Lithium, Oxygen and Magnesium Isotope Systematics of Volcanic Rocks in the Okinawa Trough: Implications for Plate Subduction Studies
Abstract
:1. Introduction
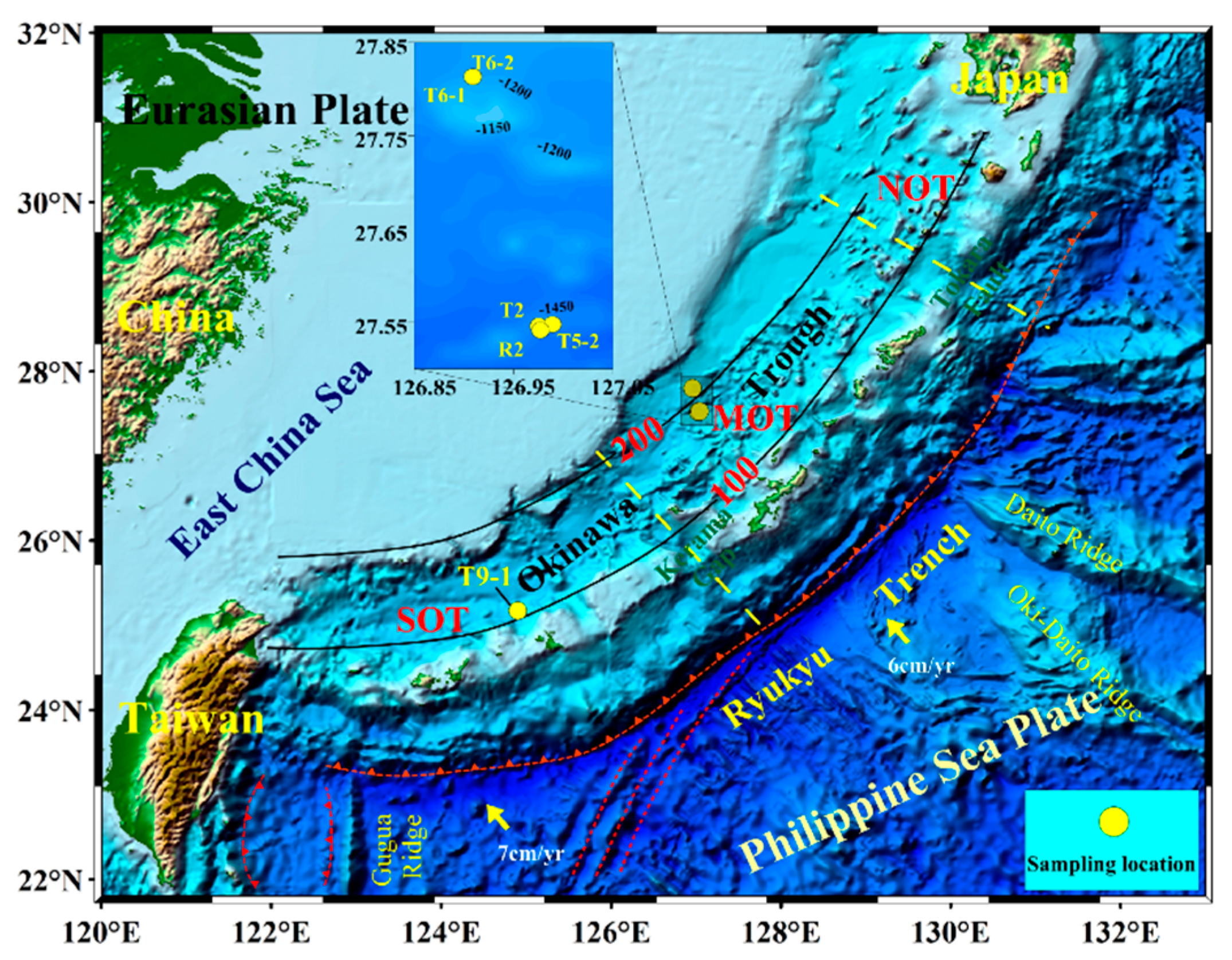
2. Geologic Setting
3. Sampling and Methods
3.1. Sample Collection
3.2. Li, O, and Mg Isotope Analyses
4. Results
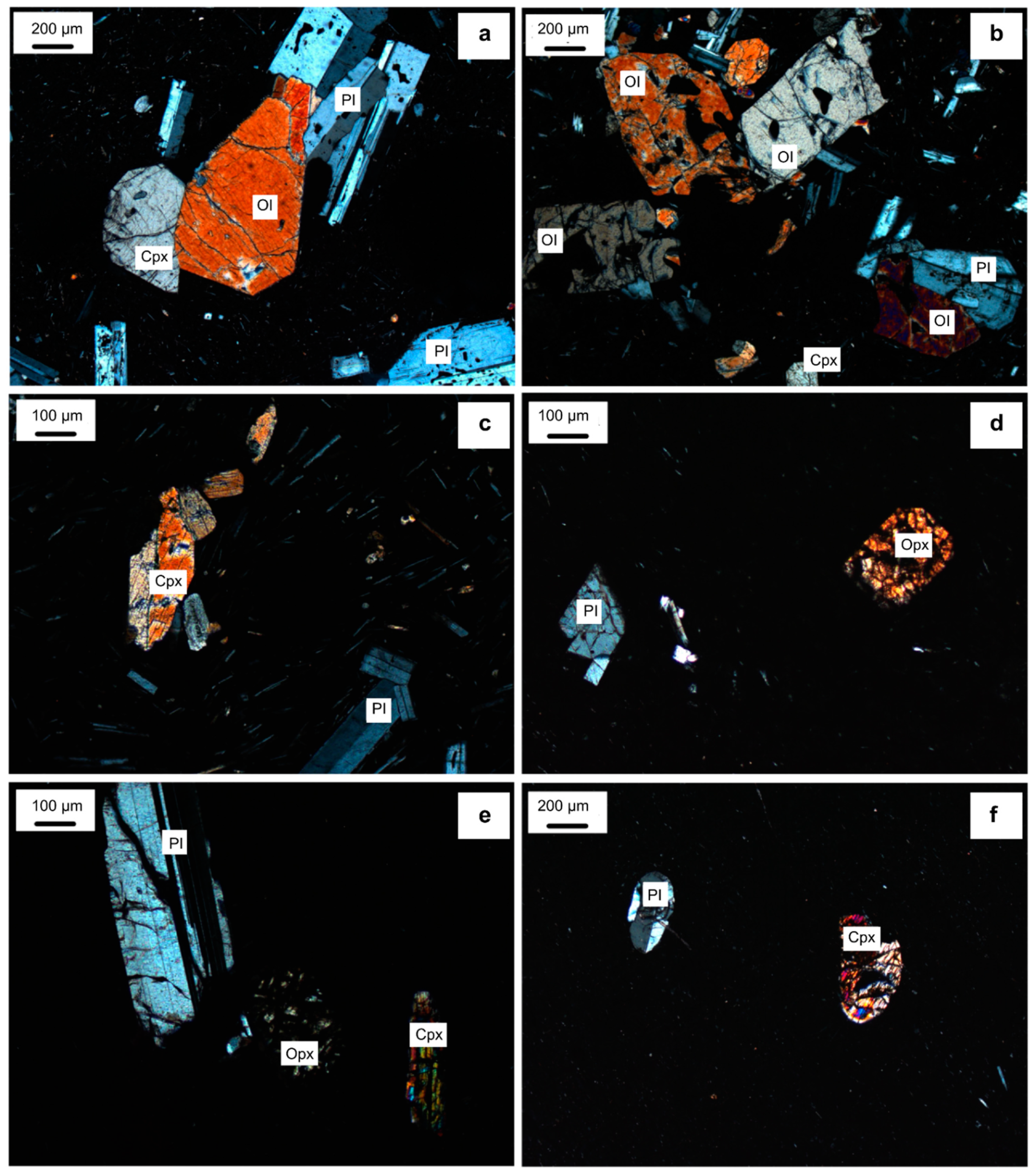
4.1. Clinopyroxene and Orthopyroxene
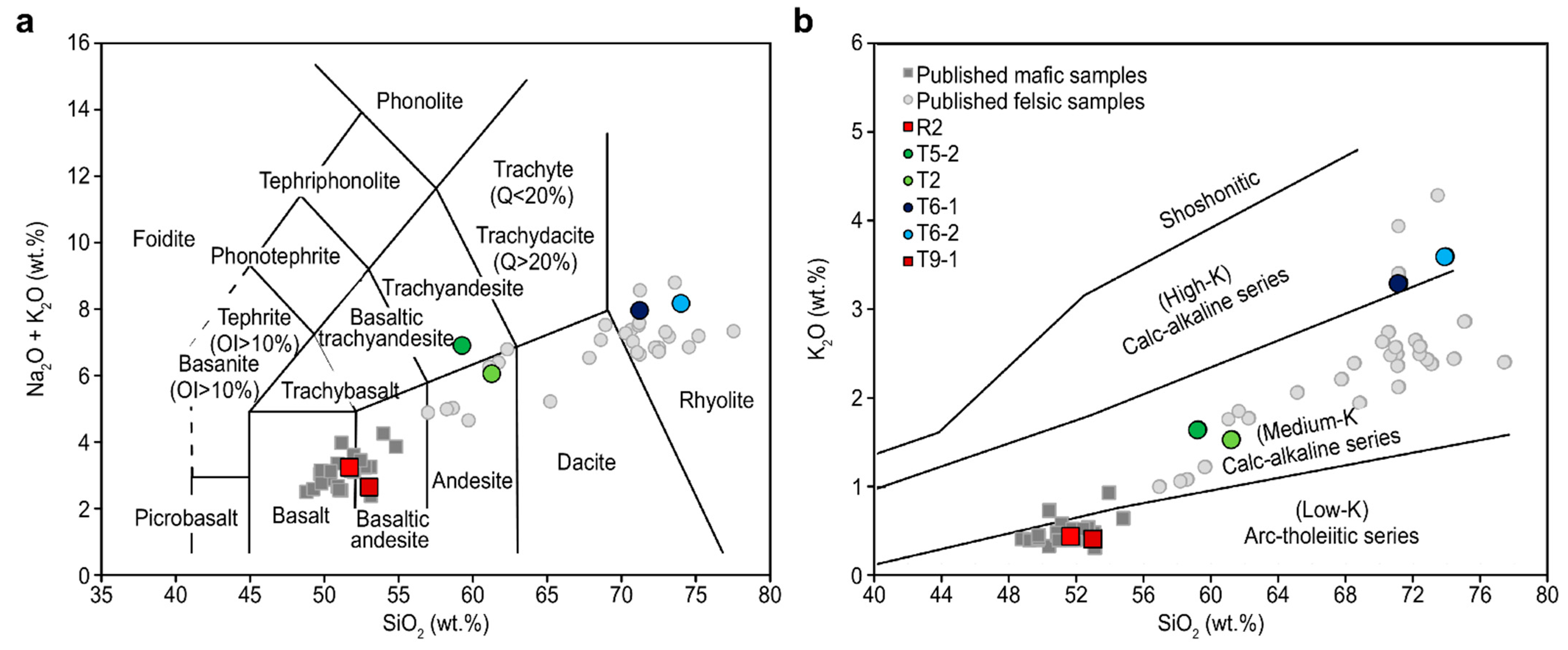
4.2. Major Element and Trace Element Compositions of the Volcanic Lavas
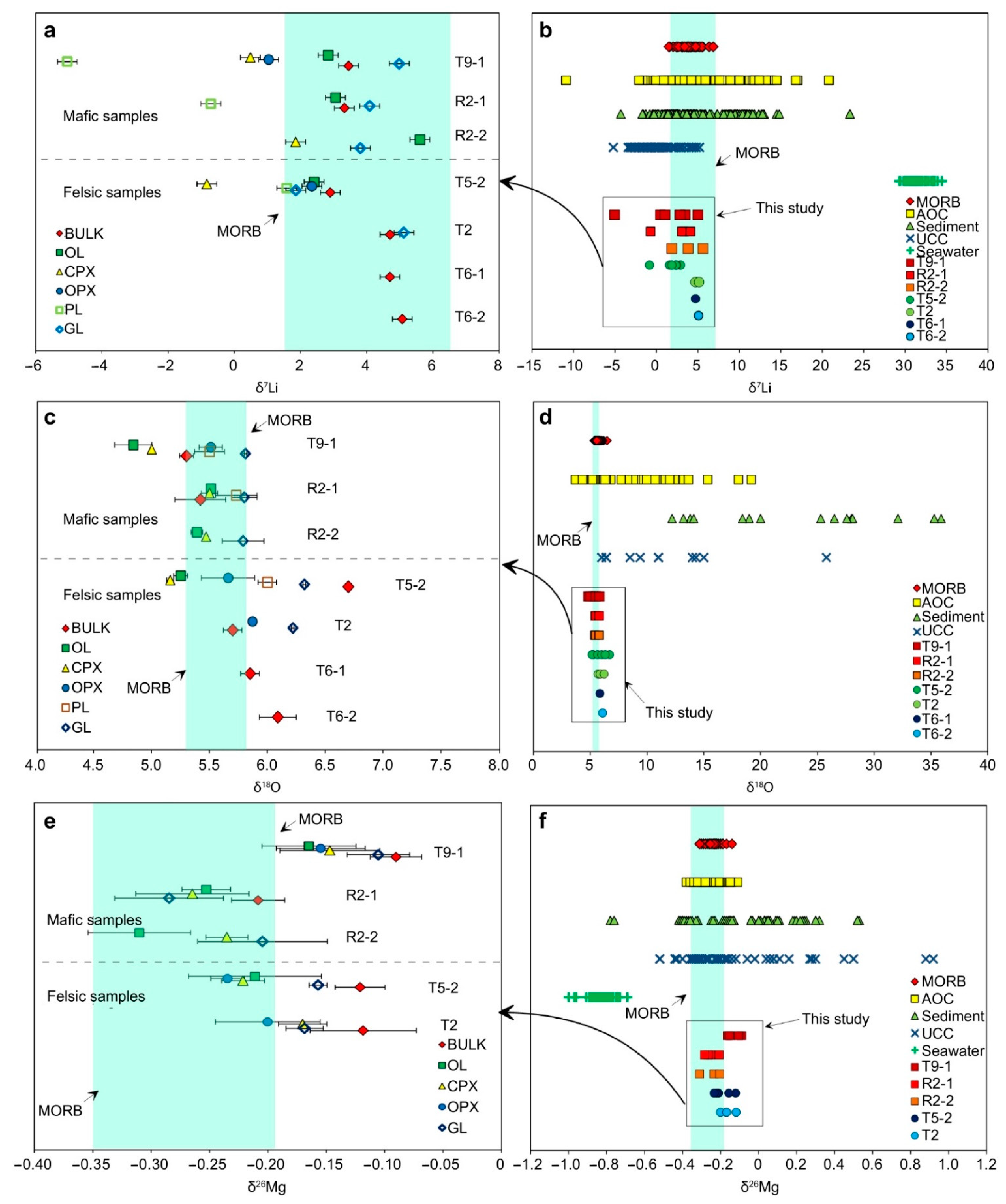
4.3. Li–O–Mg Isotope Compositions of the Volcanic Lavas and Minerals
5. Discussion
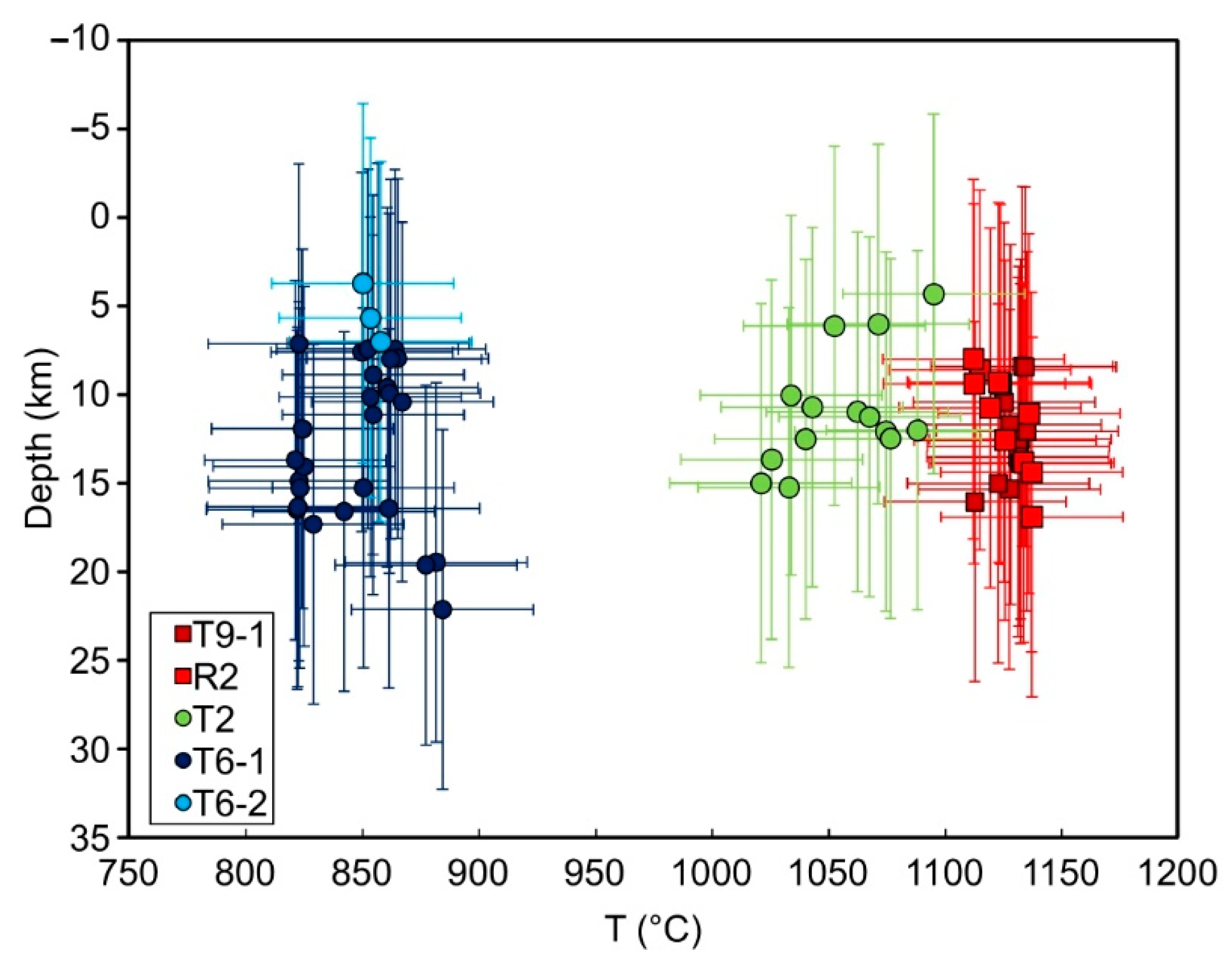
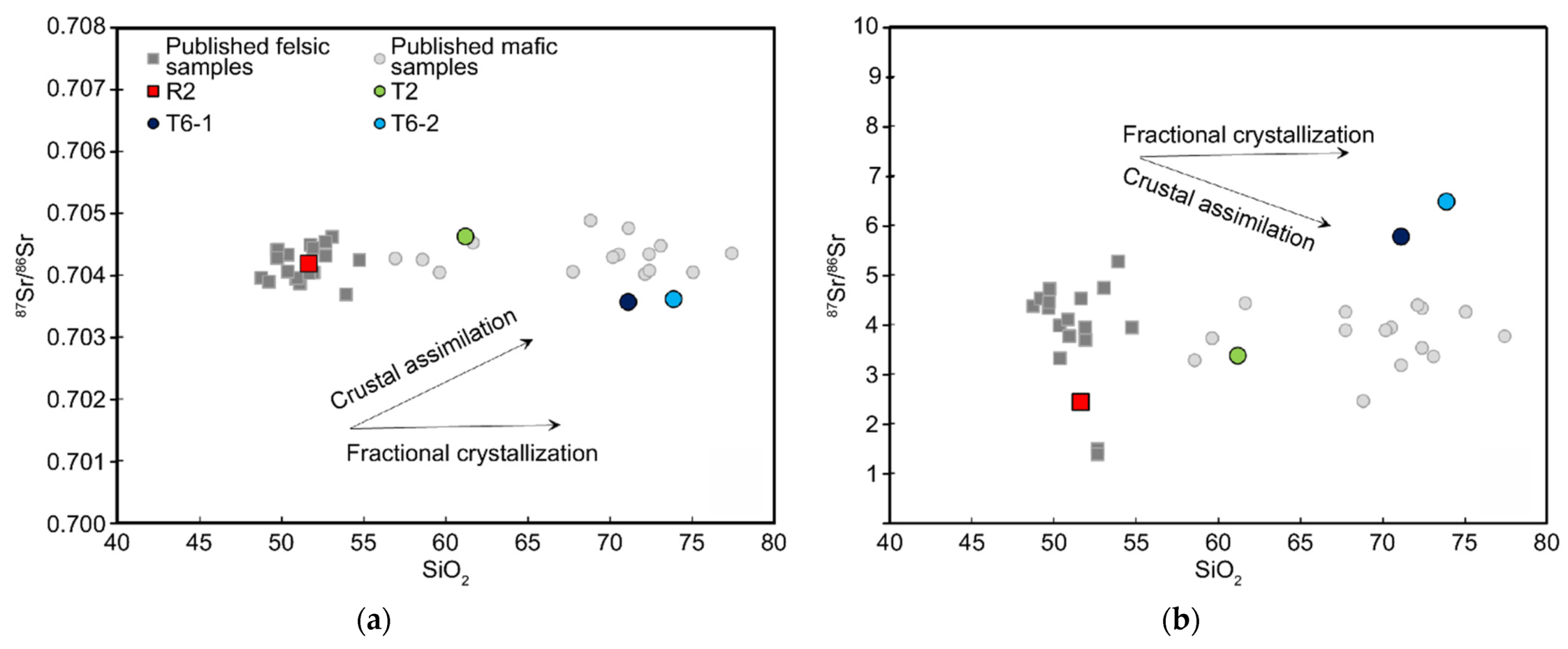
5.1. Genesis and Evolution of Magmas
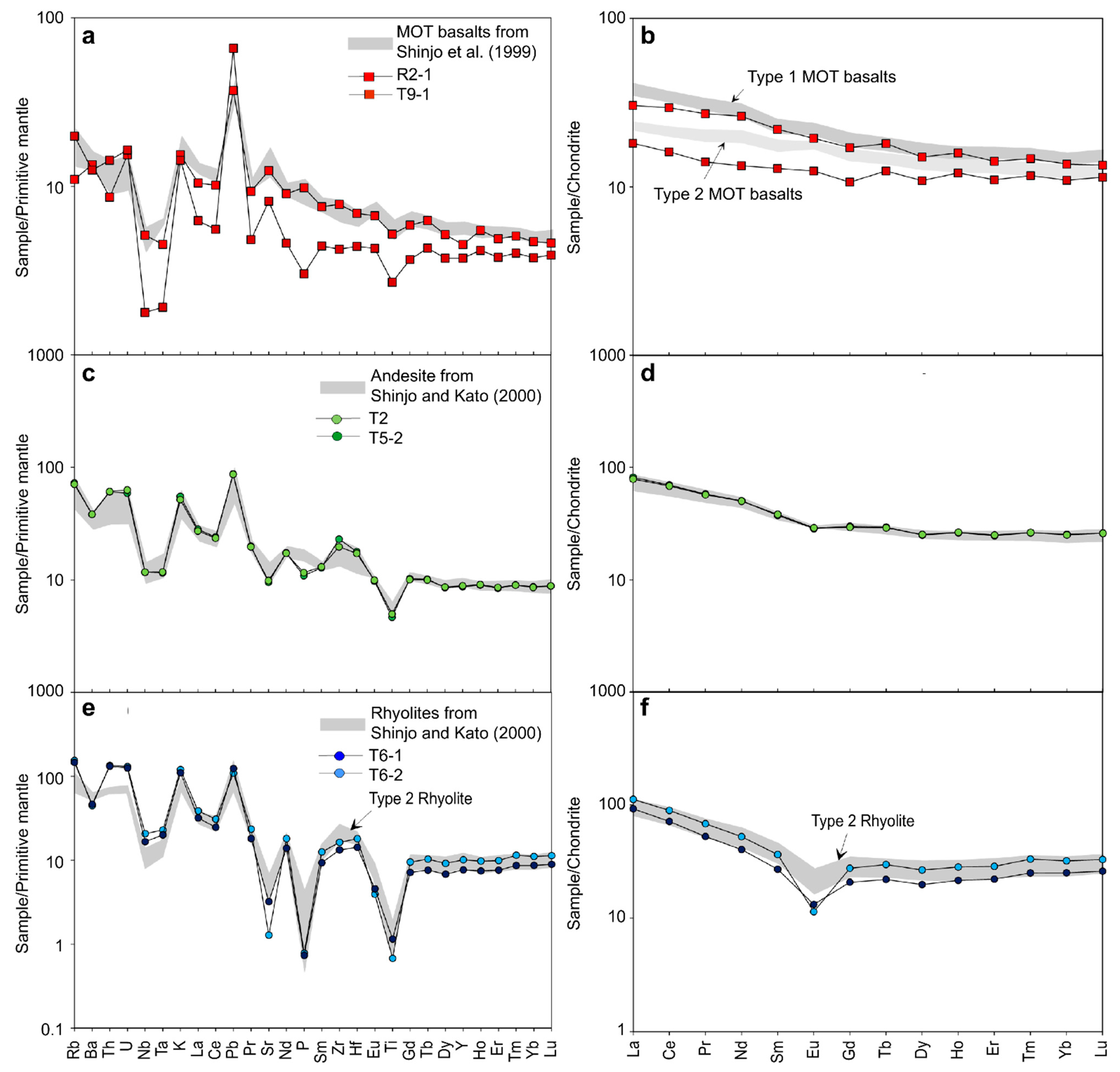
5.2. Influence of Subduction Components Inferred from Trace Elements
5.3. Subduction Input of Low-δ7Li Components to the OT Magmas
5.4. Subduction of Low-and High-δ18O Components
5.5. Contribution of High-δ26Mg Slab Fluids
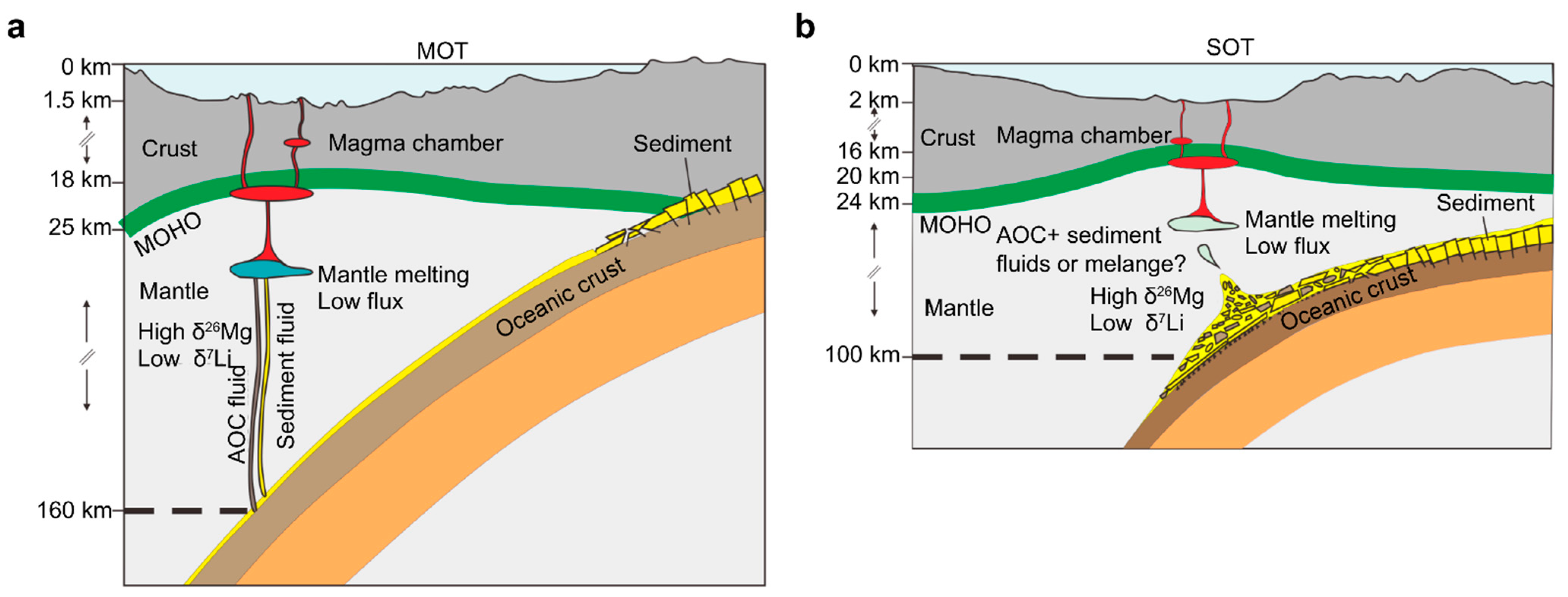
5.6. Mixing of Subduction Components
5.7. Implications of Differences in Plate Subduction
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guo, K.; Zhai, S.; Yu, Z.; Wang, S.; Zhang, X.; Wang, X. Geochemical and Sr-Nd-Pb-Li isotopic characteristics of volcanic rocks from the Okinawa Trough: Implications for the influence of subduction components and the contamination of crustal materials. J. Mar. Syst. 2018, 180, 140–151. [Google Scholar] [CrossRef]
- Bindeman, I.N.; Eiler, J.M.; Yogodzinski, G.M.; Tatsumi, Y.; Stern, C.R.; Grove, T.L.; Portnyagin, M.; Hoernle, K.; Danyushevsky, L.V. Oxygen isotope evidence for slab melting in modern and ancient subduction zones. Earth Planet. Sci. Lett. 2005, 235, 480–496. [Google Scholar] [CrossRef]
- Chan, L.H.; Kastner, M. Lithium isotopic compositions of pore fluids and sediments in the Costa Rica subduction zone: Implications for fluid processes and sediment contribution to the arc volcanoes. Earth Planet. Sci. Lett. 2000, 183, 275–290. [Google Scholar] [CrossRef]
- Chan, L.H.; Leeman, W.P.; You, C.F. Lithium isotopic composition of Central American volcanic arc lavas: Implications for modification of subarc mantle by slab-derived fluids: Correction. Chem. Geol. 2002, 182, 293–300. [Google Scholar] [CrossRef]
- Chen, Y.X.; Schertl, H.P.; Zheng, Y.F.; Huang, F.; Zhou, K.; Gong, Y.Z. Mg–O isotopes trace the origin of Mg-rich fluids in the deeply subducted continental crust of Western Alps. Earth Planet. Sci. Lett. 2016, 456, 157–167. [Google Scholar] [CrossRef]
- Huang, P.; Li, A.; Jiang, H. Geochemical features and their geological implications of volcanic rocks from the northern and middle Okinawa Trough. Acta Petrol. Sin. 2006, 22, 1703–1712. [Google Scholar]
- Ito, E.; Stern, R.J. Oxygen- and strontium-isotopic investigations of subduction zone volcanism: The case of the Volcano Arc and the Marianas Island Arc. Earth Planet. Sci. Lett. 1986, 76, 312–320. [Google Scholar] [CrossRef]
- Niu, Y.; Wilson, M.; Humphreys, E.R.; O’Hara, M.J. A trace element perspective on the source of ocean island basalts (OIB) and fate of subducted ocean crust (SOC) and mantle lithosphere (SML). Episodes 2012, 35, 310–327. [Google Scholar] [CrossRef] [Green Version]
- Pearce, J.A.; Peate, D.W. Tectonic implications of the composition of volcanic ARC magmas. Annu. Rev. Earth Planet. Sci. 1995, 23, 251–285. [Google Scholar] [CrossRef]
- Plank, T.; Langmuir, C.H. The chemical composition of subducting sediment and its consequences for the crust and mantle. Chem. Geol. 1998, 145, 325–394. [Google Scholar] [CrossRef]
- Shinjo, R. Geochemistry of high Mg andesites and the tectonic evolution of the Okinawa Trough–Ryukyu arc system. Chem. Geol. 1999, 157, 69–88. [Google Scholar] [CrossRef]
- Yang, Y.Z.; Wang, Y.; Ye, R.S.; Li, S.Q.; He, J.F.; Siebel, W.; Chen, F. Petrology and geochemistry of Early Cretaceous A-type granitoids and late Mesozoic mafic dikes and their relationship to adakitic intrusions in the lower Yangtze River belt, Southeast China. Int. Geol. Rev. 2017, 59, 62–79. [Google Scholar] [CrossRef]
- Taylor, B.; Martinez, F. Back-arc basin basalt systematics. Earth Planet. Sci. Lett. 2003, 210, 481–497. [Google Scholar] [CrossRef]
- White, W.M.; Duncan, R.A. Geochemistry and geochronology of the Society Islands: New evidence for deep mantle recycling. In Earth Processes: Reading the Isotopic Code; Basu, A., Hart, S., Eds.; American Geophysical Union: Washington, DC, USA, 1996; pp. 183–206. [Google Scholar]
- Duan, X.; Sun, H.; Yang, W.; Su, B.; Xiao, Y.; Hou, Z.; Shi, H. Melt–peridotite interaction in the shallow lithospheric mantle of the North China Craton: Evidence from melt inclusions in the quartz-bearing orthopyroxene-rich websterite from Hannuoba. Int Geol. Rev. 2014, 56, 448–472. [Google Scholar] [CrossRef]
- Class, C.; Miller, D.M.; Goldstein, S.L.; Langmuir, C.H. Distinguishing melt and fluid subduction components in Umnak Volcanics, Aleutian Arc. Geochem. Geophys Geosyst 2000, 1, 1004. [Google Scholar] [CrossRef]
- Elliott, T.; Plank, T.; Zindler, A.; White, W.; Bourdon, B. Element transport from slab to volcanic front at the Mariana arc. J. Geophys. Res. Solid Earth 1997, 102, 14991–15019. [Google Scholar] [CrossRef]
- George, R.; Turner, S.; Hawkesworth, C.; Morris, J.; Nye, C.; Ryan, J.; Zheng, S.H. Melting processes and fluid and sediment transport rates along the Alaska-Aleutian arc from an integrated U-Th-Ra-Be isotope study. J. Geophys. Res. Solid Earth 2003, 108, 2252. [Google Scholar] [CrossRef] [Green Version]
- Plank, T. Constraints from thorium/lanthanum on sediment recycling at subduction zones and the evolution of the continents. J. Pet. 2005, 46, 921–944. [Google Scholar] [CrossRef] [Green Version]
- Singer, B.S.; Jicha, B.R.; Leeman, W.P.; Rogers, N.W.; Thirlwall, M.F.; Ryan, J.; Nicolaysen, K.E. Along-strike trace element and isotopic variation in Aleutian Island arc basalt: Subduction melts sediments and dehydrates serpentine. J. Geophys. Res. 2007, 112, B06206. [Google Scholar] [CrossRef] [Green Version]
- Brenan, J.M.; Ryerson, F.J.; Shaw, H.F. The role of aqueous fluids in the slab-to-mantle transfer of boron, beryllium, and lithium during subduction: Experiments and models. Geochim. Cosmochim. Acta 1998, 62, 3337–3347. [Google Scholar] [CrossRef]
- Chan, L.; Edmond, J.; Thompson, G.; Gillis, K. Lithium isotopic composition of submarine basalts: Implications for the lithium cycle in the oceans. Earth Planet. Sci. Lett. 1992, 108, 151–160. [Google Scholar] [CrossRef]
- Teng, F.Z.; Rudnick, R.L.; McDonough, W.F.; Gao, S.; Tomascak, P.B.; Liu, Y. Lithium isotopic composition and concentration of the deep continental crust. Chem. Geol. 2008, 255, 47–59. [Google Scholar] [CrossRef]
- Wunder, B.; Meixner, A.; Romer, R.L.; Heinrich, W. Temperature-dependent isotopic fractionation of lithium between clinopyroxene and high-pressure hydrous fluids. Contrib Miner. Pet. 2006, 151, 112–120. [Google Scholar] [CrossRef]
- Bouman, C.; Elliott, T.; Vroon, P.Z. Lithium inputs to subduction zones. Chem. Geol. 2004, 212, 59–79. [Google Scholar] [CrossRef]
- Chan, L.H.; Alt, J.C.; Teagle, D.A.H. Lithium and lithium isotope profiles through the upper oceanic crust: A study of seawater–basalt exchange at ODP Sites 504B and 896A. Earth Planet. Sci. Lett. 2002, 201, 187–201. [Google Scholar] [CrossRef]
- Chan, L.H.; Leeman, W.P.; Plank, T. Lithium isotopic composition of marine sediments. Geochem. Geophys Geosyst 2006, 7, Q06005. [Google Scholar] [CrossRef]
- Zack, T.; Tomascak, P.B.; Rudnick, R.L.; Dalpé, C.; McDonough, W.F. Extremely light Li in orogenic eclogites: The role of isotope fractionation during dehydration in subducted oceanic crust. Earth Planet. Sci. Lett. 2003, 208, 279–290. [Google Scholar] [CrossRef]
- Marschall, H.R.; von Strandmann, P.A.E.P.; Seitz, H.M.; Elliott, T.; Niu, Y. The lithium isotopic composition of orogenic eclogites and deep subducted slabs. Earth Planet. Sci. Lett. 2007, 262, 563–580. [Google Scholar] [CrossRef]
- Xiao, Y.; Hoefs, J.; Hou, Z.; Simon, K.; Zhang, Z. Fluid/rock interaction and mass transfer in continental subduction zones: Constraints from trace elements and isotopes (Li, B, O, Sr, Nd, Pb) in UHP rocks from the Chinese Continental Scientific Drilling Program, Sulu, East China. Contrib. Miner. Pet. 2011, 162, 797–819. [Google Scholar] [CrossRef] [Green Version]
- Xiao, Y.; Sun, H.; Gu, H.; Huang, J.; Li, W.; Liu, L. Fluid/melt in continental deep subduction zones: Compositions and related geochemical fractionations. Sci. China Earth Sci. 2015, 58, 1457–1476. [Google Scholar] [CrossRef]
- Ishikawa, T.; Nakamura, E. Origin of the slab component in arc lavas from across-arc variation of B and Pb isotopes. Nature 1994, 370, 205–208. [Google Scholar] [CrossRef]
- Moriguti, T.; Nakamura, E. Across-arc variation of Li isotopes in lavas and implications for crust/mantle recycling at subduction zones. Earth Planet. Sci. Lett. 1998, 163, 167–174. [Google Scholar] [CrossRef]
- Nakamura, E.; Campbell, I.H.; Sun, S.S. The influence of subduction processes on the geochemistry of Japanese alkaline basalts. Nature 1985, 316, 55–58. [Google Scholar] [CrossRef]
- Benton, L.D.; Ryan, J.G.; Savov, I.P. Lithium abundance and isotope systematics of forearc serpentinites, Conical Seamount, Mariana forearc: Insights into the mechanics of slab-mantle exchange during subduction. Geochem. Geophys. Geosyst. 2004, 5, Q08J12. [Google Scholar] [CrossRef] [Green Version]
- Honma, H.; Kusakabe, M.; Kagami, H.; Iizumi, S.; Sakai, H.; Kodama, Y.; Kimura, M. Major and trace element chemistry and D/H, 18O/16O, 87Sr/86Sr and 143Nd/144Nd ratios of rocks from the spreading center of the Okinawa Trough, a marginal back-arc basin. Geochem. J. 1991, 25, 121–136. [Google Scholar] [CrossRef] [Green Version]
- Macpherson, C.G.; Hilton, D.R.; Mattey, D.P.; Sinton, J.M. Evidence for an 18O-depleted mantle plume from contrasting 18O/16O ratios of back-arc lavas from the Manus Basin and Mariana Trough. Earth Planet. Sci. Lett. 2000, 176, 171–183. [Google Scholar] [CrossRef]
- Macpherson, C.G.; Mattey, D.P. Oxygen isotope variations in Lau Basin lavas. Chem. Geol. 1998, 144, 177–194. [Google Scholar] [CrossRef]
- Huang, J.; Ke, S.; Gao, Y.; Xiao, Y.; Li, S. Magnesium isotopic compositions of altered oceanic basalts and gabbros from IODP site 1256 at the East Pacific Rise. Lithos 2015, 231, 53–61. [Google Scholar] [CrossRef]
- Li, S.G.; Yang, W.; Ke, S.; Meng, X.; Tian, H.; Xu, L.; He, Y.; Huang, J.; Wang, X.C.; Xia, Q.; et al. Deep carbon cycles constrained by a large-scale mantle Mg isotope anomaly in eastern China. Natl. Sci. Rev. 2017, 4, 111–120. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.J.; Teng, F.Z.; Li, S.G.; Hong, J.A. Magnesium isotopic systematics of mafic rocks during continental subduction. Geochim Cosmochim Acta 2014, 143, 34–48. [Google Scholar] [CrossRef]
- Wang, S.J.; Teng, F.Z.; Williams, H.M.; Li, S.G. Magnesium isotopic variations in cratonic eclogites: Origins and implications. Earth Planet. Sci. Lett. 2012, 359-360, 219–226. [Google Scholar] [CrossRef] [Green Version]
- Xiao, Y.; Teng, F.Z.; Zhang, H.F.; Yang, W. Large magnesium isotope fractionation in peridotite xenoliths from eastern North China craton: Product of melt–rock interaction. Geochim Cosmochim Acta 2013, 115, 241–261. [Google Scholar] [CrossRef]
- Yang, W.; Teng, F.Z.; Zhang, H.F.; Li, S.G. Magnesium isotopic systematics of continental basalts from the North China craton: Implications for tracing subducted carbonate in the mantle. Chem. Geol. 2012, 328, 185–194. [Google Scholar] [CrossRef]
- Shimoda, G.; Tatsumi, Y.; Nohda, S.; Ishizaka, K.; Jahn, B.M. Setouchi high-Mg andesites revisited: Geochemical evidence for melting of subducting sediments. Earth Planet. Sci. Lett. 1998, 160, 479–492. [Google Scholar] [CrossRef]
- Shu, Y.; Nielsen, S.G.; Zeng, Z.; Shinjo, R.; Blusztajn, J.; Wang, X.; Chen, S. Tracing subducted sediment inputs to the Ryukyu arc-Okinawa Trough system: Evidence from thallium isotopes. Geochim Cosmochim Acta 2017, 217, 462–491. [Google Scholar] [CrossRef]
- Guo, K.; Zeng, Z.G.; Chen, S.; Zhang, Y.X.; Qi, H.Y.; Ma, Y. The influence of a subduction component on magmatism in the Okinawa Trough: Evidence from thorium and related trace element ratios. J. Asian Earth Sci. 2017, 145, 205–216. [Google Scholar] [CrossRef]
- Li, Z.G.; Chu, F.Y.; Dong, Y.H.; Liu, J.Q.; Chen, L. Geochemical constraints on the contribution of Louisville seamount materials to magmagenesis in the Lau back-arc basin, SW Pacific. Int. Geol. Rev. 2015, 57, 978–997. [Google Scholar] [CrossRef]
- Pi, J.L.; You, C.F.; Wang, K.L. The influence of Ryukyu subduction on magma genesis in the Northern Taiwan Volcanic Zone and Middle Okinawa Trough—Evidence from boron isotopes. Lithos 2016, 260, 242–252. [Google Scholar] [CrossRef]
- Shinjo, R.; Chung, S.L.; Kato, Y.; Kimura, M. Geochemical and Sr-Nd isotopic characteristics of volcanic rocks from the Okinawa Trough and Ryukyu Arc: Implications for the evolution of a young, intracontinental back arc basin. J. Geophys Res. Solid Earth 1999, 104, 10591–10608. [Google Scholar] [CrossRef]
- Chang-Hwa, C.; Typhoon, L.; Yuch-Ning, S.; Cheng-Hong, C.; Wen-Yu, H. Magmatism at the onset of back-arc basin spreading in the Okinawa Trough. J. Volcanol. Geotherm. Res. 1995, 69, 313–322. [Google Scholar] [CrossRef]
- Zengqian, H.; Zaw, K.; Yanhe, L.; Qiling, Z.; Zhigang, Z.; Urabe, T. Contribution of magmatic fluid to the active hydrothermal system in the JADE Field, Okinawa trough: Evidence from fluid inclusions, oxygen and helium isotopes. Int. Geol. Rev. 2010, 47, 420–437. [Google Scholar] [CrossRef]
- Zeng, Z.; Yu, S.; Wang, X.; Fu, Y.; Yin, X.; Zhang, G.; Wang, X.; Chen, S. Geochemical and isotopic characteristics of volcanic rocks from the northern East China Sea shelf margin and the Okinawa Trough. Acta Oceanol. Sin. 2010, 29, 48–61. [Google Scholar] [CrossRef]
- Lee, C.S.; Shor, G.G.; Bibee, L.D.; Lu, R.S.; Hilde, T.W.C. Okinawa Trough: Origin of a back-arc basin. Mar. Geol. 1980, 35, 219–241. [Google Scholar] [CrossRef]
- Seno, T.; Maruyama, S. Paleogeographic reconstruction and origin of the Philippine Sea. Tectonophysics 1984, 102, 53–84. [Google Scholar] [CrossRef]
- Seno, T.; Stein, S.; Gripp, A.E. A model for the motion of the Philippine Sea Plate consistent with NUVEL-1 and geological data. J. Geophys. Res. Solid Earth 1993, 98, 17941–17948. [Google Scholar] [CrossRef]
- Hoang, N.; Uto, K. Upper mantle isotopic components beneath the Ryukyu arc system: Evidence for ‘back-arc’ entrapment of Pacific MORB mantle. Earth Planet. Sci. Lett. 2006, 249, 229–240. [Google Scholar] [CrossRef]
- Ishizuka, H.; Kawanobe, Y.; Sakai, H. Petrology and geochemistry of volcanic rocks dredged from the Okinawa Trough, an active back-arc basin. Geochem. J. 1990, 24, 75–92. [Google Scholar] [CrossRef]
- Han, B.; Zhang, X.H.; Pei, J.X.; Zhang, W.G. Characteristics of crust-mantle in the East China Sea and adjacent regions. Prog. Geophys. 2007, 22, 376–382. [Google Scholar]
- Iwasaki, T.; Hirata, N.; Kanazawa, T.; Melles, J.; Suyehiro, K.; Urabe, T.; Möller, L.; Makris, J.; Shimamura, H. Crustal and upper mantle structure in the Ryukyu Island Arc deduced from deep seismic sounding. Geophys. J. Int. 1990, 102, 631–651. [Google Scholar] [CrossRef] [Green Version]
- Klingelhoefer, F.; Lee, C.S.; Lin, J.Y.; Sibuet, J.C. Structure of the southernmost Okinawa Trough from reflection and wide-angle seismic data. Tectonophys 2009, 466, 281–288. [Google Scholar] [CrossRef] [Green Version]
- Liu, B.; Li, S.Z.; Suo, Y.H.; Li, G.X.; Dai, L.M.; Somerville, I.D.; Guo, L.L.; Zhao, S.J.; Yu, S. The geological nature and geodynamics of the Okinawa Trough, Western Pacific. Geol. J. 2016, 51, 416–428. [Google Scholar] [CrossRef]
- Nakahigashi, K.; Shinohara, M.; Suzuki, S.; Hino, R.; Shiobara, H.; Takenaka, H.; Nishino, M.; Sato, T.; Yoneshima, S.; Kanazawa, T. Seismic structure of the crust and uppermost mantle in the incipient stage of back arc rifting—Northernmost Okinawa Trough. Geophys Res. Lett. 2004, 31, L02614. [Google Scholar] [CrossRef]
- Sibuet, J.C.; Hsu, S.K.; Shyu, C.T.; Liu, C.S. Structural and kinematic evolutions of the Okinawa Trough backarc basin. In Backarc Basins: Tectonics and Magmatism; Taylor, B., Ed.; Springer: Boston, MA, USA, 1995; pp. 343–379. [Google Scholar]
- Arai, R.; Kodaira, S.; Yuka, K.; Takahashi, T.; Miura, S.; Kaneda, Y. Crustal structure of the southern Okinawa Trough: Symmetrical rifting, submarine volcano, and potential mantle accretion in the continental back-arc basin. J. Geophys Res. Solid Earth 2017, 122, 622–641. [Google Scholar] [CrossRef]
- Furukawa, M.; Tokuyama, H.; Abe, S.; Nishizawa, A.; Kinoshita, H. Report on DELP 1988 cruises in the Okinawa Trough: Part 2. seismic reflection studies in the southwestern part of the Okinawa Trough. Bull. Earthq Res. Inst. Univ. Tokyo 1991, 66, 17–36. [Google Scholar]
- Letouzey, J.; Kimura, M. Okinawa Trough genesis: Structure and evolution of a backarc basin developed in a continent. Mar. Pet. Geol. 1985, 2, 111–130. [Google Scholar] [CrossRef]
- Sibuet, J.C.; Deffontaines, B.; Hsu, S.K.; Thareau, N.; Le Formal, J.P.; Liu, C.S. Okinawa trough backarc basin: Early tectonic and magmatic evolution. J. Geophys Res. Solid Earth 1998, 103, 30245–30267. [Google Scholar] [CrossRef] [Green Version]
- Argus, D.F.; Gordon, R.G.; DeMets, C. Geologically current motion of 56 plates relative to the no-net-rotation reference frame. Geochem. Geophys Geosyst 2011, 12, Q11001. [Google Scholar] [CrossRef] [Green Version]
- Kubo, A.; Fukuyama, E. Stress field along the Ryukyu Arc and the Okinawa Trough inferred from moment tensors of shallow earthquakes. Earth Planet. Sci. Lett. 2003, 210, 305–316. [Google Scholar] [CrossRef]
- Pezzopane, S.K.; Wesnousky, S.G. Large earthquakes and crustal deformation near Taiwan. J. Geophys Res. 1989, 94, 7250–7264. [Google Scholar] [CrossRef]
- Li, W.; Yang, Z.; Wang, Y.; Zhang, B. The petrochemical features of the volcanic rocks in the Okinawa Trough and their geological significance. Acta Petrol. Sin. 1997, 13, 538–550. [Google Scholar]
- Shinjo, R.; Kato, Y. Geochemical constraints on the origin of bimodal magmatism at the Okinawa Trough, an incipient back-arc basin. Lithos 2000, 54, 117–137. [Google Scholar] [CrossRef]
- Li, X.; Zeng, Z.; Chen, S.; Ma, Y.; Yang, H.; Zhang, Y.; Chen, Z. Geochemical and Sr-Nd-Pb isotopic compositions of volcanic rocks from the Iheya Ridge, the middle Okinawa Trough: Implications for petrogenesis and a mantle source. Acta Oceanol Sin. 2018, 37, 73–88. [Google Scholar] [CrossRef]
- Chen, Z.; Zeng, Z.; Wang, X.; Zhang, Y.; Yin, X.; Chen, S.; Ma, Y.; Li, X.; Qi, H. Mineral chemistry indicates the petrogenesis of rhyolite from the southwestern Okinawa Trough. J. Ocean. Univ. China 2017, 16, 1097–1108. [Google Scholar] [CrossRef]
- Ishikawa, M.; Sato, H.; Furukawa, M.; Kimura, M.; Kato, Y.; Tsugaru, R.; Shimamura, K. Report on DELP 1988 cruises in the Okinawa Trough: Part 6. petrology of volcanic rocks. Bull. Earthq. Res. Inst. Univ. Tokyo 1991, 66, 151–177. [Google Scholar]
- Gao, Y.; Casey, J.F. Lithium isotope composition of ultramafic geological reference materials JP-1 and DTS-2. Geostand Geoanal Res. 2012, 36, 75–81. [Google Scholar] [CrossRef]
- Rudnick, R.L.; Tomascak, P.B.; Njo, H.B.; Gardner, L.R. Extreme lithium isotopic fractionation during continental weathering revealed in saprolites from South Carolina. Chem. Geol. 2004, 212, 45–57. [Google Scholar] [CrossRef]
- Flesch, G.D.; Anderson, A.R.; Svec, H.J. A secondary isotopic standard for 6Li/7Li determinations. Int. J. Mass Spectrom. Ion. Phys. 1973, 12, 265–272. [Google Scholar] [CrossRef]
- Zheng, Y.F.; Wang, Z.R.; Li, S.G.; Zhao, Z.F. Oxygen isotope equilibrium between eclogite minerals and its constraints on mineral Sm-Nd chronometer. Geochim. Cosmochim. Acta 2002, 66, 625–634. [Google Scholar] [CrossRef]
- Zheng, Y.F.; Fu, B.; Li, Y.; Xiao, Y.; Li, S. Oxygen and hydrogen isotope geochemistry of ultrahigh-pressure eclogites from the Dabie Mountains and the Sulu terrane. Earth Planet. Sci. Lett. 1998, 155, 113–129. [Google Scholar] [CrossRef]
- Gong, B.; Zheng, Y.F.; Chen, R.X. TC/EA-MS online determination of hydrogen isotope composition and water concentration in eclogitic garnet. Phys. Chem. Min. 2007, 34, 687–698. [Google Scholar] [CrossRef]
- An, Y.; Wu, F.; Xiang, Y.; Nan, X.; Yu, X.; Yang, J.; Yu, H.; Xie, L.; Huang, F. High-precision Mg isotope analyses of low-Mg rocks by MC-ICP-MS. Chem. Geol. 2014, 390, 9–21. [Google Scholar] [CrossRef]
- Putirka, K.; Condit, C.D. Cross section of a magma conduit system at the margin of the Colorado Plateau. Geology 2003, 31, 701–704. [Google Scholar] [CrossRef]
- Putirka, K.D. Thermometers and barometers for volcanic systems. Rev. Mineral. Geochem. 2008, 69, 61–120. [Google Scholar] [CrossRef]
- Irvine, T.N.; Baragar, W.R.A. A guide to the chemical classification of the common volcanic rocks. Can. J. Earth Sci. 1971, 8, 523–548. [Google Scholar] [CrossRef]
- Le Maitre, R.W. A classification of Igneous Rocks and Glossary of Terms: Recommendations of the International Union of Geological Sci.ences Subcommission on the Systematics of Igneous Rocks; Blackwell: Oxford, UK, 1989. [Google Scholar]
- Roberts, M.P.; Clemens, J.D. Origin of high-potassium, talc-alkaline, I-type granitoids. Geology 1993, 21, 825–828. [Google Scholar] [CrossRef]
- Hu, Y.; Niu, Y.; Li, J.; Ye, L.; Kong, J.; Chen, S.; Zhang, Y.; Zhang, G. Petrogenesis and tectonic significance of the late Triassic mafic dikes and felsic volcanic rocks in the East Kunlun Orogenic Belt, Northern Tibet Plateau. Lithos 2016, 245, 205–222. [Google Scholar] [CrossRef] [Green Version]
- Sun, S.S.; McDonough, W.F. Chemical and isotopic systematics of oceanic basalts: Implications for mantle composition and processes. Geol. Soc. Lond. Spec. Publ. 1989, 42, 313–345. [Google Scholar] [CrossRef]
- Elliott, T.; Thomas, A.; Jeffcoate, A.; Niu, Y. Lithium isotope evidence for subduction-enriched mantle in the source of mid-ocean-ridge basalts. Nature 2006, 443, 565–568. [Google Scholar] [CrossRef]
- Nishio, Y.; Nakai, S.i.; Ishii, T.; Sano, Y. Isotope systematics of Li, Sr, Nd, and volatiles in Indian Ocean MORBs of the Rodrigues Triple Junction: Constraints on the origin of the DUPAL anomaly. Geochim. Cosmochim. Acta 2007, 71, 745–759. [Google Scholar] [CrossRef]
- Tomascak, P.B.; Langmuir, C.H.; le Roux, P.J.; Shirey, S.B. Lithium isotopes in global mid-ocean ridge basalts. Geochim. Cosmochim. Acta 2008, 72, 1626–1637. [Google Scholar] [CrossRef]
- Brant, C.; Coogan, L.A.; Gillis, K.M.; Seyfried, W.E.; Pester, N.J.; Spence, J. Lithium and Li-isotopes in young altered upper oceanic crust from the East Pacific Rise. Geochim. Cosmochim. Acta 2012, 96, 272–293. [Google Scholar] [CrossRef]
- James, R.H.; Rudnicki, M.D.; Palmer, M.R. The alkali element and boron geochemistry of the Escanaba Trough sediment-hosted hydrothermal system. Earth Planet. Sci. Lett. 1999, 171, 157–169. [Google Scholar] [CrossRef]
- Leeman, W.P.; Tonarini, S.; Chan, L.H.; Borg, L.E. Boron and lithium isotopic variations in a hot subduction zone—the southern Washington Cascades. Chem. Geol. 2004, 212, 101–124. [Google Scholar] [CrossRef]
- Zhang, L.; Chan, L.H.; Gieskes, J.M. Lithium isotope geochemistry of pore waters from ocean drilling program Sites 918 and 919, Irminger Basin. Geochim. Cosmochim. Acta 1998, 62, 2437–2450. [Google Scholar] [CrossRef]
- Teng, F.Z.; McDonough, W.F.; Rudnick, R.L.; Dalpé, C.; Tomascak, P.B.; Chappell, B.W.; Gao, S. Lithium isotopic composition and concentration of the upper continental crust. Geochim. Cosmochim. Acta 2004, 68, 4167–4178. [Google Scholar] [CrossRef]
- Cooper, K.M.; Eiler, J.M.; Sims, K.W.W.; Langmuir, C.H. Distribution of recycled crust within the upper mantle: Insights from the oxygen isotope composition of MORB from the Australian-Antarctic Discordance. Geochem. Geophys. Geosyst. 2009, 10, Q12004. [Google Scholar] [CrossRef] [Green Version]
- Eiler, J.M.; Schiano, P.; Kitchen, N.; Stolper, E.M. Oxygen-isotope evidence for recycled crust in the sources of mid-ocean-ridge basalts. Nature 2000, 403, 530–534. [Google Scholar] [CrossRef]
- Ito, E.; White, W.M.; Göpel, C. The O, Sr, Nd and Pb isotope geochemistry of MORB. Chem. Geol. 1987, 62, 157–176. [Google Scholar] [CrossRef]
- Gregory, R.T.; Taylor, H.P. An oxygen isotope profile in a section of Cretaceous oceanic crust, Samail Ophiolite, Oman: Evidence for δ18O buffering of the oceans by deep (>5 km) seawater-hydrothermal circulation at mid-ocean ridges. J. Geophys Res. Solid Earth 1981, 86, 2737–2755. [Google Scholar] [CrossRef] [Green Version]
- Staudigel, H.; Davies, G.R.; Hart, S.R.; Marchant, K.M.; Smith, B.M. Large scale isotopic Sr, Nd and O isotopic anatomy of altered oceanic crust: DSDP/ODP sites417/418. Earth Planet. Sci. Lett. 1995, 130, 169–185. [Google Scholar] [CrossRef]
- Alt, J.; Shanksiii, W. Stable isotope compositions of serpentinite seamounts in the Mariana forearc: Serpentinization processes, fluid sources and sulfur metasomatism. Earth Planet. Sci. Lett. 2006, 242, 272–285. [Google Scholar] [CrossRef]
- Simon, L.; Lécuyer, C. Continental recycling: The oxygen isotope point of view. Geochem. Geophys Geosyst. 2005, 6, Q08004. [Google Scholar] [CrossRef]
- Bourdon, B.; Tipper, E.T.; Fitoussi, C.; Stracke, A. Chondritic Mg isotope composition of the Earth. Geochim. Cosmochim. Acta 2010, 74, 5069–5083. [Google Scholar] [CrossRef]
- Teng, F.Z.; Li, W.Y.; Ke, S.; Marty, B.; Dauphas, N.; Huang, S.; Wu, F.Y.; Pourmand, A. Magnesium isotopic composition of the Earth and chondrites. Geochim. Cosmochim. Acta 2010, 74, 4150–4166. [Google Scholar] [CrossRef]
- Teng, F.Z.; Hu, Y.; Chauvel, C. Magnesium isotope geochemistry in arc volcanism. Proc. Natl. Acad. Sci. USA 2016, 113, 7082–7087. [Google Scholar] [CrossRef] [Green Version]
- Li, W.Y.; Teng, F.Z.; Ke, S.; Rudnick, R.L.; Gao, S.; Wu, F.Y.; Chappell, B.W. Heterogeneous magnesium isotopic composition of the upper continental crust. Geochim. Cosmochim. Acta 2010, 74, 6867–6884. [Google Scholar] [CrossRef]
- Eiler, J.M. Oxygen isotope variations of basaltic lavas and upper mantle rocks. Rev. Miner. Geochem. 2001, 43, 319–364. [Google Scholar] [CrossRef] [Green Version]
- Mattey, D.; Lowry, D.; Macpherson, C. Oxygen isotope composition of mantle peridotite. Earth Planet. Sci. Lett. 1994, 128, 231–241. [Google Scholar] [CrossRef]
- Beattie, P. Olivine-melt and orthopyroxene-melt equilibria. Contrib. Miner. Pet. 1993, 115, 103–111. [Google Scholar] [CrossRef]
- Davis, B.T.C.; Boyd, F.R. The join Mg2Si2O6-CaMgSi2O6at 30 kilobars pressure and its application to pyroxenes from kimberlites. J. Geophys Res. 1966, 71, 3567–3576. [Google Scholar] [CrossRef]
- Rudnick, R.L.; Gao, S. Composition of the continental crust. In Treatise on Geochemistry; Holland, H.D., Turekian, K.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2003; Volume 3, pp. 1–64. [Google Scholar]
- Park, S.H.; Lee, S.M.; Kamenov, G.D.; Kwon, S.T.; Lee, K.Y. Tracing the origin of subduction components beneath the South East rift in the Manus Basin, Papua New Guinea. Chem. Geol. 2010, 269, 339–349. [Google Scholar] [CrossRef]
- Hong, L.B.; Zhang, Y.H.; Qian, S.P.; Liu, J.Q.; Ren, Z.Y.; Xu, Y.G. Constraints from melt inclusions and their host olivines on the petrogenesis of Oligocene-Early Miocene Xindian basalts, Chifeng area, North China Craton. Contrib. Miner. Pet. 2013, 165, 305–326. [Google Scholar] [CrossRef]
- Sun, H.; Xiao, Y.; Gao, Y.; Lai, J.; Hou, Z.; Wang, Y. Fluid and melt inclusions in the Mesozoic Fangcheng basalt from North China Craton: Implications for magma evolution and fluid/melt-peridotite reaction. Contrib. Miner. Pet. 2013, 165, 885–901. [Google Scholar] [CrossRef]
- Zhang, Y.; Zeng, Z.; Li, X.; Yin, X.; Wang, X.; Chen, S.; Li, S. High-potassium volcanic rocks from the Okinawa Trough: Implications for a cryptic potassium-rich and DUPAL-like source. Geol. J. 2018, 53, 1755–1766. [Google Scholar] [CrossRef]
- Prægel, N.O.; Holm, P.M. Lithospheric contributions to high-MgO basanites from the Cumbre Vieja Volcano, La Palma, Canary Islands and evidence for temporal variation in plume influence. J. Volcanol. Geotherm. Res. 2006, 149, 213–239. [Google Scholar] [CrossRef]
- Zhang, H.F.; Sun, M. Geochemistry of Mesozoic basalts and mafic dikes, southeastern North China Craton, and tectonic implications. Int. Geol. Rev. 2002, 44, 370–382. [Google Scholar] [CrossRef]
- Zhang, J.J.; Zheng, Y.F.; Zhao, Z.F. Geochemical evidence for interaction between oceanic crust and lithospheric mantle in the origin of Cenozoic continental basalts in east-central China. Lithos 2009, 110, 305–326. [Google Scholar] [CrossRef]
- Liu, Y.; Gao, S.; Gao, C.; Zong, K.; Hu, Z.; Ling, W. Garnet-rich granulite xenoliths from the Hannuoba basalts, North China: Petrogenesis and implications for the Mesozoic crust-mantle interaction. J. Earth Sci. 2010, 21, 669–691. [Google Scholar] [CrossRef]
- Moriguti, T.; Shibata, T.; Nakamura, E. Lithium, boron and lead isotope and trace element systematics of Quaternary basaltic volcanic rocks in northeastern Japan: Mineralogical controls on slab-derived fluid composition. Chem. Geol. 2004, 212, 81–100. [Google Scholar] [CrossRef]
- Tomascak, P.B.; Widom, E.; Benton, L.D.; Goldstein, S.L.; Ryan, J.G. The control of lithium budgets in island arcs. Earth Planet. Sci. Lett. 2002, 196, 227–238. [Google Scholar] [CrossRef] [Green Version]
- Grove, T.; Parman, S.; Bowring, S.; Price, R.; Baker, M. The role of an H2O-rich fluid component in the generation of primitive basaltic andesites and andesites from the Mt. Shasta region, N California. Contrib. Mineral. Petrol. 2002, 142, 375–396. [Google Scholar] [CrossRef]
- Handley, H.K.; Macpherson, C.G.; Davidson, J.P.; Berlo, K.; Lowry, D. Constraining fluid and sediment contributions to subduction-related magmatism in Indonesia: Ijen volcanic complex. J. Pet. 2007, 48, 1155–1183. [Google Scholar] [CrossRef] [Green Version]
- Magna, T.; Wiechert, U.; Grove, T.L.; Halliday, A.N. Lithium isotope fractionation in the southern Cascadia subduction zone. Earth Planet. Sci. Lett. 2006, 250, 428–443. [Google Scholar] [CrossRef]
- Decitre, S.; Deloule, E.; Reisberg, L.; James, R.; Agrinier, P.; Mével, C. Behavior of Li and its isotopes during serpentinization of oceanic peridotites. Geochem. Geophys Geosyst 2002, 3, 1–20. [Google Scholar] [CrossRef] [Green Version]
- Lui-Heung, C.; Gieskes, J.M.; Chen-Feng, Y.; Edmond, J.M. Lithium isotope geochemistry of sediments and hydrothermal fluids of the Guaymas Basin, Gulf of California. Geochim. Cosmochim. Acta 1994, 58, 4443–4454. [Google Scholar] [CrossRef]
- Tomascak, P.B.; Carlson, R.W.; Shirey, S.B. Accurate and precise determination of Li isotopic compositions by multi-collector sector ICP-MS. Chem. Geol. 1999, 158, 145–154. [Google Scholar] [CrossRef]
- Chan, L.H.; Frey, F.A. Lithium isotope geochemistry of the Hawaiian plume: Results from the Hawaii Scientific Drilling Project and Koolau Volcano. Geochem. Geophys. Geosyst. 2003, 4, 8707. [Google Scholar] [CrossRef]
- Ryan, J.G.; Langmuir, C.H. The systematics of lithium abundances in young volcanic rocks. Geochim. Cosmochim. Acta 1987, 51, 1727–1741. [Google Scholar] [CrossRef]
- Bindeman, I.N.; Ponomareva, V.V.; Bailey, J.C.; Valley, J.W. Volcanic arc of Kamchatka: A province with high-δ18O magma sources and large-scale 18O/16O depletion of the upper crust. Geochim. Cosmochim. Acta 2004, 68, 841–865. [Google Scholar] [CrossRef]
- Bindeman, I. Oxygen isotopes in mantle and crustal magmas as revealed by single crystal analysis. Rev. Miner. Geochem. 2008, 69, 445–478. [Google Scholar] [CrossRef]
- Auer, S.; Bindeman, I.; Wallace, P.; Ponomareva, V.; Portnyagin, M. The origin of hydrous, high-δ18O voluminous volcanism: Diverse oxygen isotope values and high magmatic water contents within the volcanic record of Klyuchevskoy volcano, Kamchatka, Russia. Contrib Miner. Pet. 2009, 157, 209–230. [Google Scholar] [CrossRef]
- Dorendorf, F.; Wiechert, U.; Wörner, G. Hydrated sub-arc mantle: A source for the Kluchevskoy volcano, Kamchatka/Russia. Earth Planet. Sci. Lett. 2000, 175, 69–86. [Google Scholar] [CrossRef] [Green Version]
- Perkins, G.B.; Sharp, Z.D.; Selverstone, J. Oxygen isotope evidence for subduction and rift-related mantle metasomatism beneath the Colorado Plateau–Rio Grande rift transition. Contrib. Miner. Pet. 2006, 151, 633–650. [Google Scholar] [CrossRef] [Green Version]
- Harmon, R.S.; Hoefs, J. Oxygen isotope heterogeneity of the mantle deduced from global 18 O systematics of basalts from different geotectonic settings. Contrib Miner. Pet. 1995, 120, 95–114. [Google Scholar] [CrossRef]
- Wang, Z.; Eiler, J. Insights into the origin of low-δ18O basaltic magmas in Hawaii revealed from in situ measurements of oxygen isotope compositions of olivines. Earth Planet. Sci. Lett. 2008, 269, 377–387. [Google Scholar] [CrossRef]
- Wei, C.S.; Zheng, Y.F.; Zhao, Z.F.; Valley, J.W. Oxygen and neodymium isotope evidence for recycling of juvenile crust in northeast China. Geology 2002, 30, 375–378. [Google Scholar] [CrossRef] [Green Version]
- Muehlenbachs, K. Alteration of the oceanic crust and the 18O history of seawater. In Stable Isotopes in High Temperature Geological Processes; Valley, J.W., Taylor, H.P., O’Neil, J.R., Eds.; De Gruyter: Berlin, Germany, 1986; pp. 425–444. [Google Scholar]
- Cooper, K.M.; Eiler, J.M.; Asimow, P.D.; Langmuir, C.H. Oxygen isotope evidence for the origin of enriched mantle beneath the mid-Atlantic ridge. Earth Planet. Sci. Lett. 2004, 220, 297–316. [Google Scholar] [CrossRef]
- Demény, A.; Vennemann, T.W.; Hegner, E.; Nagy, G.; Milton, J.A.; Embey-Isztin, A.; Homonnay, Z.; Dobosi, G. Trace element and C–O–Sr–Nd isotope evidence for subduction-related carbonate–silicate melts in mantle xenoliths (Pannonian Basin, Hungary). Lithos 2004, 75, 89–113. [Google Scholar] [CrossRef]
- Eiler, J.M.; Farley, K.A.; Valley, J.W.; Hauri, E.; Craig, H.; Hart, S.R.; Stolper, E.M. Oxygen isotope variations in ocean island basalt phenocrysts. Geochim. Cosmochim. Acta 1997, 61, 2281–2293. [Google Scholar] [CrossRef]
- Xia, Q.K.; Dallai, L.; Deloule, E. Oxygen and hydrogen isotope heterogeneity of clinopyroxene megacrysts from Nushan Volcano, SE China. Chem. Geol. 2004, 209, 137–151. [Google Scholar] [CrossRef]
- Liu, P.P.; Teng, F.Z.; Dick, H.J.B.; Zhou, M.F.; Chung, S.L. Magnesium isotopic composition of the oceanic mantle and oceanic Mg cycling. Geochim. Cosmochim. Acta 2017, 206, 151–165. [Google Scholar] [CrossRef] [Green Version]
- Handler, M.R.; Baker, J.A.; Schiller, M.; Bennett, V.C.; Yaxley, G.M. Magnesium stable isotope composition of Earth’s upper mantle. Earth Planet. Sci. Lett. 2009, 282, 306–313. [Google Scholar] [CrossRef]
- Ionov, D.A. Petrology of mantle wedge lithosphere: New data on supra-subduction zone peridotite xenoliths from the andesitic Avacha volcano, Kamchatka. J. Pet. 2010, 51, 327–361. [Google Scholar] [CrossRef]
- Teng, F.Z.; Wadhwa, M.; Helz, R.T. Investigation of magnesium isotope fractionation during basalt differentiation: Implications for a chondritic composition of the terrestrial mantle. Earth Planet. Sci. Lett. 2007, 261, 84–92. [Google Scholar] [CrossRef]
- Yang, W.; Teng, F.Z.; Zhang, H.F. Chondritic magnesium isotopic composition of the terrestrial mantle: A case study of peridotite xenoliths from the North China craton. Earth Planet. Sci. Lett. 2009, 288, 475–482. [Google Scholar] [CrossRef]
- Wimpenny, J.; Yin, Q.Z.; Tollstrup, D.; Xie, L.W.; Sun, J. Using Mg isotope ratios to trace Cenozoic weathering changes: A case study from the Chinese Loess Plateau. Chem. Geol. 2014, 376, 31–43. [Google Scholar] [CrossRef]
- Li, W.Y.; Teng, F.Z.; Xiao, Y.; Huang, J. High-temperature inter-mineral magnesium isotope fractionation in eclogite from the Dabie orogen, China. Earth Planet. Sci. Lett. 2011, 304, 224–230. [Google Scholar] [CrossRef]
- Von Strandmann, P.A.E.P.; Elliott, T.; Marschall, H.R.; Coath, C.; Lai, Y.J.; Jeffcoate, A.B.; Ionov, D.A. Variations of Li and Mg isotope ratios in bulk chondrites and mantle xenoliths. Geochim. Cosmochim. Acta 2011, 75, 5247–5268. [Google Scholar] [CrossRef]
- Hacker, B.R. H2O subduction beyond arcs. Geochem. Geophys. Geosyst. 2008, 9, Q03001. [Google Scholar] [CrossRef] [Green Version]
- Magni, V.; Faccenna, C.; van Hunen, J.; Funiciello, F. How collision triggers backarc extension: Insight into Mediterranean style of extension from 3-D numerical models. Geology 2014, 42, 511–514. [Google Scholar] [CrossRef] [Green Version]
- Peacock, S.M.; Wang, K. Seismic consequences of warm versus cool subduction metamorphism: Examples from southwest and northeast Japan. Science 1999, 286, 937–939. [Google Scholar] [CrossRef] [Green Version]
- Van Keken, P.E.; Hacker, B.R.; Syracuse, E.M.; Abers, G.A. Subduction factory: 4. Depth-dependent flux of H2O from subducting slabs worldwide. J. Geophys. Res. 2011, 116, B01401. [Google Scholar] [CrossRef] [Green Version]
- Zheng, Y.F.; Chen, R.X.; Xu, Z.; Zhang, S.B. The transport of water in subduction zones. Sci. China Earth Sci. 2016, 59, 651–682. [Google Scholar] [CrossRef]
- Ko, Y.T.; Kuo, B.Y.; Wang, K.L.; Lin, S.C.; Hung, S.H. The southwestern edge of the Ryukyu subduction zone: A high Q mantle wedge. Earth Planet. Sci. Lett. 2012, 335–336, 145–153. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeng, Z.; Li, X.; Zhang, Y.; Qi, H. Lithium, Oxygen and Magnesium Isotope Systematics of Volcanic Rocks in the Okinawa Trough: Implications for Plate Subduction Studies. J. Mar. Sci. Eng. 2022, 10, 40. https://doi.org/10.3390/jmse10010040
Zeng Z, Li X, Zhang Y, Qi H. Lithium, Oxygen and Magnesium Isotope Systematics of Volcanic Rocks in the Okinawa Trough: Implications for Plate Subduction Studies. Journal of Marine Science and Engineering. 2022; 10(1):40. https://doi.org/10.3390/jmse10010040
Chicago/Turabian StyleZeng, Zhigang, Xiaohui Li, Yuxiang Zhang, and Haiyan Qi. 2022. "Lithium, Oxygen and Magnesium Isotope Systematics of Volcanic Rocks in the Okinawa Trough: Implications for Plate Subduction Studies" Journal of Marine Science and Engineering 10, no. 1: 40. https://doi.org/10.3390/jmse10010040
APA StyleZeng, Z., Li, X., Zhang, Y., & Qi, H. (2022). Lithium, Oxygen and Magnesium Isotope Systematics of Volcanic Rocks in the Okinawa Trough: Implications for Plate Subduction Studies. Journal of Marine Science and Engineering, 10(1), 40. https://doi.org/10.3390/jmse10010040






