1. Introduction
Worldwide, agriculture substantially relies on grassland utilization. Feeding high amounts of forage to ruminants is beneficial with regard to maintaining rumen function and reduced competition with resources for human nutrition [
1]. Moreover, utilization of forage produced on farm can be advantageous over imported concentrate in terms of both cost and nutrient cycles.
In many countries, conservation of herbage plays a key role, either to supply forage for winter feeding or as year-round feed in stall-feeding systems. Ensiling is often favored to conserve herbage in humid and temperate regions due to a reduced period between cutting and harvesting [
2]. However, traditional conservation as hay has gained renewed interest in grassland-dominated regions specialized in the production of dairy products with different quality labels, such as protected-designation-of-origin (PDO) hard cheese types (e.g., Gruyère cheese [
3]). These labels often offer higher milk payment but prohibit the feeding of silage, e.g., because of concerns regarding lowered cheese processing quality caused by clostridia contamination [
4]. Haymaking in the field requires constant weather conditions for several days, which causes some uncertainty for the production of high-quality forage. A way to reduce the time in the field is conservation as barn-dried hay where the fresh herbage is put on a ventilation just after wilting in the field for some days [
5].
However, conservation of forages—and particularly, ensiling—can have significant effects on crude protein (CP) composition of the forage and N utilization by the animal [
6,
7]. This is mainly due to the fact that much of the original true protein (TP) is degraded to non-protein N (NPN) during ensiling. Crude protein degradation in dried forages is generally less pronounced than in silages [
8]. True protein concentration as an indicator of protein degradation during conservation is routinely included in silage quality evaluation by many laboratories. However, a more detailed fractionation of feed CP according to the Cornell Net Carbohydrate and Protein System (CNCPS [
9,
10]) could provide a better understanding of the effect of the conservation method on herbage quality. The distribution of CP fractions per se can reveal potential conservation-induced changes in herbage CP. The underlying concept of different ruminal solubilities further allows the CP fractions to be used in regression equations to estimate ruminally undegraded feed CP (RUP) values for a variety of feedstuffs, including forages [
8,
11].
There is a long history of research on forage conservation including N utilization in silage feeding [
12]. However, there is a lack of targeted research on the conservation of herbage from temperate regions focusing on the relationship between conservation method and N balance in animals, as well as detailed descriptions of CP composition and protein value of the feed.
Therefore, the aim of this study was to determine the effect of three different conservation methods (i.e., ensiling, barn-drying, field-drying) of herbage on N balance and utilization in lactating dairy cows. We hypothesized that N utilization would be improved by feeding hay compared to silage due to its lower concentration of NPN. The differently conserved herbages were further characterized regarding CP composition and protein value, including CP fractionation and estimation of ruminal CP degradation and intestinal protein digestibility.
4. Discussion
The feed characteristics of the differently conserved herbages reflected typical effects of the conservation method, such as lower and higher concentrations of DM and CP fraction A, respectively, in silages compared to hay. A trend towards lower CP and higher fiber concentrations from SI over BH to FH could be related to longer wilting time, increased mechanical processing, and an associated loss of leaf material. Generally, the production of barn-dried hay can result in considerably lower DM losses from cutting to feeding compared to field-dried hay and, in some cases, also to silage [
28]. The duration until inhibition of respiration either by anaerobic conditions in silage or by low moisture in hay has a large impact on forage quality [
28,
29]. Consequently, NEL concentrations were the highest for SI and the lowest for FH, which underwent the longest time until stable DM conditions were reached.
The silage had a relatively high DM concentration and, therefore, the fermentation process was limited, as reflected in the low concentration of lactic acid. However, silage fermentation quality was “very good” when assessed with the scheme of the German Agricultural Society (DLG [
30]) based on the concentrations of acetic acid and butyric acid and the pH value. Fermentation quality is linked to DM concentration in grass silages, which is why higher DM concentrations can increase feed intake [
31]. A considerably higher feed intake for hay compared to silage has been reported [
32,
33]. However, the effect is dependent on a variety of characteristics often related to silage quality [
34], not clearly demonstrated by literature data [
2] and, in the current study, was visible only for BH but not for FH. The intake by sheep was higher for barn-dried hay compared to field-dried hay, possibly due to higher OM digestibility [
35]. In the current study, apparent total tract OM digestibility was not significantly different between FH and BH, but it was higher for SI compared to FH. This could be related to lower concentration (NDF) and higher digestibility (ADF) of fiber in SI. Higher CP (or N) digestibility and, specifically, degradation in the rumen [
36] may also have contributed to higher OM digestibility in SI, but quantitative aspects of ruminal OM or CP degradation were not investigated here. However, ruminal VFA concentrations were analyzed. Friggens et al. [
37] discussed considerable differences in the molar proportions of VFA in the ruminal fluid when feeding silage versus hay. In other studies, moderate effects on single VFA were observed [
38,
39]. In contrast, neither total concentrations nor molar proportions of VFA were affected by the conservation method in the current study. The lack of effect may be due to the silage being relatively dry and restrictedly fermented and thus yielding lower propionate proportions of VFA compared to extensively fermented silages [
39]. The proportions of acetate and propionate were higher and lower, respectively, than reported by other authors when comparing hay and silage feeding [
38,
39], presumably due to an at least moderate supplementation of concentrate in the latter studies.
In addition to VFA, the relative abundances of ruminal bacteria were evaluated via quantitative PCR.
Prevotella spp. represented the majority of bacteria, consistent with earlier observations [
40]. The higher abundance in the ruminal fluid from cows fed SI compared to FH could be related to better accessibility of feed protein, as
Prevotella are known for proteolytic and peptidolytic activity [
41].
B. fibrisolvens also displays proteolytic activity [
41] and was elevated through feeding FH compared to SI, but its overall relative abundances were low. Carbohydrate-degrading bacteria
F. succinognenes,
R. albus, and
R. flavefaciens showed together a high relative abundance, presumably reflecting the forage-only diet. The conservation method affected
R. albus and
R. flavefaciens relative abundances, but the effect was not consistent.
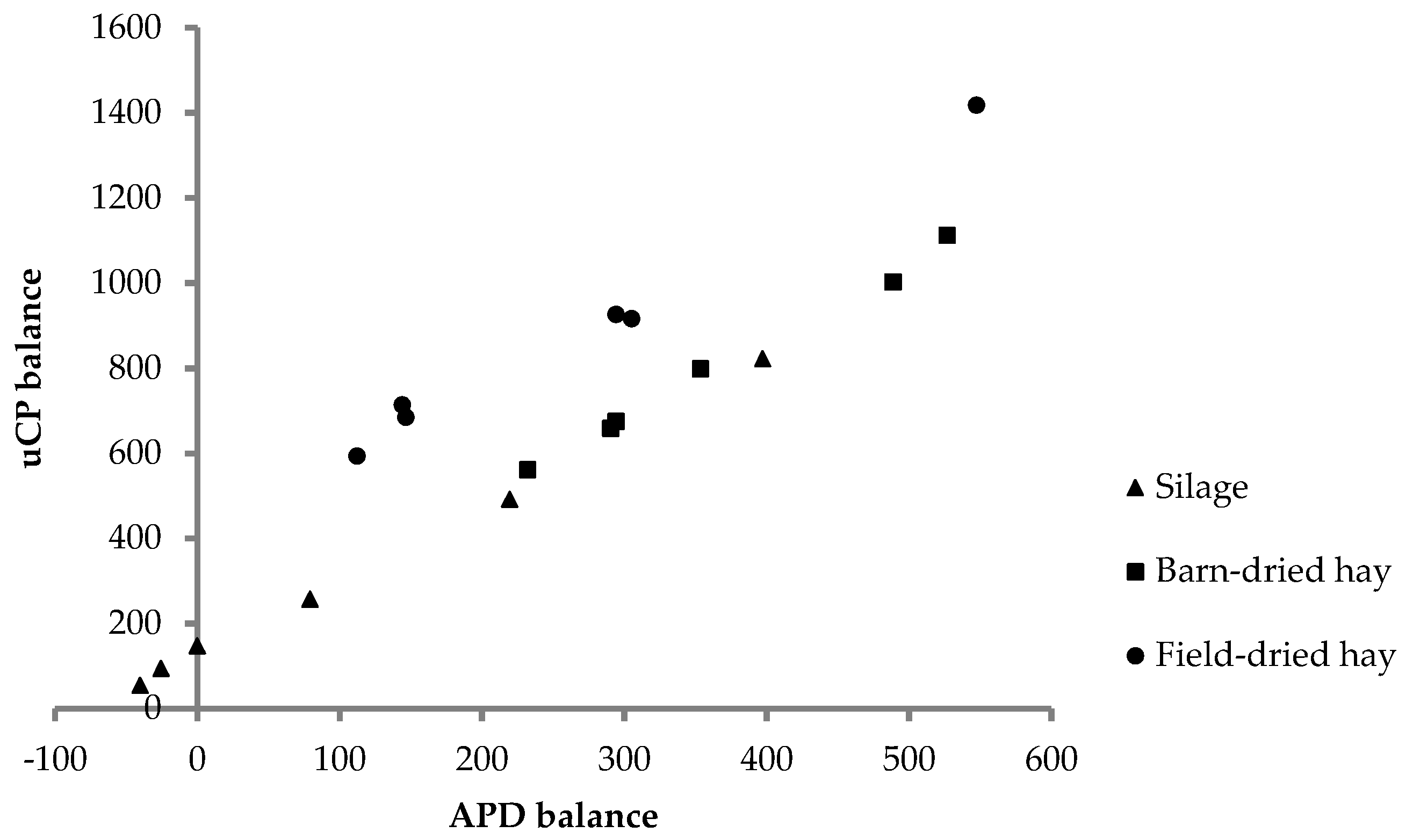
The focus of our study was on N balance and utilization. Dry matter intake of feed from all conservation methods was high, given the cows were in late lactation. Combined with the high dietary CP concentrations, this led to high intake of N. Further, intake of uCP calculated from the concentration of uCP estimated in vitro and feed intake exceeded uCP requirements [
27] (
Figure 1). Similarly, APD intake was in excess with respect to the requirements [
26], except for two cows in two periods (
Figure 1).
As a consequence of excess dietary N intake, urinary N excretion amounted to around 0.5 of N intake. Urine as the main route of surplus N excretion has been observed for various diet compositions (e.g., [
42,
43,
44]). Regulatory N excretion via urine is in the form of urea [
45], which could also be observed in the current experiment, where feeding FH resulted in both the lowest N intake and the lowest UUN excretion. In contrast, UNUN excretion was similar for all treatments and as such not affected by different N intakes. The observed UNUN excretion was also very close to the value of 51.9 g/d estimated by Spek et al. [
46]. Moreover, UNUN was around 3 g/kg DMI and thus consistent with the values reported in a literature review by Pfeffer et al. [
45], who concluded that UNUN excretion mostly is <4 g/kg DMI.
Similar to UNUN, fecal N excretion did not differ between treatments and was approximately 10 g/kg DMI. This value is in line with collated literature data and can be viewed as obligatory and not related to the regulation of N in the body pool [
45]. However, fecal N excretion expressed as proportion of N intake decreases with higher N intake [
47]. Thus, a higher percentage of fecal N excretion related to N intake for FH was most likely an artifact of slight differences in feed intake and CP concentration of the herbages, resulting in lower daily N intake for FH. In contrast to fecal N excretion, the proportion of UNUN in urinary N was not significantly affected by the treatment, which is not consistent with the concept of UNUN seen as obligatory excretion. The fact that less N had to be disposed of when FH was fed was visible not only in UUN excretion but also in lower urea concentrations in serum and milk of cows fed FH.
Even though differences in N intake certainly explain a significant share of the observed effects on urea concentrations, feed protein characteristics may also play a role. Field-dried hay displayed the lowest apparent total tract digestibility of N. This is in line with the lower potential of prececal CP digestibility indicated by the enzymatic in vitro method. Moreover, RUP values estimated from CP fractionation and in vitro incubation in
S. griseus protease solution indicated lower ruminal CP degradability for FH, which was also reflected in lower ruminal ammonia concentration for FH compared to BH. However, SI displayed higher concentrations of CP, which in addition contained a higher proportion of NPN. This should theoretically have led to higher ruminal ammonia concentrations for SI compared to FH, which was not the case. A possible explanation could be the fact that the ruminal fluid was collected before the morning feeding. At this time point, ammonia rapidly released from dietary NPN can already be absorbed. Moreover, significant amounts of soluble N fractions from silage, including non-ammonia NPN, may escape from the rumen [
48]. In contrast, protein degradation in BH and FH will have proceeded more steadily.
Crude protein fractionation revealed the highest proportion of fraction B3 in FH. In a study by Edmunds et al. [
8], 60% of the variation in RUP in silages and dried forages could be assigned to differences in CP fraction B3. In sheep, Verbič et al. [
36] found a lower ruminal CP degradability of hay compared to differently prepared silages from the same parent material. However, it cannot be determined if the observed results indicating lower N turnover and clearance for FH were due to lower N intake, lower ruminal CP degradability, or a combined effect. While the effect of increased dietary RUP concentration is debated, reducing N supply is a commonly recommended measure to reduce N excretion and increase NUE [
47], also in grass-based diets [
49,
50].
Feeding SI and BH resulted in similar N intake. The observed pattern of CP fractions and in vitro and chemical RUP estimation indicated that BH delivered higher amounts of RUP to the animals, which could have led to the observed tendencies for higher milk N excretion and milk protein yield in cows fed BH compared to SI. However, there was also a tendency towards a higher intake of feed and particularly digestible OM for BH compared to SI. On the other hand, neither partitioning of N excretion nor urea concentrations in milk and serum differed between feeding BH and SI, contradictory to our hypothesis that N utilization would be improved by feeding hay compared to silage. True protein in SI still contributed >500 g/kg CP. Much lower TP concentrations can be reached as a result of protein breakdown even in well-fermented silages [
51]. The silage produced in this experiment was relatively dry due to constant dry weather conditions during the wilting period on the field. Possibly, stronger effects of ensiling compared to drying of herbage could have been expected if silage with lower DM concentrations had been produced. In particular, concentrations of TP, RUP, and uCP may be lower in silages with lower DM concentrations [
52]. Furthermore, DM concentrations in grass-clover silages are positively correlated to the duodenal flow of microbial CP [
53]. However, even if RUP supply was actually different between SI and BH, excess supply of feed CP by both treatments may have prevented possible positive effects of an increased dietary RUP concentration on NUE [
54].
The enzymatic estimation revealed similar but low IPD values for herbage from all three conservation methods. This indicates that a large proportion of RUP consisted of fiber-bound N and, hence, was not accessible for enzymatic digestion in the small intestine. However, IPD was lower than the values for grass products reported in the literature [
55,
56], but it has to be noted that methods differed. Edmunds et al. [
57] demonstrated that the AA pattern of forage protein is altered during ruminal incubation but does not widely differ between RUP from differently conserved forages. For the current study, this would imply that only total supply and not quality in terms of intestinal digestibility and AA pattern of RUP differed between forages.
Overall, NUE was low, as N excreted in milk was only 20–22% of N intake. These values correspond well to the efficiency of N utilization observed for the lower quartile in collated data of Calsamiglia et al. [
58]. Interestingly, the CP concentration in forage that Calsamiglia et al. [
58] estimated for this quartile was almost equal to the CP concentration of the conserved forages in the current experiment. For diets mainly based on grass silage, NUE estimated from collated feeding trial data was 27.7% [
59]. However, the reported minimum and maximum NUE values were as low as 16.0 and as high as 40.2%, respectively [
59]. Reports of NUE in dairy cows receiving only conserved forage are scarce. Shingfield et al. [
39] observed slightly higher NUE for hay compared to differently treated silages prepared from the same mixed swards (timothy and meadow fescue). However, concentrate supplementation was part of the experiment, and the level of NUE was around 30%. A similar mean value was demonstrated for cows fed grass-clover silage supplemented with concentrate [
60]. Low NUE of around 20–25% were also reported for cows grazing ryegrass pasture with only moderate concentrate supplementation [
61].
The utilization of N seems particularly low, given the fact that adequate or surplus supply of APD and uCP was accompanied by a negative N balance. Moreover, N intake of cows largely exceeded the requirements to maintain a stable N balance calculated by Pfeffer et al. [
45]. A negative N balance indicates a mobilization of body protein. This can occur during non-sufficient dietary supply of N, when AA from the skeletal muscle protein are used for milk protein synthesis [
62]. However, in the current experiment, a shortage of dietary N supply was precisely not likely, and thus AA from degraded body protein would not have been essential for milk protein synthesis. Instead, it is more likely that AA from skeletal muscle protein were used for energy supply [
62], and the amino group of AA was disposed of as urea and excreted via urine. In fact, except for one cow, all cows lost body weight over the course of the complete trial (body weight change from −49.9 to +1.9 kg, average −23.6 kg). Milk yields were moderate, but high milk protein and fat concentrations elevated ECM. The requirements of NEL [
27] were not met by the actual intake for five of the six cows, and the mean estimated NEL balance was −9.2 MJ/d. Hence, the assumptions of Pfeffer et al. [
45] regarding N supply to maintain a stable N balance were not met in this study. Negative energy balance is of major significance during early lactation, when substantial amounts of body protein can be mobilized along with body fat despite sufficient dietary CP supply [
63]. However, the proportion of mobilized body protein in total mobilized tissue decreases fast after parturition, and protein balance can become positive after four weeks of lactation [
64]. In contrast, the cows in the current experiment were in late lactation, where energy supply under most feeding regimes is not limited.
Balancing dietary energy and protein supply to maximize N utilization is primarily discussed concerning ruminal metabolism [
65]. Energy supply matching N supply may lead to the efficient use of N for microbial growth and help in capturing rapidly released ammonia, e.g., in silages. However, the current results should also be seen in the light of adequate postabsorptive energy supply, which may improve AA uptake in the mammary gland independently from protein supply [
66]. In this regard, Tamminga [
67] discussed postabsorptive N losses due to an imbalance between energy and AA availability at the tissue level. This also has a practical implication for herbage-dominated feeding systems without supplementary concentrate. These systems can result in a “high metabolic load in high-yielding dairy cows during early lactation” [
68]. Although the cows in the current study were not in early lactation, and milk yields were moderate, the loss of body weight and a negative NEL balance point to the fact that high metabolic loads may have occurred nonetheless.
When feeding only forage, balancing the supply of energy and CP is a challenge. Harvest and conservation of herbage are weather-dependent and thus offer limited opportunities to modify both energy and CP concentrations to the desired level. From a study with grass silages, Dewhurst et al. [
69] clearly concluded that in order to maximize the utilization of grass silage N, crops with higher energy and lower CP concentrations are needed. The results from the current experiments underline this conclusion and further indicate that it can be extended to herbage conserved as hay. Similar suggestions have been made with regard to pasture systems, where high N concentrations in ryegrass and clover result in high N losses [
49]. Energy may become first limiting, and N be used less efficiently when cows are fed pasture without supplementation [
70].