Fatty Acids Produced by Neofusicoccum vitifusiforme and N. parvum, Fungi Associated with Grapevine Botryosphaeria Dieback
Abstract
1. Introduction
2. Materials and Methods
2.1. General Experimental Procedures
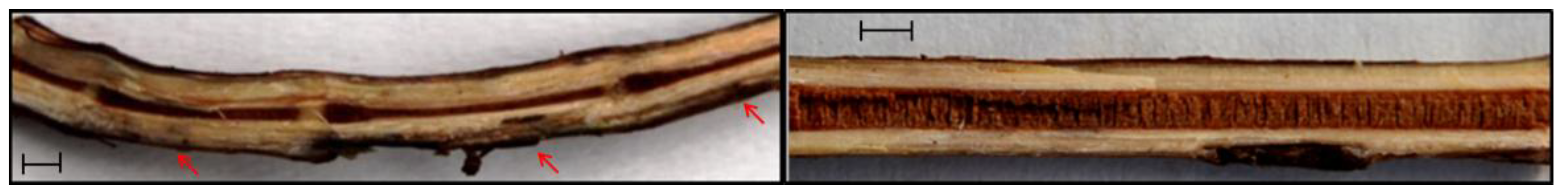
2.2. Fungal Strain and Pathogenicity Test
2.3. Culture Filtrate Production
2.4. Extraction and Purification Processes
2.4.1. Neofusicoccum vitifusiforme
2.4.2. Neofusicoccum parvum
2.5. Fatty Acids Methylation
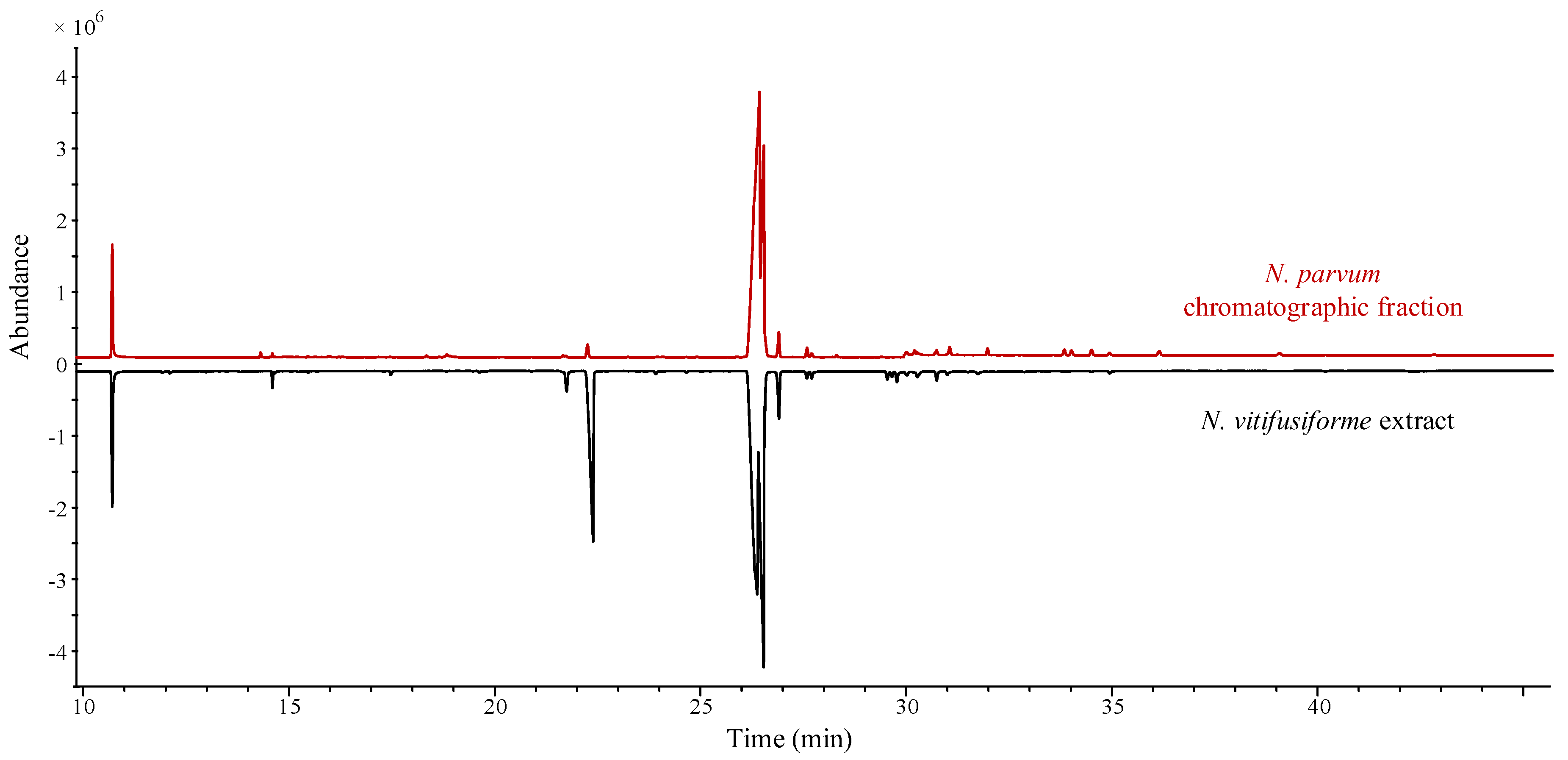
2.6. Qualitative and Quantitative Analysis of Fatty Acids
2.7. Phytotoxicity Bioassays
3. Results
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- 2017 World Vitiviniculture Situation-OIV Statistical report on World Vitiviniculture. Available online: http://www.oiv.int/public/medias/5479/oiv-en-bilan-2017 (accessed on 1 September 2018).
- Jayawardena, R.S.; Purahong, W.; Zhang, W.; Wubet, T.; Li, X.; Liu, M.; Zhao, W.; Hyde, K.D.; Liu, J.; Yan, J. Biodiversity of fungi on Vitis vinifera L. revealed by traditional and high-resolution culture-independent approaches. Fungal Div. 2018, 90, 1–84. [Google Scholar] [CrossRef]
- Úrbez-Torres, J.R.; Adams, P.; Kamas, J.; Gubler, W.D. Identification, incidence, and pathogenicity of fungal species associated with grapevine dieback in Texas. Am. J. Enol. Vitic. 2009, 60, 497–507. [Google Scholar]
- Mugnai, L.; Graniti, A.; Surico, G. Esca (black measles) and brown wood-streaking: Two old and elusive diseases of grapevines. Plant Dis. 1999, 83, 404–418. [Google Scholar] [CrossRef]
- Bertsch, C.; Ramírez-Suero, M.; Magnin-Robert, M.; Larignon, P.; Chong, J.; Abou-Mansour, E.; Spagnolo, A.; Clement, C.; Fontaine, F. Grapevine trunk diseases: Complex and still poorly understood. Plant Pathol. 2013, 62, 243–265. [Google Scholar] [CrossRef]
- Úrbez-Torres, J.R. The status of Botryosphaeriaceae species infecting grapevine. Phytopathol. Mediterr. 2011, 50, S5–S45. [Google Scholar]
- Larignon, P.; Fulchic, R.; Cere, L.; Dubos, B. Observation on black dead arm in French vineyards. Phytopathol. Mediterr. 2001, 40, S336–S342. [Google Scholar]
- Yan, J.Y.; Xie, Y.; Zhang, W.; Wang, Y.; Liu, J.K.; Hyde, K.D.; Seem, R.C.; Zang, G.Z.; Wang, Z.Y.; Yao, S.W.; et al. Species of Botryosphaeriaceae involved in grapevine dieback in China. Fungal Divers. 2013, 61, 221–236. [Google Scholar] [CrossRef]
- Mondello, V.; Songy, A.; Battiston, E.; Pinto, C.; Coppin, C.; Trotel-Aziz, P.; Clément, C.; Mugnai, L.; Fontaine, F. Grapevine Trunk Diseases: A review of fifteen years of trials for their control with chemicals and biocontrol agents. Plant Dis. 2018, 102, 1189–1217. [Google Scholar] [CrossRef]
- Burruano, S.; Mondello, V.; Conigliaro, G.; Alfonzo, A.; Spagnolo, A.; Mugnai, L. Grapevine decline in Italy caused by Lasiodiplodia theobromae. Phytopathol. Mediterr. 2008, 47, 132–136. [Google Scholar]
- Mondello, V.; Lo Piccolo, S.; Conigliaro, G.; Alfonzo, A.; Torta, L.; Burruano, S. First report of Neofusiccoccum vitifusiforme and presence of other Botryosphaeriaceae species associated with Botryosphaeria dieback of grapevine in Sicily (Italy). Phytopathol. Mediterr. 2013, 52, 388–396. [Google Scholar]
- Andolfi, A.; Basso, S.; Giambra, S.; Conigliano, G.; Lo Piccolo, S.; Alves, A.; Burruano, S. Lasiolactols A and B produced by the grapevine fungal pathogen Lasiodiplodia mediterranea. Chem. Biodivers. 2016, 13, 395–402. [Google Scholar] [CrossRef]
- Burruano, S.; Giambra, S.; Mondello, V.; DellaGreca, M.; Basso, S.; Tuzi, A.; Andolfi, A. Naphthalenone polyketides produced by Neofusicoccum parvum, a fungus associated with grapevine Botryosphaeria dieback. Phytopathol. Mediterr. 2016, 55, 197–206. [Google Scholar]
- Larignon, P.; Dubos, B. Fungi associated with esca disease in grapevine. Eur. J. Plant Pathol. 1997, 103, 147–157. [Google Scholar] [CrossRef]
- Abou-Mansour, E.; Débieux, J.L.; Ramírez-Suero, M.; Bénard-Gellon, M.; Magnin-Robert, M.; Spagnolo, A.; Chong, J.; Farine, S.; Bertsch, C.; L’Haridon, F.; et al. Larignon. Phytotoxic metabolites from Neofusicoccum parvum, a pathogen of Botryosphaeria dieback of grapevine. Phytochemistry 2015, 115, 207–215. [Google Scholar] [CrossRef]
- Andolfi, A.; Mugnai, L.; Luque, J.; Surico, G.; Cimmino, A.; Evidente, A. Phytotoxins produced by fungi associated with grapevine trunk diseases. Toxins 2011, 3, 1569–1605. [Google Scholar] [CrossRef]
- Martos, S.; Andolfi, A.; Luque, J.; Mugnai, L.; Surico, G.; Evidente, A. Production of phytotoxic metabolites by five species of Botryosphaeriaceae causing decline on grapevines, with special interest in the species Neofusicoccum luteum and N. parvum. Eur. J. Plant Pathol. 2008, 121, 451–461. [Google Scholar] [CrossRef]
- Evidente, A.; Punzo, B.; Andolfi, A.; Cimmino, A.; Melck, D.; Luque, J. Lipophilic phytotoxins produced by Neofusicoccum parvum, a grapevine canker agent. Phytopathol. Mediterr. 2010, 49, 74–79. [Google Scholar]
- Ramírez-Suero, M.; Bénard-Gellon, M.; Chong, J.; Laloue, H.; Stempien, E.; Abou-Mansour, E.; Fontaine, F.; Larignon, P.; Mazet-Kieffer, F.; Farine, S.; et al. Extracellular compounds produced by fungi associated with Botryosphaeria dieback induce differential defence gene expression patterns and necrosis in Vitis vinifera cv. Chardonnay cells. Protoplasma 2014, 251, 1417–1426. [Google Scholar] [CrossRef]
- Bénard-Gellon, M.; Farine, S.; Goddard, M.L.; Schmitt, M.; Stempien, E.; Pensec, F.; Laloue, H.; Mazet-Kieffer, F.; Fontaine, F.; Larignon, P.; et al. Toxicity of extracellular proteins from Diplodia seriata and Neofusicoccum parvum involved in grapevine Botryosphaeria dieback. Protoplasma 2015, 252, 679–687. [Google Scholar] [CrossRef]
- Andolfi, A.; Maddau, L.; Cimmino, A.; Linaldeddu, B.T.; Franceschini, A.; Serra, S.; Basso, S.; Merck, D.; Evidente, A. Cyclobotryoxide, a phytotoxic metabolite produced by the plurivorous pathogen Neofusicoccum australe. J. Nat. Prod. 2012, 75, 1785–1791. [Google Scholar] [CrossRef]
- Andolfi, A.; Maddau, L.; Cimmino, A.; Linaldeddu, B.T.; Basso, S.; Deidda, A.; Serra, S.; Evidente, A.; Lasiojasmonates, A.-C. Three jasmonic acid esters produced by Lasiodiplodia sp., a grapevine pathogen. Phytochemistry 2014, 103, 145–153. [Google Scholar] [CrossRef]
- Uranga, C.C.; Beld, J.; Mrse, A.; Córdova-Guerrero, I.; Burkart, M.D.; Hernández-Martínez, R. Fatty acid esters produced by Lasiodiplodia theobromae function as growth regulators in tobacco seedlings. Biochem. Biophys. Res. Commun. 2016, 472, 339–345. [Google Scholar] [CrossRef]
- Kopka, J.; Schauer, N.; Krueger, S.; Birkemeyer, C.; Usadel, B.; Bergmüller, E.; Dormann, P.; Weckwerth, Y.G.; Fernie, A.R.; Steinhauser, D.; et al. GMD@ CSB. DB: The Golm metabolome database. Bioinformatics 2004, 21, 1635–1638. [Google Scholar] [CrossRef]
- AMDIS NET. Available online: http://www.amdis.net/ (accessed on 18 July 2018).
- Sparkman, O.D.; Penton, Z.E.; Kitson, F.G. Gas Chromatography and Mass Spectrometry: A Practical Guide, 2nd ed.; Elsevier Inc.: Burlington, MA, USA, 2011; ISBN 978-0-12-373628-4978-0-12-373628-4. [Google Scholar]
- Nieva-Echevarría, B.; EncarnaciónGoicoechea, M.; Manzanos, J.; Guillén, M.D. A method based on 1H NMR spectral data useful to evaluate the hydrolysis level in complex lipid mixtures. Food Res. Int. 2014, 66, 379–387. [Google Scholar] [CrossRef]
- Salvatore, M.M.; Nicoletti, R.; Salvatore, F.; Naviglio, D.; Andolfi, A. GC-MS approaches for the screening of metabolites produced by marine-derived Aspergillus. Marine Chem. 2018, 206, 19–33. [Google Scholar] [CrossRef]
- Spagnolo, A.; Larignon, P.; Magnin-Robert, M.; Hovasse, A.; Cilindre, C.; van Dorsselaer, A.; Clement, C.; Schaeffer-Reiss, C.; Fontaine, F. Flowering as the most highly sensitive period of grapevine (Vitis vinifera L. cv Mourvèdre) to the botryosphaeria dieback agents Neofusicoccum parvum and Diplodia seriata infection. Int. J. Mol. Sci. 2014, 15, 9644–9669. [Google Scholar] [CrossRef]
- van Niekerk, J.M.; Crous, P.W.; Groenewald, J.Z.; Fourie, P.H.; Halleen, F. DNA phylogeny, morphology and pathogenicity of Botryosphaeria species on grapevines. Mycologia 2004, 96, 781–798. [Google Scholar] [CrossRef]
- Luque, J.; Martos, S.; Aroca, A.; Raposo, R.; Garcia-Figueres, F. Symptoms and fungi associated with declining mature grapevine plants in northeast Spain. J. Plant Pathol. 2009, 381–390. [Google Scholar]
- Candolfi-Arballo, O.; Valenzuela-Solano, C.; Gubler, W.D.; Hernández-Martínez, R. Botryosphaeriaceae species associated with grapevine decline in Mexico. Phytopathol. Mediterr. 2010, 49, 105–106. [Google Scholar]
- Schaller, A.; Stintzi, A. Enzymes in jasmonate biosynthesis–structure, function, regulation. Phytochemistry 2009, 70, 1532–1538. [Google Scholar] [CrossRef]
- Husain, A.; Ahmad, A.; Agrawal, P.K. (−)-jasmonic acid, a phytotoxic substance from Botryodiplodiatheobromae: Characterization by nmr spectroscopic methods. J. Nat. Prod. 1993, 56, 2008–2011. [Google Scholar] [CrossRef]
- Chanclud, E.; Morel, J.B. Plant hormones: A fungal point of view. Mol. Plant Pathol. 2016, 17, 1289–1297. [Google Scholar] [CrossRef]
- Félix, C.; Salvatore, M.M.; DellaGreca, M.; Meneses, R.; Duarte, A.S.; Salvatore, F.; Naviglio, D.; Gallo, M.; Jorrín-Novo, J.V.; Alves, A.; et al. Production of toxic metabolites by two strains of Lasiodiplodia theobromae, isolated from a coconut tree and a human patient. Mycologia 2018, 110, 642–653. [Google Scholar] [CrossRef]
- Tsukada, K.; Takahashi, K.; Nabeta, K. Biosynthesis of jasmonic acid in a plant pathogenic fungus, Lasiodiplodiatheobromae. Phytochemistry 2010, 71, 2019–2023. [Google Scholar] [CrossRef]
- Farmer, E.E.; Ryan, C.A. Octadecanoid precursors of jasmonic acid activate the synthesis of wound-inducible proteinase inhibitors. Plant Cell 1992, 4, 129–134. [Google Scholar] [CrossRef]
- Fitton, A.; Goa, K.L. Azelaic acid. In Drugs; Springer: Berlin, Germany, 1991; Volume 41, pp. 780–798. ISBN 0012-6667. [Google Scholar]
| N. vitifusiforme | N. parvum | |
|---|---|---|
| Azelaic acid | ✓ | - |
| Palmitoleic acid | ✓ | - |
| Palmitic acid | ✓ | - |
| Linoleic acid | ✓ | ✓ |
| Elaidic acid | ✓ | ✓ |
| Stearic acid | ✓ | ✓ |
| Code | Name | KI 1 | Abundance (%) in the Extract of N. vitifusiforme (B8) | Abundance (%) in the Extract of N. parvum (B19) 2 |
|---|---|---|---|---|
| - | Azelaic acid | 1548 | 0.5 | - |
| 16:1n-7 | Palmitoleic acid | 1903 | 1.5 | - |
| 16:0 | Palmitic acid | 1926 | 10.4 | - |
| 18:2n-6 | Linoleic acid | 2092 | 48.2 | 0.96 |
| 18:1 | Elaidic acid | 2109 | 38.0 | 0.33 |
| 18:0 | Stearic acid | 2125 | 1.4 | 0.01 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salvatore, M.M.; Giambra, S.; Naviglio, D.; DellaGreca, M.; Salvatore, F.; Burruano, S.; Andolfi, A. Fatty Acids Produced by Neofusicoccum vitifusiforme and N. parvum, Fungi Associated with Grapevine Botryosphaeria Dieback. Agriculture 2018, 8, 189. https://doi.org/10.3390/agriculture8120189
Salvatore MM, Giambra S, Naviglio D, DellaGreca M, Salvatore F, Burruano S, Andolfi A. Fatty Acids Produced by Neofusicoccum vitifusiforme and N. parvum, Fungi Associated with Grapevine Botryosphaeria Dieback. Agriculture. 2018; 8(12):189. https://doi.org/10.3390/agriculture8120189
Chicago/Turabian StyleSalvatore, Maria Michela, Selene Giambra, Daniele Naviglio, Marina DellaGreca, Francesco Salvatore, Santella Burruano, and Anna Andolfi. 2018. "Fatty Acids Produced by Neofusicoccum vitifusiforme and N. parvum, Fungi Associated with Grapevine Botryosphaeria Dieback" Agriculture 8, no. 12: 189. https://doi.org/10.3390/agriculture8120189
APA StyleSalvatore, M. M., Giambra, S., Naviglio, D., DellaGreca, M., Salvatore, F., Burruano, S., & Andolfi, A. (2018). Fatty Acids Produced by Neofusicoccum vitifusiforme and N. parvum, Fungi Associated with Grapevine Botryosphaeria Dieback. Agriculture, 8(12), 189. https://doi.org/10.3390/agriculture8120189








