Effect of Forage Moringa oleifera L. (moringa) on Animal Health and Nutrition and Its Beneficial Applications in Soil, Plants and Water Purification
Abstract
1. Introduction
2. Description of Moringa oleifera
2.1. Nutritional Composition of the Moringa oleifera
2.2. Phytochemicals of the Moringa oleifera
3. Uses of Moringa in Soil and Plants
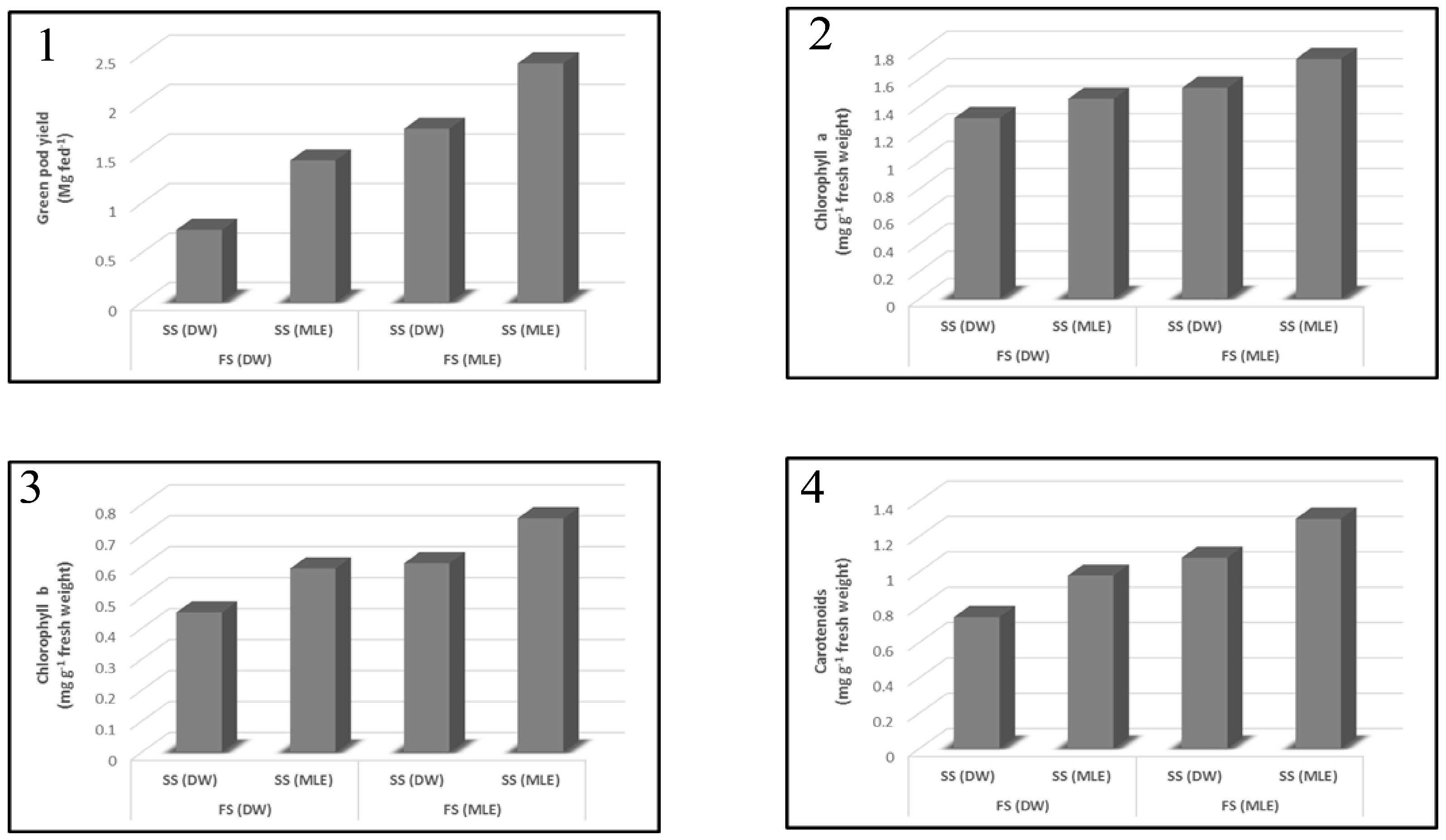
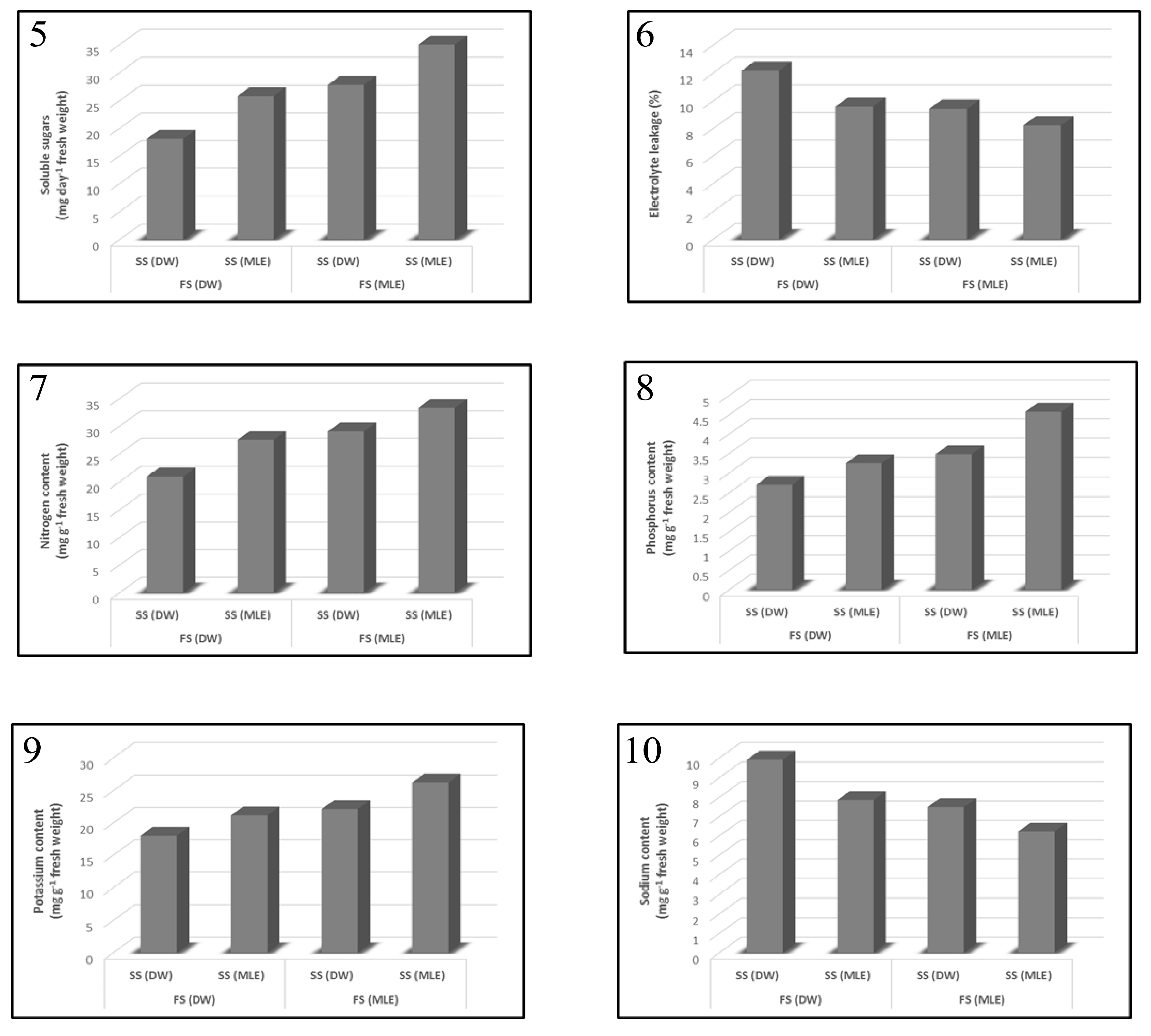
3.1. Effect of Moringa oleifera on Plant Growth Characteristics and Yield
3.2. Effect of Moringa oleifera on Physicochemical Attributes
3.3. Applications of Moringa oleifera in Water Treatment and Purification
3.4. Effect of Moringa on Heavy Metal Accumulation in Water and Soil
3.5. Uses of Moringa in Plant Disease Management
4. Uses of Moringa in the Animal and Poultry Industry
4.1. Inclusion of Moringa oleifera Leaf in Poultry Diets
4.2. Anticoccidial Effect of Moringa oleifera on Poultry Parasitic Diseases
4.3. Antiviral Activity of Moringa oleifera on Poultry Viral Disease
4.4. Antibacterial Effect of Moringa oleifera on Poultry Bacterial Disease
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ahossi, P.K.; Dougnon, J.T.; Kiki, P.S.; Houessionon, J.M. Effects of Tridax procumbens powder on zootechnical, biochemical parameters and carcass characteristics of Hubbard broiler chicken. J. Anim. Health Prod. 2016, 4, 15–21. [Google Scholar]
- Ciftci, M.; Ertas, O.N.; Guler, T. Effect of vitamin E and vitamin C dietary supplementation on egg production and egg quality of laying hens exposed to a chronic stress. Rev. Med. Vet. 2005, 156, 107–111. [Google Scholar]
- Nghonjuyi, N.W.; Tiambo, C.K.; Kimbi, H.K.; Manka’a, C.N.; Juliano, R.S.; Lisita, F. Efficacy of ethanolic extract of Carica papaya leaves as a substitute of sulphanomide for the control of coccidiosis in KABIR chickens in Cameroon. J. Anim. Health Prod. 2015, 3, 21–27. [Google Scholar] [CrossRef]
- Cross, D.E.; Mcdevith, R.M.; Hillman, K.; Agamovic, T. The effect of herbs and their associated essential oils on performance, digestibilities and gut microflora in chickens 7 to 28 days of age. Br. Poult. Sci. 2007, 4, 496–506. [Google Scholar] [CrossRef] [PubMed]
- Soliman, K.M.; Badea, R.I. Effect of oil extracted from some medicinal plants on different mycotoxigenic fungi. Food Chem. Toxicol. 2002, 40, 1669–1675. [Google Scholar] [CrossRef]
- Burtis, M.; Bucar, F. Antioxidant activity of Nigella sativa essential oil. Phytother. Res. 2000, 14, 323–328. [Google Scholar] [CrossRef]
- Abd El-Hack, M.E.; Alagawany, M. Performance, egg quality, blood profile, immune function and antioxidant enzyme activities in laying hens fed diets with thyme powder. J. Anim. Feed Sci. 2015, 24, 127–133. [Google Scholar] [CrossRef]
- Abd El-Hack, M.E.; Alagawany, M.; Farag, M.R.; Tiwari, R.; Kumaragurubaran, K.; Dhama, K.; Zorriehzahra, J.; Adel, M. Beneficial Impacts of Thymol Essential Oil on Health and Production of Animals, Fish and Poultry: A review. J. Essent. Oil Res. 2016, 28. [Google Scholar] [CrossRef]
- Maroni, H.M. Studying the effects of pesticides on humans (International Commission on Occupational Health). In Proceedings of the 9th International workshop, International Centre for Pesticide Safety, Busto Garolfo, Milan, Italy, 2–4 May 1990. [Google Scholar]
- Adandonon, A.; Aveling, T.A.S.; Labuschagne, N.; Tamo, M. Biocontrol agents in combination with Moringa oleifera extract for integrated control of Sclerotium-caused cowpea damping-off and stem rot. Eur. J. Plant Pathol. 2006, 115, 409–418. [Google Scholar] [CrossRef]
- Farag, M.R.; Alagawany, M.; Badr, M.M.; Khalil, S.R.; El-Kholy, M.S. An overview of Jatropha curcas meal induced productive and reproductive toxicity in Japanese quail: Potential mechanisms and heat detoxification. Theriogenology 2018, 113, 208–220. [Google Scholar] [CrossRef] [PubMed]
- Dahot, M.U. Vitamin contents of the flowers and seeds of Moringa oleifera. Pak. J. Biochem. 1988, 21, 1–2. [Google Scholar]
- Fahey, J.W.; Zalcmann, A.T.; Talalay, P. The chemical diversity and distribution of glucosinolates and isothiocyanates among plants. Phytochemistry 2001, 56, 5–51. [Google Scholar] [CrossRef]
- Yang, R.; Chang, L.C.; Hsu, J.C.; Weng, B.B.C.; Palada, M.C.; Chadha, M.L.; Levasseur, V. Nutritional and functional properties of Moringa leaves—from Germplasm, to plant, to food, to health. Moringa Nutr. Plant Resour. Strateg. Stand. Mark. Better Impact Nutr. Afr. 2006, 11, 16–18. [Google Scholar]
- Ali, G.H.; El-Taweel, G.E.; Ali, M.A. The cytotoxicity and antimicrobial efficiency of Moringa oleifera seeds extracts. Int. J. Environ. Stud. 2004, 61, 699–708. [Google Scholar] [CrossRef]
- Adline, J.; Devi, J. A study on phytochemical screening and antibacterial activity of Moringa oleifera. Int. J. Res. Appl. 2014, 2, 169–176. [Google Scholar]
- Fuglie, L.J. The Miracle Tree: Moringa oleifera, Natural Nutrition for the Tropics; Church World Service: Elkhart, IN, USA, 1999; p. 172. [Google Scholar]
- Sultana, N.; Alimon, A.R.; Haque, K.S.; Sazili, A.Q.; Yaakub, H.; Hossain, S.M.J. The effect of cutting interval on yield and nutrient composition of different plant fractions of Moringa oleifera tree. J. Food Agric. Environ. 2014, 12, 599–604. [Google Scholar]
- Olson, M.E.; Carlquist, S. Stem and root anatomical correlations with life form diversity, ecology and systematics in Moringa (Moringaceae). Bot. J. Linn. Soc. 2001, 135, 315–348. [Google Scholar] [CrossRef]
- Olson, M.E. Combining data from DNA sequences and morphology for a phylogeny of Moringaceae (Brassicales). Syst. Bot. 2002, 27, 55–73. [Google Scholar]
- Shahzad, U.; Khan, M.A.; Jaskani, M.J.; Khan, I.A.; Korban, S.S. Genetic diversity and population structure of Moringa oleifera. Conserv. Genet. 2013, 14, 1161–1172. [Google Scholar] [CrossRef]
- Fahey, J.W. Moringa oleifera: A Review of the Medical Evidence for Its Nutritional, Therapeutic and Prophylactic Properties. Trees Life J. 2005, 1, 1–15. [Google Scholar]
- Berger, M.R.; Habs, M.; Jahn, S.A.A.; Schmalhl, D. Toxicological assessment of seeds from Moringa oleifera and Moringa stenopetala, two highly efficient primary coagulants for domestic water treatment of tropical waters. East Afr. Med. J. 1984, 61, 712–717. [Google Scholar] [PubMed]
- Sultana, B.; Anwar, F. Flavonols (kaempeferol, quercetin, myricetin) contents of selected fruits, vegetables and medicinal plants. Food Chem. 2008, 108, 879–884. [Google Scholar] [CrossRef] [PubMed]
- Gopalakrishnan, L.; Doriya, K.; Kumar, D.S. Moringa oleifera: A review on nutritive importance and its medicinal application. Food Sci. Hum. Wellness 2016, 5, 49–56. [Google Scholar] [CrossRef]
- Aregheore, E.M. Intake and digestibility of Moringa oleifera-batiki grass mixtures for growing goats. Small Rumin. Res. 2002, 46, 23–28. [Google Scholar] [CrossRef]
- Richter, N.; Perumal, S.; Becker, K. Evaluation of nutritional quality of Moringa (Moringa oleifera Lam.) leaves as an alternative protein source for Nile tilapia (Oreochromis niloticus L.). Aquaculture 2003, 217, 599–611. [Google Scholar] [CrossRef]
- Rubanza, C.; Shem, M.; Otsyina, E.; Bakengesa, S.; Ichinohe, T.; Fujihara, T. Polyphenolics and tannins effect on in vitro digestibility of selected Acacia species leaves. Anim. Feed Sci. Technol. 2005, 119, 129–142. [Google Scholar] [CrossRef]
- Asaolou, V.; Binuomote, R.; Akinlade, J.; Aderinola, O.; Oyelami, O. Intake and growth performance of West African dwarf goats fed Moringa oleifera, Gliricidia Sepium and Leucaena leucocephala dried leaves as supplements to cassava peels. J. Biol. Agric. Healthc. 2012, 2, 76–88. [Google Scholar]
- Makkar, H.P.S.; Becker, K. Nutritional value and antinutritional components of whole and ethanol extracted of Moringa oleifera leaves. Anim. Feed Sci. Technol. 1996, 63, 211–228. [Google Scholar] [CrossRef]
- Grubben, G.; Denton, O. Plant Resources of Tropical Africa; Earthprint Limited: Stevenage, UK, 2004; pp. 56–60. [Google Scholar]
- Bose, C.K. Nerve growth factor, follicle stimulating hormone receptor and epithelial ovarian cancer. Med. Hypotheses 2004, 5, 917–918. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, J.; Guha, G.; Bhattacharya, B. Powder microscopy of bark–poison used for abortion: Moringa pterygosperma gaertn. J. Indian Forensic Sci. 1978, 17, 47–50. [Google Scholar] [PubMed]
- Faizi, S.; Siddiqui, B.S.; Saleem, R.; Siddiqui, S.; Aftab, K.; Gilani, A.H. Isolation and structure elucidation of new nitrile and mustard oil glycosides from Moringa oleifera and their effect on blood pressure. J. Nat. Prod. 1994, 57, 1256–1261. [Google Scholar] [CrossRef] [PubMed]
- Morton, J.F. The Horseradish Tree, Moringa Pterygosperma (Moringaceae)—A boon to arid lands? Econ. Bot. 1991, 45, 318–333. [Google Scholar] [CrossRef]
- Mazumder, U.K.; Gupta, M.; Chakrabarti, S.; Pal, D. Evaluation of hematological and hepatorenal functions of methanolic extract of Moringa oleifera Lam root treated mice. Indian J. Exp. Biol. 1999, 37, 612–614. [Google Scholar] [PubMed]
- Bennett, R.; Mellon, F.; Foidl, N.; Pratt, J.; Dupont, M.; Perkins, L.; Kroon, P. Profiling glucosinolates and phenolics in vegetative and reproductive tissues of the multi-purpose trees Moringa oleifera L. (Horseradish tree) and Moringa stenopetala L. J. Agric. Food Chem. 2003, 51, 3546–3553. [Google Scholar] [CrossRef] [PubMed]
- Anwar, F.; Latif, S.; Ashraf, M.; Gilani, A.H. Moringa oleifera: A food plant with multiple medicinal uses. Phytother. Res. 2007, 21, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A. Moringa oleifera Lam. (Sahijan)—A plant with a plethora of diverse therapeutic benefits: An updated retrospection. Med. Aromat. Plants 2012, 1, 101. [Google Scholar] [CrossRef]
- Al-Asmari, A.K.; Albalawi, S.M.; Athar, M.T.; Khan, A.Q.; Al-Shahrani, H.; Islam, M. Moringa oleifera as an anti-cancer agent against breast and colorectal cancer cell lines. PLoS ONE 2015, 10. [Google Scholar] [CrossRef] [PubMed]
- Yameogo, C.; Bengaly, M.; Savadogo, A.; Nikiema, P.; Traore, S. Determination of chemical composition and nutritional values Moringa oleifera leaves. Pak. J. Nutr. 2011, 10, 264–268. [Google Scholar] [CrossRef]
- Siddhuraju, P.; Becker, K. Antioxidant properties of various solvent extracts of total phenolic constituents from three different agro-climatic origins of drumstick tree (Moringa oleifera Lam.). J. Agric. Food Chem. 2003, 15, 2144–2155. [Google Scholar] [CrossRef] [PubMed]
- Abbas, T.; Ahmed, M. Use of Moringa oleifera seeds in broilers diet and its effects on the performance and carcass characteristics. Int. J. Appl. Poult. Res. 2012, 1, 1–4. [Google Scholar]
- Pakade, V.; Cukrowska, E.; Chimuka, L. Comparison of antioxidant activity of Moringa oleifera and selected vegetables in South Africa. S. Afr. J. Sci. 2013, 109, 3–4. [Google Scholar] [CrossRef]
- Anwar, F.; Bhanger, M. Analytical characterization of Moringa oleifera seed oil grown in temperate regions of Pakistan. J. Agric. Food Chem. 2003, 51, 6558–6563. [Google Scholar] [CrossRef] [PubMed]
- Sreelatha, S.; Padma, P. Antioxidant activity and total phenolic content of Moringa oleifera leaves in two stages of maturity. Plant Foods Hum. Nutr. 2009, 64, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Verma, J.; Dubey, N.K. Prospectives of botanicals and microbial products as pesticides of tomorrow. Curr. Sci. 1999, 76, 172–179. [Google Scholar]
- Jaafaru, M.S.; Nordin, N.; Shaari, K.; Rosli, R.; Abdull Razis, A.F. Isothiocyanate from Moringa oleifera seeds mitigates hydrogen peroxide-induced cytotoxicity and preserved morphological features of human neuronal cells. PLoS ONE 2018, 13. [Google Scholar] [CrossRef]
- Yasmeen, A.; Basra, S.M.A.; Ahmad, R.; Wahid, A. Performance of late sown wheat in response to foliar application of Moringa oleifera Lam. leaf extract. Chil. J. Agric. Res. 2012, 2, 92–97. [Google Scholar] [CrossRef]
- Rehman, H.; Nawaz, M.Q.; Basra, S.M.A.; Afzal, I.; Yasmeen, A.; Hassan, F.U. Seed priming influence on early crop growth, phenological development and yield performance of linola (Linum usitatissimum L.). J. Integr. Agric. 2014, 13, 990–996. [Google Scholar] [CrossRef]
- Rady, M.M.; Bhavya, V.C.; Howladar, S.M. Common bean (Phaseolus vulgaris L.) seedlings overcome NaCl stress as a result of presoaking in Moringa oleifera leaf extract. Sci. Hortic. 2013, 162, 63–70. [Google Scholar] [CrossRef]
- Salem, J.M. In vitro propagation of Moringa oleifera L. under salinity and ventilation conditions. Genet. Plant Physiol. 2016, 6, 54–64. [Google Scholar]
- Rashid, U.; Anwar, F.; Moser, B.R.; Knothe, G. Moringa oleifera oil: A possible source of biodiesel. Bioresour. Technol. 2008, 99, 8175–8179. [Google Scholar] [CrossRef] [PubMed]
- Bashir, K.A.; Waziri, A.F.; Musa, D.D. Moringa oleifera, a potential miracle tree; a review. IOSR J. Pharm. Biol. Sci. 2016, 11, 25–30. [Google Scholar]
- Fuglie, L. New uses of Moringa studied in Nicaragua: Moringa leaf concentrate. ECHO Dev. Notes 2009, 68, 1–8. [Google Scholar]
- Howladar, S.M. A novel Moringa oleifera leaf extract can mitigate the stress effects of salinity and cadmium in bean (Phaseolus vulgaris L.) plants. Ecotoxicol. Environ. Saf. 2014, 100, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Rady, M.M.; Mohamed, G.F.; Abdalla, A.M.; Ahmed Yasmin, H.M. Integrated application of salicylic acid and Moringa oleifera leaf extract alleviates the salt-induced adverse effects in common bean plants. Int. J. Agric. Technol. 2015, 11, 1595–1614. [Google Scholar]
- Osman, H.; Abohassan, A.A. Moringa: The Strategic Tree for the Third Century; King Abdulaziz University Publishing Center: Jeddah, Saudi Arabia, 2015; ISBN 7-746-06-9960. [Google Scholar]
- Amirigbal, M.; Nadeemakbar, A.; Abbas, R.; Khan, H.; Maqsood, Q. Response of Canola to foliar application of Moring (Moringa oleifera L.) and Brassica (Brassica napus L.) water extracts. Int. J. Agric. Crop Sci. 2014, 14, 1431–1433. [Google Scholar]
- Muhammed, R.; Olurokooba, M.; Akinyaju, J.; Kambai, E. Evaluation of different concentrations and frequency of foliar application of Moringa extract on growth and yield of onions. Agroresearch 2013, 13, 196–205. [Google Scholar] [CrossRef]
- Azooz, M.M.; Shaddad, M.A.; Abdel-Latef, A.A. The accumulation and compartmentation of proline in relation to salt tolerance of three sorghum cultivars. Ind. J. Plant Physiol. 2004, 9, 1–8. [Google Scholar]
- Afzal, I.; Hussain, B.; Basra, S.M.A.; Rehman, H. Priming with MLE reduces imbibitional chilling injury in spring maize. Seed Sci. Technol. 2012, 40, 271–276. [Google Scholar] [CrossRef]
- Basra, S.M.A.; Iftikhar, M.N.; Afzal, I. Potential of moringa (Moringa oleifera) leaf extract as priming agent for hybrid maize seeds. Int. J. Agric. Biol. 2011, 13, 1006–1010. [Google Scholar]
- Moyo, B.; Masika, P.J.; Hugo, A.; Muchenje, V. Nutritional characterization of Moringa (Moringa oleifera Lam) leaves. Afr. J. Biotech. 2011, 10, 12925–12933. [Google Scholar]
- Taiz, L.; Zeiger, E. Plant Physiology; Sinauer Associates: Sunderland, MA, USA, 2010. [Google Scholar]
- Desoky, E.M.; Merwad, A.M.; Elrys, A.S. Response of pea plants to natural bio-stimulants under soil salinity stress. Am. J. Plant Physiol. 2017, 12, 28–37. [Google Scholar]
- Rady, M.M.; Mohamed, G.F. Modulation of salt stress effects on the growth, physio-chemical attributes and yields of Phaseolus vulgaris L. plants by the combined application of salicylic acid and Moringa oleifera leaf extract. Sci. Hortic. 2015, 193, 105–113. [Google Scholar] [CrossRef]
- Basra, S.M.A.; Afzal, I.; Anwar, S.; Shafique, M.; Haq, A.; Majeed, K. Effect of different seed invigoration techniques on wheat (Triticum aestivum L.) seeds sown under saline and non-saline conditions. J. Seed Technol. 2005, 28, 36–45. [Google Scholar]
- Nouman, W.; Siddiqui, M.T.; Basra, S.M.A.; Farooq, H.; Zubair, M.; Gull, T. Biomass production and nutritional quality of Moringa oleifera as field crop. Turk. J. Agric. For. 2013, 37, 410–419. [Google Scholar] [CrossRef]
- Azra, Y. Exploring the Potential of Moringa (Moringa Oleifera) Leaf Extract as a Natural Plant Growth Enhancer. Ph.D. Thesis, University of Agriculture, Faisalabad, Pakistan, 2011. [Google Scholar]
- Hanafy, R. Using Moringa oleifera leaf extract as a bio-fertilizer for drought stress mitigation of Glycine max L. plants. Egypt. J. Bot. 2017, 57, 281–292. [Google Scholar]
- Dietrich, J.T.; Kaminek, V.; Belvins, D.G.; Reinbett, T.M.; Morris, R.D. Changes in cytokinins and cytokinin oxidase activity in developing maize kernel and the effects of exogenous cytokinin on kernel development. Plant Physiol. Biochem. 1995, 33, 327–336. [Google Scholar]
- Stevens, J.; Senaratna, T.; Sivasithamparam, K. Salicylic acid induces salinity tolerance in tomato (Lycopersicon esculentum cv. Roma): Associated changes in gas exchange, water relations and membrane stabilization. Plant Growth Regul. 2006, 49, 77–83. [Google Scholar]
- Gonzalez, L.; Gonzalez-Vilar, M. Determination of relative water content. In Handbook of Plant Ecophysiology Techniques; Reigosa, M.J., Ed.; Springer: Dordrecht, The Netherlands, 2001; pp. 207–212. [Google Scholar]
- Hebers, K.; Sonnewald, V. Altered gene expression: Brought about by inter and pathogen interactions. J. Plant Res. 1998, 111, 323–328. [Google Scholar] [CrossRef]
- Hernández, J.A.; Francisco, F.J.; Corpas, G.M.; Gómez, L.A.; Del Río, F.S. Salt induced oxidative stresses mediated by activated oxygen species in pea leaves mitochondria. Physiol. Plant. 1993, 89, 103–110. [Google Scholar] [CrossRef]
- Del Río, L.A.; Sandalio, L.M.; Corpas, F.J.; Palma, J.M.; Barroso, J.B. Reactive oxygen species and reactive nitrogen species in peroxisomes, production scavenging and role in cell signaling. Plant Physiol. 2006, 141, 330–335. [Google Scholar] [CrossRef] [PubMed]
- Zaki, S.F.; Rady, M.M. Moringa oleifera leaf extract improves growth, physio-chemical attributes, antioxidant defence system and yields of salt-stressed Phaseolus vulgaris L. plants. Int. J. Chem. Technol. Res. 2015, 8, 120–134. [Google Scholar]
- Foyer, C.H. The role of ascorbate in plants, interactions with photosynthesis and regulatory significance. In Active Oxygen, Oxidative Stress and Plant Metabolism; American Society of Plant Physiology: Monona Drive, MD, USA, 1991. [Google Scholar]
- Tetley, R.M.; Thimann, K.V. The metabolism of oat leaves during senescence: I. Respiration, carbohydrate metabolism and the action of cytokinins. Plant Physiol. 1974, 54, 294–303. [Google Scholar] [CrossRef] [PubMed]
- Bichi, M.H. A Review of the Applications of Moringa oleifera Seeds Extract in Water Treatment. Civ. Environ. Res. 2013, 3, 1–8. [Google Scholar]
- Kansal, S.K.; Kumari, A. Potential of M. oleifera for the treatment of water and wastewater. Chem. Rev. 2014, 114, 4993–5010. [Google Scholar] [CrossRef] [PubMed]
- Deeba, F.; Abbas, N.; Butt, T.; Imtiaz, N.; Khan, R.A.; Ahsan, M.M. Utilization of Moringa oleifera seeds for treatment of canal and industrial waste water-an alternative sustainable solution for developing countries. J. Biodivers. Environ. Sci. 2015, 7, 54–60. [Google Scholar]
- Pritchard, M.; Craven, T.; Mkandawire, T.; Edmondson, A.; O’neill, J. A study of the parameters affecting the effectiveness of Moringa oleifera in drinking water purification. Phys. Chem. Earth Parts A/B/C 2010, 35, 791–797. [Google Scholar] [CrossRef]
- Barth, H.; Habs, M.; Klute, R.; Muller, S.; Bernard, T. Anwendung Von naturlichen Wirkstoffen aus Moringa oliefera Lam Samen zur rinkwasseruabereitung. Chem. Ztg. 1982, 1, 75–78. (In German) [Google Scholar]
- Adedapo, A.; Mogbojuri, O.; Emikpe, B. Safety evaluations of the aqueous extract of the leafs of Moringa oleifera in rats. J. Med. Plants Res. 2009, 3, 586–591. [Google Scholar]
- Ali, E.N. Application of Moringa Seeds Extract in Water Treatment. Ph.D. Thesis, International Islamic University, Kuala Lumpur, Malaysia, 2010. [Google Scholar]
- Fahmi, M.R.; Najib, N.W.A.Z.; Ping, P.C.; Hamidin, N. Mechanism of turbidity and hardness removal in hard water sources by using Moringa oleifera. J. Appl. Sci. 2011, 11, 2947–2953. [Google Scholar] [CrossRef]
- Idris, M.A.; Jami, M.S.; Hammed, A.M.; Jamal, P. Moringa oleifera seed extract: A review on its environmental applications. Int. J. Appl. Environ. Sci. 2016, 11, 1469–1486. [Google Scholar]
- Lea, M. Bioremediation of turbid surface water using seed extract from Moringa oleifera Lam. (drumstick) tree. Curr. Protoc. Microbiol. 2010, 16, 1–2. [Google Scholar]
- Muyibi, S.A.; Okuofu, C.A. Softening hard well waters with Moringa oleifera seed extracts. Int. J. Environ. Stud. 1996, 50, 247–257. [Google Scholar] [CrossRef]
- Santos, A.F.S.; Paiva, P.M.G.; Teixeira, J.C.; Brito, A.G.; Coelho, L.C.B.B.; Nogueira, R. Coagulant properties of Moringa oleifera protein preparations: Application to humic acid removal. Environ. Technol. 2013, 33, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Marandi, R.; Bakhtiar Sepehr, S.M. Removal of orange 7 dye from wastewater used by natural adsorbent of Moringa oleifera seeds. Amer. J. Environ. Eng. 2012, 1, 1–9. [Google Scholar] [CrossRef]
- Prasad, R.K. Color removal from distillery spent wash through coagulation using Moringa oleifera seeds: Use of optimum response surface methodology. J. Hazard. Mater. 2009, 165, 804–811. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, S.; Othman, Z.; Ahmad, A.L. Pretreatment of palm oil mill effluent (POME) using Moringa oleifera seeds as natural coagulant. J. Hazard. Mater. 2007, 145, 120–126. [Google Scholar] [CrossRef] [PubMed]
- James, A.; Zikankuba, V. Moringa oleifera a potential tree for nutrition security in sub-Sahara Africa. Am. J. Res. Commun. 2017, 5, 1–12. [Google Scholar]
- Ferreira, R.S.; Napoleão, T.H.; Santos, A.F.S.; Sá, R.A.; Carneiro-da-Cunha, M.G.; Morais, M.M.C.; Silva-Lucca, R.A.; Oliva, M.L.V.; Coelho, L.C.B.B.; Paiva, P.M.G. Coagulant and antibacterial activities of the water-soluble seed lectin from Moringa oleifera. Appl. Microbiol. 2011, 53, 186–192. [Google Scholar] [CrossRef] [PubMed]
- Nkurunziza, T.; Nduwayezu, J.B.; Banadda, E.N.; Nhapi, I. The effect of turbidity levels and Moringa oleifera concentration on the effectiveness of coagulation in water treatment. Water Sci. Technol. 2009, 59, 1551–1558. [Google Scholar] [CrossRef] [PubMed]
- Sivakumar, D. Adsorption study on municipal solid waste leachate using Moringa oleifera seed. Int. J. Environ. Sci. Technol. 2013, 10, 113–124. [Google Scholar] [CrossRef]
- Thakur, S.S.; Choubey, S. Assessment of coagulation efficiency of Moringa oleifera and Okra for treatment of turbid water. Arch. Appl. Sci. Res. 2014, 6, 24–30. [Google Scholar]
- Alsharaa, A.; Basheer, C.; Adio, S.; Alhooshani, K.; Lee, H. Removal of haloethers, trihalomethanes and haloketones from water using Moringa oleifera seeds. Int. J. Environ. Sci. Technol. 2016, 13, 2609–2618. [Google Scholar] [CrossRef]
- Muthuraman, G.; Sasikala, S. Removal of turbidity from drinking water using natural coagulants. J. Ind. Eng. Chem. 2014, 20, 1727–1731. [Google Scholar] [CrossRef]
- Orwa, C.; Mutua, A.; Kindt, R.; Jamnadass, R.; Anthony, S. Agroforestree Database: A Tree Species Reference and Selection Guide Version 4.0 [CD-ROM]; World Agro-forestry Centre: Nairobi, Kenya, 2009; pp. 335–336. [Google Scholar]
- Aruna, M.; Srilatha, N. Water clarification using Moringa oleifera Lam. seed as a natural coagulant. Curr. Biot. 2012, 5, 472–486. [Google Scholar]
- Egbuikwem, P.; Sangodoyin, A. Coagulation efficacy of Moringa oleifera seed extract compared to alum for removal of turbidity and E. coli in three different water sources. Eur. Int. J. Sci. Technol. 2013, 2, 13–20. [Google Scholar]
- Preston, K.; Lantagne, D.; Kotlarz, N.; Jellison, K. Turbidity and chlorine demand reduction using alum and moringa flocculation before household chlorination in developing countries. J. Water Health 2010, 8, 60–70. [Google Scholar] [CrossRef] [PubMed]
- Baig, T.H.; Garcia, A.E.; Tiemann, K.J.; Paso, E. Adsorption of heavy metal ions by the biomass of Solanum elaeagnifolium (silverleaf night-shade). In Proceedings of the 1999 conference on Hazardous Waste Research, Missouri, MO, USA, 24–27 May 1999. [Google Scholar]
- Acheampong, M.A.; Pereira, J.P.C.; Meulepas, R.J.W.; Lens, P.N.L. Kinetics modelling of Cu(II) biosorption on to coconut shell and Moringa oleifera seeds from tropical regions. Environ. Technol. 2012, 33, 409–417. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Kumari, P.; Srivastava, M.M.; Srivastava, S. Ternary biosorption studies of Cd(II), Cr(III) and Ni(II) on shelled Moringa oleifera seeds. Bioresour. Technol. 2007, 98, 474–477. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P. Removal of Cd(II) and Pb(II) from aqueous environment using Moringa oleifera seeds as biosorbent: A low cost and ecofriendly technique for water purification. Trans. Indian Inst. Met. 2008, 61, 107–110. [Google Scholar] [CrossRef]
- Kumari, P.; Sharma, P.; Srivastava, S.; Srivastava, M.M. Biosorption studies on shelled Moringa oleifera Lamarck seed powder: Removal and recovery of arsenic from aqueous system. Int. J. Miner. Process. 2006, 78, 131–139. [Google Scholar] [CrossRef]
- Kumar, V.K.; Rubha, M.N.; Manivasagan, M.; Babu, R.; Balaji, P. Moringa oleifera—The Nature’s Gift. Univ. J. Environ. Res. Technol. 2012, 2, 203–209. [Google Scholar]
- Sajidu, S.M.; Henry, E.M.T.; Kwamdera, G.; Mataka, L. Removal of lead, iron and cadmium ions by means of polyelectrolytes of the Moringa oleifera whole seed kernel. WIT Trans. Ecol. Environ. 2006, 80, 1–8. [Google Scholar]
- Abirami, M.; Rohini, C. Comparative study on the treatment of turbid water using Moringa oleifera and Alum as coagulants. In Proceedings of the International Conference on Emerging Trends in Engineering, Science and Sustainable Technology, Thudupathi, India, 5–6 April 2018. [Google Scholar]
- Maina, I.W.; Obuseng, V.; Nareetsile, F. Use of Moringa oleifera (Moringa) seed pods and Sclerocarya birrea (Morula) nut shells for removal of heavy metals from wastewater and borehole water. J. Chem. 2016. [Google Scholar] [CrossRef]
- Shan, T.C.; Al Matar, M.; Makky, E.A.; Ali, E.N. The use of Moringa oleifera seed as a natural coagulant for wastewater treatment and heavy metals removal. Appl. Water Sci. 2017, 7, 1369–1376. [Google Scholar] [CrossRef]
- Pagnanelli, F.; Mainelli, S.; Veglio, F.; Toro, L. Heavy metal removal by olive pomace: Biosorbent characterization and equilibrium modeling. Chem. Eng. Sci. 2003, 58, 4709–4717. [Google Scholar] [CrossRef]
- Reddy, D.H.K.; Ramana, D.K.V.; Seshaiah, K.; Reddy, A.V.R. Biosorption of Ni(II) from aqueous phase by Moringa oleifera bark, a low cost biosorbent. Desalination 2011, 268, 150–157. [Google Scholar] [CrossRef]
- Reddy, D.H.K.; Seshaiaha, K.; Reddy, A.V.R.; Leec, S.M. Optimization of Cd(II), Cu(II) and Ni(II) biosorption by chemically modified Moringa oleifera leaves powder. Carbohydr. Polym. 2012, 88, 1077–1086. [Google Scholar] [CrossRef]
- Ogle, H. Disease Management: Chemicals. Available online: https://www.appsnet.org/Publications/Brown_Ogle/24%20Control-chemicals%20(HJO).pdf (accessed on 7 August 2018).
- El-Mougy, N.; Abdel-Kader, L. Vegetables root rot disease management by an integrated control measures under greenhouse and plastic houses conditions in Egypt—A review. Int. J. Eng. Innov. Technol. 2012, 6, 241–248. [Google Scholar]
- Najar, A.G.; Anwar, A.; Masoodi, L.; Khar, M.S. Evaluation of native biocontrol agents against Fusarium solani f. sp. melongenae causing wilt disease of brinjal in Kashmir. J. Phytol. 2011, 3, 31–34. [Google Scholar]
- Satish, S.; Mohana, D.C.; Raghavendra, M.P.; Raveesha, K.A. Antifungal activity of some plant extracts against important seed borne pathogens of Aspergillus sp. J. Agric. Technol. 2007, 3, 109–119. [Google Scholar]
- Kuri, S.K.; Islam, R.M.; Mondal, U. Antifungal potentiality of some botanical extracts against important seedborne fungal pathogen associated with brinjal seeds, Solanum melongena L. J. Agric. Technol. 2011, 7, 1139–1153. [Google Scholar]
- Mariani, C.; Braca, A.; Vitalini, S.; Tommasi, N.D.; Visioli, F.; Fico, G. Flavonoid characterization and in vitro antioxidant activity of Aconitum anthora L. (Ranunculaceae). Phytochemistry 2008, 69, 1220–1226. [Google Scholar] [CrossRef] [PubMed]
- Saavedra Gonzalez, Y.R.; van der Maden, E.C.L.J. Opportunities for Development of the Moringa Sector in Bangladesh: Desk-Based Review of the Moringa Value Chains in Developing Countries and End-Markets in Europe; Centre for Development Innovation: Wageningen, WD, USA, 2015. [Google Scholar]
- Thanaa, S.H.M.; Kassim, N.E.; AbouRayya, M.S.; Abdalla, A.M. Influence of foliar application with moringa (Moringa oleifera L.) leaf extract on yield and fruit quality of Hollywood plum cultivar. J. Hortic. 2017, 4. [Google Scholar] [CrossRef]
- Balogun, S.O.; Idowu, A.A.; Ojiako, F.O. Evaluation of the effects of four plant materials and Fernazzan D on the mycelial growth of Aspergillus flavus isolated from stored maize grains. Plant Sci. 2004, 4, 105–114. [Google Scholar]
- Makkar, H.P.S.; Becker, K. Nutrients and antiquality factors in different morphological parts of the Moringa oleifera tree. J. Agric. Sci. 1997, 128, 311–322. [Google Scholar] [CrossRef]
- Hussain, S.; Malik, F.; Mahmood, S. Review: An exposition of medicinal preponderance of Moringa oleifera (Lank.). Pak. J. Pharm. Sci. 2014, 27, 397–403. [Google Scholar] [PubMed]
- Goss, M.; Mafongoya1, P.; Gubba, A. Moringa oleifera extracts effect on Fusarium solani and Rhizoctonia solani growth. Asian Res. J. Agric. 2017, 6, 1–10. [Google Scholar] [CrossRef]
- Chen, M. Elucidation of bactericidal effects incurred by Moringa oleifera and Chitosan. J. U.S. SJWP 2009, 4, 65–79. [Google Scholar]
- Viera, G.H.F.; Mourão, J.A.; Ângelo, Â.M.; Costa, R.A.; Vieira, R.H.S.D.F. Antibacterial effect (in vitro) of Moringa oleifera and Annona muricata against Gram positive and Gram negative bacteria. Rev. Inst. Med. Trop. Sao Paulo 2010, 52, 129–132. [Google Scholar] [CrossRef] [PubMed]
- Jahn, S.A.; Musnad, H.A.; Burgstaller, H. The tree that purifies water: Cultivating multipurpose Moringaceae in the Sudan. Unasylva 1986, 38, 23–28. [Google Scholar]
- Holetz, F.B.; Greisiele, L.P.; Neviton, R.S.; Diógenes, A.G.C.; Celso, V.N.; Benedito, P.D.F. Screening of some plants used in the Brazilian folk medicine for the treatment of infectious diseases. Mem. Inst. Oswaldo Cruz 2002, 97, 1027–1031. [Google Scholar] [CrossRef] [PubMed]
- Bukar, A.; Uba, A.; Oyeyi, T.I. Antimicrobial profile of Moringa oleifera lam. Extracts against some food born microorganism. Bayero J. Pure Appl. Sci. 2010, 3, 43–48. [Google Scholar]
- Abalaka, M.E.; Daniyan, S.Y.; Oyeleke, S.B.; Adeyemo, S.O. The antibacterial evaluation of Moringa oleifera leaf extracts on selected bacterial pathogens. J. Microbiol. Res. 2012, 2, 1–4. [Google Scholar] [CrossRef]
- Food and Agricultural Organization (FAO). Moringa Traditional Crop of the Month. Available online: http://www.fao.org/traditional-crops/moringa/en/2014 (accessed on 7 August 2018).
- Jabeen, R.; Shahid, M.; Jamil, A.; Ashraf, M. Microscopic of the antimicrobial activity of seed extracts of Moringa. Pak. J. Bot. 2008, 40, 1349–1358. [Google Scholar]
- Chollom, S.C.; Agada, G.O.A.; Gotep, J.G.; Mwankon, S.E.; Dus, P.C.; Bot, Y.S.; Nyango, D.Y.; Singnap, C.L.; Fyaktu, E.J.; Okwori, A.E.J. Investigation of aqueous extract of Moringa oleifera lam seed for antiviral activity against newcastle disease virus in ovo. J. Med. Plants Res. 2012, 6, 3870–3875. [Google Scholar] [CrossRef]
- Donkor, A.; Glover, R.; Addae, K.; Kubi, K. Estimating the nutritional value of the leaves of Moringa oleifera on poultry. Food Nutr. Sci. 2013, 4, 1077–1083. [Google Scholar]
- Ayssiwede, S.B.; Zanmenou, J.C.; Issa, Y.; Hane, M.B.; Dieng, A.; Chrysostome, C.A.A.M.; Houinato, M.R.; Hornick, J.L.; Missohou, A. Nutrient composition of some unconventional and local feed resources available in Senegal and recoverable in indigenous chickens or animal feeding. Pak. J. Nutr. 2011, 10, 707–717. [Google Scholar] [CrossRef]
- Olugbemi, T.S.; Mutayoba, S.K.; Lekule, F.P. Moringa oleifera leaf meal as a hypocholesterolemic agent in laying hen diets. Livest. Res. Rural Dev. 2010, 22, 84. [Google Scholar]
- Abou-Elezz, F.; Sarmiento-Franco, L.; Santos-Ricalde, R.; Solorio-Sanchez, F. Nutritional effects of dietary inclusion of Leucaena leucocephala and Moringa oleifera leaf meal on Rhode Island Red hens’ performance. Cuban J. Agric. Sci. 2011, 45, 163–169. [Google Scholar]
- Kakengi, A.; Kaijage, J.; Sarwatt, S.; Mutayoba, S.; Shem, M.; Fujihara, T. Effect of Moringa oleifera leaf meal as a substitute for sunflower seed meal on performance of laying hens in Tanzania. Int. J. Poult. Sci. 2007, 9, 363–367. [Google Scholar]
- Safa, M.; Tazi, E. Effect of feeding different levels of Moringa oleifera leaf meal on the performance and carcass quality of broiler chicks. Int. J. Sci. Res. 2014, 3, 147–151. [Google Scholar]
- Gadzirayi, C.T.; Masamha, B.; Mupangwa, J.F.; Washaya, S. Performance of broiler chickens fed on mature Moringa oleifera leaf meal as a protein supplement to soyabean meal. Int. J. Poult. Sci. 2012, 11, 5–10. [Google Scholar] [CrossRef]
- Kaijage, J.; Sarwatt, S.; Mutayoba, S. Moringa oleifera leaf meal can improve quality characteristics and consumer preference of marketable eggs. Numer. Proc. Pap. 2004, 126–129. [Google Scholar]
- Etalem, T.; Getachew, A.; Mengistu, U.; Tadelle, D. Moringa oleifera leaf meal as an alternative protein feed ingredient in broiler ration. Int. J. Poult. Sci. 2013, 12, 289–297. [Google Scholar]
- Jung, S.; Choe, J.; Kim, B.; Yun, H.; Kruk, Z.; Jo, C. Effect of dietary mixture of garlic acid and linoleic acid on antioxidative potential and quality of breast meat from broilers. Meat Sci. 2010, 86, 520–526. [Google Scholar] [CrossRef] [PubMed]
- Pennington, J.; Fisher, R. Classification of fruits and vegetables. J. Food Compos. Anal. 2009, 22, 23–31. [Google Scholar] [CrossRef]
- Mutayoba, S.; Mutayoba, B.; Okot, P. The performance of growing pullets fed diets with varying energy and leucaena leaf meal levels. Livest. Res. Rural Dev. 2003, 15, 350–357. [Google Scholar]
- Ebenebe, C.; Anigbogu, C.; Anizoba, M.; Ufele, A. Effect of various levels of moringa leaf meal on the egg quality of Isa Brown Breed of layers. Adv. Life Sci. Technol. 2013, 14, 1–6. [Google Scholar]
- Adeniji, A.; Lawal, M. Effects of replacing groundnut cake with Moringa oleifera leaf meal in the diets of grower rabbits. Int. J. Mol. Vet. Res. 2012, 2, 8–13. [Google Scholar]
- Gadzirayi, C.T.; Mupangwa, J.F. The nutritive evaluation and utilisation of Moringa oleifera in indigenous and broiler chicken production: A review. Greener J. Agric. Sci. 2014, 14, 15–21. [Google Scholar]
- Lu, W.; Wang, J.; Zhang, H.J.; Wu, S.G.; Qi, G.H. Evaluation of Moringa oleifera leaf in laying hens: Effects on laying performance, egg quality, plasma biochemistry and organ histopathological indices. Ital. J. Anim. Sci. 2016, 15, 658–665. [Google Scholar] [CrossRef]
- Ola-Fadunsin, S.D.; Ademola, I.O. Direct effects of Moringa oleifera Lam (Moringaceae) acetone leaf extract on broiler chickens naturally infected with Eimeria species. Trop. Anim. Health Prod. 2013, 45, 1423–1428. [Google Scholar] [CrossRef] [PubMed]
- Dillard, C.J.; German, J.B. Phytochemicals: Nutraceuticals and human health: A review. J. Sci. Food Agric. 2000, 80, 1744–1756. [Google Scholar] [CrossRef]
- Allen, P.C.; Lydon, J.; Danforth, H.D. Effects of components of Artemisia annua on coccidian infections in chickens. Poult. Sci. 1997, 76, 1156–1163. [Google Scholar] [CrossRef] [PubMed]
- Luqman, S.; Srivastava, S.; Kumar, R.; Maurya, A.K.; Chanda, D. Experimental assessment of Moringa oleifera leaf and fruit for its antistress, antioxidant and scavenging potential using in vitro and in vivo assays. Evid.-Based Complement. Altern. Med. 2012. [Google Scholar] [CrossRef] [PubMed]
- Sonaiya, E.B.; Swan, S.E.J. Manual Small-Scale Poultry Production Technical Guide; Food and agriculture organization of the United Nation: Rome, Italy, 2014. [Google Scholar]
- Gueye, E.F. Newcastle disease in Family Poultry: Prospects for its control through Ethnovetrinary Medicine. Livest. Res. Rural Dev. 2002, 14, 25–29. [Google Scholar]
- Ferreira, P.M.P.; Farias, D.F.; Oliveira, J.T.A.; Carvalho, A.F.U. Moringa oleifera: Bioactive compounds and nutritional potential. Rev. Nutr. 2008, 21, 431–437. [Google Scholar] [CrossRef]
- Davis, L.; Kuttan, G. Suppressive effect of cyclophosphamide- induced toxicity by Withania somnifera extract in mice. J. Ethnopharmacol. 1998, 62, 209–221. [Google Scholar] [CrossRef]
- Bouzoubaa, K.; Lemainguer, K.; Bell, J.G. Village chickens as reservoir of Salmonella pullorum and Salmonella gallinarum in Morocco. Prev. Vet. Med. 1992, 12, 95–100. [Google Scholar] [CrossRef]
- Abiodun, B.S.; Adedeji, A.S.; Taiwo, O.; Gbenga, A. Effects of Moringa oleifera root extract on the performance and serum biochemistry of Escherichia coli challenged broiler chicks. J. Agric. Sci. 2015, 60, 505–513. [Google Scholar] [CrossRef]
- Patel, J.P. Antibacterial activity of methanolic and acetone extract of some medicinal plants used in India folklore. Int. J. Phytomed. 2011, 3, 261–269. [Google Scholar]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abd El-Hack, M.E.; Alagawany, M.; Elrys, A.S.; Desoky, E.-S.M.; Tolba, H.M.N.; Elnahal, A.S.M.; Elnesr, S.S.; Swelum, A.A. Effect of Forage Moringa oleifera L. (moringa) on Animal Health and Nutrition and Its Beneficial Applications in Soil, Plants and Water Purification. Agriculture 2018, 8, 145. https://doi.org/10.3390/agriculture8090145
Abd El-Hack ME, Alagawany M, Elrys AS, Desoky E-SM, Tolba HMN, Elnahal ASM, Elnesr SS, Swelum AA. Effect of Forage Moringa oleifera L. (moringa) on Animal Health and Nutrition and Its Beneficial Applications in Soil, Plants and Water Purification. Agriculture. 2018; 8(9):145. https://doi.org/10.3390/agriculture8090145
Chicago/Turabian StyleAbd El-Hack, Mohamed E., Mahmoud Alagawany, Ahmed S. Elrys, El-Sayed M. Desoky, Hala M. N. Tolba, Ahmed S. M. Elnahal, Shaaban S. Elnesr, and Ayman A. Swelum. 2018. "Effect of Forage Moringa oleifera L. (moringa) on Animal Health and Nutrition and Its Beneficial Applications in Soil, Plants and Water Purification" Agriculture 8, no. 9: 145. https://doi.org/10.3390/agriculture8090145
APA StyleAbd El-Hack, M. E., Alagawany, M., Elrys, A. S., Desoky, E.-S. M., Tolba, H. M. N., Elnahal, A. S. M., Elnesr, S. S., & Swelum, A. A. (2018). Effect of Forage Moringa oleifera L. (moringa) on Animal Health and Nutrition and Its Beneficial Applications in Soil, Plants and Water Purification. Agriculture, 8(9), 145. https://doi.org/10.3390/agriculture8090145









