | 0.00 | 5-13 | 0.58 | 0.45 | 11-12 | 26.94 | 0.00 |
| 3-5 | 2.15 | 0.15 | 6-7 | 3.17 | 0.08 | 11-13 | 1.24 | 0.27 |
| 3-6 | 0.27 | 0.61 | 6-8 | 0.59 | 0.44 | 12-13 | 1.68 | 0.20 |
| Variable * | Communalities | Component | ||||
|---|---|---|---|---|---|---|
| Initial | Extraction | PC1 | PC2 | PC3 | PC4 | |
| 1-2 | 1.000 | 0.829 | 0.213 | 0.513 | −0.293 | 0.624 |
| 1-3 | 1.000 | 0.908 | 0.494 | 0.500 | 0.362 | 0.026 |
| 1-4 | 1.000 | 0.972 | 0.638 | 0.612 | 0.089 | −0.094 |
| 1-5 | 1.000 | 0.981 | 0.509 | 0.531 | −0.252 | 0.603 |
| 1-6 | 1.000 | 0.960 | 0.297 | 0.744 | −0.112 | −0.151 |
| 1-8 | 1.000 | 0.957 | 0.221 | 0.827 | 0.041 | 0.046 |
| 1-9 | 1.000 | 0.974 | −0.688 | 0.584 | −0.275 | 0.046 |
| 1-11 | 1.000 | 0.890 | 0.843 | 0.346 | −0.081 | 0.158 |
| 1-12 | 1.000 | 0.882 | 0.883 | 0.249 | −0.051 | 0.067 |
| 1-13 | 1.000 | 0.870 | 0.832 | 0.266 | −0.014 | 0.283 |
| 2-3 | 1.000 | 0.927 | 0.639 | 0.360 | 0.469 | −0.147 |
| 2-5 | 1.000 | 0.820 | 0.645 | 0.387 | −0.088 | 0.417 |
| 2-6 | 1.000 | 0.942 | 0.345 | 0.590 | 0.075 | −0.426 |
| 2-9 | 1.000 | 0.966 | −0.818 | 0.355 | −0.156 | −0.210 |
| 2-10 | 1.000 | 0.967 | −0.609 | 0.309 | 0.619 | 0.060 |
| 2-11 | 1.000 | 0.850 | 0.849 | 0.024 | 0.160 | −0.233 |
| 2-12 | 1.000 | 0.895 | 0.828 | −0.092 | 0.154 | −0.310 |
| 2-13 | 1.000 | 0.810 | 0.853 | −0.078 | 0.230 | −0.099 |
| 3-4 | 1.000 | 0.892 | 0.685 | 0.439 | 0.048 | −0.199 |
| 3-9 | 1.000 | 0.955 | −0.816 | 0.251 | −0.431 | −0.033 |
| 3-10 | 1.000 | 0.930 | −0.752 | 0.178 | 0.146 | 0.254 |
| 4-8 | 1.000 | 0.946 | −0.615 | −0.137 | −0.132 | 0.186 |
| 4-9 | 1.000 | 0.989 | −0.906 | −0.062 | −0.255 | 0.107 |
| 4-10 | 1.000 | 0.989 | −0.830 | −0.161 | 0.177 | 0.327 |
| 5-7 | 1.000 | 0.867 | −0.143 | −0.060 | −0.135 | −0.399 |
| 5-8 | 1.000 | 0.915 | −0.368 | 0.196 | 0.338 | −0.660 |
| 5-9 | 1.000 | 0.970 | −0.900 | 0.083 | −0.078 | −0.310 |
| 5-10 | 1.000 | 0.976 | −0.790 | −0.007 | 0.507 | −0.161 |
| 6-9 | 1.000 | 0.930 | −0.704 | −0.080 | −0.154 | 0.189 |
| 6-10 | 1.000 | 0.967 | −0.497 | −0.260 | 0.464 | 0.432 |
| 7-8 | 1.000 | 0.893 | −0.373 | 0.037 | 0.151 | −0.166 |
| 7-9 | 1.000 | 0.990 | −0.789 | 0.059 | −0.041 | −0.015 |
| 7-10 | 1.000 | 0.982 | −0.681 | −0.015 | 0.413 | 0.168 |
| 8-11 | 1.000 | 0.940 | 0.544 | −0.532 | −0.122 | 0.081 |
| 8-13 | 1.000 | 0.906 | 0.527 | −0.597 | −0.074 | 0.151 |
| 9-10 | 1.000 | 0.945 | 0.358 | −0.111 | 0.784 | 0.366 |
| 9-11 | 1.000 | 0.930 | 0.914 | −0.253 | 0.132 | 0.063 |
| 9-12 | 1.000 | 0.960 | 0.914 | −0.287 | 0.165 | 0.019 |
| 9-13 | 1.000 | 0.969 | 0.901 | −0.292 | 0.176 | 0.131 |
| 10-11 | 1.000 | 0.954 | 0.823 | −0.230 | −0.362 | −0.182 |
| 10-12 | 1.000 | 0.967 | 0.850 | −0.261 | −0.318 | −0.204 |
| 10-13 | 1.000 | 0.947 | 0.840 | −0.271 | −0.321 | −0.087 |
| 11-12 | 1.000 | 0.427 | −0.339 | 0.091 | −0.272 | −0.099 |
| Morphological Variables + | Mean ± SE | p Value | |
|---|---|---|---|
| Chelon auratus | Chelon saliens | ||
| Otolith weight (OW) (g) | 0.03858 ± 0 | 0.03887 ± 0 | 0.899 |
| Otolith length (OL) (mm) | 7.4913 ± 0.1 | 7.68819 ± 0.07 | 0.112 |
| Otolith height (OH) (mm) | 3.84127 ± 0.08 | 3.43848 ± 0.05 | 0.000 * |
| Area (mm2) | 20.62899 ± 0.74 | 20.26439 ± 0.35 | 0.619 |
| Perimeters (mm) | 19.62229 ± 0.38 | 19.4461 ± 0.18 | 0.636 |
| OH/OL | 0.51417 ± 0.01 | 0.44796 ± 0.01 | 0.000 * |
| Aspect ratio (OL/OH) | 1.964 ± 0.04 | 2.2473 ± 0.03 | 0.000 * |
| Rectangularity (A/(OL × OH)) | 0.71783 ± 0.02 | 0.77017 ± 0.01 | 0.030 * |
| Compactness (P2/A) | 18.84561 ± 0.42 | 18.72685 ± 0.18 | 0.765 |
| Otolith Shape indices—form factor = 4πA/p2 | 0.67247 ± 0.01 | 0.67292 ± 0.01 | 0.973 |
| Otolith Shape indices—roundness = 4A/(π(OL)2) | 0.46658 ± 0.01 | 0.437 ± 0.01 | 0.015 * |
| Otolith Shape indices—circularity(P2/A) | 18.84561 ± 0.42 | 18.72685 ± 0.18 | 0.765 |
| ellipticity (E = (OL − OH/OL + OH)) | 0.32239 ± 0.01 | 0.38218 ± 0.01 | 0.000 * |
| Variable + | Communalities | Component | |||
|---|---|---|---|---|---|
| Initial | Extraction | PC1 | PC2 | PC3 | |
| Otolith weight (OW) (g) | 1 | 0.537 | 0.035 | 0.149 | 0.193 |
| Otolith length (OL) (mm) | 1 | 0.908 | 0.082 | 0.055 | 0.174 |
| Otolith height (OH) (mm) | 1 | 0.945 | −0.168 | 0.146 | 0.118 |
| Area (mm2) | 1 | 0.858 | 0.003 | 0.068 | 0.354 |
| Perimeters (mm) | 1 | 0.811 | 0.079 | 0.166 | 0.253 |
| OH/OL | 1 | 0.980 | −0.199 | 0.111 | −0.016 |
| Aspect ratio | 1 | 0.971 | 0.200 | −0.102 | 0.004 |
| Rectangularity | 1 | 0.986 | 0.122 | −0.126 | 0.188 |
| Compactness | 1 | 0.971 | 0.104 | 0.266 | −0.105 |
| Form factor | 1 | 0.928 | −0.098 | −0.264 | 0.134 |
| Roundness | 1 | 0.988 | −0.080 | −0.015 | 0.216 |
| Circularity | 1 | 0.971 | 0.104 | 0.266 | −0.105 |
| Ellipticity | 1 | 0.984 | 0.199 | −0.113 | 0.014 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bakhshalizadeh, S.; Abbasi, K.; Rostamzadeh Liafuie, A.; Bani, A.; Pavithran, A.; Tiralongo, F. Morphometric Analyses of Phenotypic Plasticity in Habitat Use in Two Caspian Sea Mullets. J. Mar. Sci. Eng. 2022, 10, 1398. https://doi.org/10.3390/jmse10101398
Bakhshalizadeh S, Abbasi K, Rostamzadeh Liafuie A, Bani A, Pavithran A, Tiralongo F. Morphometric Analyses of Phenotypic Plasticity in Habitat Use in Two Caspian Sea Mullets. Journal of Marine Science and Engineering. 2022; 10(10):1398. https://doi.org/10.3390/jmse10101398
Chicago/Turabian StyleBakhshalizadeh, Shima, Keyvan Abbasi, Adeleh Rostamzadeh Liafuie, Ali Bani, Anu Pavithran, and Francesco Tiralongo. 2022. "Morphometric Analyses of Phenotypic Plasticity in Habitat Use in Two Caspian Sea Mullets" Journal of Marine Science and Engineering 10, no. 10: 1398. https://doi.org/10.3390/jmse10101398
APA StyleBakhshalizadeh, S., Abbasi, K., Rostamzadeh Liafuie, A., Bani, A., Pavithran, A., & Tiralongo, F. (2022). Morphometric Analyses of Phenotypic Plasticity in Habitat Use in Two Caspian Sea Mullets. Journal of Marine Science and Engineering, 10(10), 1398. https://doi.org/10.3390/jmse10101398






