Effects of Seed Fraction on Sowing Quality and Yield of Three-Line Hybrid Maize
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design and Sample Collection
2.2. Laboratory Experiments
2.3. Field Experiments
2.4. Measurements
2.5. Weather Conditions
2.6. Statistical Analysis
3. Results
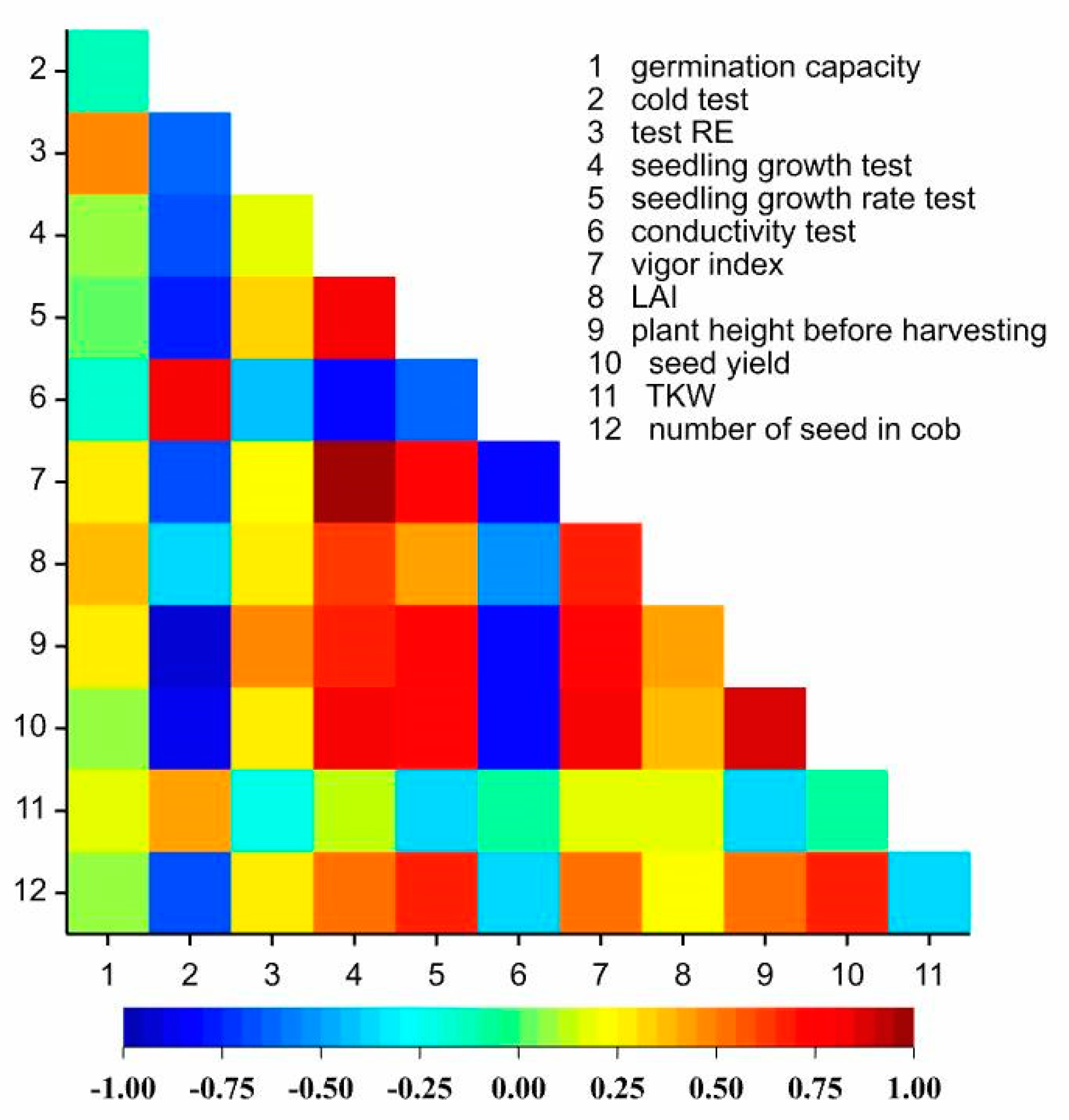
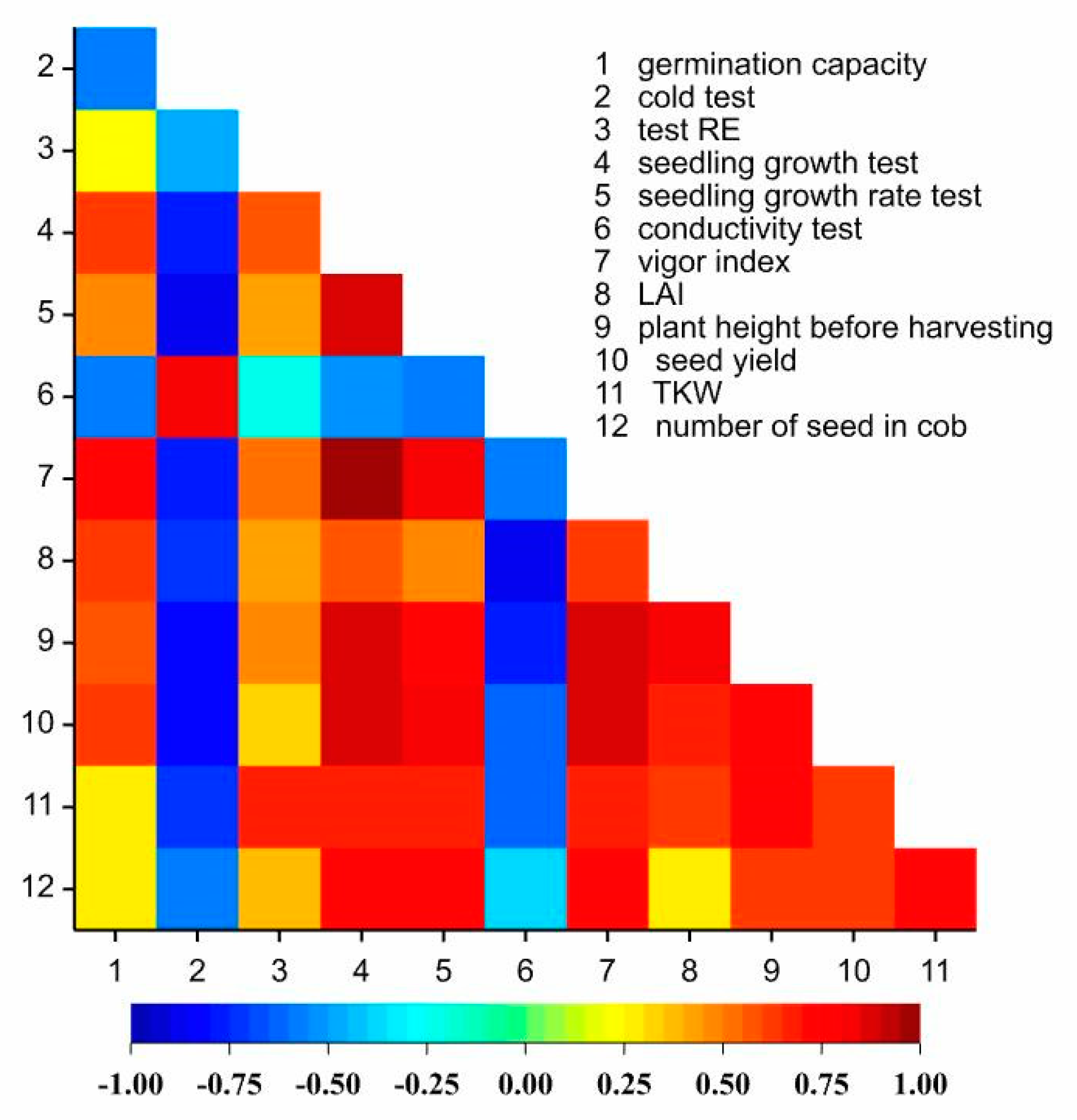
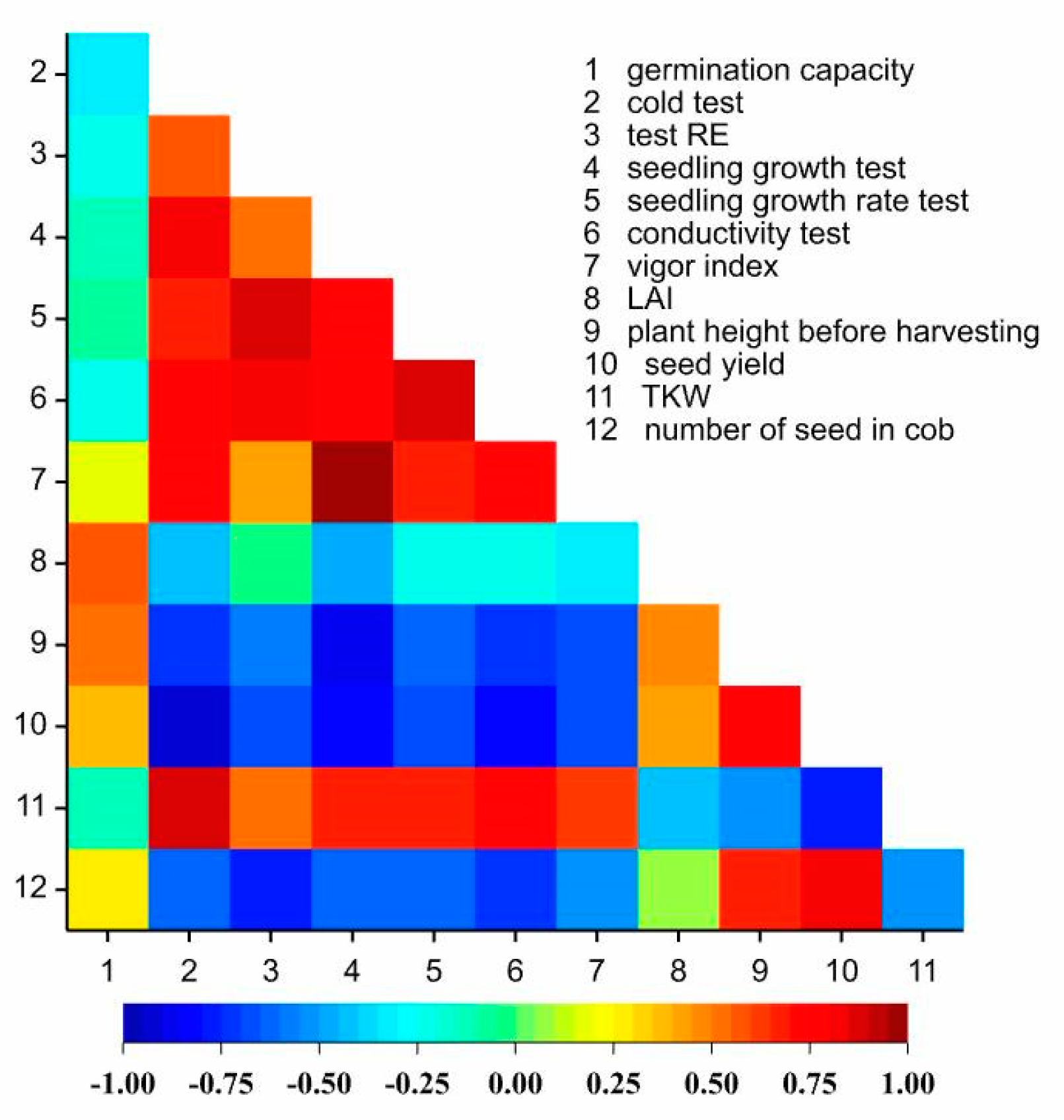
3.1. Seed Value of Kernels
3.2. LAI and SPAD
3.3. Seed Yield and Components
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- FAO. FAOSTAT Database. Food and Agriculture Organization of the United Nations 2023. Available online: www.fao.org/faostat/en/#data/QCL (accessed on 20 January 2025).
- Alam, A.; Ahmed, S.; Begum, M.; Sultan, M. Heterosis and combining ability for grain yield and its contributing characters in maize. Bangladesh J. Agric. Res. 2008, 33, 375–379. [Google Scholar] [CrossRef]
- Matin, M.Q.I.; Rasul, M.G.; Islam, A.K.M.A.; Mian, M.K.; Ivy, N.A.; Ahmed, J.U. Combining ability and heterosis in maize (Zea mays L.). Am. J. BioSci. 2016, 4, 84–90. [Google Scholar]
- Job, A.; Iseghohi, I.O.; Igyuve, M.T.; Olayiwola, R.; Akinwale, R.; Ojo, G.O.; Abe, A.; Obisesan, O.; Ogundare, E. Performance of early-maturing topcross maize hybrids across multi-environments. J. Plant Breed. Crop Sci. 2024, 16, 15–26. [Google Scholar]
- Troyer, A.F.; Wellin, E.J. Heterosis decreasing in hybrids: Yield test inbreds. Crop Sci. 2009, 49, 1969–1976. [Google Scholar] [CrossRef]
- Denis, T.; Gales, D.; Chiriac, G.; Raus, L.; Jitareanu, G. Impact of plowing on some soil physical properties under hybrid seed corn production. ProEnviron. ProMediu 2013, 6, 183–186. [Google Scholar]
- Omar, S.; Tarnawa, A.; Kende, Z.; Abd, G.R.; Kassai, M.K.; Jolánkai, M. Germination characteristics of different maize inbred hybrids and their parental lines. Cereal Res. Commun. 2022, 50, 1229–1236. [Google Scholar] [CrossRef]
- Omar, S.; Abd Ghani, R.; Khalid, N.; Jolánkai, M.; Tarnawa, Á.; Percze, A.; Mikó, P.P.; Kende, Z. Effects of Seed Quality and Hybrid Type on Maize Germination and Yield in Hungary. Agriculture 2023, 13, 1836. [Google Scholar] [CrossRef]
- Abera, T.; Debele, T.; Wegary, D. Effects of varieties and nitrogen fertilizer on yield and yield components of maize on farmers field in mid altitude areas of Western Ethiopia. Int. J. Agron. 2017, 2017, 4253917. [Google Scholar] [CrossRef]
- Atta, S.K.; Ayyad, V.F.; El Sherif, L.M.; Abo El-khair, R.R. The role of modern varieties in increasing the total production of the summer yellow maize crop in Egypt. Egypt. J. Agric. Res. 2022, 100, 228–238. [Google Scholar]
- Nawaz, R.; Riaz, U.; Gouhar, H.; Mukhtar, M.; Arshad, A.; Hussain, A.; Amjad, I. Harnessing genetic diversity for sustainable maize production. J. Phys. Biomed. Biol. Sci. 2023, 2023, 15. [Google Scholar]
- Chen, F.; Qiu, H.; Zhao, Y.; Wei, X.; Wan, X. Impact of new maize variety adoption on yield and fertilizer input in China: Implications for sustainable food and agriculture. Agric. Syst. 2024, 218, 104004. [Google Scholar] [CrossRef]
- Król-Badziak, A.; Kozyra, J.; Rozakis, S. Assessment of Suitability Area for Maize Production in Poland Related to the Climate Change and Water Stress. Sustainability 2024, 16, 852. [Google Scholar] [CrossRef]
- Anders, A.; Markowski, P.; Konopka, S.; Kaliniewicz, Y.; Lipinski, A.J.; Choszcz, D.J. Effect of seeding rate on selected physical parameters and biomass yield of maize. Chil. J. Agric. Res. 2020, 80, 171–180. [Google Scholar] [CrossRef]
- Sulewska, H.; Śmiatacz, K.; Szymańska, G.; Panasiewicz, K.; Bandurska, H.; Głowicka-Wołoszyn, R. Seed size effect on yield quantity and quality of maize (Zea mays L.) cultivated in South East Baltic region. Zemdirb. Agric. 2014, 101, 35–40. [Google Scholar] [CrossRef]
- Tabakovic, M.; Simic, M.; Stanisavljevic, R.; Milivojevic, M.; Secanski, M.; Postic, D. Effects of shape and size of hybrid maize seed on germination and vigour of different genotypes. Chil. J. Agric. Res. 2020, 80, 381–392. [Google Scholar] [CrossRef]
- Dolapo, B.A.; Modi, A.T. Germination Characteristics of SC701 Maize Hybrid According to Size and Shape at Different Temperature Regimes. Plant Prod. Sci. 2015, 18, 514–521. [Google Scholar]
- Zhang, M.; Zheng, H.; Jin, L.; Xing, L.; Zou, J.; Zhang, L.; Liu, C.; Chu, J.; Xu, M.; Wang, L. miR169o and ZmNF-YA13 act in concert to coordinate the expression of ZmYUC1 that determines seed size and weight in maize kernels. New Phytol. 2022, 235, 2270–2284. [Google Scholar] [CrossRef] [PubMed]
- Dai, D.; Ma, Z.; Song, R. Maize endosperm development. J. Integr. Plant Biol. 2021, 63, 613–627. [Google Scholar] [CrossRef]
- Akpodiete, O.N.; Lale, N.E.S.; Umeozor, O.C.; Zakka, U. Role of physical characteristics of the seed on the stability of resistance of maize varieties to maize weevil (Sitophilus zeamais Motschulsky). J. Environ. Sci. Toxicol. Food Technol. 2015, 9, 60–66. [Google Scholar]
- ISTA. ISTA Handbook on Seedling Evaluation; International Seed Testing Association, ISTA: Bassersdorf, Switzerland, 2013. [Google Scholar]
- Dąbrowska, B.; Pokojska, H.; Suchorska-Tropiło, K. Laboratory Methods of Seed Evaluation; SGGW: Warszawa, Poland, 2000; p. 91. (In Polish) [Google Scholar]
- Kabała, C.; Charzyński, P.; Chodorowski, J.; Drewnik, M.; Glina, B.; Greinert, A.; Hulisz, P.; Jankowski, M.; Jonczak, J.; Łabaz, B.; et al. Polish Soil Classification, 6th edition–principles, classification scheme and correlations. Soil Sci. Annu. 2019, 70, 71–97. [Google Scholar] [CrossRef]
- IUSS Working Group WRB. World Reference Base for Soil Resources, Update 2015 International Soil Classification System for Naming Soils and Creating Legends for Soil Maps; FAO: Rome, Italy, 2014; Volume 106, p. 182. [Google Scholar]
- SAS Institute. SAS/STAT User’s Guide, 7th ed.; Inst Inc.: Cary, NC, USA, 1999. [Google Scholar]
- Finch-Savage, W.E.; Bassel, G.W. Seed vigour and crop establishment: Extending performance beyond adaptation. J. Exp. Bot. 2016, 67, 567–591. [Google Scholar] [CrossRef] [PubMed]
- Domergue, J.B.; Abadie, C.; Limami, A.; Way, D.; Tcherkez, G. Seed quality and carbon primary metabolism. Plant Cell Environ. 2019, 42, 2776–2788. [Google Scholar] [CrossRef] [PubMed]
- Mehta, B.K.; Chauhan, H.S.; Basu, S.; Anand, A.; Baveja, A.; Zunjare, R.U.; Muthusamy, V.; Singh, A.K.; Hissain, F. Mutant crtRB1 gene negates the unfavourable effects of opaque2 gene on germination and seed vigour among shrunken2-based biofortified sweet corn genotypes. Funct. Plant Biol. 2024, 51, FP23179. [Google Scholar] [CrossRef]
- Parus, A.; Framski, G.; Rypniewski, W.; Panasiewicz, K.; Szulc, P.; Myszka, K.; Zgoła-Grześkowiak, A.; Ławniczak, Ł.; Chrzanowski, Ł. Plant growth promoting N-alkyltropinium bromides enhance seed germination, biomass accumulation and photosynthesis parameters of maize (Zea mays). New J. Chem. 2019, 43, 5805–5812. [Google Scholar] [CrossRef]
- Domin, M.; Kluza, F.; Góral, D.; Nazarewicz, S.; Kozłowicz, K.; Szmigielski, M.; Ślaska-Grzywna, B. Germination Energy and Capacity of Maize Seeds Following Low-Temperature Short Storage. Sustainability 2020, 12, 46. [Google Scholar] [CrossRef]
- Chauhan, H.S.; Muthusamy, V.; Rashmi, T.; Basu, S.; Anand, A.; Mehta, B.K.; Gain, N.; Zunjare, R.U.; Singh, A.K.; Gupta, H.S.; et al. Characterization of crtRB1-and vte4-based biofortified sweet corn inbreds for seed vigour and physico-biochemical traits. J. Appl. Genet. 2022, 63, 651–662. [Google Scholar] [CrossRef]
- Oliveira, T.L.; Pinho, R.G.V.; Santos, H.O.; Silva, K.M.; Pereira, E.; Souza, J.L. Biochemical changes and physiological quality of corn seeds subjected to different chemical treatments and storage times. J. Seed Sci. 2020, 42, e202042038. [Google Scholar] [CrossRef]
- Basu, S.; Groot, S.P.C. Seed Vigor and Invigoration. In Seed Science and Technology; Dadlani, M., Yadava, D.K., Eds.; Springer: Singapore, 2023. [Google Scholar] [CrossRef]
- Panasiewicz, K.; Koziara, W.; Sulewska, H. The effect of cereal seed dressing on their vigor after one-year storage. Prog. Plant Prot. 2009, 49, 256–259. (In Polish) [Google Scholar]
- Panasiewicz, K.; Faligowska, A.; Pociejewska, M. Sowing value and vigor of two winter wheat varieties depending on storage conditions. Sci. Nat. Technol. 2012, 6, 1–8. [Google Scholar]
- Panasiewicz, K.; Faligowska, A.; Szymańska, G. Evaluation of sowing value and vigor of dressed grain of Winter triticale and rye depending on the cultivar and period of storage. Progress. Plant Prot. 2012, 52, 651–656. [Google Scholar]
- Ponichtera, P. The sowing value of tansy Phacelia (Phacelia tanacetifolia Benth.) seeds in the long-term storage. Agric. Eng. 2016, 20, 153–159. [Google Scholar] [CrossRef]
- Ludwig, V.; Berghetti, M.R.P.; Rossato, F.P.; Wendt, L.M.; Schultz, E.E.; Both, V.; Brackmann, A. Impact of Controlled Atmosphere Storage on Physiological Quality of Soybean Seed. J. Stored Prod. Res. 2021, 90, 101749. [Google Scholar] [CrossRef]
- Sulewska, H.; Koziara, W.; Panasiewicz, K.; Ptaszyńska, G. Evaluation of maize seed sowing value in two years storage period with arm, cold and field tests. Acta Sci. Pol. Agric. 2009, 8, 21–31. [Google Scholar]
- Ambika, S.; Manonmani, V.; Somasundaram, G. Review on effect of seed size on seedling vigor and seed yield. Res. J. Seed Sci. 2014, 7, 31–38. [Google Scholar] [CrossRef]
- Panasiewicz, K.; Koziara, W.; Sulewska, H. Parameters of vigor tests depending on seed size of some grain cereals. Fragm. Agron. 2008, 25, 297–306. (In Polish) [Google Scholar]
- Msuya, D.G.; Stefano, J. Responses of Maize (Zea mays) seed germination capacity and vigour to seed selection based on size of cob and selective threshing. World J. Agric. Sci. 2010, 6, 683–688. [Google Scholar]
- Bieniek, J.; Zawada, J.; Molendowski, F.; Komarnicki, P.; Kwietniak, K. Evaluation of the quality of the technological line for prcessing corn cobs and seeds. Agric. Eng. 2013, 4, 17–25. [Google Scholar]
- Molatudi, R.L.; Mariga, I.K. The effect of maize seed size and depth of planting on seedling emergence and seedling vigour. J. Appl. Sci. Res. 2009, 5, 2234–2237. [Google Scholar]
- Yusuf, C.S.; Makate, N.; Jacob, R. Effect of seed size on germination and early growth of maize (Zea mays). Int. J. Sci. Res. Publ. 2014, 4, 159–161. [Google Scholar]
- Gageanu, I.; Gheorghe, G.; Persu, C.; Vladut, N.V.; Cujbescu, D.; Matache, M.G.; Voicea, I.; Ion, G.C.; Tabarasu, A.M.; Boruz, S.P.; et al. Contributions to the Process of Calibrating Corn Seeds Using a Calibrator with Cylindrical Sieves. Appl. Sci. 2023, 13, 9927. [Google Scholar] [CrossRef]
- Sulewska, H.; Koziara, W. Evaluation of sowing value and yielding potential of three fractions of maize seeds treated with Biochikol 020 PC. J. Res. Appl. Agric. Eng. 2006, 51, 178–182. [Google Scholar]
- Tabakovic, M.; Oro, V.; Stanisavlevic, R.; Strbanovis, R.; Secanski, M. Quality assessment of hybrid maize seeds according to their shape and size. J. Process Energy Agric. 2021, 25, 28–31. [Google Scholar] [CrossRef]
- Uździcka, B.; Juszczak, R.; Sakowska, K.; Olejnik, J. The relationship between the LAI index and spectral vegetation indices on the example of selected crop species. Water Environ. Rural. Areas. 2012, 2, 283–311. (In Polish) [Google Scholar]
- Szulc, P.; Rybus-Zając, M.; Jagła, M. Influence of Nitrogen Dose, Type of Nitrogen Fertilizer and Method of Its Application on Plant Health of Maize Hybrids (Zea mays L.). EJPAU 2014, 17, 10. Available online: http://www.ejpau.media.pl/volume17/issue2/art-10.html (accessed on 5 February 2019).
- Kimmelshue, C.L.; Goggi, A.S.; Moore, K.J. Single-Plant Grain Yield in Corn (Zea mays L.) Based on Emergence Date, Seed Size, Sowing Depth, and Plant to Plant Distance. Crops 2022, 2, 62–86. [Google Scholar] [CrossRef]
- Kadłubiec, W.; Kuriata, R. Multitrait analysis of grain yield formation in inbred lines and F1 hybrids of maize. Biul. IHAR. 2004, 231, 419–424. (In Polish) [Google Scholar] [CrossRef]
- Gąsiorowska, B.; Makarewicz, A.; Płaza, A.; Nowosielska, A. Grain yield and characteristics of corn cobs sown at different dates. Fragm. Agron. 2009, 26, 55–63. (In Polish) [Google Scholar]
- Wasilewski, P.; Gałęzewski, L.; Jaskulska, I.; Mądry, A.; Różniak, M. The effect of seed size on growth and yield of spring forms of rye and wheat. Acta Sci. Pol. Agric. 2014, 13, 81–88. [Google Scholar]
- Ptaszyńska, G.; Sulewska, H. Variability of yielding of maize hybrids of different earliness in climatic conditions of central Wielkopolska. Acta Sci. Pol. Agric. 2008, 7, 93–103. (In Polish) [Google Scholar]
- Liu, N.; Du, Y.; Warburton, M.L.; Xiao, Y.; Yan, J. Phenotypic plasticity contributes to maize adaptation and heterosis. Mol. Biol. Evol. 2021, 38, 1262–1275. [Google Scholar] [CrossRef]
- Li, D.; Chen, Z.; Wang, M.; Leiser, W.L.; Weiß, T.M.; Zhao, Z. Dissecting the phenotypic response of maize to low phosphorus soils by field screening of a large diversity panel. Euphytica 2021, 217, 12. [Google Scholar] [CrossRef]
- Li, D.; Zhou, Z.; Lu, X.; Jiang, Y.; Li, G.; Li, J.; Wang, H.; Chen, S.; Li, X.; Würschum, T.; et al. Genetic Dissection of Hybrid Performance and Heterosis for Yield-Related Traits in Maize. Front. Plant Sci. 2021, 12, 774478. [Google Scholar] [CrossRef] [PubMed]

| Year | Months | Average | |||||
|---|---|---|---|---|---|---|---|
| IV | V | VI | VII | VIII | IX | ||
| 2013 | 0.4 | 2.2 | 2.2 | 0.8 | 0.5 | 1.9 | 1.3 |
| 2014 | 1.5 | 1.8 | 0.6 | 0.7 | 1.0 | 0.8 | 1.1 |
| 2015 | 0.6 | 0.6 | 1.3 | 1.4 | 0.5 | 0.6 | 0.8 |
| 1958–2012 | 1.2 | 1.4 | 1.1 | 1.4 | 1.1 | 1.0 | - |
| Seed Fraction | Germination Energy [%] | Germination Capacity [%] | Share of Abnormally Germinating Kernels [%] | Share of Healthy Non-Germinating Kernels [%] |
|---|---|---|---|---|
| I—small (244 g) | 94.7 c * | 95.5 b | 2.9 a | 1.6 a |
| II—medium (296 g) | 97.6 b | 97.9 a | 1.5 a | 0.6 b |
| III—large (334 g) | 99.0 a | 98.7 a | 1.0 a | 0.3 b |
| IV—very large (396 g) | 94.8 c | 97.3 a | 0.4 a | 0.5 b |
| Seed Fraction | Cold Test [%] | Radicle Growth (RE) [%] | Seedling Growth Test [cm] | Seedling Growth Rate Test [mg] | Conductivity Test [µS cm g−1] | Vigor Index |
|---|---|---|---|---|---|---|
| I—small (244 g) | 86.1 ab * | 88.3 c | 4.30 bc | 14.1 ab | 4.84 d | 411 b |
| II—medium (296 g) | 85.8 a | 82.0 b | 4.13 b | 16.0 b | 4.00 c | 405 b |
| III—large (334 g) | 90.7 b | 90.9 c | 4.64 c | 17.7 c | 3.33 a | 457 c |
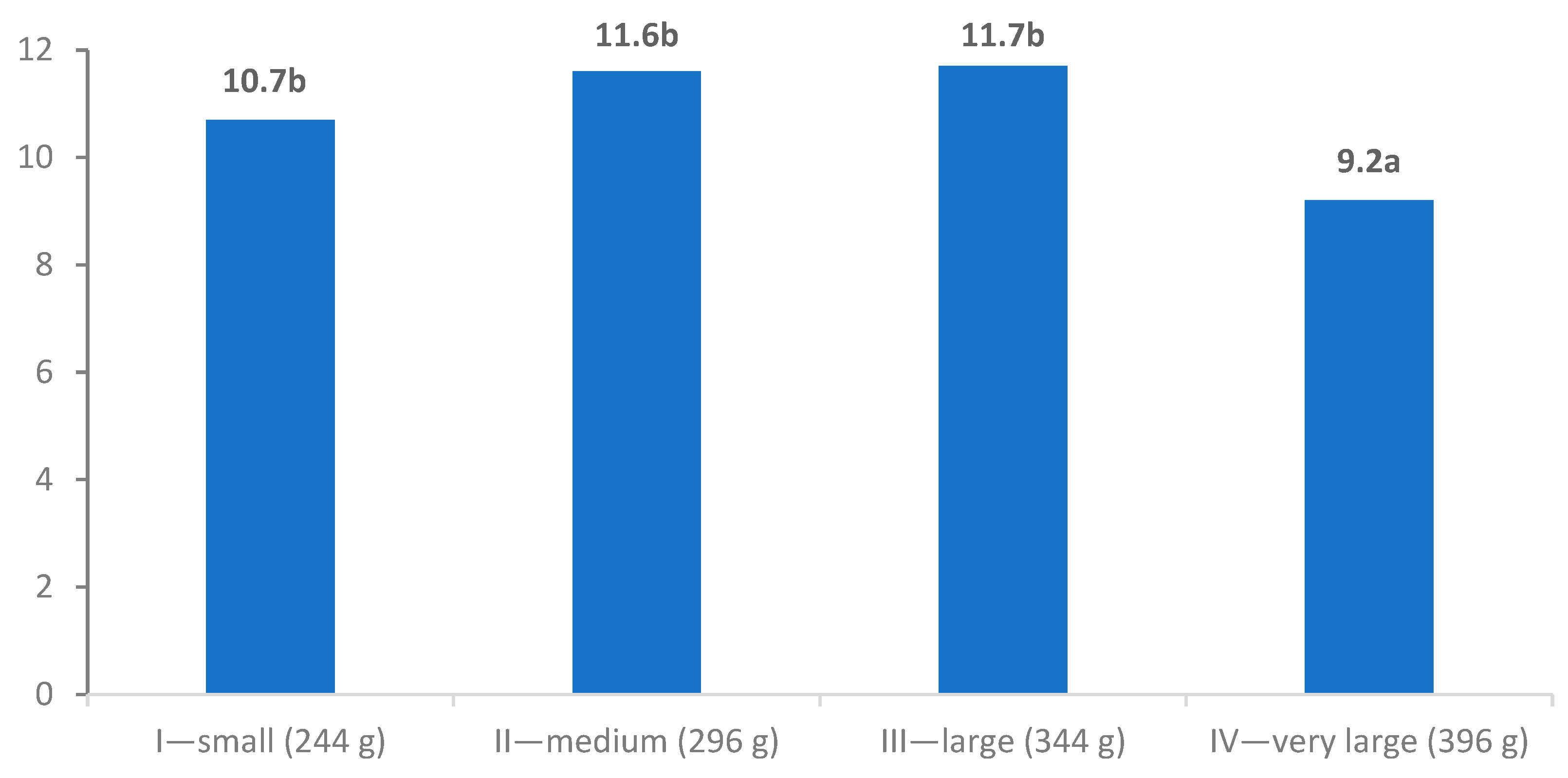
| IV—very large (396 g) | 88.4 ab | 72.7 a | 3.23 a | 13.2 a | 3.51 b | 308 a |
| Seed Fraction | SPAD [BBCH 30] | SPAD [BBCH 67] | LAI |
|---|---|---|---|
| I—small (244 g) | 491 a * | 766 a | 3.4 a |
| II—medium (296 g) | 505 a | 772 a | 3.5 a |
| III—large (334 g) | 487 a | 796 a | 3.3 a |
| IV—very large (396 g) | 489 a | 781 a | 3.5 a |
| Seed Fraction | Number of Cobs [pcs m2] | TKW [g] | Number of Kernels in Cob [pcs.] | Seed Yield [dt ha−1] |
|---|---|---|---|---|
| I—small (244 g) | 7.7 a * | 343.9 b | 500.9 b | 97.4 a |
| II—medium (296 g) | 8.2 a | 355.6 a | 533.7 a | 100.8 a |
| III—large (334 g) | 7.8 a | 345.4 b | 518.9 b | 106 a |
| IV—very large (396 g) | 7.9 a | 346.1 ab | 540.8 a | 104.4 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Panasiewicz, K.; Sobieszczański, R.; Ratajczak, K.; Faligowska, A.; Szymańska, G.; Bocianowski, J.; Kolanoś, A.; Pretkowski, R. Effects of Seed Fraction on Sowing Quality and Yield of Three-Line Hybrid Maize. Agriculture 2025, 15, 972. https://doi.org/10.3390/agriculture15090972
Panasiewicz K, Sobieszczański R, Ratajczak K, Faligowska A, Szymańska G, Bocianowski J, Kolanoś A, Pretkowski R. Effects of Seed Fraction on Sowing Quality and Yield of Three-Line Hybrid Maize. Agriculture. 2025; 15(9):972. https://doi.org/10.3390/agriculture15090972
Chicago/Turabian StylePanasiewicz, Katarzyna, Rafał Sobieszczański, Karolina Ratajczak, Agnieszka Faligowska, Grażyna Szymańska, Jan Bocianowski, Anna Kolanoś, and Rafał Pretkowski. 2025. "Effects of Seed Fraction on Sowing Quality and Yield of Three-Line Hybrid Maize" Agriculture 15, no. 9: 972. https://doi.org/10.3390/agriculture15090972
APA StylePanasiewicz, K., Sobieszczański, R., Ratajczak, K., Faligowska, A., Szymańska, G., Bocianowski, J., Kolanoś, A., & Pretkowski, R. (2025). Effects of Seed Fraction on Sowing Quality and Yield of Three-Line Hybrid Maize. Agriculture, 15(9), 972. https://doi.org/10.3390/agriculture15090972











