Potential of Baled Silage to Preserve White Grape Pomace for Ruminant Feeding
Abstract
1. Introduction
2. Materials and Methods
2.1. By-Product
2.2. Experimental Design
2.3. Microbiology
2.4. Fermentation Metabolites
2.5. Physico-Chemical Parameters and Nutritional Composition
2.6. Fatty Acid Profile
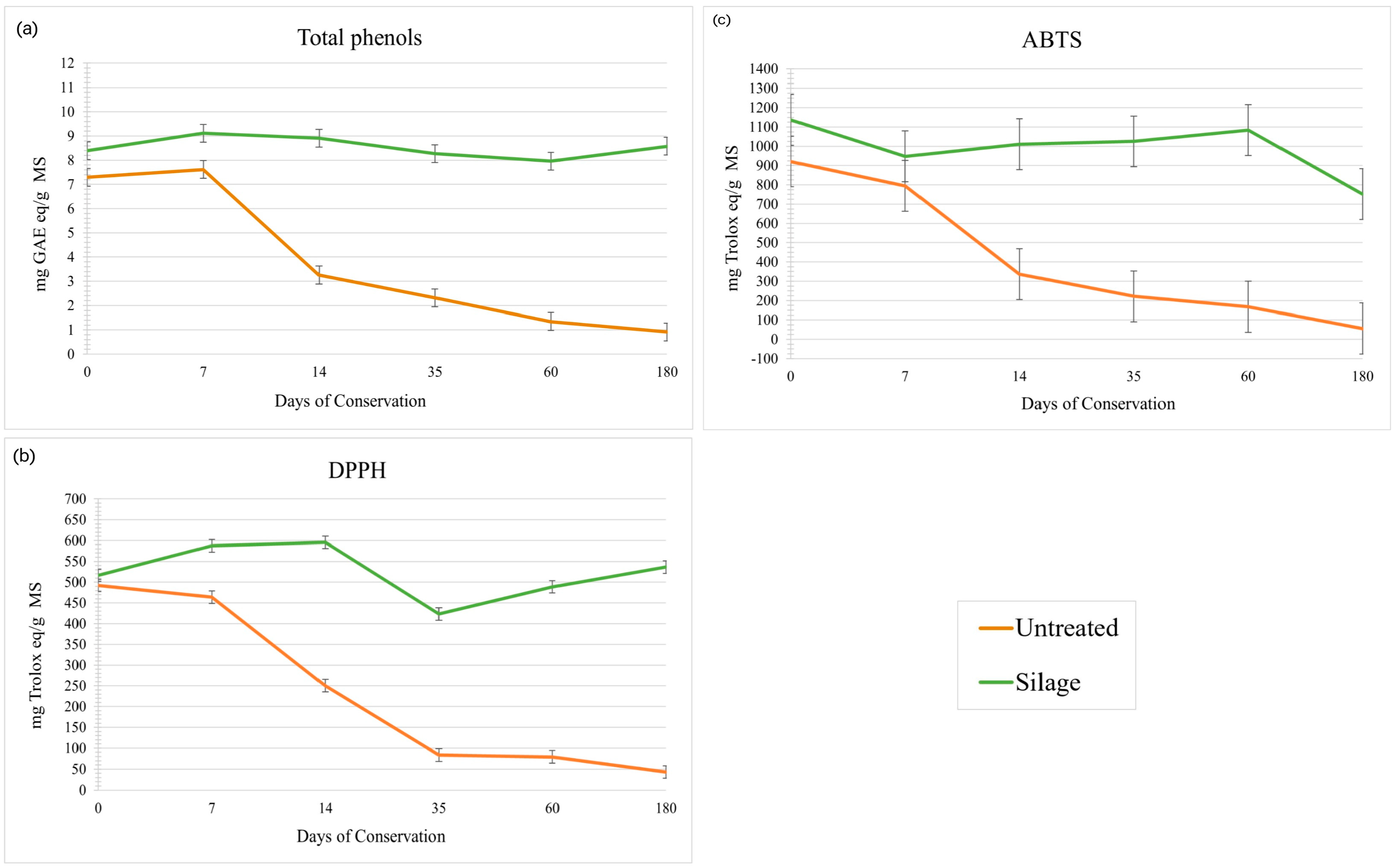
2.7. Bioactive Properties
2.8. Statistical Analysis
2.9. Other Key Aspects
3. Results
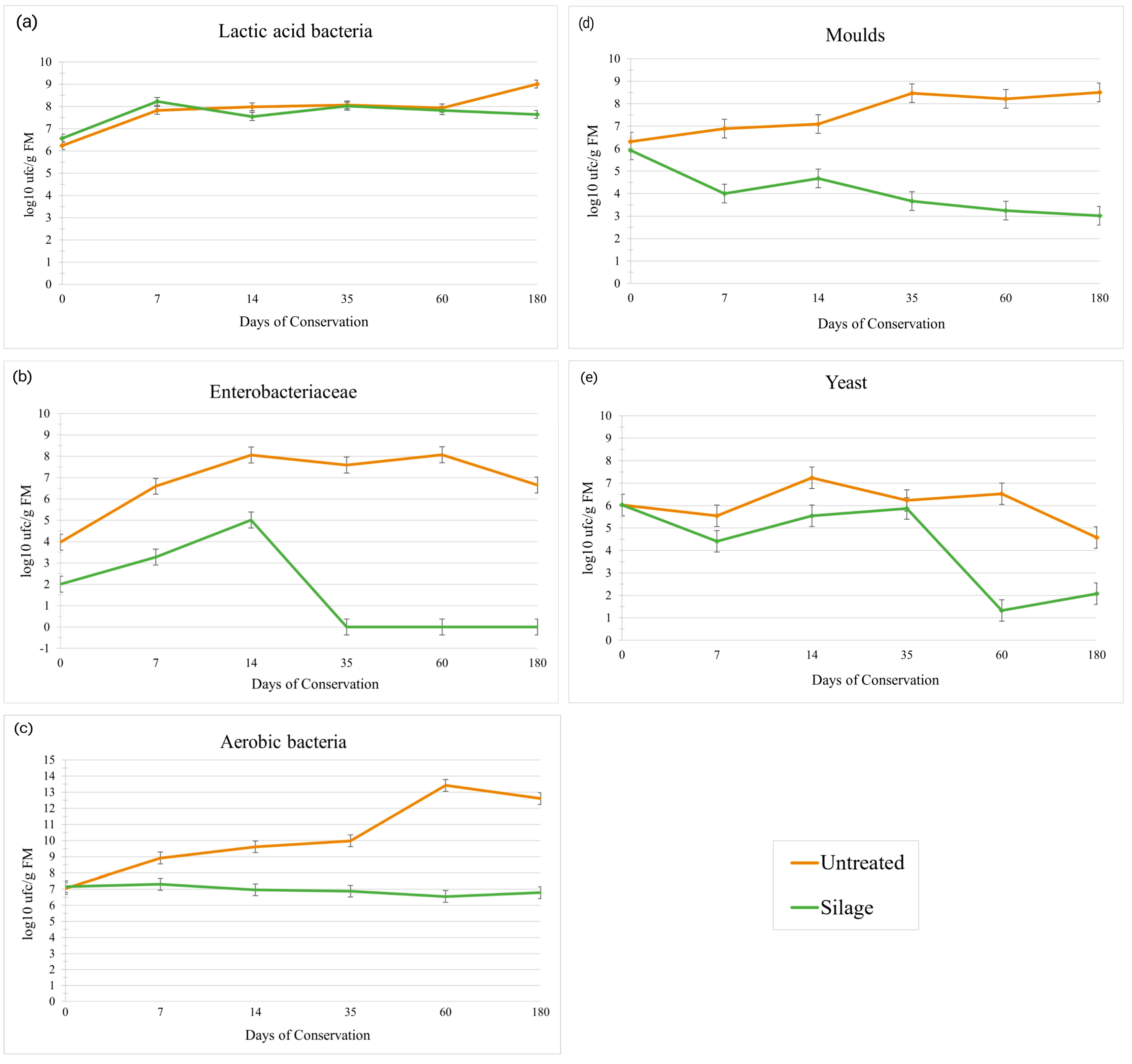
3.1. Microbiology
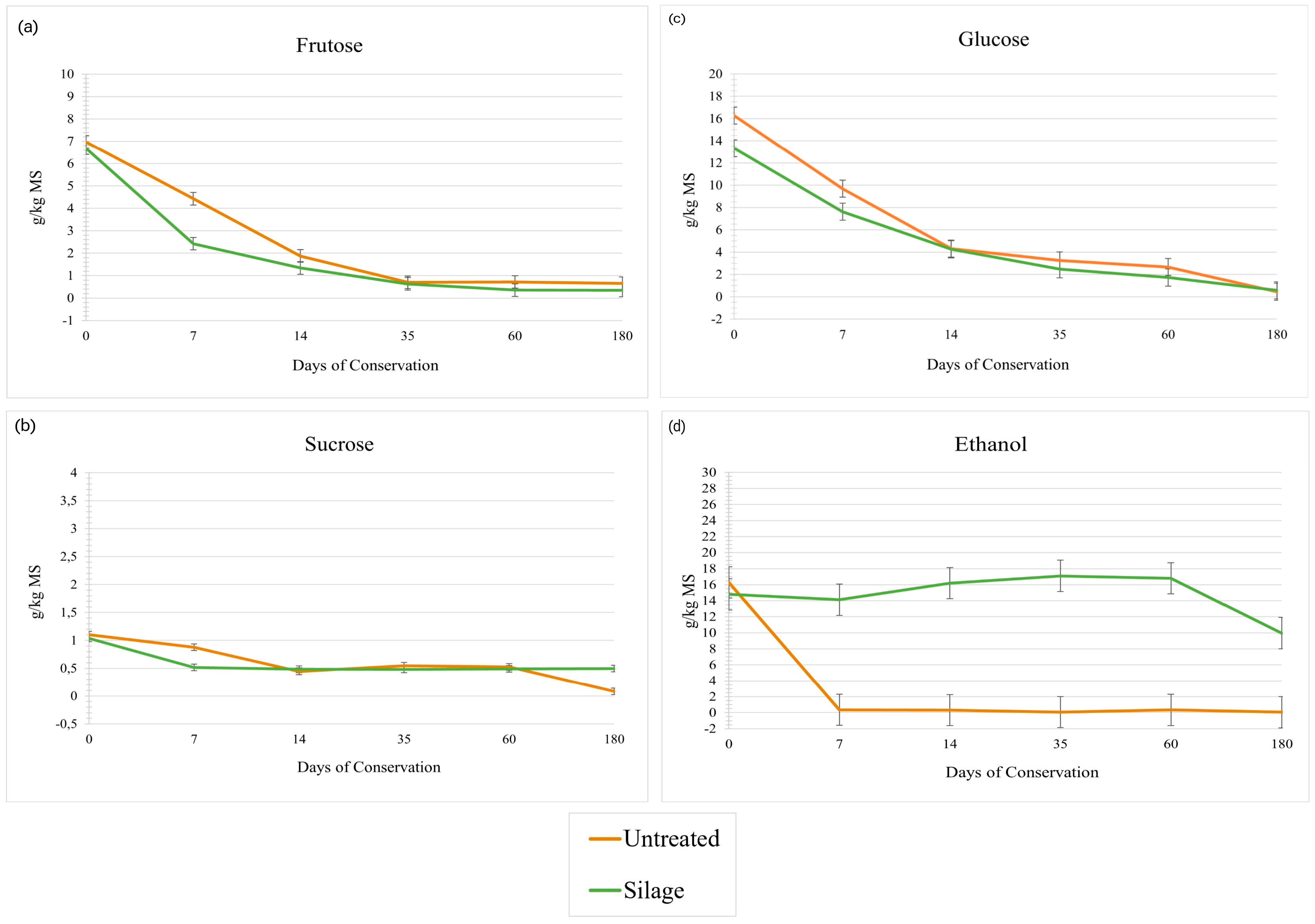
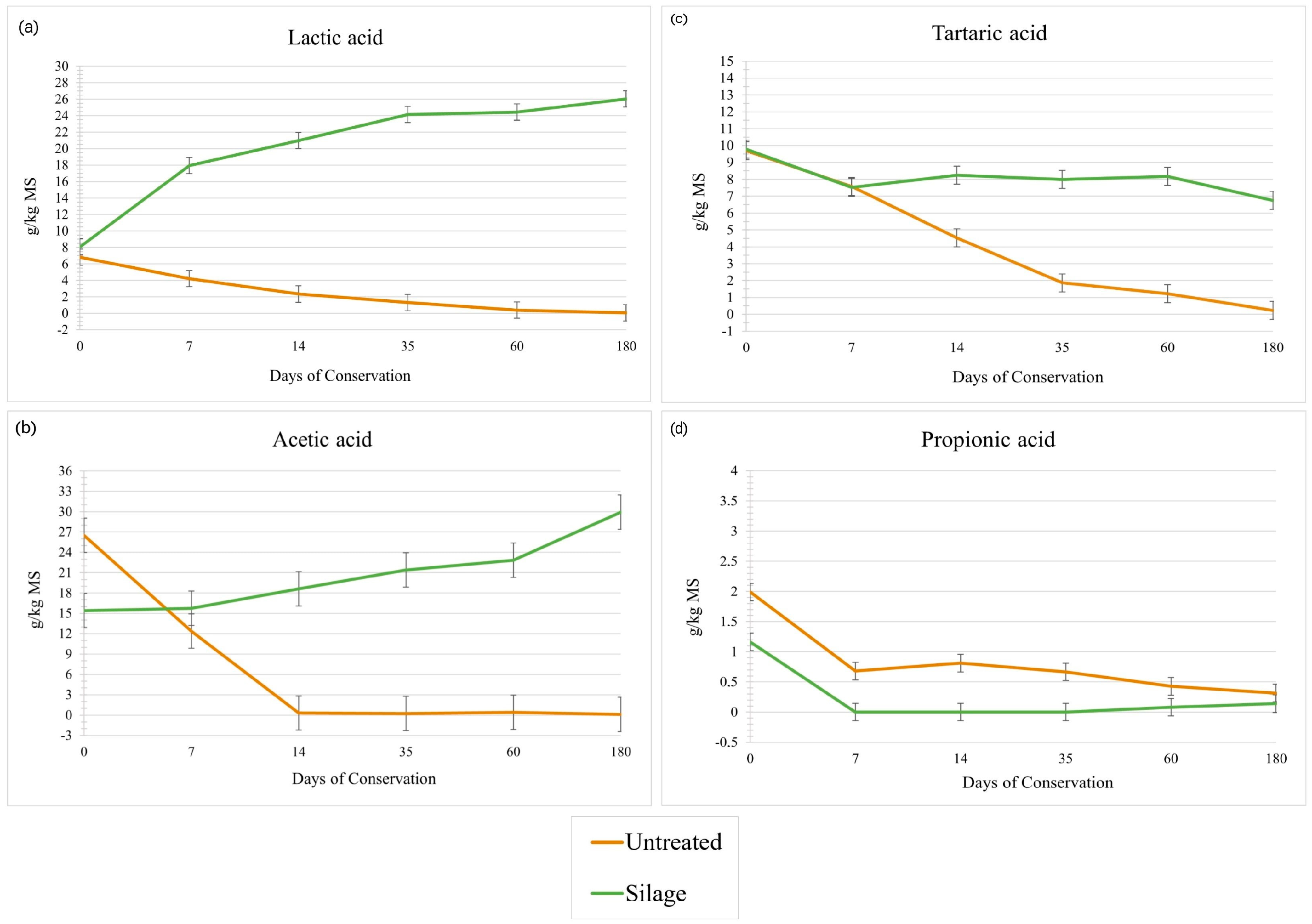
3.2. Sugars and Fermentation Metabolites
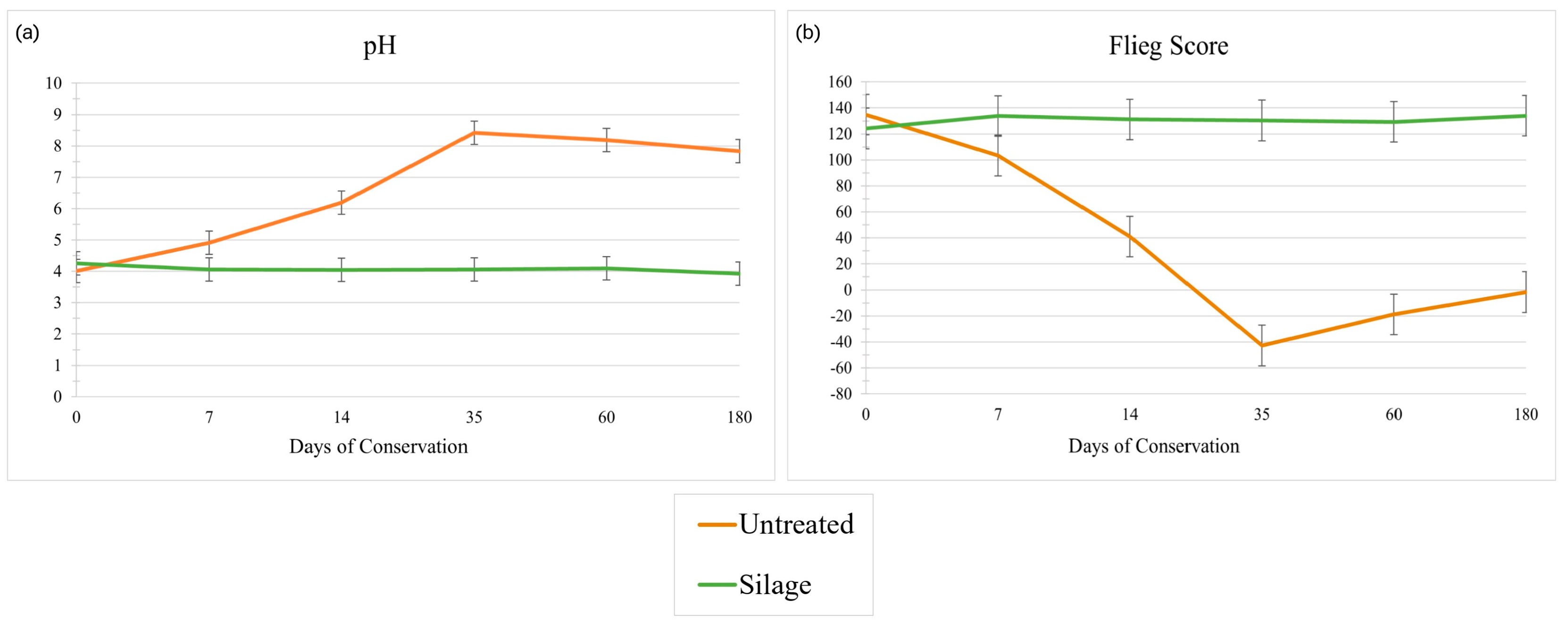
3.3. Physico-Chemical Properties and Nutritional Composition
3.4. Fatty Acid Profile
3.5. Bioactive Properties
3.6. Other Key Aspects
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ABTS | 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonate) |
| ADF | Acid Detergent Fiber |
| ADL | Acid Detergent Lignin |
| AOAC | Association of Analytical Communities |
| CP | Crude Protein |
| DM | Dry Matter |
| DPPH | 1,1-diphenyl-2-picrylhydrazyl |
| EC | European Commission |
| EE | Ether Extract |
| FAME | Fatty Acid Methyl Ester |
| FM | Fresh Matter |
| GAE | Gallic Acid Equivalent |
| HH | Hypocholesterolemic/Hypercholesterolemic Ratio |
| IA | Index of Atherogenicity |
| IT | Index of Thrombogenicity |
| LAB | Lactic Acid Bacteria |
| MRL | Maximum Residue Limit |
| MUFA | Monounsaturated Fatty Acid |
| NDF | Neutral Detergent Fiber |
| POD | Protected Designation of Origin |
| PUFA | Polyunsaturated Fatty Acid |
| SDGs | Sustainable Development Goals |
| SEM | Standard Error of the Mean |
| SFA | Saturated Fatty Acid |
| TP | Total Phenolic Content |
| UFL | Milk Forage Unit |
References
- Organización Internacional de la Viña y el Vino (OIV). Actualidad de la Coyuntura del Sector Vitivinícola Mundial en 2023. Available online: https://www.oiv.int/public/medias/8780/es-state-of-the-world-vine-and-wine-sector-february-2025.pdf (accessed on 27 February 2025).
- Taladrid, D.; Laguna, L.; Bartolomé, B.; Moreno-Arribas, M.V. Applications and new uses of winemaking byproducts. Intercompany 2019, 22, 42–45. [Google Scholar]
- Bordiga, M.; Travaglia, F.; Locatelli, M.; Arlorio, M.; y Coïsson, J.D. Spent grape pomace as a still potential by-product. Int. J. Food Sci. Technol. 2015, 50, 2022–2031. [Google Scholar] [CrossRef]
- Bordiga, M.; Travaglia, F.; Locatelli, M. Valorisation of grape pomace: An approach that is increasingly reaching its maturity—A review. Int. J. Food Sci. Technol. 2019, 54, 933–942. [Google Scholar] [CrossRef]
- Tangolar, S.G.; Özogul, F.; Tangolar, S.; Yağmur, C. Tocopherol content in fifteen grape varieties obtained using a rapid HPLC method. J. Food Compos. Anal. 2011, 24, 481–486. [Google Scholar] [CrossRef]
- Galanakis, C.M. Recovery of high added-value components from food wastes: Conventional, emerging technologies, and commercialized applications. Trends Food Sci. Technol. 2012, 26, 68–87. [Google Scholar] [CrossRef]
- Harbeoui, H.; Rebey, I.B.; Ouerghemmi, I.; Wannes, W.A.; Zemni, H.; Zoghlami, N.; Khan, N.A.; Ksouri, R.; Tounsi, M.S. Biochemical characterization and antioxidant activity of grape (Vitis vinifera L.) seed oils from nine Tunisian varieties. J. Food Biochem. 2017, 42, 1–12. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations [FAO]. The State of Food Security and Nutrition in the World 2023. Urbanization, Agrifood Systems Transformation, and Healthy Diets Across the Rural-Urban Continuum; FAO: Rome, Italy, 2023; Available online: https://openknowledge.fao.org/items/445c9d27-b396-4126-96c9-50b335364d01 (accessed on 12 February 2025).
- Marques, J.G.O.; de Oliveira Silva, R.; Barioni, L.G.; Hall, J.A.J.; Fossaert, C.; Tedeschi, L.O.; Moran, D. Evaluating environmental and economic trade-offs in cattle feed strategies using multiobjective optimization. Agric. Syst. 2022, 195, 103308. [Google Scholar] [CrossRef]
- Makkar, H.P.S. Effects and fate of tannins in ruminant animals, adaptation to tannins and strategies to overcome detrimental effects of feeding tannin rich feeds. Small Rumin. Res. 2003, 49, 241–256. [Google Scholar] [CrossRef]
- Frutos, P.; Hervás, G.; Giráldez, F.J.; Mantecón, A.R. Review: Tannins and ruminant nutrition. Span. J. Agric. Res. 2004, 2, 191–202. [Google Scholar] [CrossRef]
- Patra, A.K.; Saxena, J. Exploitation of dietary tannins to improve rumen metabolism and ruminant nutrition. J. Sci. Food Agric. 2011, 91, 24–37. [Google Scholar] [CrossRef]
- Martins Flores, D.R.; Patrícia da Fonseca, A.F.; Schmitt, J.; José Tonetto, C.; Rosado Junior, A.G.; Hammerschmitt, R.K.; Nörnberg, J.L. Lambs fed with increasing levels of grape pomace silage: Effects on meat quality. Small Rumin. Res. 2021, 195, 106234. [Google Scholar] [CrossRef]
- Monllor, P.; Zemzmi, J.; Muelas, R.; Roca, A.; Sendra, E.; Romero, G.; Díaz, J.R. Long-Term Feeding of Dairy Goats with 40% Artichoke By-Product Silage Preserves Milk Yield, Nutritional Composition, and Animal Health Status. Animals 2023, 13, 3585. [Google Scholar] [CrossRef]
- Dentinho, M.T.P.; Paulos, K.; Costa, C.; Costa, J.; Fialho, L.; Cachucho, L. Silages of agro-industrial by-products in lamb *-diets—Effect on growth performance, carcass, meat quality and in vitro methane emissions. Anim. Feed Sci. Technol. 2023, 298, 115603. [Google Scholar] [CrossRef]
- Molosse, V.L.; Deolindo, G.L.; Lago, R.V.P.; Cécere, B.G.O.; Zotti, C.A.; Vedovato, M.; da Silva, A.S. The effects of the inclusion of ensiled and dehydrated grape pomace in beef cattle diet: Growth performance, health, and economic viability. Anim. Feed Sci. Technol. 2023, 302, 115671. [Google Scholar] [CrossRef]
- Baker, L.; Bender, J.; Ferguson, J.; Rassler, S.; Pitta, D.; Chann, S.; Dou, Z. Leveraging dairy cattle to upcycle culled citrus fruit for emission mitigation and resource co-benefits: A case study. Resour. Conserv. Recycl. 2024, 203, 107452. [Google Scholar] [CrossRef]
- Mogodiniyai, K.; Rustas, B.O.; Spörndly, R.; Udén, P. Prediction Models for Silage Fermentation Products Based on Crop Composition under Strict Anaerobic Conditions: A Meta-Analysis. J. Dairy Sci. 2013, 96, 6644–6649. [Google Scholar] [CrossRef]
- Monllor, P.; Romero, G.G.; Muelas, R.; Sandoval-Castro, C.A.; Díaz, J.R. Ensiling Process in Commercial Bales of Horticultural By-Products from Artichoke and Broccoli. Animals 2020, 10, 831. [Google Scholar] [CrossRef]
- Hassan, M.; Belanche, A.; Jiménez, E.; Rivelli, I.; Martín-García, A.I.; Margolles, A.; Yáñez-Ruiz, D.R. Evaluation of the nutritional value and presence of minerals and pesticides residues in agro-industrial by-products to replace conventional ingredients of small ruminant diets. Small Rumin. Res. 2023, 229, 107117. [Google Scholar] [CrossRef]
- Hartinger, T.; Gruber, T.; Fliegerová, K.; Terler, G.; Zebeli, Q. Mixed ensiling with by-products and silage additives significantly valorizes drought-impaired whole-crop corn. Anim. Feed Sci. Technol. 2024, 309, 115899. [Google Scholar] [CrossRef]
- Teow, C.C.; Truong, V.D.; McFeeters, R.F.; Thompson, R.L.; Pecota, K.V.; Yencho, G.C. Antioxidant activities, phenolic contents and carotenoids of sweet potato genotypes with varying flesh colors. Food Chem. 2007, 103, 829–838. [Google Scholar] [CrossRef]
- Łozicki, A.; Koziorzebska, A.; Halika, G.; Dymnicka, M.; Arkuszewska, E.; Niemiec, T.; Bogdan, J. Effect of Pumpkin Silage (Cucurbita maxima D.) with Dried Sugar Beet Pulp on the Content of Bioactive Compounds in Silage and Its Antioxidant Potential. Anim. Feed Sci. Technol. 2015, 206, 108–113. [Google Scholar] [CrossRef]
- Kabir, M.; Alam, M.; Hossain, M.; Ferdaushi, Z. Effect of feeding probiotic fermented rice straw-based total mixed ration on production, blood parameters and faecal microbiota of fattening cattle. J. Anim. Health Prod. 2022, 10, 190–197. [Google Scholar] [CrossRef]
- Qiu, Y.; Zhao, H.; He, X.; Zhu, F.; Zhang, F.; Liu, B.; Liu, Q. Effects of fermented feed of Pennisetum giganteum on growth performance, oxidative stress, immunity and gastrointestinal microflora of Boer goats under thermal stress. Front. Microbiol. 2023, 13, 1030262. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; You, Y.; Huang, W.; Zhan, J. The High-Value and Sustainable Utilization of Grape Pomace: A Review. Food Chem. X 2024, 24, 101845. [Google Scholar] [CrossRef]
- Massaro Junior, F.L.; Bumbieris Junior, V.; Zanin, E.; Mizubuti, I.Y. Effect of storage time and use of additives on the quality of grape pomace silages. J. Food Process. Preserv. 2020, 44, e14373. [Google Scholar] [CrossRef]
- Díaz, J.R.; Fenoll, J.; Fenoll, A.; Romero, G.; Sendra, E. Procedimiento de Fabricación de Microsilos a Partir de Alcachofas (Cynara scolymus L.) para la Alimentación Animal. U.S. Patent ES2607220B1, 2018. [Google Scholar]
- Arias, C.; Oliete, B.; Seseña, S.; Jiménez, L.; Palop, L.; Pérez-Guzmán, M.D.; Arias, R. Importance of on-farm management practices on lactate-fermenting Clostridium spp. spore contamination of total mixed ration of Manchega ewe feeding. Small Rumin. Res. 2016, 139, 39–45. [Google Scholar] [CrossRef]
- Liu, F.X.; Fu, S.F.; Bi, X.F.; Chen, F.; Liao, X.J.; Hu, X.S.; Wu, J.H. Physico-chemical and antioxidant properties of four mango (Mangifera indica L.) cultivars in China. Food Chem. 2013, 138, 396–405. [Google Scholar] [CrossRef]
- Association of Official Analytical Chemists. Official Methods of Analysis, 16th ed.; Association of Official Analytical Chemists: Washington, DC, USA, 1999. [Google Scholar]
- Kilic, A. Silo Feed: Instruction, Education and Application Proposals; Bilgehan Press: Izmir, Turkey, 1986. [Google Scholar]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for dietary neutral detergent fibre and non-starch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Licitra, G.; Hernandez, T.M.; Van Soest, P.J. Standardization of Procedures for Nitrogen Fractionation of Ruminant Feeds. Anim. Feed Sci. Technol. 1996, 57, 347–358. [Google Scholar] [CrossRef]
- ISO 10520:1997; Native Starch—Determination of Starch Content—Ewers Polarimetric Method. International Organization for Standardization (ISO): Geneva, Switzerland, 1997.
- Kramer, J.K.G.; Fellner, V.; Dugan, M.E.R.; Sauer, F.D.; Mossoba, M.M.; Yurawecz, M.P. Evaluación de catalizadores ácidos y básicos en la metilación de ácidos grasos de la leche y el rumen, con especial énfasis en dienos conjugados y ácidos grasos trans totales. Lipids 1997, 32, 1219–1228. [Google Scholar] [CrossRef]
- Chen, J.; Liu, H. Nutritional Indices for Assessing Fatty Acids: A Mini-Review. Int. J. Mol. Sci. 2020, 21, 5695. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Jeong, S.W.; Lee, C.Y. Antioxidant capacity of phenolic phytochemicals from various cultivars of plums. Food Chem. 2003, 81, 321–326. [Google Scholar] [CrossRef]
- Cheng, Z.; Moore, J.; Yu, L. Relative High-Throughput DPPH Radical Scavenging Capacity Assay. J. Agric. Food Chem. 2006, 54, 7429–7436. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C.L.W.T. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Leite, A.V.; Malta, L.G.; Riccio, M.F.; Eberlin, M.N.; Pastore, G.M.; Maróstica Júnior, M.R. Antioxidant Potential of Rat Plasma by Administration of Freeze-Dried Jaboticaba Peel (Myrciaria jaboticaba Vell Berg). J. Agric. Food Chem. 2011, 59, 2277–2283. [Google Scholar] [CrossRef] [PubMed]
- INRA. Feeding of Cattle, Sheep, and Goats; Jarrige, R., Ed.; Institut National de la Recherche Agronomique (INRA): Paris, France, 1988; pp. 142–144. [Google Scholar]
- McDonald, P.; Henderson, A.R.; Heron, S.J.E. The Biochemistry of Silage; Chalcombe Publications: Marlow Bottom, UK, 1991. [Google Scholar]
- Cai, Y.; Benno, Y.; Ogawa, M.; Ohmomo, S.; Kumai, S.; Nakase, T. Influence of Lactobacillus spp. from an Inoculant and of Weissella and Leuconostoc spp. from Forage Crops on Silage Fermentation. Appl. Environ. Microbiol. 1998, 64, 2982–2987. [Google Scholar] [CrossRef]
- Weinberg, Z.G.; Muck, R.E. New trends and opportunities in the development and use of inoculants for silage. FEMS Microbiol. Rev. 1996, 19, 53–68. [Google Scholar] [CrossRef]
- Ke, W.C.; Yang, F.Y.; Undersander, D.J.; Guo, X.S. Fermentation characteristics, aerobic stability, proteolysis, and lipid composition of alfalfa silage ensiled with apple or grape pomace. Anim. Feed Sci. Technol. 2015, 202, 12–19. [Google Scholar] [CrossRef]
- De Bellis, P.; Maggiolino, A.; Albano, C.; De Palo, P.; Blando, F. Ensiling Grape Pomace with and Without Addition of a Lactiplantibacillus plantarum Strain: Effect on Polyphenols and Microbiological Characteristics, in vitro Nutrient Apparent Digestibility, and Gas Emission. Front. Vet. Sci. 2022, 9, 808293. [Google Scholar] [CrossRef]
- Woolford, M.K. The Silage Fermentation; Microbiological Series; Marcel Dekker Inc.: New York, NY, USA; Basel, Switzerland, 1984; Volume 14. [Google Scholar]
- Muller, T.; Behrendt, U.; Muller, M. Antagonistic activity in plant-associated lactic acid bacteria. Microbiol. Res. 1996, 151, 63–70. [Google Scholar] [CrossRef]
- Fang, X.; Li, Y.; Guo, W.; Ke, W.; Bi, S.; Guo, X.; Zhang, Y. Lactobacillus delbrueckii subsp. bulgaricus F17 and Leuconostoc lactis H52 supernatants delay the decay of strawberry fruits: A microbiome perspective. Food Funct. 2019, 10, 435–447. [Google Scholar] [CrossRef]
- Ni, K.; Wang, F.; Zhu, B.; Yang, J.; Zhou, G.; Pan, Y.; Tao, Y.; Zhong, J. Effects of lactic acid bacteria and molasses additives on the microbial community and fermentation quality of soybean silage. Bioresour. Technol. 2017, 238, 706–715. [Google Scholar] [CrossRef]
- Moselhy, M.A.; Borba, J.P.; Borba, A.E.S. Production of high-quality silage from invasive plants plus agro-industrial by-products with or without bacterial inoculation. Biocatal. Agric. Biotechnol. 2022, 39, 102251. [Google Scholar] [CrossRef]
- Du, Z.; Yamasaki, S.; Oya, T.; Nguluve, D.; Euridse, D.; Tinga, B.; Macome, F.; Cai, Y. Microbial network and fermentation modulation of napier grass and sugarcane top silage in Southern Africa. Microbiol. Spectr. 2023, 12, e03032-23. [Google Scholar] [CrossRef] [PubMed]
- Kung, L.; Shaver, R.D.; Grant, R.J.; Schmidt, R.J. Silage review: Interpretation of chemical, microbial, and organoleptic components of silages. J. Dairy Sci. 2018, 101, 4020–4033. [Google Scholar] [CrossRef] [PubMed]
- Kung, L.; Sheperd, A.C.; Smagala, A.M.; Endres, K.M.; Bessett, C.A.; Ranjit, N.K.; Glancey, J.L. The effect of preservatives based on propionic acid on the fermentation and aerobic stability of corn silage and a total mixed ration. J. Dairy Sci. 1998, 81, 1322–1330. [Google Scholar] [CrossRef]
- Bolsen, K.K.; Whitlock, L.A.; Wistuba, T.; Pope, R.V. Effect of level of surface spoilage on the nutritive value of whole crop maize silage diets. In Proceedings of the 10th International Symposium of Forage Conservation, Brno, Czech Republic, 11 September 2001; pp. 174–175. [Google Scholar] [CrossRef]
- Ahmadi, F.; Lee, Y.H.; Lee, W.H.; Oh, Y.; Park, K.; Kwak, W.S. Long-term anaerobic conservation of fruit and vegetable discards without or with moisture adjustment after aerobic preservation with sodium metabisulfite. Waste Manag. 2019, 87, 258–267. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Tian, J.; Zhang, Q.; Jiang, Y.; Wu, Z.; Yu, Z. Effects of mixing red clover with alfalfa at different ratios on dynamics of proteolysis and protease activities during ensiling. J. Dairy Sci. 2018, 101, 8954–8964. [Google Scholar] [CrossRef]
- Tahir, M.; Li, J.; Xin, Y.; Wang, T.; Chen, C.; Zhong, Y.; Zhang, L.; Liu, H.; He, Y.; Wen, X.; et al. Response of fermentation quality and microbial community of oat silage to homofermentative lactic acid bacteria inoculation. Front. Microbiol. 2023, 13, 1091394. [Google Scholar] [CrossRef]
- Niu, Y.; Guo, Y.; Huang, R.; Niu, J.; Wang, Y.; Zhang, P.; Lu, Q.; Zhang, W. Fermentative profile and bacterial community structure of whole-plant triticale silage (Triticosecale Wittmack) with or without the addition of Streptococcus bovis and Lactiplantibacillus plantarum. mSphere 2025, 10, e0089424. [Google Scholar] [CrossRef]
- Vasta, V.; Nudda, A.; Cannas, A.; Lanza, M.; Priolo, A. Alternative feed resources and their effects on the quality of meat and milk from small ruminants. Anim. Feed Sci. Technol. 2008, 146, 223–246. [Google Scholar] [CrossRef]
- Sun, Q.; Gao, F.; Yu, Z.; Tao, Y.; Zhao, S.; Cai, Y. Fermentation quality and chemical composition of shrub silage treated with lactic acid bacteria inoculants and cellulase additives. Anim. Sci. J. 2011, 82, 811–819. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Ke, W.; Ding, Z.; Xu, D.; Wang, M.; Chen, M.; Guo, X. Microbial mechanisms of using feruloyl esterase-producing Lactobacillus plantarum A1 and grape pomace to improve fermentation quality and mitigate ruminal methane emission of ensiled alfalfa for cleaner animal production. J. Environ. Manag. 2022, 308, 114637. [Google Scholar] [CrossRef]
- Megías, M.D.; Meneses, M.; Madrid, J.; Hernández, F.; Martínez-Teruel, A.; Cano, J.A. Nutritive, fermentative and environmental characteristics of silage of two industrial broccoli (Brassica oleracea, var. Itálica) by-products for ruminant feed. Int. J. Agric. Biol. 2014, 16, 307–313. [Google Scholar]
- Arias Carrasquillo, F. Fermentative Characteristics and Aerobic Stability of Two Tropical Corn Varieties and Guinea Grass Silage at Different Maturity Stages. Master’s Thesis, University of Puerto Rico, Mayagüez, Puerto Rico, 1998. [Google Scholar]
- Zhong, R.; Zhao, C.; Feng, P.; Wang, Y.; Zhao, X.; Luo, D.; Fang, Y. Effects of feeding ground versus pelleted total mixed ration on digestion, rumen function and milk production performance of dairy cows. Int. J. Dairy Technol. 2020, 73, 22–30. [Google Scholar] [CrossRef]
- Driehuis, F.; van Wikselaar, P.V. The occurrence and prevention of ethanol fermentation in high-dry-matter grass silage. J. Sci. Food Agric. 2000, 80, 711–718. [Google Scholar] [CrossRef]
- Li, M.; Su, J.; Yang, H.; Feng, L.; Wang, M.; Xu, G.; Shao, J.; Ma, C. Grape Tartaric Acid: Chemistry, Function, Metabolism, and Regulation. Horticulturae 2023, 9, 1173. [Google Scholar] [CrossRef]
- Robinson, P.H.; Swanepoel, N.; Heguy, J.M.; Price, T.; Meyer, D.M. ‘Shrink’ losses in commercially sized corn silage piles: Quantifying total losses and where they occur. Sci. Total Environ. 2016, 542, 530–539. [Google Scholar] [CrossRef]
- Chen, L.; Yuan, X.; Li, J.; Wang, S.; Dong, Z.; Shao, T. Effect of lactic acid bacteria and propionic acid on conservation characteristics, aerobic stability and in vitro gas production kinetics and digestibility of whole-crop corn-based total mixed ration silage. J. Integr. Agric. 2017, 16, 1592–1600. [Google Scholar] [CrossRef]
- da Silva, É.B.; Liu, X.; Mellinger, C.; Gressley, T.F.; Stypinski, J.D.; Moyer, N.A.; Kung, L. Effect of dry matter content on the microbial community and on the effectiveness of a microbial inoculant to improve the aerobic stability of corn silage. J. Dairy Sci. 2022, 105, 5024–5043. [Google Scholar] [CrossRef]
- Muck, R.E. Dry matter level effects on alfalfa silage quality. I. Nitrogen transformations. Trans. ASAE 1987, 30, 7. [Google Scholar] [CrossRef]
- Kulyk, M.F.; Zhukov, V.P.; Obertiukh, Y.V.; Vyhovska, I.O.; Honchar, L.O.; Skoromna, O.I.; Tkachenko, T.Y.; Zelinska, I.P. Experimental substantiation of new criteria for silage quality evaluation. Feed. Feed Prod. 2019, 88, 99–106. [Google Scholar] [CrossRef]
- Shinners, K.J.; Wepner, A.D.; Muck, R.E.; Weimer, P.J. Aerobic and Anaerobic Storage of Single-Pass Chopped Corn Stover. BioEnergy Res. 2011, 4, 61–75. [Google Scholar] [CrossRef]
- Suksombat, W.; Lounglawan, P. Silage from agricultural by-products in Thailand: Processing and storage. Asian-Australas. J. Anim. Sci. 2004, 17, 473–478. [Google Scholar] [CrossRef]
- Ramzan, H.N.; Tanveer, A.; Maqbool, R.; Akram, H.M.; Mirza, M.A. Use of sugarcane molasses as an additive can improve the silage quality of sorghum-sudangrass hybrid. Pakistan J. Agric. Sci. 2022, 59, 75–81. [Google Scholar] [CrossRef]
- D’Alessandro, A.G.; Dibenedetto, R.S.; Skoufos, I.; Martemucci, G. Potential use of wheat straw, grape pomace, olive mill wastewater and cheese whey in mixed formulations for silage production. Agronomy 2023, 13, 2323. [Google Scholar] [CrossRef]
- Li, J.; Wan, D.; Jin, S.; Ren, H.; Wang, Y.; Huang, J.; Zhang, G. Fast treatment and recycling method of large-scale vegetable wastes. Sci. Total Environ. 2023, 892, 164308. [Google Scholar] [CrossRef]
- Zhang, T.; Li, H.; Yan, T.; Shaheen, S.M.; Niu, Y.; Xie, S.; Zhang, Y.; Abdelrahman, H.; Ali, E.F.; Bolan, N.S.; et al. Organic matter stabilization and phosphorus activation during vegetable waste composting: Multivariate and multiscale investigation. Sci. Total Environ. 2023, 891, 164608. [Google Scholar] [CrossRef]
- Chen, T.; Wang, Q.; Wang, Y.; Dou, Z.; Yu, X.; Feng, H.; Yin, J. Using fresh vegetable waste from Chinese traditional wet markets as animal feed: Material feasibility and utilization potential. Sci. Total Environ. 2023, 902, 166105. [Google Scholar] [CrossRef]
- Kearney, P.C.; Kennedy, W.K. Relationship between losses of fermentable sugars and changes in organic acids of silage. Agron. J. 1962, 54, 114–115. [Google Scholar] [CrossRef]
- Opsahl, S.; Benner, R. Characterization of carbohydrates during early diagenesis of five vascular plant tissues. Org. Geochem. 1999, 30, 83–94. [Google Scholar] [CrossRef]
- Dulf, F.V.; Vodnar, D.C.; Toşa, M.I.; Dulf, E. Simultaneous enrichment of grape pomace with γ-linolenic acid and carotenoids by solid-state fermentation with zygomycetes fungi and antioxidant potential of the bioprocessed substrates. Food Chem. 2020, 310, 125927. [Google Scholar] [CrossRef]
- Ianni, A.; Martino, G. Dietary grape pomace supplementation in dairy cows: Effect on nutritional quality of milk and its derived dairy products. Foods 2020, 9, 168. [Google Scholar] [CrossRef]
- Carmona-Jiménez, Y.; Igartuburu, J.M.; Guillén-Sánchez, D.A.; García-Moreno, M.V. Fatty Acid and Tocopherol Composition of Pomace and Seed Oil from Five Grape Varieties Southern Spain. Molecules 2022, 27, 6980. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, M.; García-García, R.M.; Arias-Álvarez, M.; Millán, P.; Febrel, N.; Formoso-Rafferty, N.; López-Tello, J.; Lorenzo, P.L.; Rebollar, P.G. Improvements in the conception rate, milk composition, and embryo quality of rabbit does after dietary enrichment with n-3 polyunsaturated fatty acids. Animal 2018, 12, 2080–2088. [Google Scholar] [CrossRef]
- Akter, A.; Li, X.; Grey, E.; Wang, S.C.; Kebreab, E. Grape pomace supplementation reduced methane emissions and improved milk quality in lactating dairy cows. J. Dairy Sci. 2025, 108, 2468–2480. [Google Scholar] [CrossRef]
- Grinari, J.M.; Nurmela, K.V.V.; Corl, B.A.; Lacy, S.H.; Chouinard, P.Y.; Bauman, D.E. Conjugated linoleic acid is synthesized endogenously in lactating dairy cows by the Δ9-desaturase. J. Nutr. 2000, 130, 2285–2291. [Google Scholar] [CrossRef] [PubMed]
- McGuire, M.A.; McGuire, M.K. Conjugated linoleic acid (CLA): A ruminant fatty acid with beneficial effects on human health. J. Anim. Sci. 2000, 77, 2527–2533. [Google Scholar] [CrossRef]
- Sealls, W.; Gonzalez, M.; Brosnan, M.J.; Black, P.N.; DiRusso, C.C. Dietary polyunsaturated fatty acids (C18:2 ω6 and C18:3 ω3) do not suppress hepatic lipogenesis. Biochim. Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2008, 1781, 406–414. [Google Scholar] [CrossRef]
- Ying, Q.; Wojciechowska, P.; Siger, A.; Kaczmarek, A.; Rudzińska, M. Phytochemical content, oxidative stability, and nutritional properties of cold-pressed unconventional edible oils. J. Food Nutr. Res. 2018, 6, 476–485. [Google Scholar] [CrossRef]
- Violante, B.; Gerbaudo, L.; Borretta, G.; Tassone, F. Effects of extra virgin olive oil supplementation at two different low doses on lipid profile in mild hypercholesterolemic subjects: A randomized clinical trial. J. Endocrinol. Investig. 2009, 32, 794–796. [Google Scholar] [CrossRef] [PubMed]
- Angelini, P.; Flores, G.A.; Piccirilli, A.; Venanzoni, R.; Acquaviva, A.; Di Simone, S.C.; Libero, M.L.; Tirillini, B.; Zengin, G.; Chiavaroli, A.; et al. Polyphenolic composition and antimicrobial activity of extracts obtained from grape processing by-products: Between green biotechnology and nutraceutical. Process Biochem. 2022, 118, 84–91. [Google Scholar] [CrossRef]
- Casazza, A.A.; Aliakbarian, B.; De Faveri, D.; Fiori, L.; Perego, P. Antioxdants from winemaking wastes: A study on extraction parameters using response surface methodology. J. Food Biochem. 2012, 36, 28–37. [Google Scholar] [CrossRef]
- Yammine, S.; Delsart, C.; Vitrac, X.; Mietton-Peuchot, M.; Ghidossi, R. Characterization of polyphenols and antioxidant potential of extracts from red and white grape pomace by subcritical water extraction. OENO One 2020, 54, 263–278. [Google Scholar] [CrossRef]
- Kammerer, D.R.; Claus, A.; Carle, R.; Schieber, A. Polyphenol screening of pomace from red and white grape varieties (Vitis vinifera L.) by HPLC-DAD-MS/MS. J. Agric. Food Chem. 2004, 52, 4360–4367. [Google Scholar] [CrossRef]
- Fitri, A.; Obitsu, T.; Sugino, T. Effect of ensiling persimmon peel and grape pomace as tannin-rich byproduct feeds on their chemical composition and in vitro rumen fermentation. Anim. Sci. J. 2021, 92, e13524. [Google Scholar] [CrossRef]
- Caetano, M.; Wilkes, M.J.; Pitchford, W.S.; Lee, S.J.; Hynd, P.I. Effect of ensiled crimped grape marc on energy intake, performance and gas emissions of beef cattle. Anim. Feed Sci. Technol. 2019, 247, 166–172. [Google Scholar] [CrossRef]
- Martin Flores, D.R.; Da Fonseca, P.A.F.; Schmitt, J.; Tonetto, C.J.; Junior, A.G.R.; Hammerschmitt, R.K.; Nörnberg, J.L. Lambs fed with increasing levels of grape pomace silage: Effects on productive performance, carcass characteristics, and blood parameters. Livest. Sci. 2020, 240, 104169. [Google Scholar] [CrossRef]
- Mu, C.; Yang, W.; Wang, P.; Zhao, J.; Hao, X.; Zhang, J. Effects of high-concentrate diet supplemented with grape seed proanthocyanidins on growth performance, liver function, meat quality, and antioxidant activity in finishing lambs. Anim. Feed Sci. Technol. 2020, 266, 114518. [Google Scholar] [CrossRef]
- Bennato, F.; Ianni, A.; Bellocci, M.; Grotta, L.; Sacchetti, G.; Martino, G. Influence of dietary grape pomace supplementation on chemical and sensory properties of ewes’ cheese. Int. Dairy J. 2023, 143, 105671. [Google Scholar] [CrossRef]
- Lonja de Cereales de Albacete. Cotizaciones de Cereal de la Semana 12. Interempresas, 20 de marzo de 2025. Available online: https://www.interempresas.net/A592011 (accessed on 20 March 2025).
- De Blas, C.; García-Rebollar, P.; Gorrachategui, M.; Mateos, G.G. FEDNA de Composición y Valor Nutritivo de Alimentos para la Fabricación de Piensos Compuestos, 4th ed.; Fundación Española para el Desarrollo de la Nutrición Animal: Madrid, Spain, 2019; ISBN 978-8409156887. [Google Scholar]
- Muck, R.E. Fermentation Characteristics of Round-Bale Silages; USDA, Agricultural Research Service, US Dairy Forage Research Center: Madison, WI, USA, 2006. [Google Scholar]
| Treatment WGP | Days of Conservation | SEM | |||||
|---|---|---|---|---|---|---|---|
| 0 | 7 | 14 | 35 | 60 | 180 | ||
| Dry Matter (g/kg) | |||||||
| Silage | 445.71 bc | 455.80 bc | 440.04 bc | 437.65 bc | 439.57 bc | 430.55 c | 1.06 |
| Untreated | 451.09 bc | 474.87 b | 418.11 c | 445.66 bc | 518.48 a | 533.02 a | |
| Organic Matter (g/kg DM) | |||||||
| Silage | 884.00 ab | 896.00 a | 878.50 ab | 898.00 a | 889.50 a | 888.50 a | 1.59 |
| Untreated | 877.50 ab | 877.00 ab | 875.50 ab | 858.50 ab | 840.50 b | 838.50 b | |
| Ash (g/kg DM) | |||||||
| Silage | 52.00 b | 50.00 b | 51.50 b | 55.50 b | 55.00 b | 69.00 b | 0.92 |
| Untreated | 55.50 b | 54.00 b | 63.00 b | 72.00 ab | 99.00 a | 81.00 a | |
| Neutral Detergent Fibre (g/kg DM) | |||||||
| Silage | 449.50 c | 488.00 bc | 551.50 abc | 553.00 abc | 499.00 bc | 405.50 c | 5.58 |
| Untreated | 547.00 abc | 565.00 abc | 436.00 c | 685.00 ab | 685.50 ab | 715.50 a | |
| Acid Detergent Fibre (g/kg DM) | |||||||
| Silage | 458.00 bcd | 453.50 bcd | 463.00 bcd | 482.50 bcd | 420.00 cd | 337.00 d | 6.24 |
| Untreated | 430.50 bcd | 482.00 bcd | 514.00 abcd | 571.50 abc | 598.00 ab | 683.50 a | |
| Acid Detergent Lignin (g/kg DM) | |||||||
| Silage | 303.85 cd | 314.30 bcd | 328.65 bc | 343.70 bc | 299.55 cd | 189.45 d | 3.88 |
| Untreated | 282.65 cd | 334.25 bc | 370.00 bc | 394.25 abc | 443.75 ab | 527.80 a | |
| Ether Extract (g/kg DM) | |||||||
| Silage | 66.00 ab | 69.00 ab | 74.00 a | 67.50 ab | 69.50 ab | 63.00 ab | 0.45 |
| Untreated | 65.00 ab | 70.50 ab | 70.00 ab | 61.50 ab | 68.00 ab | 56.00 b | |
| Crude Protein (g/kg DM) | |||||||
| Silage | 104.50 bc | 100.00 c | 106.50 abc | 107.50 abc | 105.00 bc | 121.50 ab | 0.65 |
| Untreated | 107.00 abc | 106.00 bc | 115.50 abc | 122.50 ab | 125.50 a | 125.50 a | |
| Non-Protein Nitrogen (g/kg DM) | |||||||
| Silage | 10.50 abc | 14.00 a | 12.50 ab | 9.00 abc | 8.00 abc | 10.50 abc | 0.23 |
| Untreated | 10.00 abc | 10.50 abc | 4.00 c | 5.50 bc | 7.00 abc | 8.50 abc | |
| Starch (g/kg DM) | |||||||
| Silage | 34.00 abc | 32.00 abc | 30.50 abc | 33.00 abc | 38.00 ab | 46.50 a | 0.47 |
| Untreated | 38.50 ab | 32.00 abc | 24.50 bcd | 24.50 bcd | 18.50 cd | 12.50 d | |
| Total Sugar (g/kg DM) | |||||||
| Silage | 17.00 a | 6.50 b | 4.00 b | 4.00 b | 4.00 b | 3.00 b | 0.28 |
| Untreated | 11.50 ab | 10.50 ab | 4.00 b | 3.00 b | 4.00 b | 3.00 b | |
| White Grape Pomace | SEM | ||
|---|---|---|---|
| Fatty Acid Profile | Silage | Untreated | |
| SFA | 19.33 | 19.53 | 0.44 |
| MUFA | 17.54 | 18.56 | 0.18 |
| PUFA | 63.13 | 61.90 | 0.36 |
| SCFA | 1.08 | 0.45 | 0.20 |
| MCFA | 13.15 | 13.19 | 0.36 |
| LCFA | 85.77 | 86.34 | 0.48 |
| C18:2 | 58.10 | 59.12 | 0.64 |
| C18:1 | 15.86 | 16.95 | 0.20 |
| C16:0 | 11.66 | 10.78 | 0.19 |
| C18:0 | 4.53 | 5.20 | 0.06 |
| C18:3 | 3.41 b | 2.31 a | 0.18 |
| Nutritional Health Indices | |||
| n6n3 | 18.07 a | 77.69 b | 3.42 |
| LAALA | 18.16 a | 81.02 b | 3.32 |
| UI | 148.03 | 144.85 | 0.81 |
| TFA | 0.03 | 0.08 | 0.04 |
| IA | 0.16 | 0.16 | 0.01 |
| IT | 0.34 | 0.35 | 0.00 |
| HH | 6.67 | 7.07 | 0.26 |
| Feedstuffs | ||
|---|---|---|
| Manufacturing Cost | WGP Silage | Alfalfa Hay |
| EUR/t fresh WGP | 25.00 | - |
| EUR/t baled silage manufacturing | 28.80 | - |
| EUR/t FM WGP silage | 53.80 | - |
| Selling Price | ||
| EUR/t DM | 120.89 | 307.69 |
| EUR/kg CP | 1.63 | 1.60 |
| EUR/UFL | 122.12 | 350.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Galvez-Lopez, M.; Navarro, A.; Muelas, R.; Roca, A.; Peris, C.; Romero, G.; Díaz, J.R. Potential of Baled Silage to Preserve White Grape Pomace for Ruminant Feeding. Agriculture 2025, 15, 974. https://doi.org/10.3390/agriculture15090974
Galvez-Lopez M, Navarro A, Muelas R, Roca A, Peris C, Romero G, Díaz JR. Potential of Baled Silage to Preserve White Grape Pomace for Ruminant Feeding. Agriculture. 2025; 15(9):974. https://doi.org/10.3390/agriculture15090974
Chicago/Turabian StyleGalvez-Lopez, Marina, Alfonso Navarro, Raquel Muelas, Amparo Roca, Cristofol Peris, Gema Romero, and José Ramón Díaz. 2025. "Potential of Baled Silage to Preserve White Grape Pomace for Ruminant Feeding" Agriculture 15, no. 9: 974. https://doi.org/10.3390/agriculture15090974
APA StyleGalvez-Lopez, M., Navarro, A., Muelas, R., Roca, A., Peris, C., Romero, G., & Díaz, J. R. (2025). Potential of Baled Silage to Preserve White Grape Pomace for Ruminant Feeding. Agriculture, 15(9), 974. https://doi.org/10.3390/agriculture15090974








