Smart Farming Technologies for Sustainable Agriculture: A Case Study of a Mediterranean Aromatic Farm
Abstract
1. Introduction
2. Materials and Methods
2.1. Description of the Site
2.2. Field Data Collection
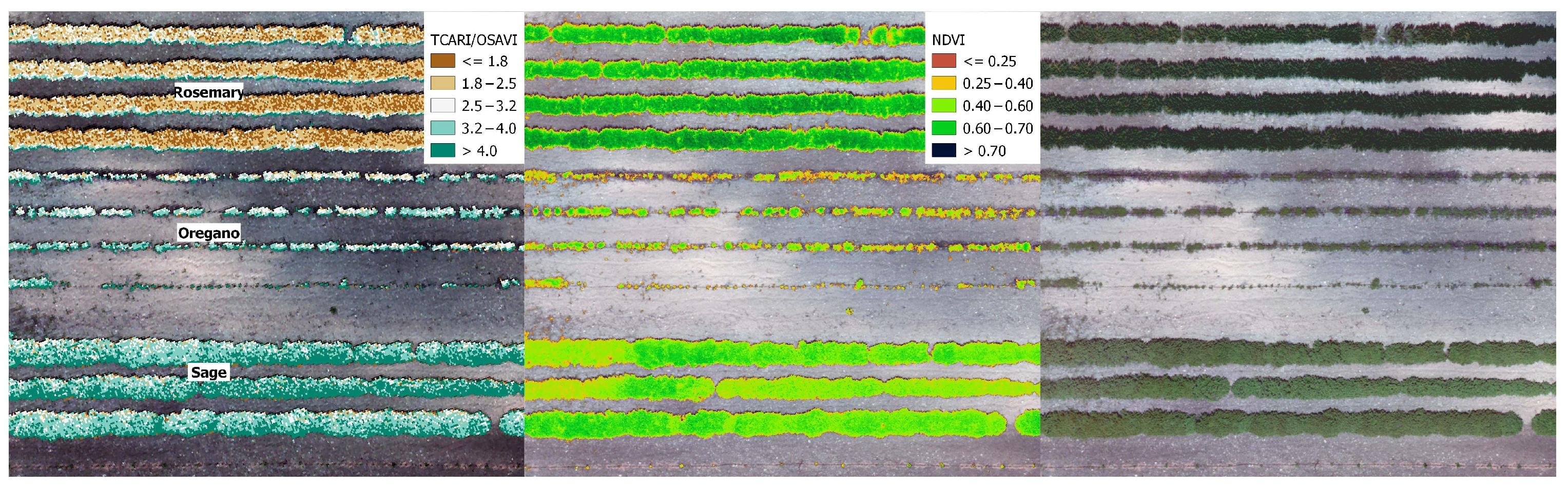
2.3. Spectral Vegetation Indices
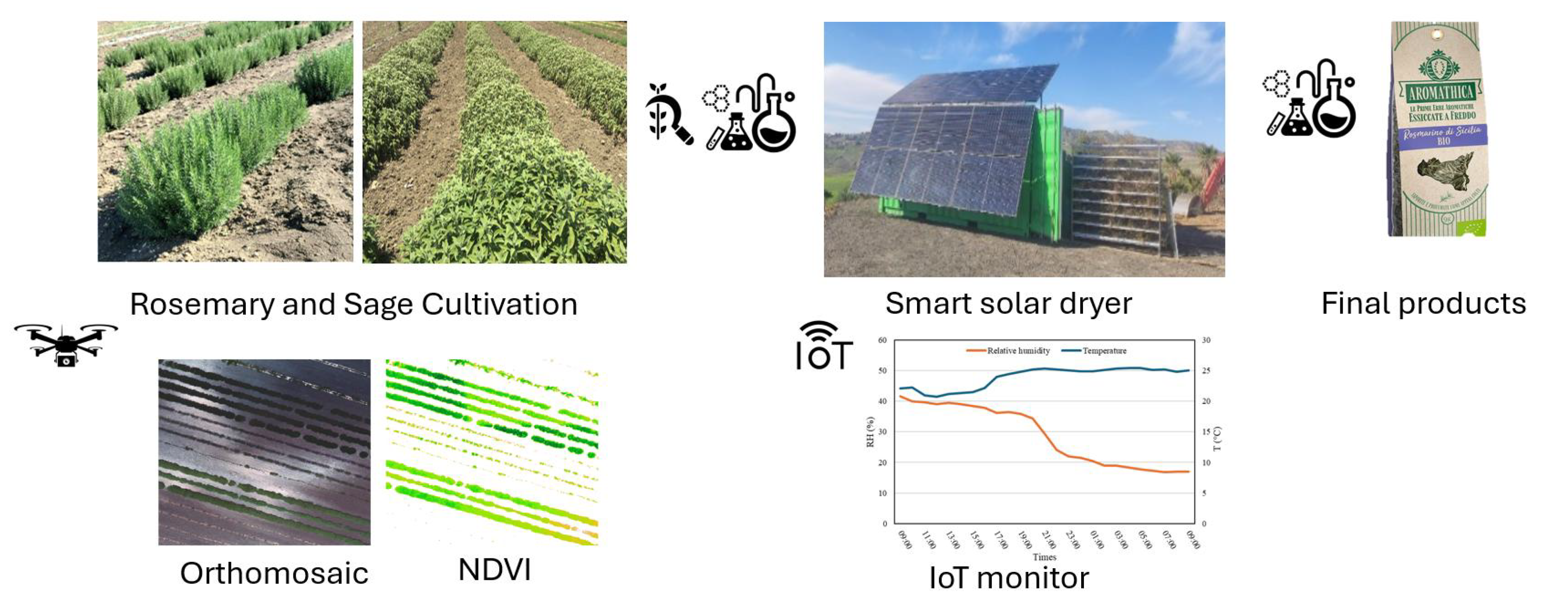
2.4. Rosemary and Sage Harvesting Time Individuation, Collecting, and Drying
2.5. A Smart Solar Dryer (SSD) WSN-Based System
2.6. Hygienic and Safety Aspects of Rosemary and Sage
2.7. Statistical Analysis
3. Results and Discussion
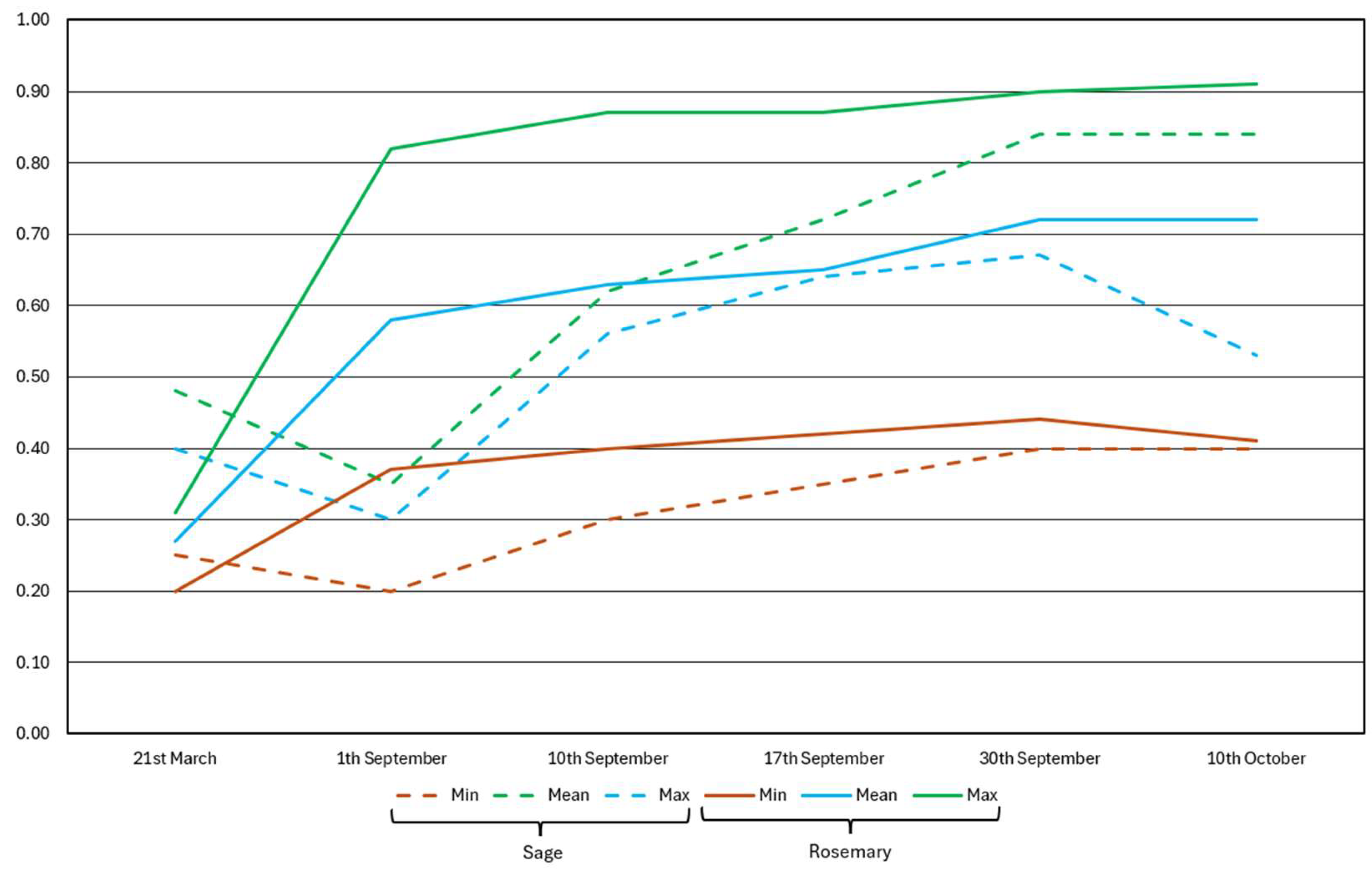
3.1. Rosemary and Sage Harvesting Time
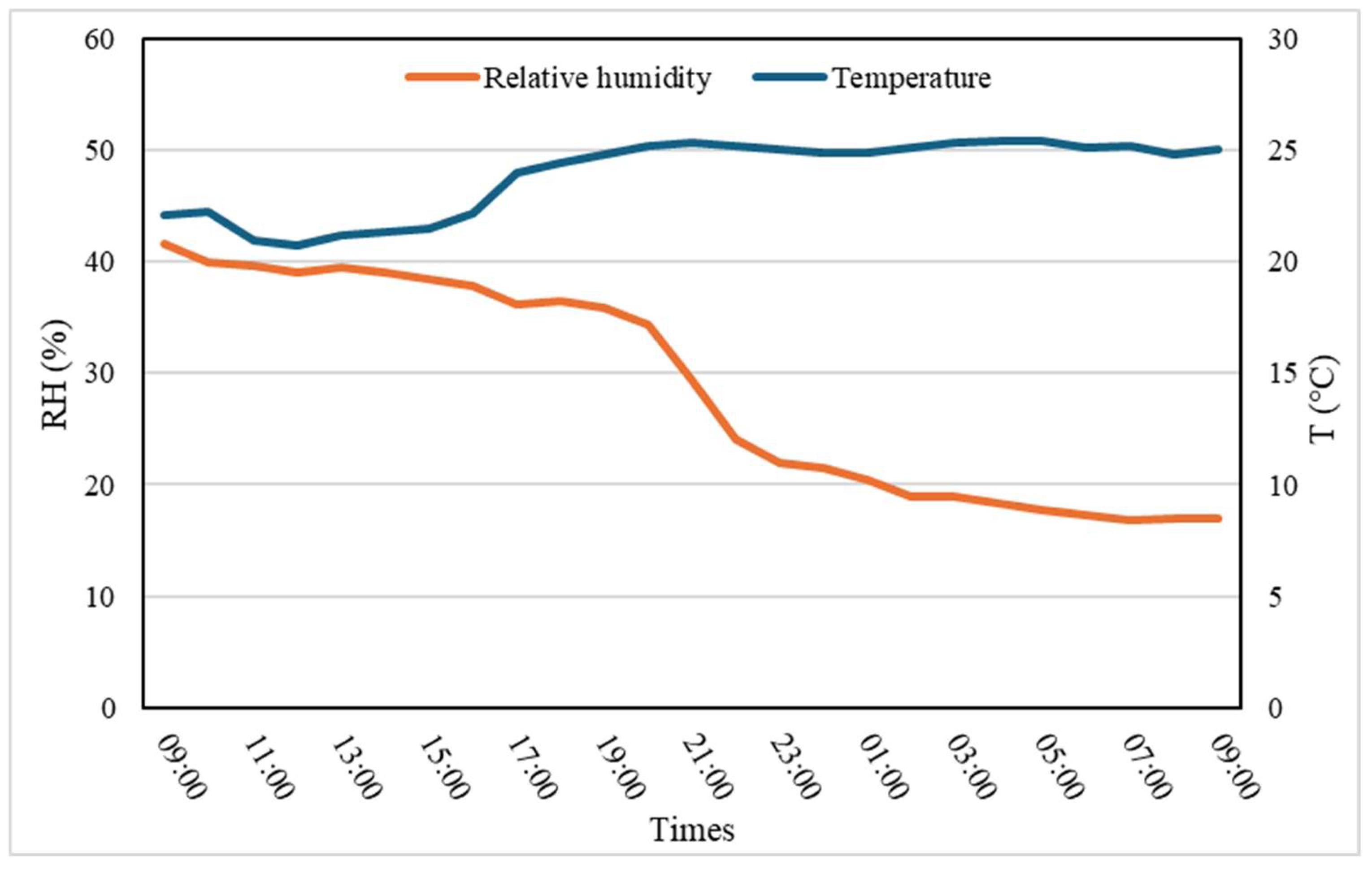
3.2. Drying Process Results
3.3. Safety Criteria for Foodstuffs
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Konfo, T.R.C.; Chabi, A.B.P.; Gero, A.A.; Lagnika, C.; Avlessi, F.; Biaou, G.; Sohounhloue, C.K.D. Recent climate-smart innovations in agrifood to enhance producer incomes through sustainable solutions. J. Agric. Food Res. 2024, 15, 100985. [Google Scholar] [CrossRef]
- Greco, C.; Comparetti, A.; Febo, P.; La Placa, G.; Mammano, M.M.; Orlando, S. Sustainable Valorisation of Biowaste for Soilless Cultivation of Salvia Officinalis in a Circular Bioeconomy. Agronomy 2020, 10, 1158. [Google Scholar] [CrossRef]
- Orlando, S.; Greco, C.; Tuttolomondo, T.; Leto, C.; Cammalleri, I.; La Bella, S. Identification of Energy Hubs for the Exploitation of Residual Biomass in an Area of Western Sicily. In Proceedings of the EUBCE 2017 Online Conference Proceedings, Stockholm, Sweden, 12–15 June 2017; pp. 64–69. [Google Scholar]
- Comparetti, A.; Febo, P.; Greco, C.; Navickas, K.; Nekrosius, A.; Orlando, S.; Venslauskas, K. Assessment of organic waste management methods through energy balance. Am. J. Appl. Sci. 2014, 11, 1631–1644. [Google Scholar] [CrossRef]
- Choruma, D.J.; Dirwai, T.L.; Mutenje, M.J.; Mustafa, M.; Chimonyo, V.G.P.; Jacobs-Mata, I.; Mabhaudhi, T. Digitalisation in agriculture: A scoping review of technologies in practice, challenges, and opportunities for smallholder farmers in sub-saharan Africa. J. Agric. Food Res. 2024, 18, 101286. [Google Scholar] [CrossRef]
- Rakholia, R.; Tailor, J.; Prajapati, M.; Shah, M.; Saini, J.R. Emerging technology adoption for sustainable agriculture in India—A pilot study. J. Agric. Food Res. 2024, 17, 101238. [Google Scholar] [CrossRef]
- Rajak, P.; Ganguly, A.; Adhikary, S.; Bhattacharya, S. Internet of Things and smart sensors in agriculture: Scopes and challenges. J. Agric. Food Res. 2023, 14, 100776. [Google Scholar] [CrossRef]
- Rodríguez, J.P.; Montoya-Munoz, A.I.; Rodriguez-Pabon, C.; Hoyos, J.; Corrales, J.C. IoT-Agro: A smart farming system to Colombian coffee farms. Comput. Electron. Agric. 2021, 190, 106442. [Google Scholar] [CrossRef]
- Kwaghtyo, D.K.; Eke, C.I. Smart farming prediction models for precision agriculture: A comprehensive survey. Artif. Intell. Rev. 2023, 56, 5729–5772. [Google Scholar] [CrossRef]
- Lykas, C.; Vagelas, I. Innovations in Agriculture for Sustainable Agro-Systems. Agronomy 2023, 13, 2309. [Google Scholar] [CrossRef]
- Taoufik, B.; Rhaimi, C.; Alomari, S.; Aljuhani, L. Design and Implementation of an Integrated IoT and Artificial Intelligence System for Smart Irrigation Management. Int. J. Adv. Soft Comput. Appl. 2024, 16, 197–218. [Google Scholar]
- Muhie, S.H. Novel approaches and practices to sustainable agriculture. J. Agric. Food Res. 2022, 10, 100446. [Google Scholar] [CrossRef]
- Lytos, A.; Lagkas, T.; Sarigiannidis, P.; Zervakis, M.; Livanos, G. Towards smart farming: Systems, frameworks and exploitation of multiple sources. Comput. Netw. 2020, 172, 107147. [Google Scholar] [CrossRef]
- Széles, A.; Huzsvai, L.; Mohammed, S.; Nyéki, A.; Zagyi, P.; Horváth, E.; Simon, K.; Arshad, S.; Tamás, A. Precision agricultural technology for advanced monitoring of maize yield under different fertilization and irrigation regimes: A case study in Eastern Hungary (Debrecen). J. Agric. Food Res. 2024, 15, 100967. [Google Scholar] [CrossRef]
- Javaid, M.; Haleem, A.; Singh, R.P.; Suman, R. Enhancing smart farming through the applications of Agriculture 4.0 technologies. Int. J. Intell. Netw. 2022, 3, 150–164. [Google Scholar] [CrossRef]
- Greco, C.; Catania, P.; Orlando, S.; Vallone, M.; Mammano, M.M. Assessment of Vegetation Indices as Tool to Decision Support System for Aromatic Crops. In Safety, Health and Welfare in Agriculture and Agro-Food Systems. SHWA 2023; Berruto, R., Biocca, M., Cavallo, E., Cecchini, M., Failla, S., Romano, E., Eds.; Lecture Notes in Civil Engineering; Springer: Cham, Switzerland, 2024; Volume 521. [Google Scholar] [CrossRef]
- Avola, G.; Di Gennaro, S.F.; Cantini, C.; Riggi, E.; Muratore, F.; Tornambè, C.; Matese, A. Remotely sensed vegetation indices to discriminate field-grown olive cultivars. Remote Sens. 2019, 11, 1242. [Google Scholar] [CrossRef]
- Deng, L.; Mao, Z.; Li, X.; Hu, Z.; Duan, F.; Yan, Y. UAV-based multispectral remote sensing for precision agriculture: A comparison between different cameras. ISPRS J. Photogramm. Remote Sens. 2018, 146, 124–136. [Google Scholar] [CrossRef]
- Jensen, J.R. Remote Sensing of the Environment: An Earth Resource Perspective, 2nd ed.; Pearson Education: Bengaluru, India, 2009. [Google Scholar]
- Pagliai, A.; Ammoniaci, M.; Sarri, D.; Lisci, R.; Perria, R.; Vieri, M.; D’arcangelo, M.E.M.; Storchi, P.; Kartsiotis, S.-P. Comparison of aerial and ground 3D point clouds for canopy size assessment in precision viticulture. Remote Sens. 2022, 14, 1145. [Google Scholar] [CrossRef]
- Lu, B.; Dao, P.D.; Liu, J.; He, Y.; Shang, J. Recent advances of hyperspectral imaging technology and applications in agriculture. Remote Sens. 2020, 12, 2659. [Google Scholar] [CrossRef]
- Wu, C.; Niu, Z.; Tang, Q.; Huang, W. Estimating Chlorophyll Content from Hyperspectral Vegetation Indices: Modeling and Validation. Agric. For. Meteorol. 2008, 148, 1230–1241. [Google Scholar] [CrossRef]
- Hunt, E.R., Jr.; Daughtry, C.S.T.; Eitel, J.U.H.; Long, D.S. Remote Sensing Leaf Chlorophyll Content Using a Visible Band Index. Agron. J. 2011, 103, 1090–1099. [Google Scholar] [CrossRef]
- Greco, C.; Agnello, A.; La Placa, G.; Mammano, M.M.; Navickas, K. Biowaste in a circular bioeconomy in Mediterranean area: A case study of compost and vermicompost as growing substrates alternative to peat. Riv. Studi Sulla Sostenibilità 2019, 2, 345–362. [Google Scholar] [CrossRef]
- Comparetti, A.; Greco, C.; Orlando, S.; Ciulla, S.; Mammano, M.M. Comparison of mechanical, assisted and manual harvest of Origanum vulgare L. Sustain. For. 2022, 14, 2562. [Google Scholar] [CrossRef]
- Comparetti, A.; Greco, C.; Mammano, M.M.; Navickas, K.; Orlando, S.; Venslauskas, K. Valorisation of urban green areas for producing renewable energy and biochar as growing substrate of Sicilian aromatic and nutraceutical species in a circular economy. Riv. Studi Sulla Sostenibilita 2019, 2 (Suppl. S2), 299–314. [Google Scholar] [CrossRef]
- Greco, C.; Comparetti, A.; Fascella, G.; Febo, P.; La Placa, G.; Saiano, F.; Mammano, M.M.; Orlando, S.; Laudicina, V.A. Effects of Vermicompost, Compost and Digestate as Commercial Alternative Peat-Based Substrates on Qualitative Parameters of Salvia officinalis. Agronomy 2021, 11, 98. [Google Scholar] [CrossRef]
- Virk, A.L.; Noor, M.A.; Fiaz, S.; Hussain, S.; Hussain, H.A.; Rehman, M.; Ahsan, M.; Ma, W. Smart farming: An overview. In Smart Village Technology; Springer: Berlin/Heidelberg, Germany, 2020; pp. 191–201. [Google Scholar]
- Mesgaran, M.B.; Madani, K.; Hashemi, H.; Azadi, P. Iran’s land suitability for agriculture. Sci. Rep. 2017, 7, 1–12. [Google Scholar] [CrossRef]
- Balafoutis, A.T.; Beck, B.; Fountas, S.; Tsiropoulos, Z.; Vangeyte, J.; van der Wal, T.; Soto-Embodas, I.; Gómez-Barbero, M.; Pedersen, S.M. Smart Farming Technologies—Description, Taxonomy and Economic Impact; Springer: Berlin/Heidelberg, Germany, 2017; pp. 21–77. ISBN 978-3-319-68713-1. [Google Scholar] [CrossRef]
- Abhinav, S.; Jain, A.; Gupta, P.; Chowdary, V. Machine learning applications for precision agriculture: A comprehensive review. IEEE Access 2021, 9, 4843–4873. [Google Scholar] [CrossRef]
- Petrović, B.; Bumbálek, R.; Zoubek, T.; Kuneš, R.; Smutný, L.; Bartoš, P. Application of precision agriculture technologies in Central Europe-review. J. Agric. Food Res. 2024, 15, 101048. [Google Scholar] [CrossRef]
- Campiotti, C.A.; Bibbiani, C.; Greco, C. Renewable energy for greenhouse agriculture. J. Sustain. Energy 2019, 20, 152–156. [Google Scholar]
- Greco, C.; Campiotti, A.; De Rossi, P.; Febo, P.; Giagnacovo, G. Energy consumption and improvement of energy efficiency for the European agricultural-food system. Riv. Studi Sulla Sostenibilità 2020, 2020, 92–103. [Google Scholar] [CrossRef]
- Greco, C.; Campiotti, A.; Latini, A.; Agnello, A.; Jotautiene, E.; Mammano, M.M. Innovation for the Italian agricultural and food industry sector. Riv. Studi Sulla Sostenibilità 2019, 2019, 115–126. [Google Scholar] [CrossRef]
- Attard, G.; Comparetti, A.; Febo, P.; Greco, C.; Massimo Mammano, M.; Orlando, S. Case study of potential production of renewable energy sources (RES) from livestock wastes in Mediterranean islands. Chem. Eng. Trans. 2017, 58, 1–7. [Google Scholar]
- Kimaro, D.; Nyangarika, A.; Kivevele, T. Uncovering socioeconomic insights of solar dryers for sustainable agricultural product preservation: A systematic review. Heliyon 2024, 10, e40726. [Google Scholar] [CrossRef] [PubMed]
- Ndukwu, M.C.; Simo-Tagne, M.; Bennamoun, L. Solar drying research of medicinal and aromatic plants: An African experience with assessment of the economic and environmental impact. Afr. J. Sci. Technol. Innov. Dev. 2021, 13, 247–260. [Google Scholar] [CrossRef]
- Kherrafi, M.A.; Benseddik, A.; Saim, R.; Bouregueba, A.; Badji, A.; Nettari, C.; Hasrane, I. Advancements in solar drying technologies: Design variations, hybrid systems, storage materials and numerical analysis: A review. Sol. Energy 2024, 270, 112383. [Google Scholar] [CrossRef]
- Bennamoun, L. Integration of photovoltaic cells in solar drying systems. Dry. Technol. 2013, 31, 1284–1296. [Google Scholar] [CrossRef]
- Rao, T.S.S.B.; & Sivalingam, M.; Sivalingam, M. Assessment of energy, exergy, environmental, and economic study of an evacuated tube solar dryer for drying Krishna Tulsi. Environ. Sci. Pollut. Res. 2023, 30, 67351–67367. [Google Scholar] [CrossRef]
- Srivastava, A.; Anand, A.; Shukla, A.; Kumar, A.; Buddhi, D.; Sharma, A. A comprehensive overview on solar grapes drying: Modeling, energy, environmental and economic analysis. Sustain. Energy Technol. Assess. 2021, 47, 101513. [Google Scholar] [CrossRef]
- Machala, M.L.; Tan, F.L.; Poletayev, A.; Khan, M.I.; Benson, S.M. Overcoming barriers to solar dryer adoption and the promise of multi-seasonal use in India. Energy Sustain. Dev. 2022, 68, 18–28. [Google Scholar] [CrossRef]
- Khallaf, A.E.M.; El-Sebaii, A. Review on drying of the medicinal plants (herbs) using solar energy applications. Heat Mass Transf. 2022, 58, 1411–1428. [Google Scholar] [CrossRef]
- Sakare, P.; Prasad, N.; Thombare, N.; Singh, R.; Sharma, S.C. Infrared Drying of Food Materials: Recent Advances. Food Eng. Rev. 2020, 12, 381–398. [Google Scholar] [CrossRef]
- Nukulwar, M.R.; Tungikar, V.B. Evaluation of drying model and quality analysis of turmeric using solar thermal system. Appl. Sol. Energy 2020, 56, 233–241. [Google Scholar] [CrossRef]
- Khan, N.; Sudhakar, K.; Mamat, R. Seaweed processing: Efficiency, kinetics, and quality attributes under solar drying. Food Chem. Adv. 2025, 6, 100859. [Google Scholar] [CrossRef]
- Radhakrishnan, G.; Breaz, T.O.; Al Mahrouqi, A.W.A.; Al Zakwani, N.A.; Al Fahdi, M.H.; Al Shuraiqi, A.S.; Al Awamri, S.A.; Al Aamri, R.S.; Karthikeyan, K.R. A Comparative Management Analysis on the Performance of Different Solar Drying Methods for Drying Vegetables and Fruits. Sustainability 2024, 16, 775. [Google Scholar] [CrossRef]
- Singh, P.; Vyas, S.; Yadav, A. Experimental comparison of open sun drying and solar drying based on evacuated tube collector. Int. J. Sustain. Energy 2019, 38, 348–367. [Google Scholar] [CrossRef]
- Monesh, S.; Shankar, A.; Ezhilarasan, S.; Tiwari, S.; Sahdev, R.K.; Tiwari, P. Comparison of different solar dryers with and without heat storage for crop drying: A review. Environ. Prog. Sustain. Energy 2024, 43, e14479. [Google Scholar]
- Munarso, S.J.; Widayanti, S.M. Advances in postharvest technology and its implementation. IOP Conf. Ser. Earth Environ. Sci. 2022, 1024, 012001. [Google Scholar] [CrossRef]
- Mammano, M.M.; Comparetti, A.; Ciulla, S.; Greco, C.; Orlando, S. A prototype of photovoltaic dryer for nutraceutical and aromatic plants. In AIIA 2022: Biosystems Engineering Towards the Green Deal; Ferro, V., Giordano, G., Orlando, S., Vallone, M., Cascone, G., Porto, S.M.C., Eds.; Lecture Notes in Civil Engineering; Springer: Cham, Switzerland, 2023; Volume 337. [Google Scholar]
- Al-Hamdani, A.; Jayasuriya, H.; Pathare, P.B.; Al-Attabi, Z. Drying Characteristics and Quality Analysis of Medicinal Herbs Dried by an Indirect Solar Dryer. Foods 2022, 11, 4103. [Google Scholar] [CrossRef]
- Yilmaz, A.; Alibas, I.; Asik, B.B. The effect of drying methods on the color, chlorophyll, total phenolic, flavonoids, and macro and micronutrients of thyme plant. J. Food Process. Preserv. 2021, 45, e15915. [Google Scholar] [CrossRef]
- Garofalo, G.; Buzzanca, C.; Ponte, M.; Barbera, M.; D’Amico, A.; Greco, C.; Mammano, M.M.; Franciosi, E.; Piazzese, D.; Guarrasi, V.; et al. Comprehensive analysis of Moringa oleifera leaves’ antioxidant properties in ovine cheese. Food Biosci. 2024, 61, 104974. [Google Scholar] [CrossRef]
- Mammano, M.M.; Comparetti, A.; Greco, C.; Orlando, S. A model of Sicilian environmentally friendly multifunctional farm for soil protection. In AIIA 2022: Biosystems Engineering Towards the Green Deal; Ferro, V., Giordano, G., Orlando, S., Vallone, M., Cascone, G., Porto, S.M.C., Eds.; Lecture Notes in Civil Engineering; Springer: Cham, Switzerland, 2023; Volume 337. [Google Scholar]
- Xue, J.; Su, B. Significant Remote Sensing Vegetation Indices: A Review of Developments and Applications. J. Sens. 2017, 1353691, 17. [Google Scholar] [CrossRef]
- Peng, Y.; Gitelson, A.A. Application of chlorophyll-related vegetation indices for remote estimation of maize productivity. Agric. For. Meteorol. 2011, 151, 1267–1276. [Google Scholar] [CrossRef]
- Zarco-Tejada, P.J.; Miller, J.R.; Mohammed, G.H.; Noland, T.L.; Sampson, P.H. Estimation of chlorophyll fluorescence under natural illumination from hyperspectral data. Int. J. Appl. Earth Obs. Geoinf. 2001, 3, 321–327. [Google Scholar] [CrossRef]
- Zarco-Tejada, P.J.; Miller, J.R.; Mohammed, G.H.; Noland, T.L. Chlorophyll fluorescence effects on vegetative apparent reflectance: I. Leaf-level measurements and model simulation. Remote Sens. Environ. 2000, 74, 582–595. [Google Scholar] [CrossRef]
- Daughtry, C.; Walthall, C.; Kim, M.; Colstoun, E.B.; McMurtrey, J.E. Estimating Corn Leaf Chlorophyll Concentration from Leaf and Canopy Reflectance. Remote Sens. Environ. 2000, 74, 229–239. [Google Scholar] [CrossRef]
- Kim, M.S.; Daughtry, C.S.; Chappelle, E.W.; McMurtrey, J.E.; Walthall, C.L. The Use of High Spectral Resolution Bands for Estimating Absorbed Photosynthetically Active Radiation (A Par); CNES: Paris, France, 1994. [Google Scholar]
- Haboudane, D.; Miller, J.R.; Tremblay, N.; Zarco-Tejada, P.J.; Dextraze, L. Integrated Narrow-Band Vegetation Indices for Prediction of Crop Chlorophyll Content for Application to Precision Agriculture. Remote Sens. Environ. 2002, 81, 416–426. [Google Scholar] [CrossRef]
- Lieth, H.; Whittaker, R.H. Primary productivity of the biosphere. In Ecological Studies; Springer: Berlin/Heidelberg, Germany, 1975; Volume 14, pp. 7–16. [Google Scholar]
- Gitelson, A.A.; Viña, A.; Verma, S.B.; Rundquist, D.C.; Arkebauer, T.J.; Keydan, G.; Leavitt, B.; Ciganda, V.; Burba, G.G.; Suyker, A.E. Relationship between gross primary production and chlorophyll content in crops: Implications for the synoptic monitoring of vegetation productivity. J. Geophys. Res. 2006, 111, D08S11. [Google Scholar] [CrossRef]
- Wu, C.; Niu, Z.; Tang, Q.; Huang, W.; Rivard, B.; Feng, J. Remote estimation of gross primary production in wheat using chlorophyll-related vegetation indices. Agric. For. Meteorol. 2009, 149, 1015–1021. [Google Scholar] [CrossRef]
- Hilker, T.; Gitelson, A.A.; Coops, N.C.; Hall, F.G.; Black, T.A. Tracking plant physiological properties from multi-angular tower-based remote sensing. Oecologia 2011, 165, 865–876. [Google Scholar] [CrossRef]
- Walters, D.T. Diagnosis of nitrogen deficiency in maize and the influence of hybrid and plant density. In North Central Extension-industry Soil Fertility Conference; NCSFC: Des Moines, IA, USA, 2003; Volume 19. [Google Scholar]
- Solari, F.; Shanahan, J.; Ferguson, R.; Schepers, J.; Gitelson, A.A. Active sensor reflectance measurements of corn nitrogen status and yield potential. Agron. J. 2008, 100, 571–579. [Google Scholar] [CrossRef]
- Liu, C.; Liu, Y.; Lu, Y.; Liao, Y.; Nie, J.; Yuan, X.; Chen, F. Use of a leaf chlorophyll content index to improve the prediction of above-ground biomass and productivity. PeerJ 2019, 6, e6240. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Catania, P.; Gaglio, R.; Orlando, S.; Settanni, L.; Vallone, M. Design and Implementation of a Smart System to Control Aromatic Herb Dehydration Process. Agriculture 2020, 10, 332. [Google Scholar] [CrossRef]
- Liguori, G.; Greco, G.; Salsi, G.; Garofalo, G.; Gaglio, R.; Barbera, M.; Greco, C.; Orlando, S.; Fascella, G.; Mammano, M.M. Effect of the gellanbased edible coating enriched with oregano essential oil on the preservation of the ‘Tardivo di Ciaculli’ mandarin (Citrus reticulata Blanco cv. Tardivo di Ciaculli). Front. Sustain. Food Syst. 2024, 8, 1334030. [Google Scholar] [CrossRef]
- Saini, R.K.; Saini, D.K.; Gupta, R.; Verma, P.; Thakur, R.; Kumar, S.; Ali Wassouf, A. Technological development in solar dryers from 2016 to 2021-A review. Renew. Sustain. Energy Rev. 2023, 188, 113855. [Google Scholar] [CrossRef]
- ISO 11290-1; Microbiology of the Food Chain—Horizontal Method for the Detection and Enumeration of Listeria monocytogenes and of Listeria spp. Part 1: Detection Method. International Organization for Standardization: Geneva, Switzerland, 2017.
- ISO 6579–1; Microbiology of the Food Chain–Horizontal Method for the Detection, Enumeration and Serotyping of Salmonella–Part 1: Detection of Salmonella spp. International Organization for Standardization: Geneva, Switzerland, 2017.
- Ibrahim, A.; Elsebaee, I.; Amer, A.; Aboelasaad, G.; El-Bediwy, A.; El-Kholy, M. Development and evaluation of a hybrid smart solar dryer. J. Food Sci. 2023, 88, 3859–3878. [Google Scholar] [CrossRef] [PubMed]
- Amer, A.; Ibrahim, A.; Shahin, A.; Elsebaee, I.; Saad, R.; Hassan, M.F.; Hassan, Z. Performance evaluation of an automated hybrid solar system dryer for drying some aromatic herbs. Dry. Technol. 2024, 42, 728–747. [Google Scholar] [CrossRef]
- Banout, J.; Ehl, P.; Havlik, J.; Lojka, B.; Polesny, Z.; Verner, V. Design and performance evaluation of a Double-pass solar drier for drying of red chilli (Capsicum annum L.). Sol. Energy 2011, 85, 506–515. [Google Scholar] [CrossRef]
- Sagoo, S.K.; Little, C.L.; Greenwood, M.; Mithani, V.; Grant, K.A.; McLauchlin, J.; de Pinna, E.; Threlfall, E.J. Assessment of the microbiological safety of dried spices and herbs from production and retail premises in the United Kingdom. Food Microbiol. 2009, 26, 39–43. [Google Scholar] [CrossRef]
- World Health Organization; FAO. Microbiological Hazards in Spices and Dried Aromatic Herbs: Meeting Report; WHO: Geneva, Switzerland, 2022; Volume 27. [Google Scholar]
- European Food Safety Authority; European Centre for Disease Prevention and Control. The European Union One Health 2021 Zoonoses Report. EFSA J. 2022, 20, e07666. [Google Scholar]
- Lee, H.; Yoon, Y. Etiological agents implicated in foodborne illness worldwide. Food Sci. Anim. Resour. 2021, 41, 1. [Google Scholar] [CrossRef]
- Cicero, A.F.G.; Fogacci, F.; Rizzoli, E.; D’Addato, S.; Borghi, C.; Brisighella Heart Study Group. Correction: Cicero et al. Long-Term Impact of Different Triple Combination Antihypertensive Medications on Blood Pressure Control, Metabolic Pattern and Incident Events: Data from the Brisighella Heart Study. J. Clin. Med. 2021, 10, 5921, Erratum in J. Clin. Med. 2022, 11, 7109. https://doi.org/10.3390/jcm11237109. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gerba, C.P.; Pepper, I.L. Microbial contaminants. In Environmental and Pollution Science; Academic Press: Cambridge, MA, USA, 2019; pp. 191–217. [Google Scholar]
- Kostecka, M.; Bojanowska, M. Mold contamination of commercially available herbal products and dietary supplements of plant origin. Ecol. Chem. Eng. A 2013, 20, 1369–1379. [Google Scholar]
- International Commission on Microbiological Specifications for Foods. Spices, herbs, and vegetable seasonings. In Microorganisms in Foods, Microbial Ecology of Food Commodities; ICMSF: Montreal, QC, Canada; pp. 360–372.
- de Sousa Lima, C.M.; Fujishima, M.A.T.; de Paula Lima, B.; Mastroianni, P.C.; de Sousa, F.F.O.; da Silva, J.O. Microbial contamination in herbal medicines: A serious health hazard to elderly consumers. BMC Complement Med. Ther. 2020, 20, 17. [Google Scholar] [CrossRef]


| Parameters | Value |
|---|---|
| Flight height | 50 m a.s.l. |
| Overlap front. and lat | 70% |
| Flight speed | 10 m s−1 |
| Camera angle | 90° |
| FOV | 62.7° |
| Flight path | 0° to the North |
| GSD | ≈2.6 cm |
| GNSS mode | RTK |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Greco, C.; Gaglio, R.; Settanni, L.; Sciurba, L.; Ciulla, S.; Orlando, S.; Mammano, M.M. Smart Farming Technologies for Sustainable Agriculture: A Case Study of a Mediterranean Aromatic Farm. Agriculture 2025, 15, 810. https://doi.org/10.3390/agriculture15080810
Greco C, Gaglio R, Settanni L, Sciurba L, Ciulla S, Orlando S, Mammano MM. Smart Farming Technologies for Sustainable Agriculture: A Case Study of a Mediterranean Aromatic Farm. Agriculture. 2025; 15(8):810. https://doi.org/10.3390/agriculture15080810
Chicago/Turabian StyleGreco, Carlo, Raimondo Gaglio, Luca Settanni, Lino Sciurba, Salvatore Ciulla, Santo Orlando, and Michele Massimo Mammano. 2025. "Smart Farming Technologies for Sustainable Agriculture: A Case Study of a Mediterranean Aromatic Farm" Agriculture 15, no. 8: 810. https://doi.org/10.3390/agriculture15080810
APA StyleGreco, C., Gaglio, R., Settanni, L., Sciurba, L., Ciulla, S., Orlando, S., & Mammano, M. M. (2025). Smart Farming Technologies for Sustainable Agriculture: A Case Study of a Mediterranean Aromatic Farm. Agriculture, 15(8), 810. https://doi.org/10.3390/agriculture15080810









