Agonistic Behaviour and Sound Production during Male–Male Varunid Crabs (Cyrtograpsus angulatus, Dana 1851) Encounters
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animal Collection and Maintenance
2.2. Experimental Setup
2.3. Data Analysis
2.3.1. Behavioural Analysis
- Moving (M): one or both crabs were walking, without contact between each other;
- Contact (C): both crabs touched each other;
- Still (S): both crabs were not moving or walking and had no contact between them.
- Body Movement (BodyMov): defined each time the crabs were still and moving chelae/legs without contact between each other;
- Agonistic Interaction between the crabs (AgoInt): defined as each time the pair of males contacted each other with their chelae, or only one of the males using its chelae to contact the other male;
- Non-agonistic Interaction between crabs (Int): defined as every time both males contacted each other without using their chelae, for example, contact through their pereopods, or one crab walking over the other crab.
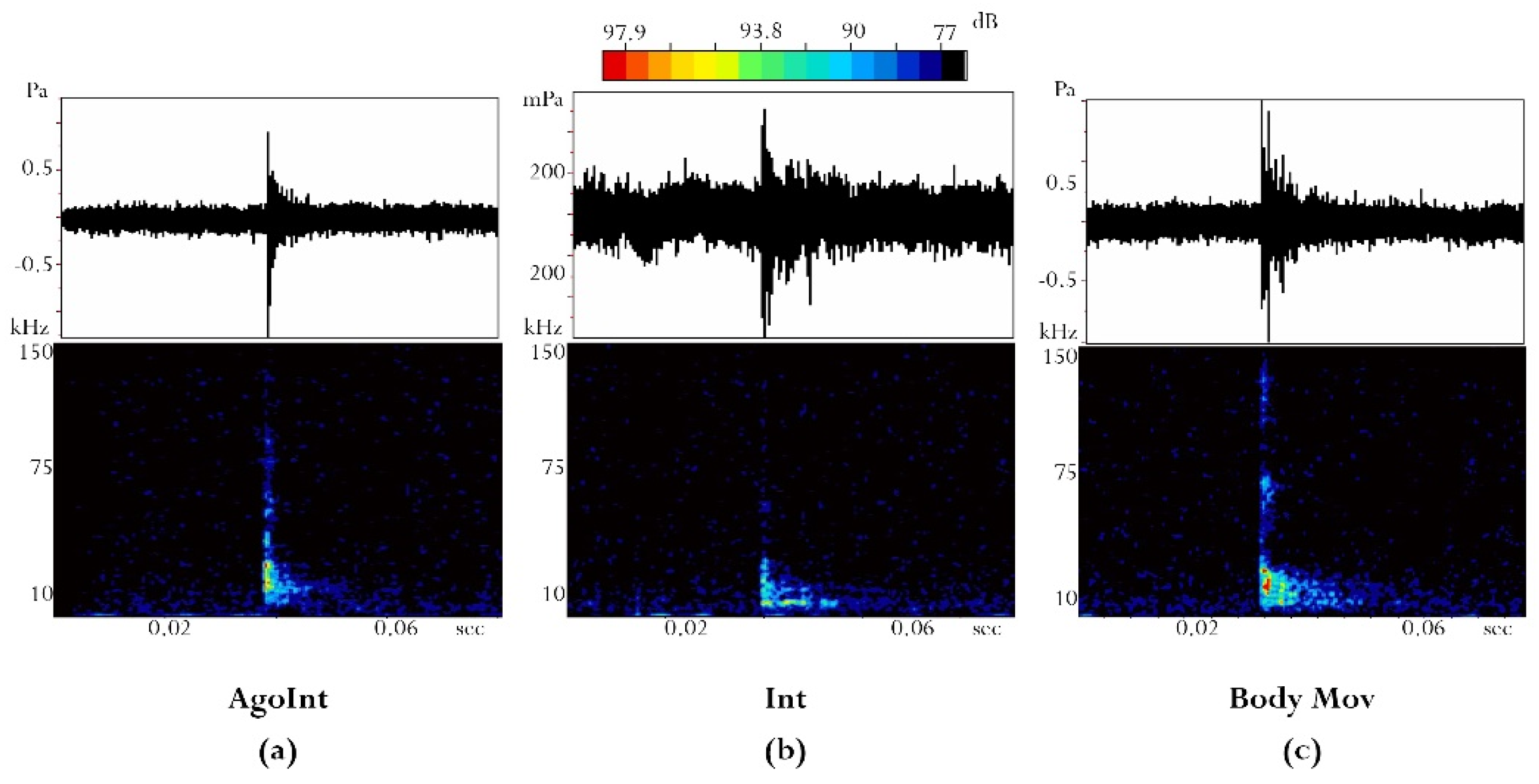
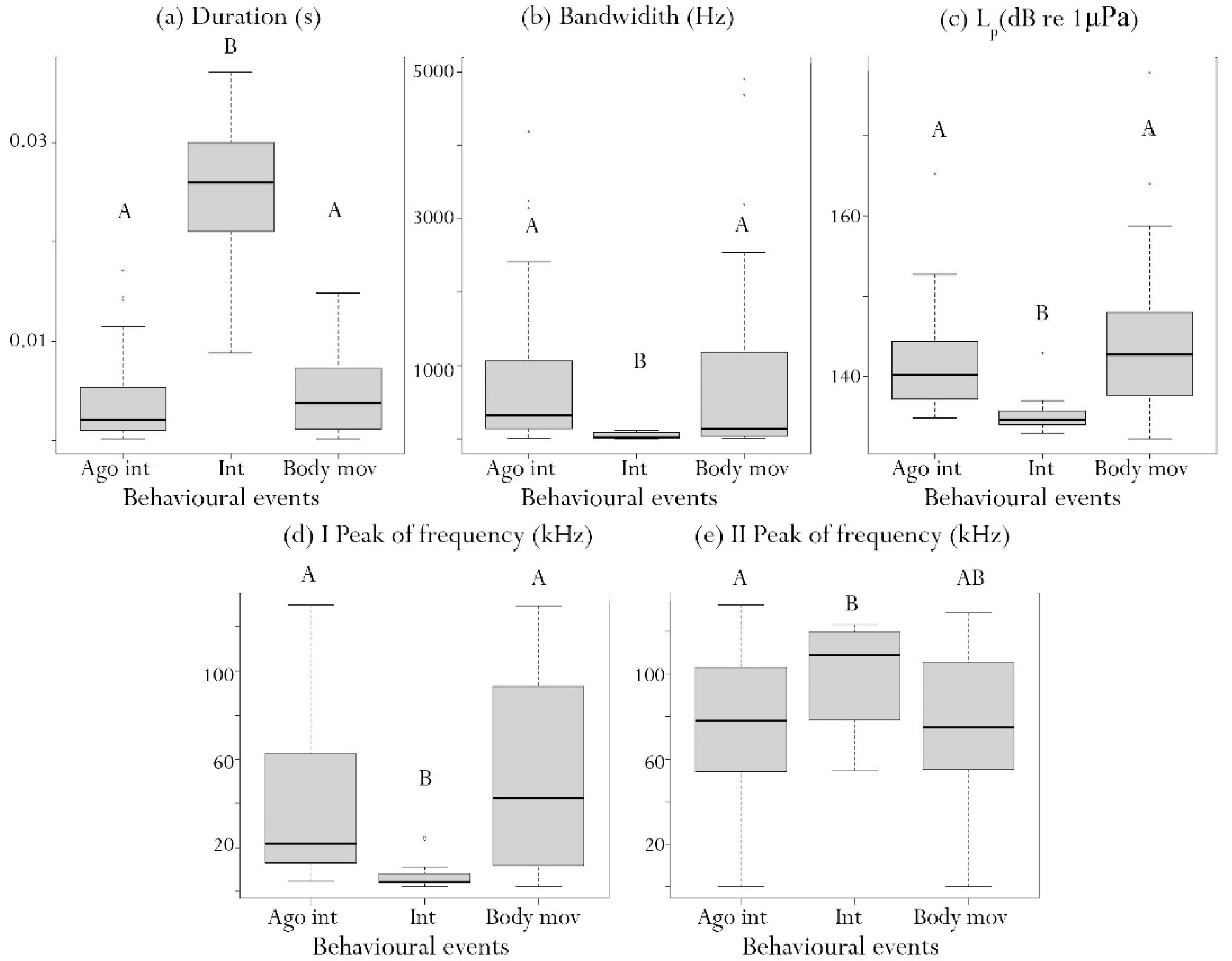
2.3.2. Acoustical Analysis
- Maximum Sound Pressure Level (Lp–dB re 1 µPa peak);
- Sound duration (s);
- 1st peak frequency (kHz);
- 2nd peak frequency (kHz);
- 3 dB bandwidth (kHz).
2.4. Statistical Analysis
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tyack, P.L. Acoustic Communication Under the Sea. In Animal Acoustic Communication: Sound Analysis and Research Methods; Hopp, S.L., Owren, M.J., Evans, C.S., Eds.; Springer: Berlin/Heidelberg, Germany, 1998; pp. 163–220. ISBN 978-3-642-76220-8. [Google Scholar]
- Xie, J.; Colonna, J.G.; Zhang, J. Bioacoustic signal denoising: A review. Artif. Intell. Rev. 2021, 54, 3575–3597. [Google Scholar] [CrossRef]
- Laiolo, P. The emerging significance of bioacoustics in animal species conservation. Biol. Conserv. 2010, 143, 1635–1645. [Google Scholar] [CrossRef]
- Shannon, G.; McKenna, M.F.; Angeloni, L.M.; Crooks, K.R.; Fristrup, K.M.; Brown, E.; Warner, K.A.; Nelson, M.D.; White, C.; Briggs, J. A synthesis of two decades of research documenting the effects of noise on wildlife. Biol. Rev. 2016, 91, 982–1005. [Google Scholar] [CrossRef]
- Popper, A.N.; Salmon, M.; Horch, K.W. Acoustic detection and communication by decapod crustaceans. J. Comp. Physiol. A 2001, 187, 83–89. [Google Scholar] [CrossRef]
- Patek, S.N.; Oakley, T.H. Comparative tests of evolutionary trade-offs in a palinurid lobster acoustic system. Evolution 2003, 57, 2082–2100. [Google Scholar]
- Bouwma, P.E.; Herrnkind, W.F. Sound production in Caribbean spiny lobster Panulirus argus and its role in escape during predatory attack by Octopus briareus. N. Z. J. Mar. Freshw. Res. 2009, 43, 3–13. [Google Scholar] [CrossRef]
- Buscaino, G.; Filiciotto, F.; Gristina, M.; Buffa, G.; Bellante, A.; Maccarrone, V.; Patti, B.; Mazzola, S. Defensive strategies of European spiny lobster Palinurus elephas during predator attack. Mar. Ecol. Prog. Ser. 2011, 423, 143–154. [Google Scholar] [CrossRef]
- Busnel, R.G. Acoustic Behaviour of Animals; Elsevier: Amsterdam, The Netherlands, 1963. [Google Scholar]
- Boon, P.; Yeo, D.; Todd, P. Sound production and reception in mangrove crabs Perisesarma spp. (Brachyura: Sesarmidae). Aquat. Biol. 2009, 5, 107–116. [Google Scholar] [CrossRef]
- Buscaino, G.; Filiciotto, F.; Gristina, M.; Bellante, A.; Buffa, G.; Di Stefano, V.; Maccarrone, V.; Tranchida, G.; Buscaino, C.; Mazzola, S. Acoustic behaviour of the European spiny lobster Palinurus elephas. Mar. Ecol. Prog. Ser. 2011, 441, 177–184. [Google Scholar] [CrossRef]
- Meyer-Rochow, V.B.; Penrose, J.D.; Oldfield, B.P.; Bailey, W.J. Phonoresponses in the rock lobster Panulirus longipes (Milne Edwards). Behav. Neural Biol. 1982, 34, 331–336. [Google Scholar] [CrossRef]
- Buscaino, G.; Gavio, A.; Galvan, D.; Filiciotto, F.; Maccarrone, V.; de Vincenzi, G.; Mazzola, S.; Orensanz, J.M. Acoustic signals and behaviour of Ovalipes trimaculatus in the context of reproduction. Aquat. Biol. 2015, 24, 61–73. [Google Scholar] [CrossRef] [Green Version]
- Filiciotto, F.; Moyano, M.P.S.; Hidalgo, F.; de Vincenzi, G.; Bazterrica, M.C.; Ceraulo, M.; Corrias, V.; Quinci, E.M.; Lorusso, M.; Mazzola, S. Underwater acoustic communication during the mating behaviour of the semi-terrestrial crab Neohelice granulata. Sci. Nat. 2019, 106, 35. [Google Scholar] [CrossRef] [PubMed]
- Guinot-Dumortier, D.; Dumortier, B. La stridulation chez les crabes. Crustaceana 1960, 1, 117–155. [Google Scholar] [CrossRef]
- Salmon, M.; Horch, K.W. Acoustic signalling and detection by semiterrestrial crabs of the family Ocypodidae. In Behavior of Marine Animals; Springer: Berlin/Heidelberg, Germany, 1972; pp. 60–96. [Google Scholar]
- McLay, C.L.; Hinnendael, F.; Lavery, S.; Riquelme-Bugueño, R. Morphological and Molecular Comparison of Hemigrapsus Crenulatus (Milne Edwards, 1837) (Brachyura: Varunidae) from New Zealand and Chile: Was Miss Rathbun Right? J. Crustac. Biol. 2011, 31, 582–589. [Google Scholar] [CrossRef]
- Sal Moyano, M.P.; Ceraulo, M.; Mazzola, S.; Buscaino, G.; Gavio, M.A. Sound production mechanism in the semiterrestrial crab Neohelice granulata (Brachyura, Varunidae). J. Acoust. Soc. Am. 2019, 146, 3466–3474. [Google Scholar] [CrossRef]
- Coquereau, L.; Grall, J.; Clavier, J.; Jolivet, A.; Chauvaud, L. Acoustic behaviours of large crustaceans in NE Atlantic coastal habitats. Aquat. Biol. 2016, 25, 151–163. [Google Scholar] [CrossRef]
- Coquereau, L.; Grall, J.; Chauvaud, L.; Gervaise, C.; Clavier, J.; Jolivet, A.; Di Iorio, L. Sound production and associated behaviours of benthic invertebrates from a coastal habitat in the north-east Atlantic. Mar. Biol. 2016, 163, 127. [Google Scholar] [CrossRef]
- Salmon, M. Coastal distribution, display and sound production by Florida fiddler crabs (Genus Uca). Anim. Behav. 1967, 15, 449–459. [Google Scholar] [CrossRef]
- Salmon, M.; Atsaides, S.P. Visual and Acoustical Signalling during Courtship by Fiddler Crabs (Genus Uca). Am. Zool. 1968, 8, 623–639. [Google Scholar] [CrossRef]
- Clayton, D. Singing and dancing in the ghost crab Ocypode platytarsus (Crustacea, Decapoda, Ocypodidae). J. Nat. Hist. 2008, 42, 141–155. [Google Scholar] [CrossRef]
- Crane, J. Combat, display and ritualization in fiddler crabs (Ocypodidae, genus Uca). Philos. Trans. R. Soc. Lond. B. Biol. Sci. 1966, 251, 459–472. [Google Scholar]
- Horch, K.W.; Salmon, M. Production, perception and reception of acoustic stimuli by semiterrestrial crabs (genus Ocypode and Uca, family Ocypodidae). Forma Functio 1969, 1, 1–25. [Google Scholar]
- Flood, A.S.; Goeritz, M.L.; Radford, C.A. Sound production and associated behaviours in the New Zealand paddle crab Ovalipes catharus. Mar. Biol. 2019, 166, 162. [Google Scholar] [CrossRef]
- Boschi, E.E. Biodiversity of marine decapod brachyurans of the Americas. J. Crustac. Biol. 2000, 20, 337–342. [Google Scholar] [CrossRef]
- Spivak, E.; Anger, K.; Luppi, T.; Bas, C.; Ismael, D. Distribution and habitat preferences of two grapsid crab species in Mar Chiquita Lagoon (Province of Buenos Aires, Argentina). Helgoländer Meeresunters. 1994, 48, 59–78. [Google Scholar] [CrossRef]
- Casariego, A.M.; Schwindt, E.; Iribarne, O. Evidence of habitat structure-generated bottleneck in the recruitment process of the SW Atlantic crab Cyrtograpsus angulatus. Mar. Biol. 2004, 145, 259–264. [Google Scholar]
- Spivak, E.D. Cangrejos estuariales del Atlántico sudoccidental (25°–41°S)(Crustacea: Decapoda: Brachyura). Investig. Mar. 1997, 25, 105–120. [Google Scholar] [CrossRef]
- Spivak, E.D.; Anger, K.; Bas, C.C.; Luppi, T.A.; Ismael, D. Size structure, sex ratio, and breeding season in two intertidal grapsid crab species from Mar Chiquita lagoon, Argentina. Rev. Nerítica 1996, 10, 7–26. [Google Scholar]
- Gavio, A.M. Hábitat, Sistemas de Apareamiento y Selección Sexual en dos Especies Simpátricas de Cyrtograpsus (Decapoda: Brachyura: Grapsidae). Ph.D. Thesis, Universidad Nacional de La Plata, La Plata, Argentinië, 2004. [Google Scholar]
- Lorusso, M.I.; Moyano, M.P.S.; Gavio, M.A.; McLay, C.L. Reproductive anatomy and receptivity of the female Cyrtograpsus angulatus (Brachyura: Varunidae): Implications for the mating system. Zool. Anz. 2020, 286, 72–80. [Google Scholar] [CrossRef]
- Daleo, P.; Luppi, T.; Mendez Casariego, A.; Escapa, M.; Ribeiro, P.; Silva, P.; Iribarne, O. The effect of size and cheliped autotomy on sexual competition between males of the mud crab Cyrtograpsus angulatus Dana. Mar. Biol. 2009, 156, 269–275. [Google Scholar] [CrossRef]
- Ceraulo, M.; Sal Moyano, M.P.; Bazterrica, M.C.; Hidalgo, F.J.; Papale, E.; Grammauta, R.; Gavio, M.A.; Mazzola, S.; Buscaino, G. Spatial and temporal variability of the soundscape in a Southwestern Atlantic coastal lagoon. Hydrobiologia 2020, 10, 2255–2277. [Google Scholar] [CrossRef]
- Sal Moyano, M.P.; Ceraulo, M.; Hidalgo, F.J.; Luppi, T.; Nuñez, J.; Radford, C.A.; Mazzola, S.; Gavio, M.A.; Buscaino, G. Effect of biological and anthropogenic sound on the orientation behavior of four species of brachyuran crabs. Mar. Ecol. Prog. Ser. 2021, 669, 107–120. [Google Scholar] [CrossRef]
- Buscaino, G.; Buffa, G.; Filiciotto, F.; Maccarrone, V.; Di Stefano, V.; Ceraulo, M.; Mazzola, S.; Alonge, G. Pulsed signal properties of free-ranging bottlenose dolphins ( Tursiops truncatus ) in the central Mediterranean Sea. Mar. Mammal Sci. 2015, 31, 891–901. [Google Scholar] [CrossRef]
- Buscaino, G.; Picciulin, M.; Canale, D.E.; Papale, E.; Ceraulo, M.; Grammauta, R.; Mazzola, S. Spatio-temporal distribution and acoustic characterization of haddock (Melanogrammus aeglefinus, Gadidae) calls in the Arctic fjord Kongsfjorden (Svalbard Islands). Sci. Rep. 2020, 10, 18297. [Google Scholar] [CrossRef] [PubMed]
- Buscaino, G.; Ceraulo, M.; Canale, D.E.; Papale, E.; Marrone, F. First evidence of underwater sounds emitted by the living fossils Lepidurus lubbocki and Triops cancriformis (Branchiopoda: Notostraca). Aquat. Biol. 2021, 30, 101–112. [Google Scholar] [CrossRef]
- Buscaino, G.; Ceraulo, M.; Alonge, G.; Pace, D.S.; Grammauta, R.; Maccarrone, V.; Bonanno, A.; Mazzola, S.; Papale, E. Artisanal fishing, dolphins, and interactive pinger: A study from a passive acoustic perspective. Aquat. Conserv. Mar. Freshw. Ecosyst. 2021, 31, 2241–2256. [Google Scholar] [CrossRef]
- Papale, E.; Alonge, G.; Caruso, F.; Grammauta, R.; Mazzola, S.; Mussi, B.; Pace, D.; Buscaino, G. The higher, the closer, the better? Influence of sampling frequency and distance on the acoustic properties of short-beaked common dolphins burst pulses in the Mediterranean Sea. Aquat. Conserv. Mar. Freshw. Ecosyst. 2019, 31, 51–60. [Google Scholar] [CrossRef]
- Staaterman, E. Passive Acoustic Monitoring in Benthic Marine Crustaceans: A New Research Frontier. In Listening in the Ocean; Au, W.W.L., Lammers, M.O., Eds.; Modern Acoustics and Signal Processing: New York, NY, USA; Springer: New York, NY, USA, 2016; pp. 325–333. ISBN 978-1-4939-3175-0. [Google Scholar]
- Li, Y.-Q.; Sun, X. Agonistic behaviors of aquatic animals. Zool. Res. 2013, 34, 214–220. [Google Scholar]
- Juanes, F.; Smith, L.D. The ecological consequences of limb damage and loss in decapod crustaceans: A review and prospectus. J. Exp. Mar. Biol. Ecol. 1995, 193, 197–223. [Google Scholar] [CrossRef]
- He, J.; Gao, Y.; Wang, W.; Xie, J.; Shi, H.; Wang, G.; Xu, W. Limb autotomy patterns in the juvenile swimming crab (Portunus trituberculatus) in earth ponds. Aquaculture 2016, 463, 189–192. [Google Scholar] [CrossRef]
- He, J.; Wang, X.J.; Yu, F.P.; Xu, W.J.; Xie, J.J.; Wang, G.S.; Wang, W.; Shi, H. The effects of number of cheliped on the ability of feeding and the competition for food of swimming crab Portunus trituberculatus. Oceanol. Limnol. Sin. 2017, 48, 609–617. [Google Scholar]
- Wu, B.; Zhao, C.; Xiong, Z.; Mu, C.; Xu, S.; Wang, D. Analysis of the agonistic behaviour and behaviour pattern of Portunus trituberculatus. Aquac. Res. 2021, 52, 2233–2242. [Google Scholar] [CrossRef]
- Goh, S.L.R.; Vishnu, H.; Ng, N.K. The sounds of fighting: Contests between violet vinegar crabs, Episesarma versicolor (Tweedie, 1940)(Decapoda: Brachyura: Sesarmidae), are resolved through acoustic communication. J. Crustac. Biol. 2019, 39, 331–341. [Google Scholar] [CrossRef]
- Olivier, S.R.; Escofet, A.; Penchaszadeh, P.E.; Orensanz, J.M. Estudios ecológicos de la región estuarial de Mar Chiquita (Buenos Aires, Argentina). I. Las comunidades bentónicas [Ecological studies of the estuary region of Mar Chiquita (Buenos Aires, Argentina). I. The benthic communities]. In Proceedings of the Anales de la Sociedad Científica Argentina; Sociedad Científica Argentina: Buenos Aires, Argentina, 1972; Volume 193, pp. 239–262. [Google Scholar]




| Replicates | Trials | Experimental Setting | Carapace M1 (mm) | Chelae M1 (mm) | Carapace M2 (mm) | Chelae M2 (mm) | Carapax Difference (mm) | Chelae Difference (mm) |
|---|---|---|---|---|---|---|---|---|
| 24 | 12 | SS | 30.9 (±3.05) | 20.8 (±3.94) | 30.9 (±3.94) | 21.5 (±4.03) | 1.6 (±1.35) | 1.4 (±1.27) |
| 12 | DS | 35.2 (±4.12) | 25.9 (±3.6) | 25.7 (±3.08) | 15.9 (±1.5) | 9.46 (±4.5) | 10.1 (±4.12) |
| Analysis of Deviance Table (Type II Wald Chi-Square Tests) | ||||
|---|---|---|---|---|
| χ2 | Degree Freedom | p-Value | ||
| Behavioural states | 96.57 | 2.00 | <0.001 | |
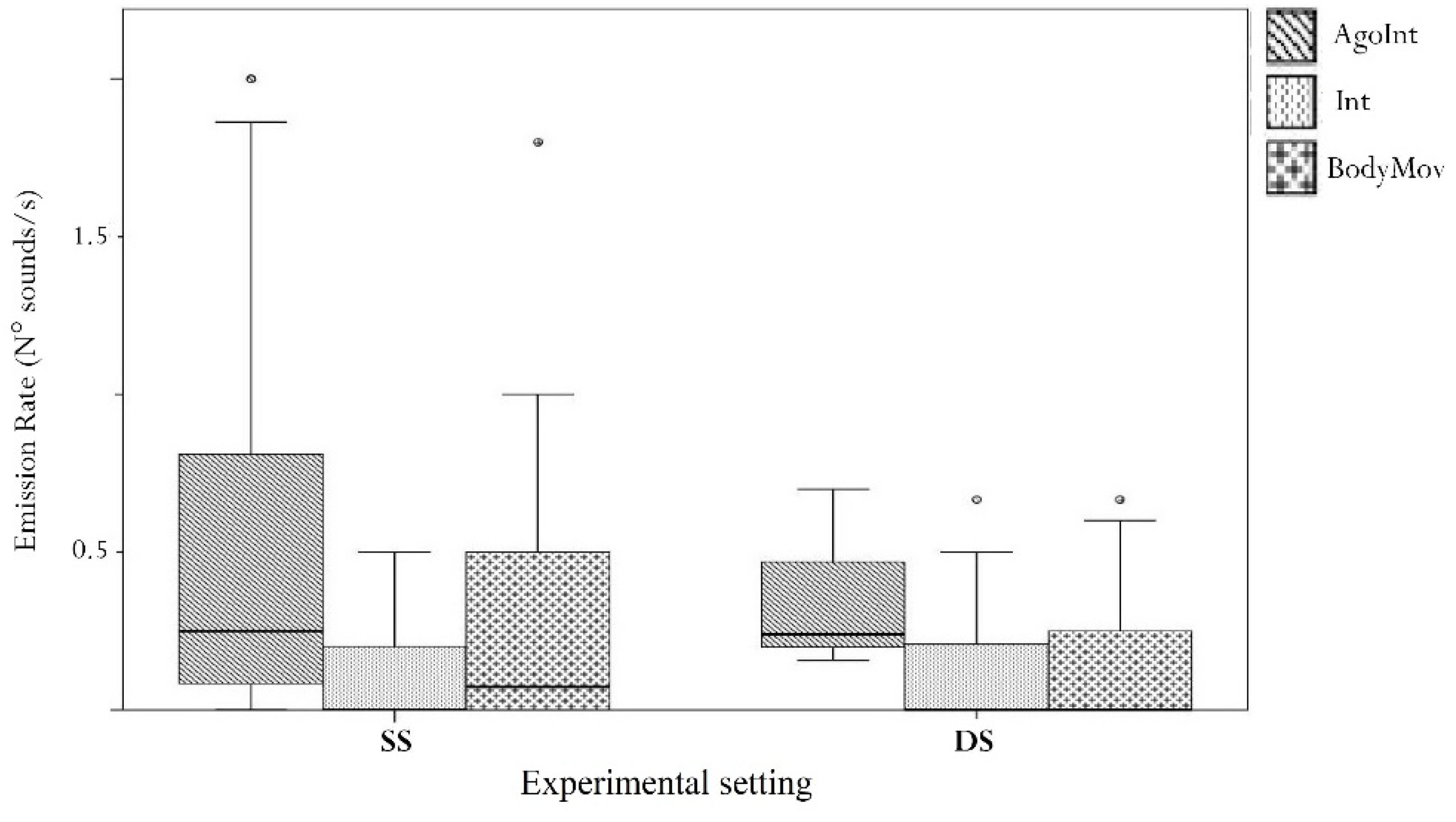
| Generalized Linear Mixed Model (GLMM), Multiple Comparisons of Means: Tukey Test Dependent Variable: Emission Rate | ||||
| Estimate | SE | z-Value | p-Value | |
| Intercept | 2.64 | 0.45 | 5.84 | <0.001 |
| Moving (M) vs. Contact (C) | 11.74 | 1.20 | 9.78 | <0.001 |
| Still (S) vs. Contact (C) | 2.06 | 1.45 | 1.42 | 0.319 |
| Still (S) vs. Moving (M) | −9.68 | 1.84 | −5.26 | <0.001 |
| Analysis of Deviance Table (Type II Wald Chi-Square Tests) | ||||
|---|---|---|---|---|
| χ2 | Degree Freedom | p-Value | ||
| Behavioural events | 20.56 | 2.00 | <0.001 | |
| Generalized Linear Mixed Model (GLMM), Multiple Comparisons of Means: Tukey Test Dependent Variable: Emission Rate | ||||
| Estimate | SE | z-Value | p-Value | |
| Intercept | −0.83 | 0.28 | −2.97 | <0.001 |
| BodyMov vs. AgoInt | 0.30 | 0.30 | 1.00 | 0.578 |
| Int vs. AgoInt | −1.14 | 0.34 | −3.31 | <0.001 |
| Int vs. BodyMov | −1.42 | 0.32 | −4.46 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ceraulo, M.; Sal Moyano, M.P.; Bazterrica, M.C.; Hidalgo, F.J.; Snitman, S.; Papale, E.; Buscaino, G.; Gavio, M.A. Agonistic Behaviour and Sound Production during Male–Male Varunid Crabs (Cyrtograpsus angulatus, Dana 1851) Encounters. J. Mar. Sci. Eng. 2022, 10, 1370. https://doi.org/10.3390/jmse10101370
Ceraulo M, Sal Moyano MP, Bazterrica MC, Hidalgo FJ, Snitman S, Papale E, Buscaino G, Gavio MA. Agonistic Behaviour and Sound Production during Male–Male Varunid Crabs (Cyrtograpsus angulatus, Dana 1851) Encounters. Journal of Marine Science and Engineering. 2022; 10(10):1370. https://doi.org/10.3390/jmse10101370
Chicago/Turabian StyleCeraulo, Maria, María Paz Sal Moyano, María Cielo Bazterrica, Fernando José Hidalgo, Solana Snitman, Elena Papale, Giuseppa Buscaino, and María Andrea Gavio. 2022. "Agonistic Behaviour and Sound Production during Male–Male Varunid Crabs (Cyrtograpsus angulatus, Dana 1851) Encounters" Journal of Marine Science and Engineering 10, no. 10: 1370. https://doi.org/10.3390/jmse10101370
APA StyleCeraulo, M., Sal Moyano, M. P., Bazterrica, M. C., Hidalgo, F. J., Snitman, S., Papale, E., Buscaino, G., & Gavio, M. A. (2022). Agonistic Behaviour and Sound Production during Male–Male Varunid Crabs (Cyrtograpsus angulatus, Dana 1851) Encounters. Journal of Marine Science and Engineering, 10(10), 1370. https://doi.org/10.3390/jmse10101370









