Comparative Analysis of Soil Biological Activity and Macroinvertebrate Diversity in Amazonian Chakra Agroforestry and Tropical Rainforests in Ecuador
Abstract
:1. Introduction
2. Materials and Methods
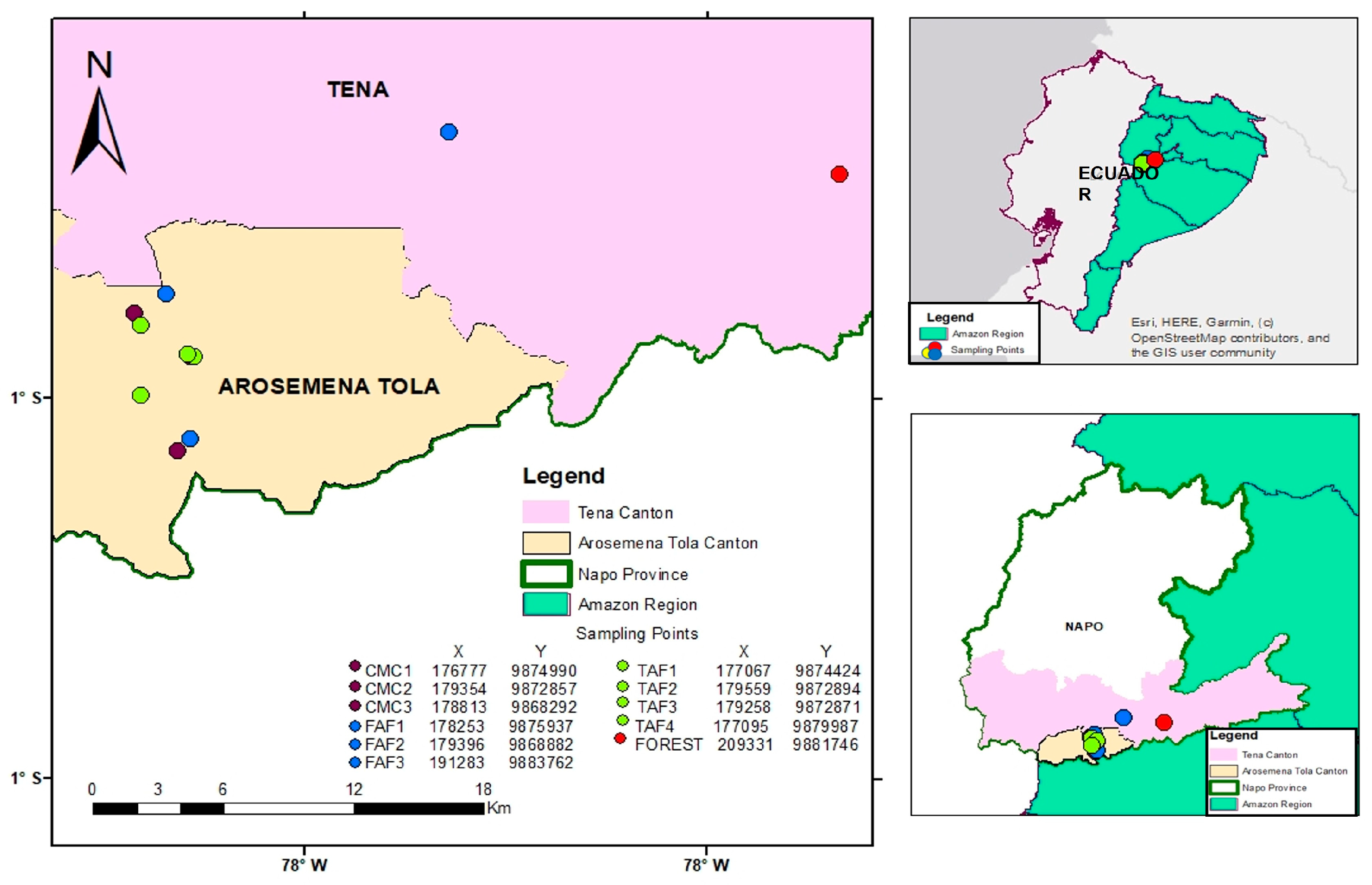
2.1. Land Uses
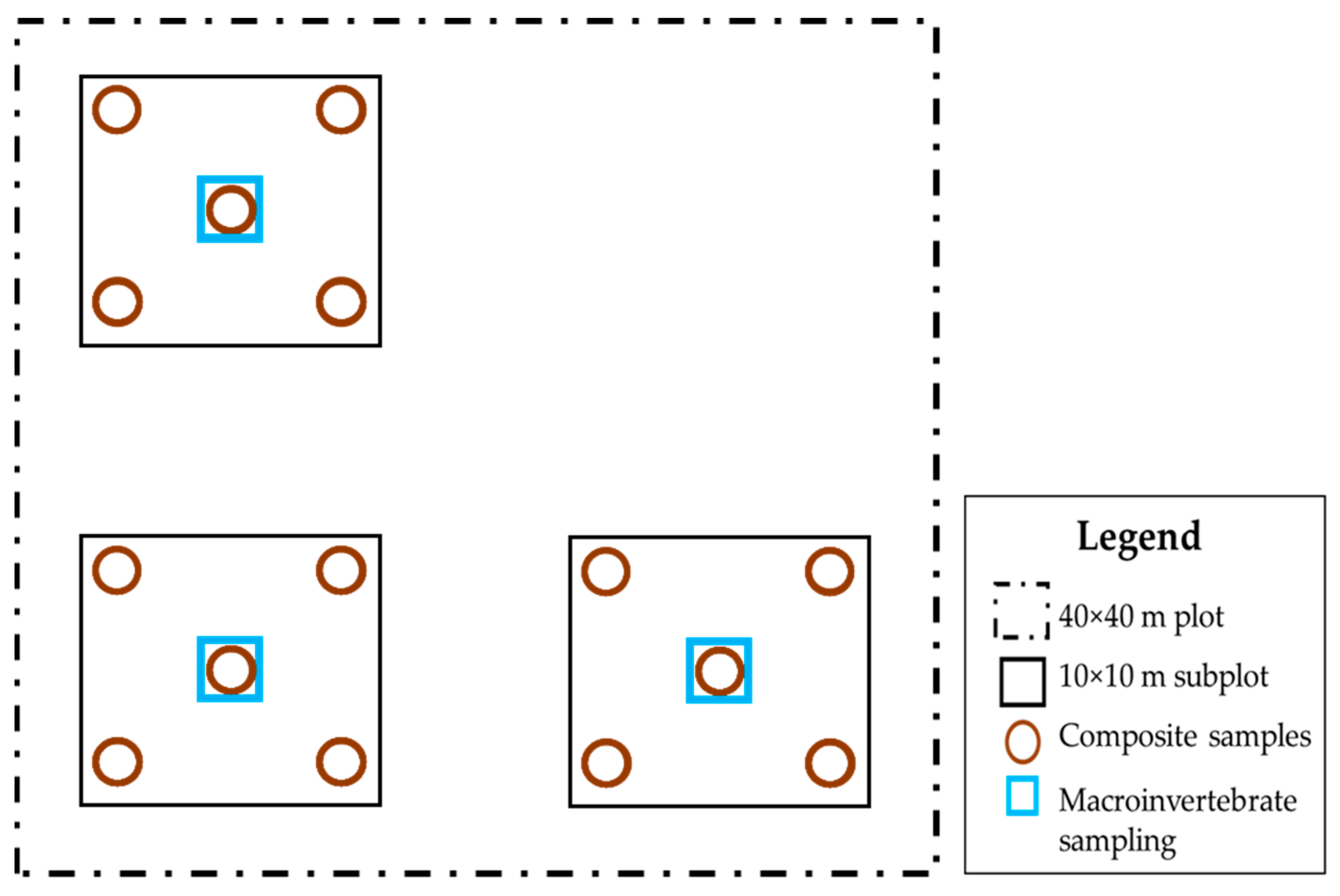
2.2. Field Sampling
2.3. Sample Analysis
2.3.1. Berlese Extraction Method
2.3.2. Manual Techniques in the Field
2.4. Biological Parameters
2.5. Enzymatic Activity
- ▪
- Dehydrogenase (DH) activity: Here, 1 g of soil was used, which was incubated in the shade at 20 °C for 20 h with 0.20 mL of 0.4% 2-(4-iodophenyl)-3-(4-nitrophenyl)-5-phenyl-2H-tetrazolium (INT) chloride as substrate. After incubation, the iodonitrotetrazolium formazan produced was extracted with 10 mL of methanol, and the absorbance was measured at 490 nm.
- ▪
- β-glucosidase (GL) activity: For this determination, 1 g of soil was used, which was incubated at 37 °C for 1 h, with 4 mL of 25 mM 4-nitrophenyl-β-d-glucopyranoside in 0.1 M modified universal buffer (MUB) pH 6.0. Then, to stop the reaction, the samples were refrigerated at 2 °C for 15 min, and the p-nitrophenol produced in the enzymatic reactions was determined at 400 nm.
- ▪
- Urease (UR) activity: Here, 0.50 g of soil was used and incubated at 30 °C for 1.5 h with 2 mL of 0.1 M pH 7.0 phosphate buffer and 0.50 mL of 1.07 M urea. The ammonia released during the hydrolytic reaction was measured spectrophotometrically at 636 nm.
- ▪
- Phosphatase (PHO) activity: Here, 1 g of soil was used, which was incubated at 37 °C for 1 h with 4 mL of 25 mM 4-nitrophenyl phosphate MUB pH 6.5. Then, to stop the reaction, the samples were refrigerated at 2 °C for 15 min, and the p-nitrophenol produced in the enzymatic reactions was determined at 398 nm.
- ▪
- Arylsulfatase (SU) activity: For this determination, 1 g of soil was used, which was incubated at 37 °C for 1 h with 4 mL of 5 mM 4-nitrophenyl sulfate in 0.5 M acetate buffer pH 5.8. Then, to stop the reaction, the samples were refrigerated at 2 °C for 15 min, and the p-nitrophenol produced in the enzymatic reactions was determined at 410 nm.
2.6. Data Analysis
3. Results
3.1. Functional Groups and Taxonomic Composition of Soil Macroinvertebrates
3.1.1. Soil Macroinvertebrate Composition Based on the Manual Method
3.1.2. Soil Macroinvertebrate Composition Based on the Berlese Extraction Method
3.1.3. Soil Macroinvertebrate Abundance and Diversity Across Land-Use Systems
3.2. Soil Quality Indicators
3.2.1. Soil Quality Indicators and Biological Parameters
3.2.2. Correlation Between Soil Properties and Biological Activity
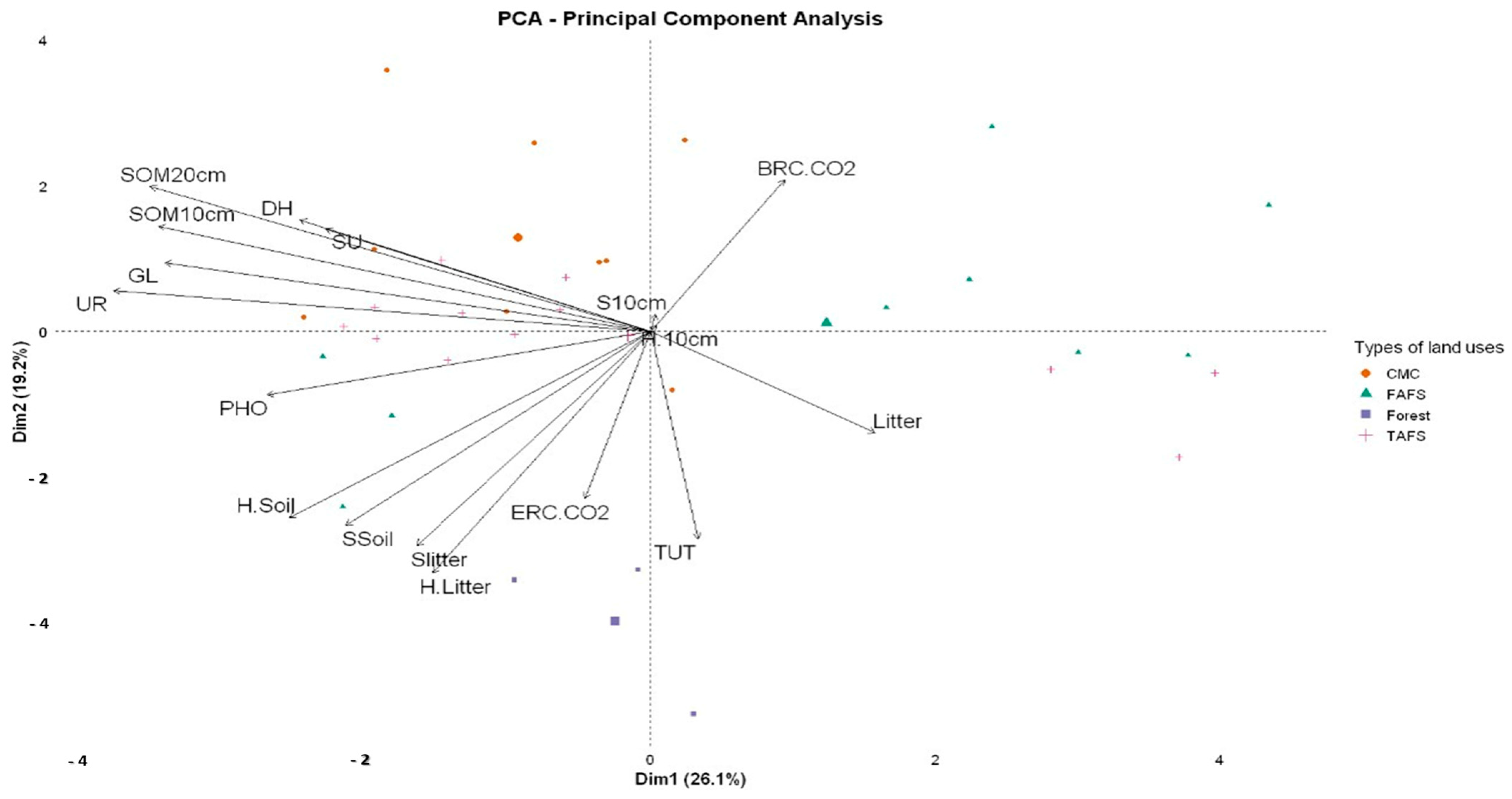
3.2.3. Principal Component Analysis (PCA) of Soil Biological Activity
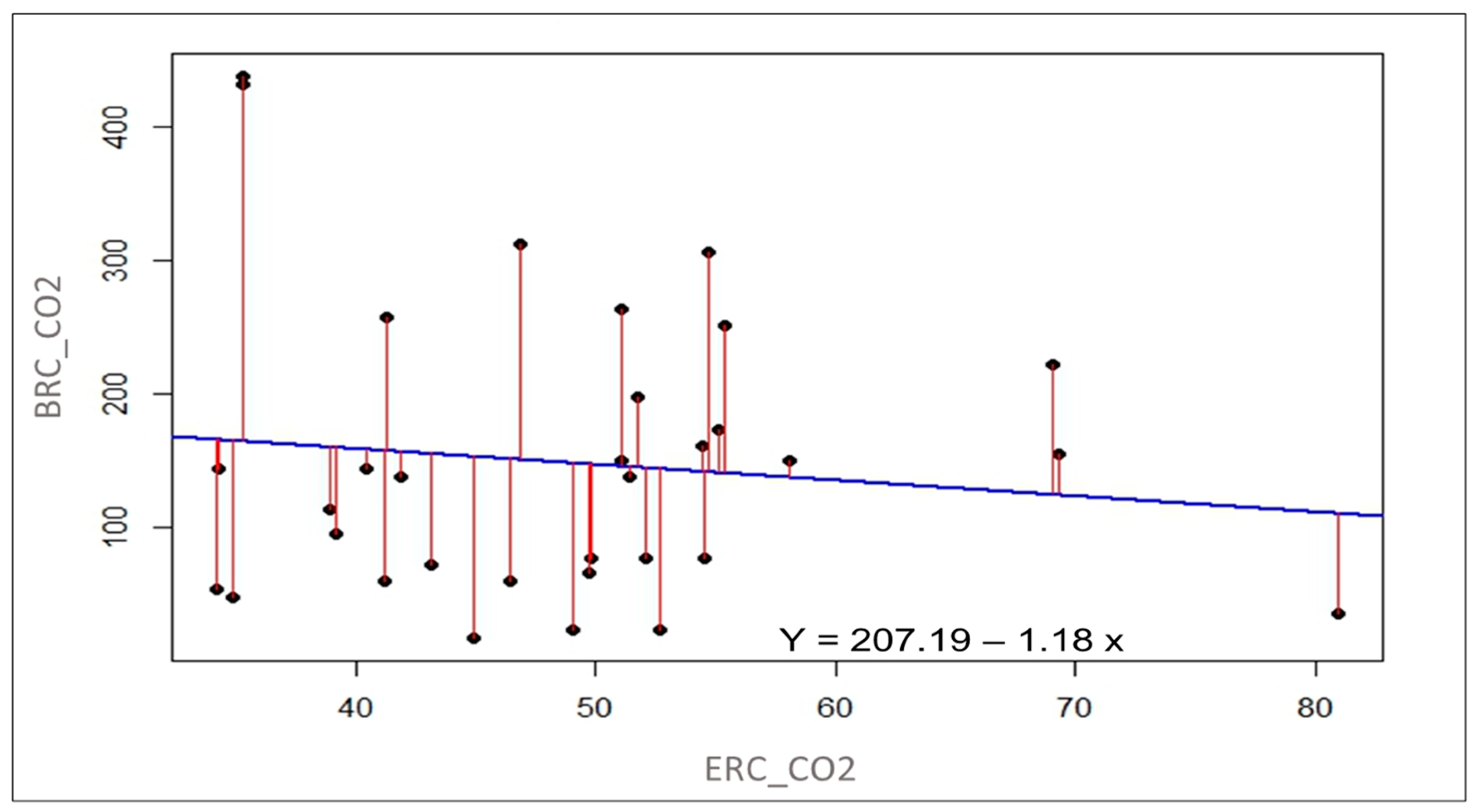
3.2.4. Regression Analysis of CO2 Respiration
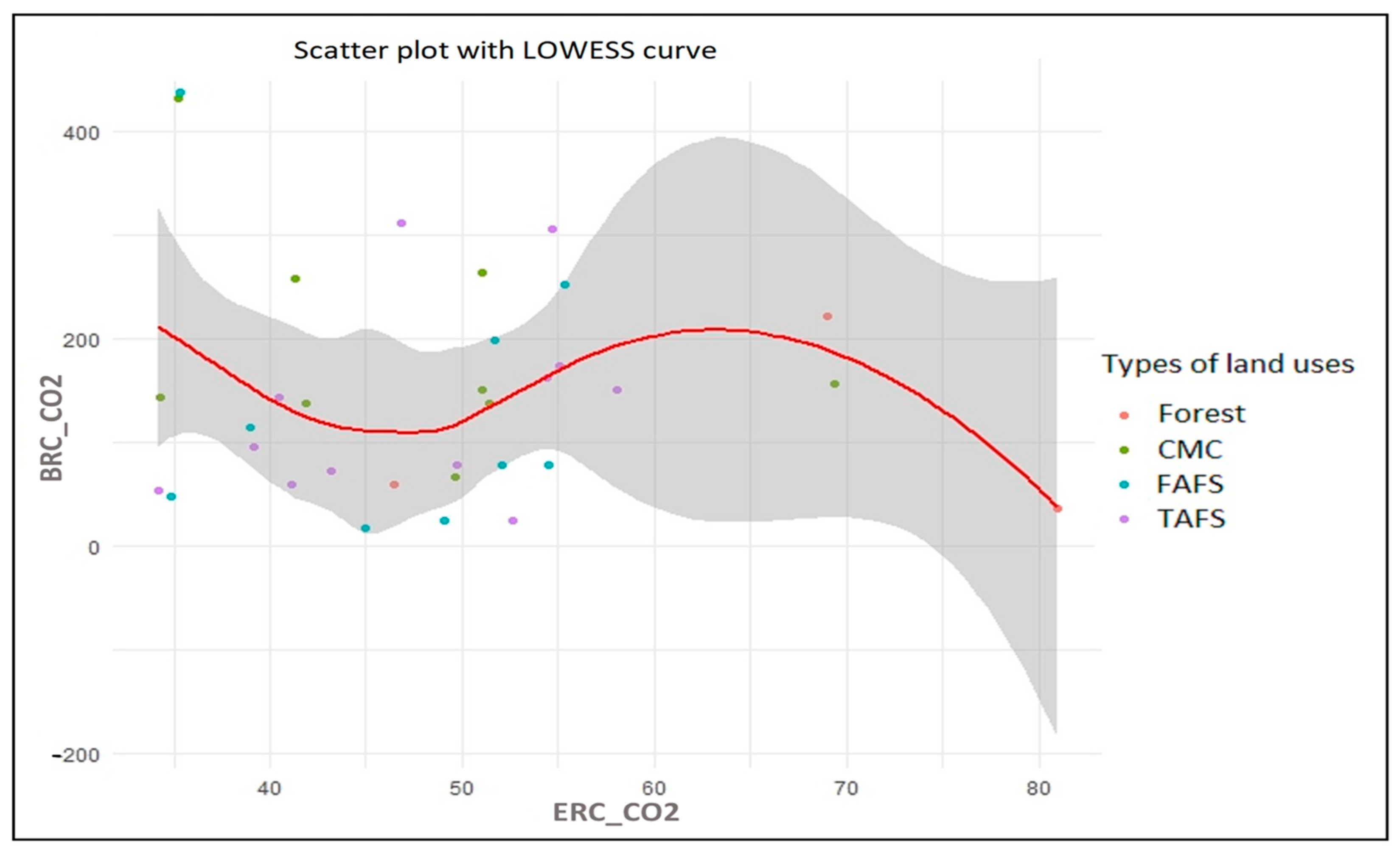
3.2.5. Locally Weighted Scatterplot (LOWESS)
4. Discussion
4.1. Effects of Land-Use Systems on Soil Biological Activity
4.2. Soil Macroinvertebrate Diversity and Functional Groups Across Land-Use Types
4.3. Functional Linkages Between Soil Organic Matter and Biological Indicator
4.4. Implications for Sustainable Soil Management and Agroforestry Practices
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Myers, N.; Mittermeier, R.A.; Mittermeier, C.G.; da Fonseca, G.A.B.; Kent, J. Biodiversity Hotspots for Conservation Priorities. Nature 2000, 403, 853–858. [Google Scholar] [CrossRef] [PubMed]
- Mittermeier, R.A.; Myers, N.; Thomsen, J.B.; da Fonseca, G.A.B.; Olivieri, S. Biodiversity Hotspots and Major Tropical Wilderness Areas: Approaches to Setting Conservation Priorities. Conserv. Biol. 1998, 12, 516–520. [Google Scholar] [CrossRef]
- Bass, M.S.; Finer, M.; Jenkins, C.N.; Kreft, H.; Cisneros-Heredia, D.F.; McCracken, S.F.; Pitman, N.C.A.; English, P.H.; Swing, K.; Villa, G.; et al. Global Conservation Significance of Ecuador’s Yasuní National Park. PLoS ONE 2010, 5, e8767. [Google Scholar] [CrossRef]
- Freitas, A.S.d; Zagatto, L.F.G.; Rocha, G.S.; Muchalak, F.; Silva, S.d.S.; Muniz, A.W.; Hanada, R.E.; Tsai, S.M. Amazonian Dark Earths Enhance the Establishment of Tree Species in Forest Ecological Restoration. Front. Soil Sci. 2023, 3, 1161627. [Google Scholar] [CrossRef]
- Torres, B.; Luna, M.; Tipán-Torres, C.; Ramírez, P.; Muñoz, J.C.; García, A. A Simplified Integrative Approach to Assessing Productive Sustainability and Livelihoods in the “Amazonian Chakra” in Ecuador. Land 2024, 13, 1–23. [Google Scholar] [CrossRef]
- Huera-Lucero, T.; Lopez-Piñeiro, A.; Torres, B.; Bravo-Medina, C. Biodiversity and Carbon Sequestration in Chakra-Type Agroforestry Systems and Humid Tropical Forests of the Ecuadorian Amazon. Forests 2024, 15, 557. [Google Scholar] [CrossRef]
- Raad Nahon, S.M.; Costa Trindade, F.; Yoshiura, C.A.; Caixeta Martins, G.; Chagas da Costa, I.R.; Oliveira Costa, P.H.; Herrera, H.; Balestrin, D.; Oliveira Godinho, T.; Marchiori, B.M.; et al. Impact of Agroforestry Practices on Soil Microbial Diversity and Nutrient Cycling in Atlantic Rainforest Cocoa Systems. Int. J. Mol. Sci. 2024, 25, 11345. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, J.L.M.; Pellizari, V.H.; Mueller, R.; Baek, K.; Jesus, E.d.C.; Paula, F.S.; Mirza, B.; Hamaoui, G.S.; Tsai, S.M.; Feigl, B.; et al. Conversion of the Amazon Rainforest to Agriculture Results in Biotic Homogenization of Soil Bacterial Communities. Proc. Natl. Acad. Sci. USA 2013, 110, 988–993. [Google Scholar] [CrossRef]
- Orczewska, A.; Dulik, A.; Długosz, P.; Depa, Ł. Intensive Agriculture vs. Invertebrate Biodiversity: A Case Study of Woodland Islets in a Matrix of Arable Land. Agriculture 2024, 14, 1400. [Google Scholar] [CrossRef]
- Pattanayak, S.; Jena, S.; Das, P.; Maitra, S.; Shankar, T.; Praharaj, S.; Mishra, P.; Mohanty, S.; Pradhan, M.; Swain, D.K.; et al. Weed Management and Crop Establishment Methods in Rice (Oryza sativa L.) Influence the Soil Microbial and Enzymatic Activity in Sub-Tropical Environment. Plants 2022, 11, 1071. [Google Scholar] [CrossRef]
- Jiang, L.; Ushio, M.; Kitayama, K. Changes of Soil Chemical Properties, Microbial Biomass and Enzymatic Activities along a Gradient of Forest Degradation in Logged over Tropical Rain Forests, Borneo. Plant Soil 2023, 485, 525–536. [Google Scholar] [CrossRef]
- Li, Y.; Han, C.; Sun, S.; Zhao, C. Effects of Tree Species and Soil Enzyme Activities on Soil Nutrients in Dryland Plantations. Forests 2021, 12, 1153. [Google Scholar] [CrossRef]
- Cahyo, A.N.; Dong, Y.; Taryono; Nugraha, Y.; Junaidi; Sahuri; Penot, E.; Hairmansis, A.; Purwestri, Y.A.; Akbar, A.; et al. Rubber-Based Agroforestry Systems Associated with Food Crops: A Solution for Sustainable Rubber and Food Production? Agriculture 2024, 14, 1038. [Google Scholar] [CrossRef]
- Ballesteros-Possú, W.; Valencia, J.C.; Navia-Estrada, J.F. Assessment of a Cocoa-Based Agroforestry System in the Southwest of Colombia. Sustainability 2022, 14, 9447. [Google Scholar] [CrossRef]
- Guo, J.; Tang, W.; Tu, H.; Zheng, J.; Wang, Y.; Yu, P.; Wang, G. Thinning Modulates the Soil Organic Carbon Pool, Soil Enzyme Activity, and Stoichiometric Characteristics in Plantations in a Hilly Zone. Forests 2024, 15, 2038. [Google Scholar] [CrossRef]
- Damasco, G.; Anhalt, M.; Perdiz, R.O.; Wittmann, F.; de Assis, R.L.; Schöngart, J.; Piedade, M.T.F.; Bacon, C.D.; Antonelli, A.; Fine, P.V.A. Certification of Açaí Agroforestry Increases the Conservation Potential of the Amazonian Tree Flora. Agrofor. Syst. 2022, 96, 407–416. [Google Scholar] [CrossRef]
- Figueira da Silva, C.; Pereira, M.G.; Gaia Gomes, J.H.; Fontes, M.A.; Ribeiro da Silva, E.M. Enzyme Activity, Glomalin, and Soil Organic Carbon in Agroforestry Systems. Floresta Ambiente 2020, 27, e20170716. [Google Scholar] [CrossRef]
- Van Noordwijk, M. Agroforestry-Based Ecosystem Services: Reconciling values of humans and nature in sustainable development. Land 2021, 10, 699. [Google Scholar] [CrossRef]
- Coq-Huelva, D.; Higuchi, A.; Alfalla-Luque, R.; Burgos-Morán, R.; Arias-Gutiérrez, R. Co-Evolution and Bio-Social Construction: The Kichwa Agroforestry Systems (Chakras) in the Ecuadorian Amazonia. Sustainability 2017, 9, 1920. [Google Scholar] [CrossRef]
- Coq-Huelva, D.; Torres-Navarrete, B.; Bueno-Suárez, C. Indigenous Worldviews and Western Conventions: Sumak kawsay and Cocoa Production in Ecuadorian Amazonia. Agric. Human Values 2018, 35, 163–179. [Google Scholar] [CrossRef]
- Ayala-Montejo, D.; Valdés-Velarde, E.; Benedicto-Valdés, G.S.; Escamilla-Prado, E.; Sánchez-Hernández, R.; Gallardo, J.F.; Martínez-Zurimendi, P. Soil Biological Activity, Carbon and Nitrogen Dynamics in Modified Coffee Agroforestry Systems in Mexico. Agronomy 2022, 12, 1794. [Google Scholar] [CrossRef]
- Reis dos Santos Bastos, T.; Anjos Bittencourt Barreto-Garcia, P.; de Carvalho Mendes, I.; Henrique Marques Monroe, P.; Ferreira de Carvalho, F. Response of Soil Microbial Biomass and Enzyme Activity in Coffee-Based Agroforestry Systems in a High-Altitude Tropical Climate Region of Brazil. Catena 2023, 230, 107270. [Google Scholar] [CrossRef]
- Rodrigues de Sousa, T.; Moreira de Carvalho, A.; Gerosa Ramos, M.L.; Duarte de Oliveira, A.; Rodrigues de Jesus, D.; Pereira da Fonseca, A.C.; Rodrigues da Costa Silva, F.; Santos Delvico, F.M.; Reis Junior, F.B.; Marchão, R.L. Dynamics of Carbon and Soil Enzyme Activities under Arabica Coffee Intercropped with Brachiaria Decumbens in the Brazilian Cerrado. Plants 2024, 13, 835. [Google Scholar] [CrossRef] [PubMed]
- Visscher, A.M.; Chavez, E.; Caicedo, C.; Tinoco, L.; Pulleman, M. Biological Soil Health Indicators Are Sensitive to Shade Tree Management in a Young Cacao (Theobroma cacao L.) Production System. Geoderma Reg. 2024, 37, e00772. [Google Scholar] [CrossRef]
- MMAyA. Guia Para La Evaluación de La Calidad Biológica de Suelos, Mesofauna; MMAyA: La Paz, Bolivia, 2020. [Google Scholar]
- Morales-Márquez, J.A.; Hernández-Hernández, R.M.; Sánchez, G.K.; Lozano, Z.; Castro, I.; Bravo, C.; Ramírez, E.; Jiménez-Ballesta, R. Soil Macroinvertebrates Community and Its Temporal Variation in a Well-Drained Savannah of the Venezuelan Llanos. Eur. J. Soil Biol. 2018, 84, 19–26. [Google Scholar] [CrossRef]
- Men, X.; Bao, Y.; Wu, M.; Liao, C.; Cheng, X. Soil Enzyme Activities Responded Differently to Short-Term Litter Input Manipulation under Coniferous and Broad-Leaved Forests in the Subalpine Area of Southwest China. For. Ecol. Manag. 2023, 546, 121360. [Google Scholar] [CrossRef]
- Silva, I.F.; Araújo Neto, S.E.d.; Kusdra, J.F. Biological Activity of Soils under Systems of Organic Farming, Agroforestry and Pasture in the Amazon. Rev. Cienc. Agron. 2014, 45, 427–432. [Google Scholar] [CrossRef]
- Piotrowska-Długosz, A.; Wilczewski, E. Effects of Catch Crops Cultivated for Green Manure on Soil C and N Content and Associated Enzyme Activities. Agriculture 2024, 14, 898. [Google Scholar] [CrossRef]
- Siwik-Ziomek, A.; Kuśmierek-Tomaszewska, R. Responses of Soil Enzymes Activities to Sprinkler Irrigation and Differentiated Nitrogen Fertilization in Barley Cultivation. Agriculture 2024, 14, 1255. [Google Scholar] [CrossRef]
- Kwiatkowski, C.A.; Harasim, E.; Feledyn-Szewczyk, B.; Antonkiewicz, J. Enzymatic Activity of Loess Soil in Organic and Conventional Farming Systems. Agriculture 2020, 10, 135. [Google Scholar] [CrossRef]
- Xu, Z.; Zhang, Z.; Peng, S.; Yuan, Y.; Wang, X. Influences of Lithium on Soil Properties and Enzyme Activities. Chemosphere 2023, 313, 137458. [Google Scholar] [CrossRef] [PubMed]
- Sarto, M.V.M.; Borges, W.L.B.; Bassegio, D.; Pires, C.A.B.; Rice, C.W.; Rosolem, C.A. Soil Microbial Community, Enzyme Activity, C and N Stocks and Soil Aggregation as Affected by Land Use and Soil Depth in a Tropical Climate Region of Brazil. Arch. Microbiol. 2020, 202, 2809–2824. [Google Scholar] [CrossRef]
- Liu, T.; Peng, D.; Tan, Z.; Guo, J.; Zhang, Y.; Liu, H. Do Stand Density and Month Regulate Soil Enzymes and the Stoichiometry of Differently Aged Larix Principis-Rupprechtii Plantations? Catena 2023, 220, 106683. [Google Scholar] [CrossRef]
- Kertesz, M.A.; Kölbener, P.; Stockinger, H.; Beil, S.; Cook, A.M. Desulfonation of Linear Alkylbenzenesulfonate Surfactants and Related Compounds by Bacteria. Appl. Environ. Microb. 1994, 60, 2296–2303. [Google Scholar] [CrossRef]
- Piotrowska-Długosz, A. Significance of Enzymes and Their Application in Agriculture. In Biocatalysis, Enzymatic Basics and Applications; Husain, Q., Ullath, M.F., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 277–308. [Google Scholar]
- Jadán, O.; Torres, B.; Günter, S. Influencia Del Uso de La Tierra Sobre Almacenamiento de Carbono En Sistemas Productivos y Bosque Primario En Napo, Reserva de Biosfera Sumaco, Ecuador. Cienc. Tecnol. 2012, 1, 173–186. [Google Scholar] [CrossRef]
- Torres, B.; Andrade, A.K.; Enriquez, F.; Luna, M.; Heredia-R, M.; Bravo, C. Estudios Sobre Medios de Vida, Sostenibilidad y Captura de Carbono En El Sistema Agroforestal Chakra Con Cacao En Comunidades de Pueblos Originarios de La Provincia de Napo: Casos de Las Asociaciones Kallari, Wiñak y Tsatsayaku, Amazonía Ecuatoriana; FAO: Quito, Ecuador, 2022; ISBN 9789942422118. [Google Scholar]
- Truelove, B. Research Methods in Weed Science. In Souther Weed Science Society, 2nd ed.; Auburn, AL, USA; 1977; Available online: https://openlibrary.org/books/OL4916817M/Research_methods_in_weed_science (accessed on 7 April 2025).
- Bravo, C.F.A. Nivel de Cobertura, Conservación de Suelos y Aguasbajo Diferentes Sistemas de Labranza. Rev. Fac. Agron. 1999, 25, 57–74. [Google Scholar]
- Bravo-Medina, C.; Lozano, Z. Evaluación de La Calidad de Los Suelos y Salud de Los Cultivos; Universidad Estatal Amazónica de Posgrado y Educación Continúa: Puyo, Ecuador, 2016. [Google Scholar]
- López-Santiago, J.G.; Casanova-Lugo, F.; Villanueva-López, G.; Díaz-Echeverría, V.F.; Solorio-Sánchez, F.J.; Martínez-Zurimendi, P.; Aryal, D.R.; Chay-Canul, A.J. Carbon Storage in a Silvopastoral System Compared to That in a Deciduous Dry Forest in Michoacán, Mexico. Agrofor. Syst. 2019, 93, 199–211. [Google Scholar] [CrossRef]
- Dantas, D.; Terra, M.d.C.N.S.; Pinto, L.O.R.; Calegario, N.; Maciel, S.M. Above and Belowground Carbon Stock in a Tropical Forest in Brazil. Acta Sci. Agron. 2020, 43, e48276. [Google Scholar] [CrossRef]
- Aryal, D.R.; Gómez-González, R.R.; Hernández-Nuriasmú, R.; Morales-Ruiz, D.E. Carbon Stocks and Tree Diversity in Scattered Tree Silvopastoral Systems in Chiapas, Mexico. Agrofor. Syst. 2019, 93, 213–227. [Google Scholar] [CrossRef]
- Caguana-Muyolema, J.A.; Román-Cáceres, D.A.; Cevallos-Rodríguez, J.P.; Roman-Robalino, D.A. Estudio Florístico En El Ecosistema Páramo de La Quebrada Galgalán, comunidad de Atillo. Polo Conoc. 2020, 5, 1020–1042. [Google Scholar] [CrossRef]
- Alberto Mora Donjuán, C.; Alanís Rodríguez, E.; Jiménez Pérez, J.; Aurelio González Tagle, M.; Israel Yerena Yamallel Luis Gerardo Cuellar Rodríguez, J. Estructura, Composición Florística y Diversidad Del Matorral Espinoso Tamaulipeco, México. Ecol. Apl. 2013, 12, 29–34. [Google Scholar] [CrossRef]
- Gallese, F.; Gismero-Rodriguez, L.; Govednik, A.; Giagnoni, L.; Lumini, E.; Suhadolc, M.; Vaccari, F.P.; Maienza, A. Soil Microarthropods as Tools for Monitoring Soil Quality: The QBS-Ar Index in Three European Agroecosystems. Agriculture 2025, 15, 89. [Google Scholar] [CrossRef]
- Saxena, K.G.; Rao, K.S. Soil Biodiversity: Inventory, Functions and Management; Bishen Singh Mahendra Pal Singh: Dehradun, India, 2016; ISBN 9788121106979. [Google Scholar]
- Gerlach, J.; Samways, M.; Pryke, J. Terrestrial Invertebrates as Bioindicators: An Overview of Available Taxonomic Groups. J. Insect Conserv. 2013, 17, 831–850. [Google Scholar] [CrossRef]
- Quiroz Medina, C.R.; Castellón, J.D.; Cea Navas, N.E.; Ortiz, M.S.; Zuniga-Gonzalez, C.A. Caracterización de La Macrofauna Edáfica En Diferentes Sistemas Agroforestales, En El Municipio de San Ramón, Departamento de Matagalpa, Nicaragua. Nexo Rev. Cient. 2021, 34, 572–582. [Google Scholar] [CrossRef]
- Hernández-Hernández, R.M.; Ramírez, E.; Castro, I.; Cano, S. Cambios En Indicadores de Calidad de Suelos de Ladera Reforestados Con Pinos (Pinus caribaea) y eucaliptos (Eucalyptus robusta). Agrociencia 2008, 42, 253–266. [Google Scholar]
- Nelson, D.W.; Sommers, L.E. Total Carbon, Organic Carbon, and Organic Matter 1. In Methods of Soil Analysis. Part 2. Chemical and Microbiological Properties-Agronomy Monograph No. 9; West Lafayette, IN, USA; Madison, WI, USA, 1982; pp. 539–579. Available online: https://acsess.onlinelibrary.wiley.com/doi/10.2134/agronmonogr9.2.2ed.c29 (accessed on 7 April 2025).
- Pla, I. Medición y Evaluación de Propiedades Fisicas de Los Suelos: Dificultades y Errores Más Frecuentes. I-Propiedades Mecánicas. Suelos Ecuat. 2011, 40, 75–93. [Google Scholar]
- Blake, G.R.; Hartge, K.H. Bulk Density. In Methods of Soil Analysis: Part 1—Physical and Mineralogical Methods; Klute, A., Ed.; SSSA Book Ser. 5.1; ASA-SSSA: Madison, WI, USA, 1986; pp. 363–375. [Google Scholar] [CrossRef]
- López-Piñeiro, A.; Albarrán, A.; Rato Nunes, J.M.; Peña, D.; Cabrera, D. Long-Term Impacts of de-Oiled Two-Phase Olive Mill Waste on Soil Chemical Properties, Enzyme Activities and Productivity in an Olive Grove. Soil Tillage Res. 2011, 114, 175–182. [Google Scholar] [CrossRef]
- Fernández-Rodríguez, D.; Fangueiro, D.P.; Abades, D.P.; Albarrán, Á.; Rato-Nunes, J.M.; López-Piñeiro, A. Direct and Residual Impacts of Olive-Mill Waste Application to Rice Soil on Greenhouse Gas Emission and Global Warming Potential under Mediterranean Conditions. Agronomy 2022, 12, 1344. [Google Scholar] [CrossRef]
- Zhao, H.; Chen, W.; Zhang, C.; Kang, D. Rapid Estimation of Seismic Intensities by Analyzing Early Aftershock Sequences Using the Robust Locally Weighted Regression Program (LOWESS). Nat. Hazards Earth Syst. Sci. 2023, 23, 3031–3050. [Google Scholar] [CrossRef]
- Visscher, A.M.; Meli, P.; Fonte, S.J.; Bonari, G.; Zerbe, S.; Wellstein, C. Agroforestry Enhances Biological Activity, Diversity and Soil-Based Ecosystem Functions in Mountain Agroecosystems of Latin America: A Meta-Analysis. Glob. Chang. Biol. 2024, 30, e17036. [Google Scholar] [CrossRef]
- Carneiro, R.G.; Figueiredo, C.C.d.; Malaquias, J.V.; Mendes, I.C. A Soil Health Assessment Tool for Vegetable Cropping Systems in Tropical Soils Based on Beta-Glucosidase, Arylsulfatase, and Soil Organic Carbon. Appl. Soil Ecol. 2024, 198, 105394. [Google Scholar] [CrossRef]
- de Vasconcelos, W.L.F.; Rodrigues, D.d.M.; Silva, R.O.C.; Alfaia, S.S. Diversity and Abundance of Soil Macrofauna in Three Land Use Systems in Eastern Amazonia. Rev. Bras. Cienc. Solo 2020, 44, e0190136. [Google Scholar] [CrossRef]
- Kuzyakov, Y.; Blagodatskaya, E. Microbial Hotspots and Hot Moments in Soil: Concept & Review. Soil Biol. Biochem. 2015, 83, 184–199. [Google Scholar] [CrossRef]
- Blagodatskaya, E.; Kuzyakov, Y. Active Microorganisms in Soil: Critical Review of Estimation Criteria and Approaches. Soil Biol. Biochem. 2013, 67, 192–211. [Google Scholar] [CrossRef]
- Zhang, S.; Li, H.; Yang, X.; Sun, B. Effects of Soil Management Regimes on Biochemical Properties of a Loess Soil. J. Soil Sci. Plant Nutr. 2015, 15, 711–725. [Google Scholar] [CrossRef]
- Ullah Khan, F.; Zaman, F.; Qu, Y.; Wang, J.; Darmorakhtievich, O.H.; Wu, Q.; Fahad, S.; Du, F.; Xu, X. Long-Term Effects of Crop Treatments and Fertilization on Soil Stability and Nutrient Dynamics in the Loess Plateau: Implications for Soil Health and Productivity. Sustainability 2025, 17, 14. [Google Scholar] [CrossRef]
- Davidson, E.A.; Janssens, I.A. Temperature Sensitivity of Soil Carbon Decomposition and Feedbacks to Climate Change. Nature 2006, 440, 165–173. [Google Scholar] [CrossRef]
- Shibu, J. Agroforestry for Ecosystem Services and Environmental Benefits: An Overview. Agrofor. Syst. 2009, 76, 1–10. [Google Scholar]
- Montagnini, F.; Nair, P.K.R. Carbon Sequestration: An Underexploited Environmental Benefit of Agroforestry Systems. In New Vistas in Agroforestry: A Compendium for 1st World Congress of Agroforestry; Springer: Berlin/Heidelberg, Germany, 2004; pp. 281–295. [Google Scholar]
- Cardinael, R.; Chevallier, T.; Barthès, B.G.; Saby, N.P.A.; Parent, T.; Dupraz, C.; Bernoux, M.; Chenu, C. Impact of Alley Cropping Agroforestry on Stocks, Forms and Spatial Distribution of Soil Organic Carbon—A Case Study in a Mediterranean Context. Geoderma 2015, 259–260, 288–299. [Google Scholar] [CrossRef]
- Nair, P.K.R.; Kumar, B.M.; Nair, V.D. Agroforestry as a Strategy for Carbon Sequestration. J. Plant Nutr. Soil Sci. 2009, 172, 10–23. [Google Scholar] [CrossRef]
| Parameter | Equation | Source | |
|---|---|---|---|
| Soil organic carbon (SOC) | [37,42,43] | (1) | |
| Litter carbon (LC) | [44] | (2) | |
| Simpson index (S) | [44,45] | (3) | |
| Shannon Wiener index (H’) | [46] | (4) |
| Functional Group | Taxa | Order | Family | Subfamily | Number Individuals |
|---|---|---|---|---|---|
| Soil engineers | Oligochaeta | Crassiclitellata | Lumbricidae | --- | 361 |
| Hexapoda | Blattodea | Termitidae | --- | 216 | |
| Hymenoptera | Formicidae | Amblyoponinae | 77 | ||
| Dolichodeninae | 23 | ||||
| Dorilynae | 10 | ||||
| Formicinae | 66 | ||||
| Myrmicinae | 310 | ||||
| Ponerinae | 43 | ||||
| Detritivores | Crustacea | Isopoda | Armadillidae | --- | 16 |
| Gastropoda | Ellobiida | Ellobiidae | Pedipedinae | 9 | |
| Hexapoda | Coleoptera (larva) | --- | --- | 61 | |
| Depredators | Myriapoda | Chilopoda | Geophilomorpha | Geophilidae | 14 |
| Chelicerata | Araneae | --- | --- | 2 | |
| Herbivores | Hexapoda | Orthoptera | Gryllotalpidae | --- | 5 |
| Hemiptera | Cicadidae | --- | 1 | ||
| Omnivores | Hexapoda | Blattodea | Blattidae | --- | 6 |
| Dermaptera | Carcinophoridae | --- | 11 | ||
| Total | 1231 | ||||
| Functional Group | Taxa | Order | Family | Subfamily | Number Individuals |
|---|---|---|---|---|---|
| Soil engineers | Hexapoda | Hymenoptera | Formicidae | Ectatominae | 43 |
| Amblyoponinae | 56 | ||||
| Dolichodeninae | 14 | ||||
| Dorilynae | 42 | ||||
| Formicinae | 101 | ||||
| Myrmicinae | 272 | ||||
| Blattodea | Termitidae | --- | 76 | ||
| Detritivores | Crustacea | Isopoda | Armadillidae | --- | 59 |
| Hexapoda | Collembola | Entomobryomorpha | --- | 1285 | |
| Poduromorpha | --- | 1354 | |||
| Coleoptera (larva) | --- | --- | 118 | ||
| Depredators | Chelicerata | Araneae | --- | --- | 39 |
| Pseudoescorpion | --- | --- | 21 | ||
| Saprophages | Myriapoda | Simphyla | Scolopendrellidae | --- | 33 |
| Hexapoda | Diplura | Endognatha | --- | 47 | |
| Protura | Eosentomidae | --- | 21 | ||
| Chelicerata | Acari | --- | --- | 1576 | |
| Omnivores | Hexapoda | Dermaptera | Carcinophoridae | --- | 38 |
| Herbivores | Thysanoptera | Aeolothripidae | --- | 24 | |
| Total | 5219 | ||||
| Method | Index | Types of Land Use | ANOVA 1 p-Value | |||
|---|---|---|---|---|---|---|
| CMC | FAFS | TAFS | Forest | |||
| Manual techniques | Ab10cm | 31.0 (±21.4) | 26.0 (±18.6) | 18.5 (±7.37) | 30.0 (±21.0) | n/s |
| Ab20cm | 10.9 (±10.7) | 11.2 (±7.93) | 14.9 (±21.6) | 10.0 (±8.89) | n/s | |
| H’10cm | 0.95 (±0.43) | 0.94 (±0.39) | 0.92 (±0.40) | 0.99 (±0.22) | n/s | |
| H’20cm | 0.87 (±0.38) | 0.93 (±0.24) | 0.63 (±0.30) | 0.86 (±0.58) | n/s | |
| S10cm | 0.53 (±0.20) | 0.52 (±0.18) | 0.49 (±0.18) | 0.53 (±0.17) | n/s | |
| S20cm | 0.50 (±0.19) | 0.54 (±0.10) | 0.39 (±0.19) | 0.49 (±0.34) | n/s | |
| Berlese | AbLitter | 95.0 (±38.4) | 81.7 (±34.5) | 93.8 (±33.5) | 99.0 (±54.7) | n/s |
| AbSoil | 67.3 b (±33.6) | 69.4 b (±21.1) | 76.6 b (±14.7) | 143 a (±24.0) | *** | |
| H’Litter | 1.57 b (±0.23) | 1.50 b (±0.34) | 1.58 b (±0.22) | 2.01 a (±0.12) | * | |
| H’Soil | 1.64 ab (±0.10) | 1.56 b (±0.31) | 1.56 b (±0.09) | 1.87 a (±0.10) | * | |
| SLitter | 0.74 (±0.08) | 0.72 (±0.08) | 0.74 (±0.06) | 0.84 (±0.02) | n/s | |
| SSoil | 0.75 (±0.03) | 0.75 (±0.06) | 0.74 (±0.03) | 0.80 (±0.03) | n/s | |
| Parameters | Types of Land Use | ANOVA 1 p-Value | |||
|---|---|---|---|---|---|
| CMC | FAFS | TAFS | Forest | ||
| ER (mg CO2 m2 ha−1) | 173 b (±39.2) | 170 b (±29.8) | 174 b (±28.3) | 240 a (±64.3) | * |
| ER (mg C-CO2 m2 ha−1) | 47.2 b (±10.7) | 46.3 b (±8.10) | 47.5 b (±7.73) | 65.5 a (±17.5) | * |
| BR (mg CO2 kg−1 soil d−1) | 711 (±398) | 508 (±502) | 499 (±343) | 389 (±371) | n/s |
| BR (mg C-CO2 kg−1 soil d−1) | 194 (±108) | 139 (±137) | 136 (±93.6) | 106 (±101) | n/s |
| SOM10cm (%) | 19.8 a (±3.88) | 9.10 b (±6.71) | 14.0 ab (±7.81) | 8.49 b (±0.38) | ** |
| SOM20cm (%) | 12.0 a (±1.22) | 5.33 b (±2.90) | 8.73 ab (±4.80) | 3.94 b (±0.26) | *** |
| TOC10cm (%) | 11.5 a (±2.25) | 5.28 b (±3.89) | 8.13 ab (±4.53) | 4.92 b (±0.22) | ** |
| TOC20cm (%) | 6.99 a (±0.71) | 3.09 b (±1.68) | 5.07 ab (±2.79) | 2.28 b (±0.15) | *** |
| Litter (Mg ha−1) | 7.33 (±5.73) | 6.37 (±2.38) | 10.1 (±4.75) | 10.4 (±0.74) | n/s |
| UR (µg NH4+/g−1 ha−1) | 298 a (±65.1) | 190 a (±94.0) | 293 a (±109) | 280 a (±52.2) | * |
| SU (µg pNP/g−1 ha−1) | 159 a (±39.4) | 114 b (±17.2) | 153 ab (±21.9) | 114 b (±9.81) | *** |
| PHO (µmol pNP/g−1 ha−1) | 3.53 a (±1.10) | 3.62 a (±1.46) | 4.66 a (±1.17) | 5.27 a (±1.03) | * |
| GL (µmol pNP/g−1 ha−1) | 0.58 (±0.08) | 0.54 (±0.13) | 0.53 (±0.14) | 0.54 (±0.05) | n/s |
| DH (µg INTF/g−1 ha−1) | 0.47 (±0.14) | 0.45 (±0.14) | 0.49 (±0.31) | 0.17 (±0.26) | n/s |
| Variables | ER | BR | SOM10cm | SOM20cm | Litter | UR | SU | PHO | GL | DH | H’10cm | S10cm | H’Litter | H’Soil | SLitter | SSoil |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ER | 1 | −0.11 | −0.08 | −0.10 | 0.08 | 0.10 | −0.08 | 0.19 | −0.03 | −0.208 | −0.12 | −0.16 | 0.26 | 0.31 | 0.19 | 0.345 |
| BR | 1 | −0.08 | −0.01 | −0.02 | 0.06 | 0.16 | 0.003 | −0.17 | −0.134 | 0.15 | 0.09 | −0.39 * | −0.34 * | −0.34 | −0.30 | |
| SOM10cm | 1 | 0.927 ** | −0.24 | 0.69 ** | 0.37 * | 0.18 | 0.53 ** | 0.383 * | −0.004 | −0.03 | 0.08 | 0.30 | 0.08 | 0.18 | ||
| SOM20cm | 1 | −0.27 | 0.77 ** | 0.52 ** | 0.21 | 0.55 ** | 0.487 ** | −0.08 | −0.08 | 0.02 | 0.15 | 0.06 | 0.07 | |||
| Litter | 1 | −0.17 | 0.10 | −0.22 | −0.48 ** | −0.39 * | −0.15 | −0.18 | 0.10 | −0.06 | 0.083 | −0.04 | ||||
| UR | 1 | 0.53 ** | 0.63 ** | 0.57 ** | 0.366 * | −0.06 | −0.07 | 0.16 | 0.33 | 0.18 | 0.24 | |||||
| SU | 1 | 0.31 | 0.38 * | 0.331 | −0.15 | −0.13 | −0.04 | 0.04 | 0.10 | −0.06 | ||||||
| PHO | 1 | 0.51 ** | 0.246 | −0.02 | −0.06 | 0.16 | 0.39 * | 0.20 | 0.30 | |||||||
| GL | 1 | 0.51 ** | −0.002 | 0.03 | 0.02 | 0.31 | 0.09 | 0.22 | ||||||||
| DH | 1 | 0.09 | 0.14 | −0.02 | −0.003 | 0.01 | 0.11 | |||||||||
| H’10cm | 1 | 0.96 ** | 0.03 | 0.18 | −0.10 | 0.12 | ||||||||||
| S10cm | 1 | 0.02 | 0.14 | −0.11 | 0.12 | |||||||||||
| H’Litter | 1 | 0.49 ** | 0.93 ** | 0.46 ** | ||||||||||||
| H’Soil | 1 | 0.39 * | 0.87 ** | |||||||||||||
| SLitter | 1 | 0.37 * | ||||||||||||||
| SSoil | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huera-Lucero, T.; Torres, B.; Bravo-Medina, C.; García-Nogales, B.; Vicente, L.; López-Piñeiro, A. Comparative Analysis of Soil Biological Activity and Macroinvertebrate Diversity in Amazonian Chakra Agroforestry and Tropical Rainforests in Ecuador. Agriculture 2025, 15, 830. https://doi.org/10.3390/agriculture15080830
Huera-Lucero T, Torres B, Bravo-Medina C, García-Nogales B, Vicente L, López-Piñeiro A. Comparative Analysis of Soil Biological Activity and Macroinvertebrate Diversity in Amazonian Chakra Agroforestry and Tropical Rainforests in Ecuador. Agriculture. 2025; 15(8):830. https://doi.org/10.3390/agriculture15080830
Chicago/Turabian StyleHuera-Lucero, Thony, Bolier Torres, Carlos Bravo-Medina, Beatriz García-Nogales, Luis Vicente, and Antonio López-Piñeiro. 2025. "Comparative Analysis of Soil Biological Activity and Macroinvertebrate Diversity in Amazonian Chakra Agroforestry and Tropical Rainforests in Ecuador" Agriculture 15, no. 8: 830. https://doi.org/10.3390/agriculture15080830
APA StyleHuera-Lucero, T., Torres, B., Bravo-Medina, C., García-Nogales, B., Vicente, L., & López-Piñeiro, A. (2025). Comparative Analysis of Soil Biological Activity and Macroinvertebrate Diversity in Amazonian Chakra Agroforestry and Tropical Rainforests in Ecuador. Agriculture, 15(8), 830. https://doi.org/10.3390/agriculture15080830








