Glassworts: From Wild Salt Marsh Species to Sustainable Edible Crops
Abstract
1. Introduction
2. Habitat and Apulian Biodiversity
3. Morphological Characteristics
4. Propagation
4.1. Gamic Propagation
4.2. In Vitro Propagation
5. Cultivation
5.1. Growth
5.2. Flowering
5.3. Salinity Tolerance, Water Relations, and Gas Exchange
6. Bioactive Compounds
7. Food Uses
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Slama, I.; Abdelly, C.; Bouchereau, A.; Flowers, T.; Savouré, A. Diversity, distribution and roles of osmoprotective compounds accumulated in halophytes under abiotic stress. Ann. Bot. 2015, 115, 433–447. [Google Scholar] [CrossRef] [PubMed]
- Ramani, B.; Reeck, T.; Debez, A.; Stelzer, R.; Huchzermeyer, B.; Schmidt, A.; Papenbrock, J. Aster tripolium L. and Sesuvium portulacastrum L.: Two halophytes, two strategies to survive in saline habitats. Plant Physiol. Biochem. 2006, 44, 395–408. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.; Buhmann, A.K.; Flowers, T.J.; Seal, C.E.; Papenbrock, J. Salicornia as a crop plant in temperate regions: Selection of genetically characterised ecotypes and optimization of their cultivation conditions. AoB Plants 2014, 6. [Google Scholar] [CrossRef] [PubMed]
- Sagi, M.; Savidov, N.A.; L’vov, N.P.; Lips, S.H. Nitrate reductase and molybdenum cofactor in annual ryegrass as affected by salinity and nitrogen source. Physiol. Plant 1997, 99, 546–553. [Google Scholar] [CrossRef]
- Flowers, T.J.; Colmer, T.D. Salinity tolerance in halophytes. New Phytol. 2008, 179, 945–963. [Google Scholar] [CrossRef]
- Jouyban, Z. The effects of salt stress on plant growth. TJAS 2012, 2, 7–10. [Google Scholar]
- Petropoulos, S.A.; Karkanis, A.; Martins, N.; Ferreira, I.C.F.R. Edible halophytes of the Mediterranean basin: Potential candidates for novel food products. Trends Food Sci. Technol. 2018, 74, 69–84. [Google Scholar] [CrossRef]
- Montesano, F.; Gattullo, C.; Parente, A.; Terzano, R.; Renna, M. Cultivation of potted sea fennel, an emerging mediterranean halophyte, using a renewable seaweed-based material as a peat substitute. Agriculture 2018, 8, 96. [Google Scholar] [CrossRef]
- He, J.; Qin, L.; Chong, E.L.C.; Choong, T.W.; Lee, S.K. Plant growth and photosynthetic characteristics of Mesembryanthemum crystallinum grown aeroponically under different blue-and red-LEDs. Plant Sci. 2017, 8, 361. [Google Scholar] [CrossRef]
- Karakas, S.; Cullu, M.A.; Dikilitaş, M. Comparison of two halophyte species (Salsola soda and Portulaca oleracea) for salt removal potential under different soil salinity conditions. Turk. J. Agric. For. 2017, 41, 183–190. [Google Scholar] [CrossRef]
- Ventura, Y.; Eshel, A.; Pasternak, D.; Sagi, M. The development of halophyte-based agriculture: Past and present. Ann. Bot. 2014, 115, 529–540. [Google Scholar] [CrossRef] [PubMed]
- Kadereit, G.; Ball, P.; Beer, S.; Mucina, L.; Sokoloff, D.; Teege, P.; Yaprak, A.E.; Freitag, H. A taxonomic nightmare comes true: Phylogeny and biogeography of glassworts (Salicornia L., Chenopodiaceae). Taxon 2007, 56, 1143–1170. [Google Scholar] [CrossRef]
- Davy, A.J.; Bishop, G.F.; Costa, C.S.B. Salicornia L. Salicornia pusilla J. Woods, S. ramosissima J. Woods, S. europaea L., S. obscura P.W. Ball & Tutin, S. nitens P.W. Ball & Tutin, S. fragilis P.W. Ball & Tutin and S. dolichostachya Moss. J. Ecol. 2001, 89, 681–707. [Google Scholar]
- Yaprak, A.E. Sarcocornia obclavata (Amaranthaceae) a new species from Turkey. Phytotaxa 2012, 49, 55–60. [Google Scholar] [CrossRef]
- Steffen, S.; Ball, P.; Mucina, L.; Kadereit, G. Phylogeny, biogeography and ecological diversification of Sarcocornia (Salicornioideae, Amaranthaceae). Ann. Bot. 2015, 115, 353–368. [Google Scholar] [CrossRef] [PubMed]
- Fuente, V.; Rufo, L.; Rodriguez, N.; Sánchez-Mata, D.; Franco, A.; Amils, R. A study of Sarcocornia A.J. Scott (Chenopodiaceae) from Western Mediterranean Europe. Plant Biosyst. 2016, 150, 343–356. [Google Scholar] [CrossRef]
- Pignatti, S. Flora d’Italia, 2nd ed.; Edagricole: Bologna, Italy, 2017; Volume 216, pp. 261–267. (In Italian) [Google Scholar]
- Corbetta, F. Lineamenti della vegetazione macrofitica dei laghi di Lesina e di Varano. G. Bot. Ital. 1970, 104, 165–191. (In Italian) [Google Scholar] [CrossRef]
- Di Pietro, R.; Dibitonto, P.; Garziano, G.; Sciandrello, S.; Wagensommer, R.P.; Medagli, P.; Tomaselli, V. Preliminary results of floristic and vegetation surveys in three coastal humid areas in the Puglia region (southern Italy). Lazaroa 2009, 30, 99. [Google Scholar]
- Castroviejo, S.; Lanz, M.; Lpez Gonzlez, G.; Montserrat, P.; Muoz Garmendia, F.; Paiva, J.; Villar, L. Flora Iberica: Plantas Vasculares de la Peninsula Iberica e Islas Baleares; CSIC: Madrid, Spain, 1990; Volume 2, pp. 526–531. [Google Scholar]
- Redondo-Gómez, S.; Mateos-Naranjo, E.; Davy, A.J.; Fernández-Muñoz, F.; Castellanos, E.M.; Luque, T.; Figueroa, M.E. Growth and photosynthetic responses to salinity of the salt-marsh shrub Atriplex portulacoides. Ann. Bot. 2007, 555–563. [Google Scholar] [CrossRef]
- Shepherd, K.; Macfarlane, T.; Colmer, T. Morphology, anatomy and histochemistry of Salicornioideae (Chenopodiaceae) fruits and seeds. Ann. Bot. 2005, 95, 917–933. [Google Scholar] [CrossRef]
- Ball, P.W. Salicornia L., in Flora of North America: North of Mexico (4): Magnoliophyta: Caryophyllidae, Part 1; Editorial Committee of the Flora of North America, Oxford University Press: Oxford, UK, 2004; ISBN 978-0-19-517389-5. [Google Scholar]
- Grouzis, M.; Berger, A.; Heim, G. Polymorphisme et germination des graineshez trois especes annuelles du genere Salicornia. OEC Plant 1976, 11, 41–45. [Google Scholar]
- Philipupillai, J.; Ungar, I.A. The effect of seed dimorph-ism on the germination and survival of Salicornia europaea L. populations. Am. J. Bot. 1984, 71, 542–549. [Google Scholar] [CrossRef]
- Harper, J.L. Population Biology of Plants; Academic Press: New York, NY, USA, 1977. [Google Scholar]
- Imbert, E. Ecological consequences and ontogeny of seed heteromorphism. Perspect. Plant Ecol. Syst. 2002, 5, 13–36. [Google Scholar] [CrossRef]
- Song, J.; Fan, H.; Zhao, Y.; Du, X.; Wang, B. Effect of salinity on germination, seedling emergence, seedling growth and ion accumulation of a euhalophyte Suaeda salsa in an intertidal zone and on saline inland. Aquat. Bot. 2008, 88, 331–337. [Google Scholar] [CrossRef]
- Gul, B.; Ansari, R.; Flowers, T.; Khan, M. Germination strategies of halophyte seeds under salinity. Environ. Exp. Bot. 2003, 92, 4–18. [Google Scholar] [CrossRef]
- Wang, F.; Xu, Y.; Wang, S.; Shi, W.; Liu, R.; Feng, G.; Song, J. Salinity effects production and salt tolerance of dimorphic seeds of Suaeda salsa. Plant Physiol. Biochem. 2015, 95, 41–48. [Google Scholar] [CrossRef]
- Ameixa, O.; Marques, B.; Fernandes, V.S.; Soares Amadeu, M.; Calado, R.; Lilleb, A.I. Dimorphic seeds of Salicornia ramosissima display contrasting germination responses under different salinities. Ecol. Eng. 2016, 87, 120–123. [Google Scholar] [CrossRef]
- Ungar, I.A. Seed banks and seed population dynamics of halophytes. Wetl. Ecol. Manag. 2001, 9, 499–510. [Google Scholar] [CrossRef]
- Khan, M.A.; Ungar, I.A. Influence of salinity and temperature on the germination of Haloxylon recurvum. Ann. Bot. 1996, 78, 547–551. [Google Scholar] [CrossRef]
- Ungar, I.A. Salinity, temperature and growth regulator effects on seed germination of Salicornia europaea L. Aquat. Bot. 1977, 3, 329–335. [Google Scholar] [CrossRef]
- Okusanya, O.T.; Ungar, I.A. The effects of time of seed production on the germination response of Spergularia marina. Physiol. Plant 1983, 59, 335–342. [Google Scholar] [CrossRef]
- Orlovsky, N.; Japakova, U.; Zhang, H.; Volis, S. Effect of salinity on seed germination, growth and ion content in dimorphic seeds of Salicornia europaea L. (Chenopodiaceae). Plant Div. 2016, 38, 183–189. [Google Scholar] [CrossRef]
- Aghaleh, M.; Niknam, V.; Ebrahimzadeh, H.; Razavi, K. Effect of salt stress on physiological and antioxidative responses in two species of Salicornia (S. persica and S. europaea). Acta Physiol. Plant. 2011, 33, 1261–1270. [Google Scholar] [CrossRef]
- Lv, S.; Jiang, P.; Chen, X.; Fan, P.; Wang, X.; Li, Y. Multiple compartmentalization of sodium conferred salt tolerance in Salicornia europaea. Plant Physiol. Biochem. 2012, 51, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Keiffer, C.H.; Ungar, I.A. The effect of extended exposure to hypersaline conditions on the germination of five inland halophyte species. Am. J. Bot. 1997, 84, 104–111. [Google Scholar] [CrossRef]
- Muscolo, A.; Panuccio, M.; Piernik, A. Ecology, Distribution and ecophysiology of Salicornia europaea L. In Sabkha Ecosystems. Volume IV: Cash Crop Halophyte and Biodiversity Conservation; Khan, M.A., Böer, B., Öztürk, M., Al Abdessalaam, T.Z., Clüsener-Godt, M., Gul, B., Eds.; Springer Publication: Dordrecht, The Netherlands, 2014; pp. 233–240. [Google Scholar]
- Lee, C.W.; Glenn, E.P.; O’Leary, J.W. In vitro propagation of Salicornia bigelovii by shoot-tip cultures. HortScience 1992, 27, 472. [Google Scholar]
- Shi, X.L.; Han, H.P.; Shi, W.L.; Li, Y.X. NaCl and TDZ are two key factors for the improvement of in vitro regeneration rate of Salicornia europaea L. J. Integr. Plant Biol. 2006, 48, 1185–1189. [Google Scholar] [CrossRef]
- Joshi, M.; Mishra, A.; Jha, B. NaCl plays a key role for in vitro micropropagation of Salicornia brachiata, an extreme halophyte. Ind. Crop Prod. 2012, 35, 313–316. [Google Scholar] [CrossRef]
- Urbano, M.; Tomaselli, V.; Bisignano, V.; Veronico, G.; Hammer, K.; Laghetti, G. Salicornia patula Duval-Jouve: From gathering of wild plants to some attempts of cultivation in Apulia region (southern Italy). Genet. Resour. Crop Evol. 2017, 64, 1465–1472. [Google Scholar] [CrossRef]
- Gunning, D. Cultivating Salicornia europaea (Marsh Samphire); Daithi O’ Murchu Marine Research Station & University College Cork: Dublin, Ireland, 2016; pp. 1–50. [Google Scholar]
- Ventura, Y.; Sagi, M. Halophyte crop cultivation: The case for Salicornia and Sarcocornia. Environ. Exp. Bot. 2013, 92, 144–153. [Google Scholar] [CrossRef]
- Rozemaa, J.; Schatb, H. Salt tolerance of halophytes, research questions reviewed in the perspective of saline agriculture. Environ. Exp. Bot. 2013, 92, 83–95. [Google Scholar] [CrossRef]
- Singh, A.; Sharma, S.; Shah, M.T. Successful cultivation of Salicornia brachiata—A sea asparagus utilizing RO reject water: A sustainable solution. Int. J. Waste Resour. 2018, 8, 322. [Google Scholar] [CrossRef]
- Bashan, Y.; Moreno, M.; Troyo, E. Growth promotion of the seawater irrigated oilseed halophyte Salicornia bigelovii inoculated with mangrove rhizosphere bacteria and halotolerant Azospirillum spp. Biol. Fertil. Soils 2000, 32, 265–272. [Google Scholar] [CrossRef]
- Reddy, M.P. Bromide tolerance in Salicornia brachiata Roxb, an obligate halophyte. Water Air Soil Pollut. 2009, 196, 151–160. [Google Scholar] [CrossRef]
- Ventura, Y.; Wuddineh, W.A.; Shpigel, M.; Samocha, T.M.; Klim, B.C.; Cohen, S.; Shemer, Z.; Santos, R.; Sagi, M. Effects of day length on flowering and yield production of Salicornia and Sarcocornia species. Sci. Hortic. 2011, 130, 510–516. [Google Scholar] [CrossRef]
- York, J.; Lu, Z.; Glenn, E.P.; John, M.E. Daylength affects floral initiation in Salicornia bigelovii Torr. Plant Biol. 2000, 41–42, (Abstract). [Google Scholar]
- Ahmad, S.; Sima, N.; Mirzaei, H.H. Effects of sodium chloride on physiological aspects of Salicornia persica growth. J. Plant Nutr. 2013, 36, 401–414. [Google Scholar] [CrossRef]
- Parida, A.K.; Jha, B.J. Physiological and biochemical responses reveal the drought tolerance efficacy of the halophyte Salicornia brachiata. J. Plant Growth Regul. 2013, 32, 342–352. [Google Scholar] [CrossRef]
- Cooper, A. The effects of salinity and waterlogging on the growth and cation uptake of salt marsh plants. New Phytol. 1982, 90, 263–275. [Google Scholar] [CrossRef]
- Katschnig, D.; Broekman, R.; Rozema, J. Salt tolerance in the halophyte Salicornia dolichostachya Moss: Growth, morphology and physiology. Environ. Exp. Bot. 2013, 92, 32–42. [Google Scholar] [CrossRef]
- Jennings, D.H. Halophytes succulence and sodium in plants—A unified theory. New Phytol. 1968, 67, 899. [Google Scholar] [CrossRef]
- Storey, R.; Wyn Jones, R.G. Responses of Atriplex spongiosa and Suaeda monoica to salinity. Plant Physiol. 1979, 63, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Pollard, A.; Wyn Jones, R.G. Enzyme activities in concentrated solutions of glycinebetaine and other solutes. Planta 1979, 144, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Jolivet, Y.; Larher, F.; Hamelin, J. Osmoregulation in halophytic higher plants-the protective effect of glycine betaine against the heat destabilization of membranes. Plant Sci. Lett. 1982, 25, 193–201. [Google Scholar] [CrossRef]
- Gorham, J.; Hughes, L.L.; Wynjones, R.G. Chemical composition of salt-marsh plants from Ynys-mon (Anglesy) - the concept of physiotypes. Plant Cell Environ. 1980, 3, 309–318. [Google Scholar] [CrossRef]
- Ayala, F.; Oleary, J.W. Growth and physiology of Salicornia bigelovii Torr. at suboptimal salinity. Int. J. Plant Sci. 1995, 156, 197–205. [Google Scholar] [CrossRef]
- Liu, X.G.; Xia, Y.G.; Wang, F.; Sun, M.; Jin, Z.J.; Wang, G.T. Analysis of fatty acid composition of Salicornia europaea L. seed oil. Food Sci. 2005, 2, 42. [Google Scholar]
- Fan, P.; Nie, L.; Jiang, P.; Feng, J.; Lv, S.; Chen, X.; Bao, H.; Guo, J.; Tai, F.; Wang, J.; et al. Transcriptome analysis of Salicornia europaea under saline conditions revealed adaptive primary metabolic pathways as early events to facilitate salt adaption. PLoS ONE 2013, 8, e80595. [Google Scholar] [CrossRef]
- Norman, H.C.; Masters, D.G.; Barrett-Lennard, E.G. Halophytes as forages in saline landscapes: Interactions between plant genotype and environment change their feeding value to ruminants. Environ. Exp. Bot. 2013, 92, 96–109. [Google Scholar] [CrossRef]
- Guil, J.L.; Rodriguez-Garcia, I.; Torija, E. Nutritional and toxic factors in selected wild edible plants. Plant Food Hum. Nutr. 1997, 51, 99–107. [Google Scholar] [CrossRef]
- Lu, D.; Zhang, M.; Wang, S.; Cai, J.; Zhou, X.; Zhu, C. Nutritional characterization and changes in quality of Salicornia bigelovii Torr. during storage. Food Sci. Technol. 2010, 43, 519–524. [Google Scholar] [CrossRef]
- Kang, S.; Kim, M.R.; Chiang, M.; Hong, J. Evaluation and comparison of functional properties of freshwater-cultivated glasswort (Salicornia herbacea L.) with naturally-grown glass- wort. Food Sci. Biotechnol. 2015, 24, 2245–2250. [Google Scholar] [CrossRef]
- Patel, S. Salicornia: Evaluating the halophytic extremophile as a food and a pharmaceutical candidate. Biotech 2016, 6, 104. [Google Scholar] [CrossRef] [PubMed]
- Isca Vera, M.S.; Seca Ana, M.L.; Pinto Diana, C.G.A.; Silva, H.; Silva Artur, M.S. Lipophilic profile of the edible halophyte Salicornia ramosissima. Food Chem. 2014, 165, 330–336. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zuo, P.; Zha, X.; Chen, X.; Zhang, R.; He, X.; Liu, C. Octacosanol enhances the proliferation and migration of human umbilical vein endothelial cells via activation of the PI3K/Akt and MAPK/Erk pathways. Lipids 2015, 50, 241–251. [Google Scholar] [CrossRef]
- Simopoulos, A.P. Omega-3 fatty acids and antioxidants in edible wild plants. Biol. Res. 2004, 37, 263–277. [Google Scholar] [CrossRef]
- Mishra, A.; Patel, M.K.; Jha, B. Non-targeted metabolomics and scavenging activity of reactive oxygen species reveal the potential of Salicornia brachiata as a functional food. J. Funct. Foods 2015, 13, 21–31. [Google Scholar] [CrossRef]
- Ksouri, R.; Ksouri, W.M.; Jallali, I.; Debez, A.; Magné, C.; Hiroko, I. Medicinal halophytes: Potent source of health promoting biomolecules with medical, nutraceutical and food applications. Crit. Rev. Biotechnol. 2012, 32, 289–326. [Google Scholar] [CrossRef]
- Zhu, J.K. Plant salt tolerance. Trends Plant Sci. 2001, 6, 66–71. [Google Scholar] [CrossRef]
- Ryu, D.S.; Kim, S.H.; Lee, D.S. Anti-proliferative effect of polysaccharides from Salicornia herbacea on induction of G2/M arrest and apoptosis in human colon cancer cells. J. Microbiol. Biotechnol. 2009, 19, 1482–1489. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Min, Z.; Yuhui, Z.; Hui, W.; Tianxing, L.; Zhihong, X. Pentadecylferulate, a potent antioxidant and antiproliferative agent from the halophyte Salicornia herbacea. Food Chem. 2013, 141, 2066–2074. [Google Scholar] [CrossRef] [PubMed]
- Essaidi, I.; Zeineb, B.; Ahmed, S.; Hayat, B.H.K.; Hervé, C.; Naoki, A.; Abdelfatteh, E.O.; Mohamed, M.C.; Nabiha, B. Phytochemical investigation of Tunisian Salicornia herbacea L., antioxidant, antimicrobial and cytochrome P450 (CYPs) inhibitory activities of its methanol extract. Food Control 2013, 32, 125–133. [Google Scholar] [CrossRef]
- Temple, J. Tasting Hawai’i with Moloka’i Chef James Temple: The Other Asparagus … Sea Asparagus. Available online: http://www.tastinghawaii.com/2013/11/the-other-asparagus-sea-asparagus.html (accessed on 21 November 2018).
- Narasimha Rao, G.M.; Reddi, B. Distribution and density of Salicornia brachiata (a potential halophyte) in Godavari Estuary. IJBPAS 2013, 2, 974–979. [Google Scholar]
- El Shaer, H.M. Halophytes and salt-tolerant plants as potential forage for ruminants in the Near East region. Small Rumin Res. 2010, 91, 3–12. [Google Scholar] [CrossRef]
- Song, S.H.; Lee, C.; Lee, S.; Park, J.M.; Lee, H.J.; Bai, D.H.; Yoon, S.S.; Choi, J.B.; Park, Y.S. Analysis of microflora profile in Korean traditional nuruk. J. Microbiol. Biotechnol. 2013, 23, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.; Chang, Y.H.; Ko, J.Y.; Jeong, Y. Physicochemical and microbial properties of the Korean traditional rice wine, Makgeolli, supplemented with banana during fermentation. Prev. Nutr. Food Sci. 2013, 18, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Seo, H.; Jeon, B.Y.; Yun, A.; Park, D.H. Effect of glasswort (Salicornia herbacea L.) on microbial community variations in the vinegar-making process and vinegar characteristics. J. Microbiol. Biotechnol. 2010, 20, 1322–1330. [Google Scholar] [CrossRef]
- Shin, M.G.; Lee, G.H. Spherical granule production from micronized saltwort (Salicornia herbacea) powder as salt substitute. Prev. Nutr. Food Sci. 2013, 18, 60–66. [Google Scholar] [CrossRef]
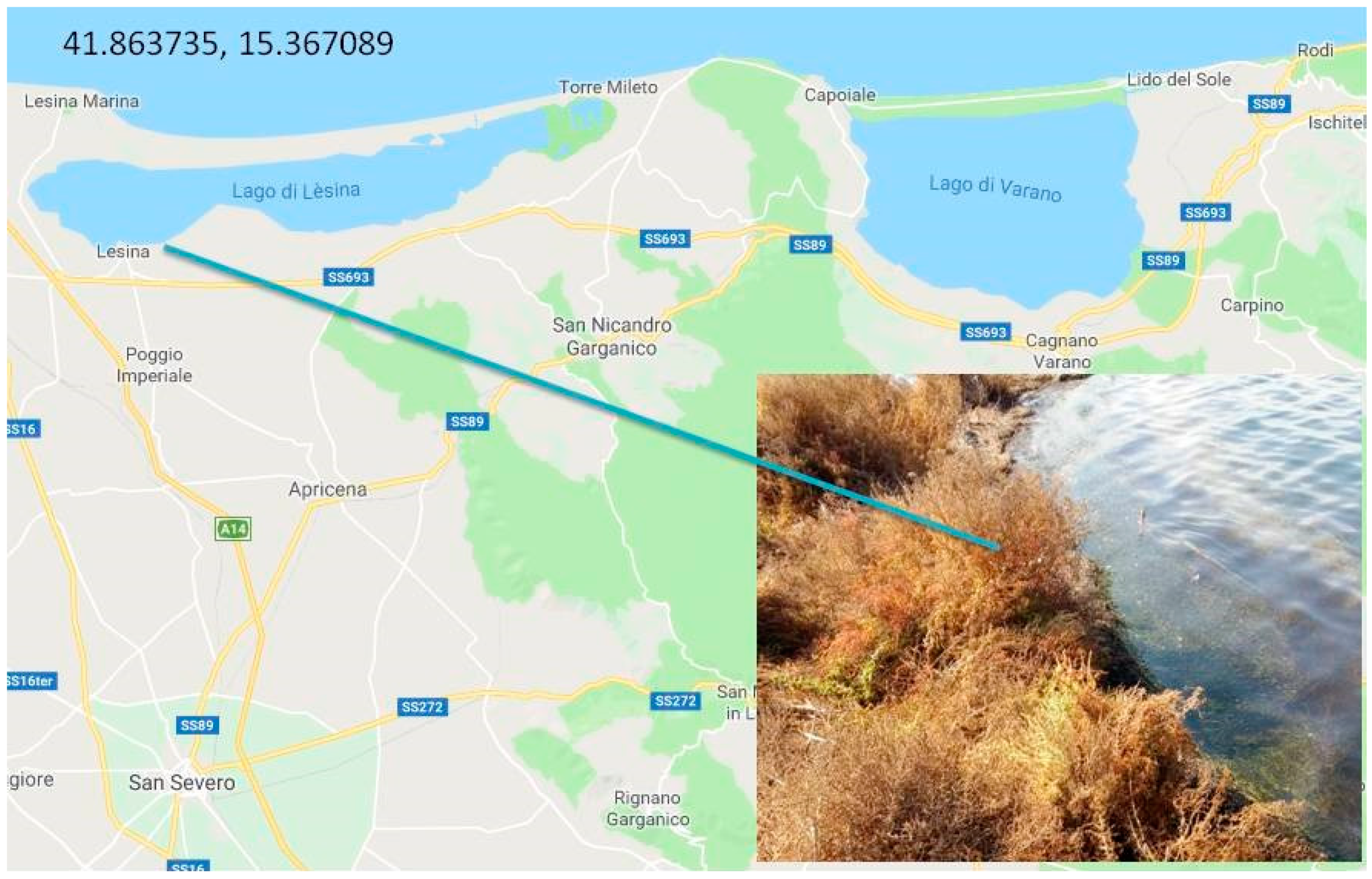
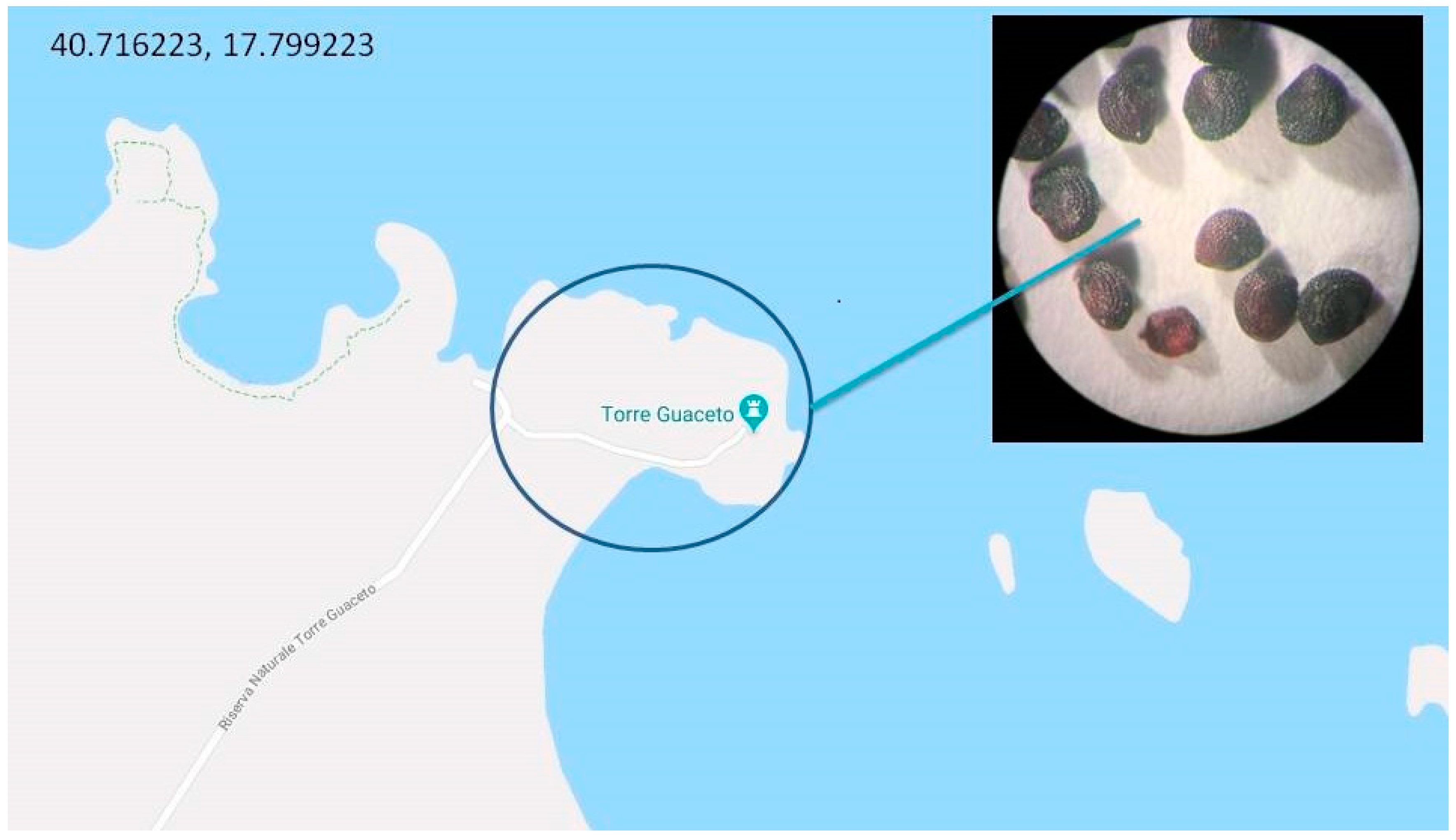
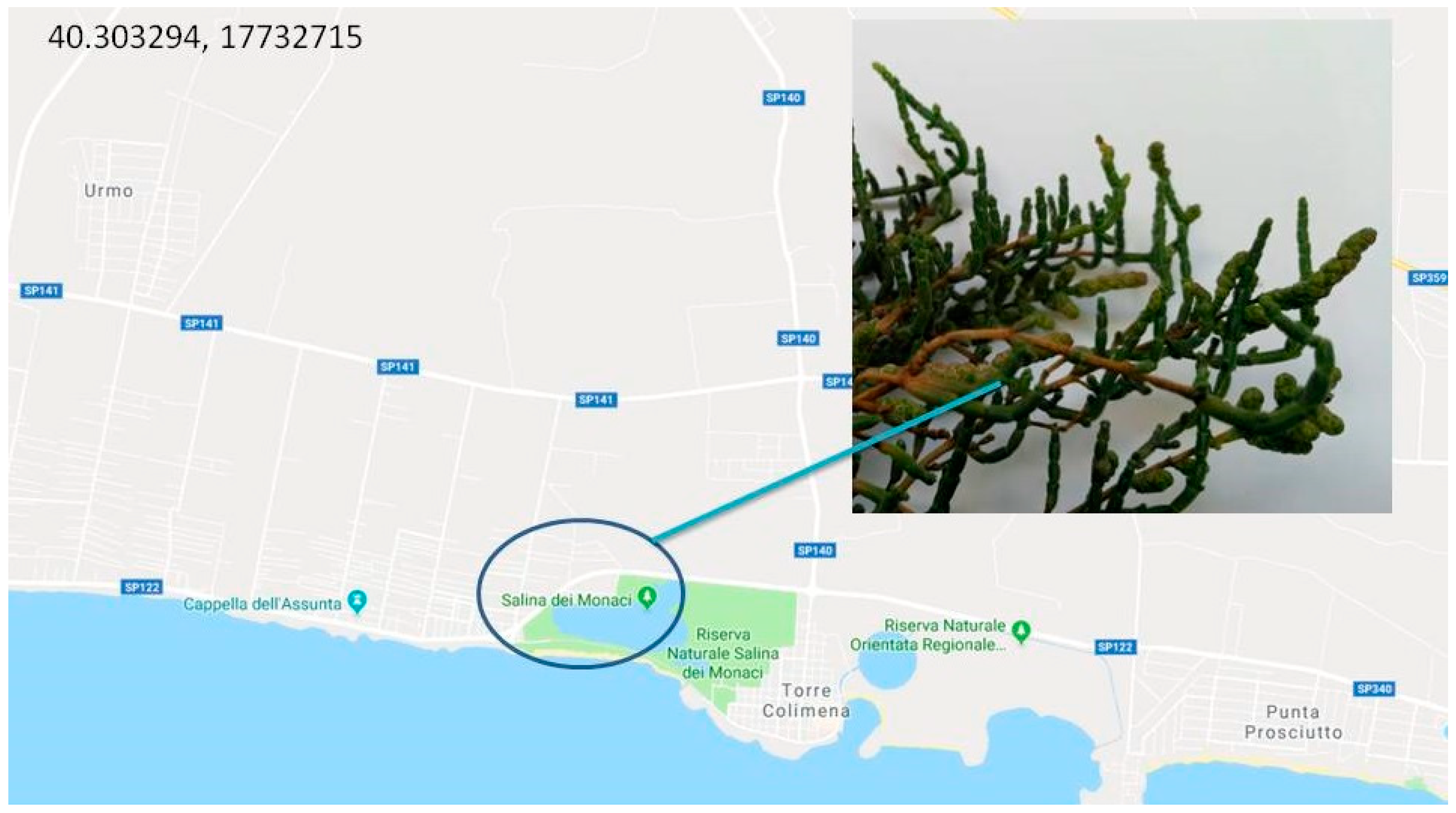


| Genus | Species | Subspecies | Origin |
|---|---|---|---|
| Salicornia | europaea L. (Syn: S. herbacea L., S. brachystachya G. F. W. Mayer, S. ramosissima J. Woods, S. patula Auct.) | europaea | From southern Spain to northern Scandinavia |
| disarticulata | Atlantic coasts of Netherlands and southern England. | ||
| marshallii | Atlantic coasts of Brittany and the Netherlands | ||
| S. | perennans Willd | perennans | North Africa and the Mediterranean region to the Baltic Sea and White Sean Asia to Yakutsk (Siberia), Japan, and Korean Peninsula |
| altaica | Altai Mountains (Russia, Mongolia) | ||
| S. | procumbens Sm. | procumbens | Mediterranean and Atlantic coasts from Morocco to Scandinavia, inland occurrences in Turkey and Ukraine |
| freitagii | Turkey (Anatolia) | ||
| pojarkovae | Coasts of the White Sea (Russia) and Barents Sea (Norway) | ||
| heterantha | Endemic in southeast European Russia (Rostov Oblast) | ||
| S. | persica Akhani | persica | Iran |
| iranica | Eastern Mediterranean and Southwest Asia | ||
| S. | dolichostachya Moss | South of Italy (Apulia), Northern European Russia: White Sea coast | |
| S. | glauca Delile (Syn: s. macrostachya Moric) | South of Italy (Apulia) | |
| S. | bigelovii Torrey | Gulf of Mexico, Atlantic coast up to Maine, S California | |
| Sarcocornia | alpini Lag. | Iberian peninsula | |
| S. | carinata Fuente, Rufo & Sánchez Mata | Spain | |
| S. | fruticosa L. | Coasts of the Mediterranean Sea and Atlantic (France) | |
| S. | hispanica Fuente, Rufo & Sánchez-Mata | Southeastern Iberian peninsula | |
| S. | lagascae Fuente, Rufo & Sánchez-Mata | Mediterranean coasts of the Iberian peninsula | |
| S. | obclavata Yaprak | Turkey | |
| S. | perennis Miller | Atlantic and Mediterranean coasts in West and South Europe and North Africa | |
| S. | pruinosa Fuente, Rufo & Sánchez-Mata | Atlantic coasts of France, Spain, and Portugal |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Loconsole, D.; Cristiano, G.; De Lucia, B. Glassworts: From Wild Salt Marsh Species to Sustainable Edible Crops. Agriculture 2019, 9, 14. https://doi.org/10.3390/agriculture9010014
Loconsole D, Cristiano G, De Lucia B. Glassworts: From Wild Salt Marsh Species to Sustainable Edible Crops. Agriculture. 2019; 9(1):14. https://doi.org/10.3390/agriculture9010014
Chicago/Turabian StyleLoconsole, Danilo, Giuseppe Cristiano, and Barbara De Lucia. 2019. "Glassworts: From Wild Salt Marsh Species to Sustainable Edible Crops" Agriculture 9, no. 1: 14. https://doi.org/10.3390/agriculture9010014
APA StyleLoconsole, D., Cristiano, G., & De Lucia, B. (2019). Glassworts: From Wild Salt Marsh Species to Sustainable Edible Crops. Agriculture, 9(1), 14. https://doi.org/10.3390/agriculture9010014








