Innovative Techniques for Managing Dollar Spot in Warm- and Cool-Season Turfgrasses: The Case of UV-B and UV-C Irradiations
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design, Plant, and Fungal Material
2.2. UV Treatments
2.3. Dark Green Colour Index Determination
2.4. Extraction and Determination of Antioxidant Activity on Plant Material
2.5. Extraction and Quantification of Photosynthetic Pigments
2.6. Statistical Analysis
3. Results
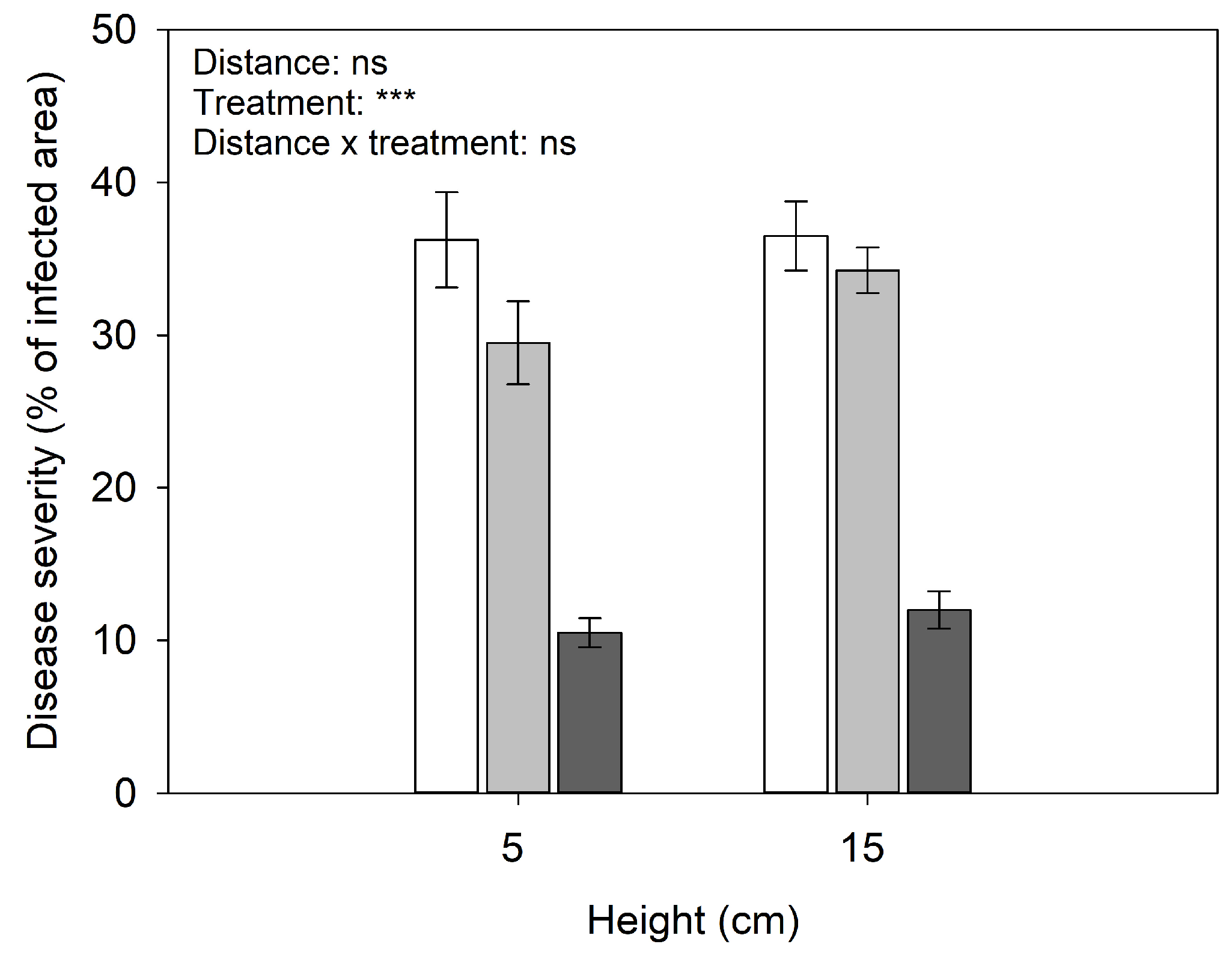
3.1. Evaluation of In Vitro and In Vivo Treatments
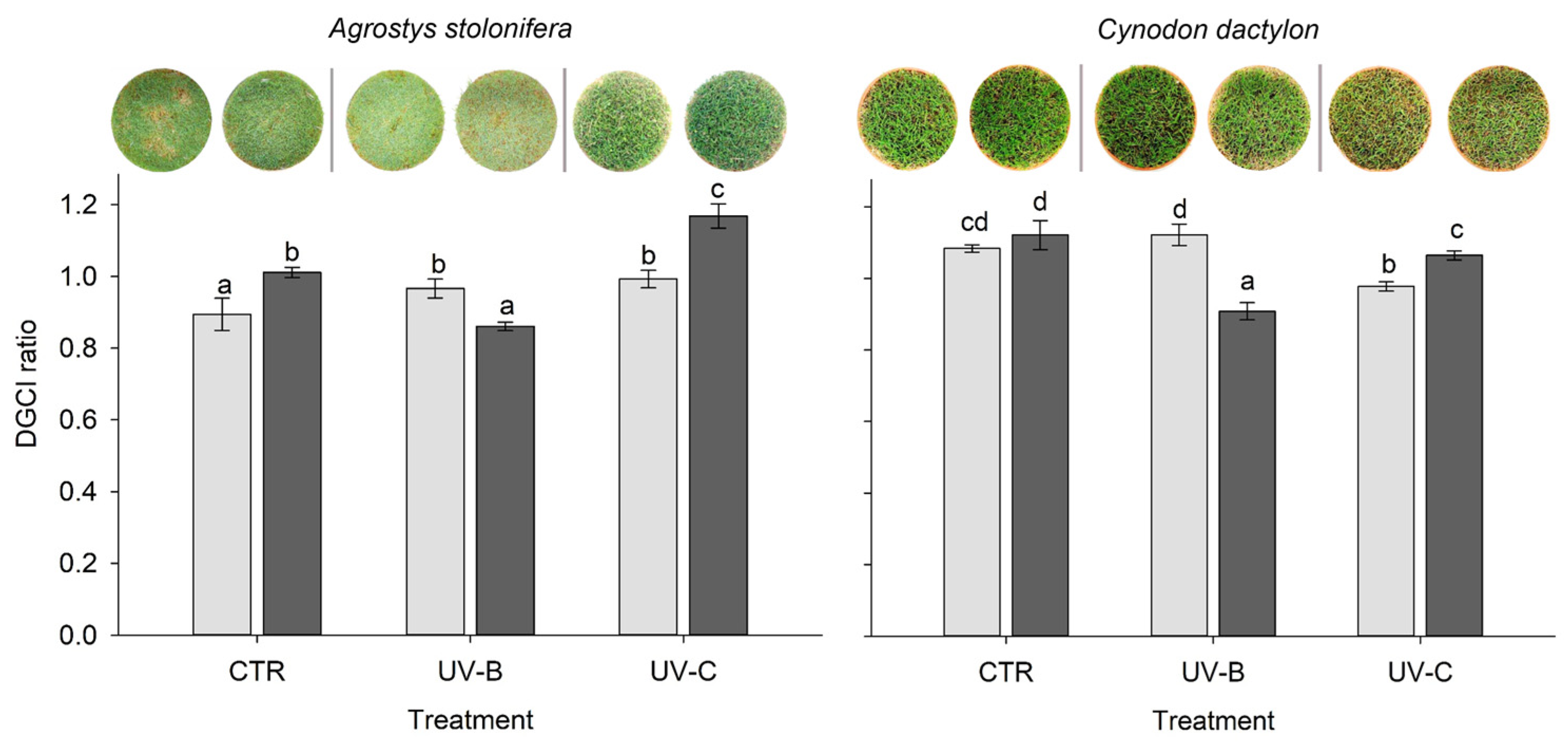
3.2. Dark Green Colour Index
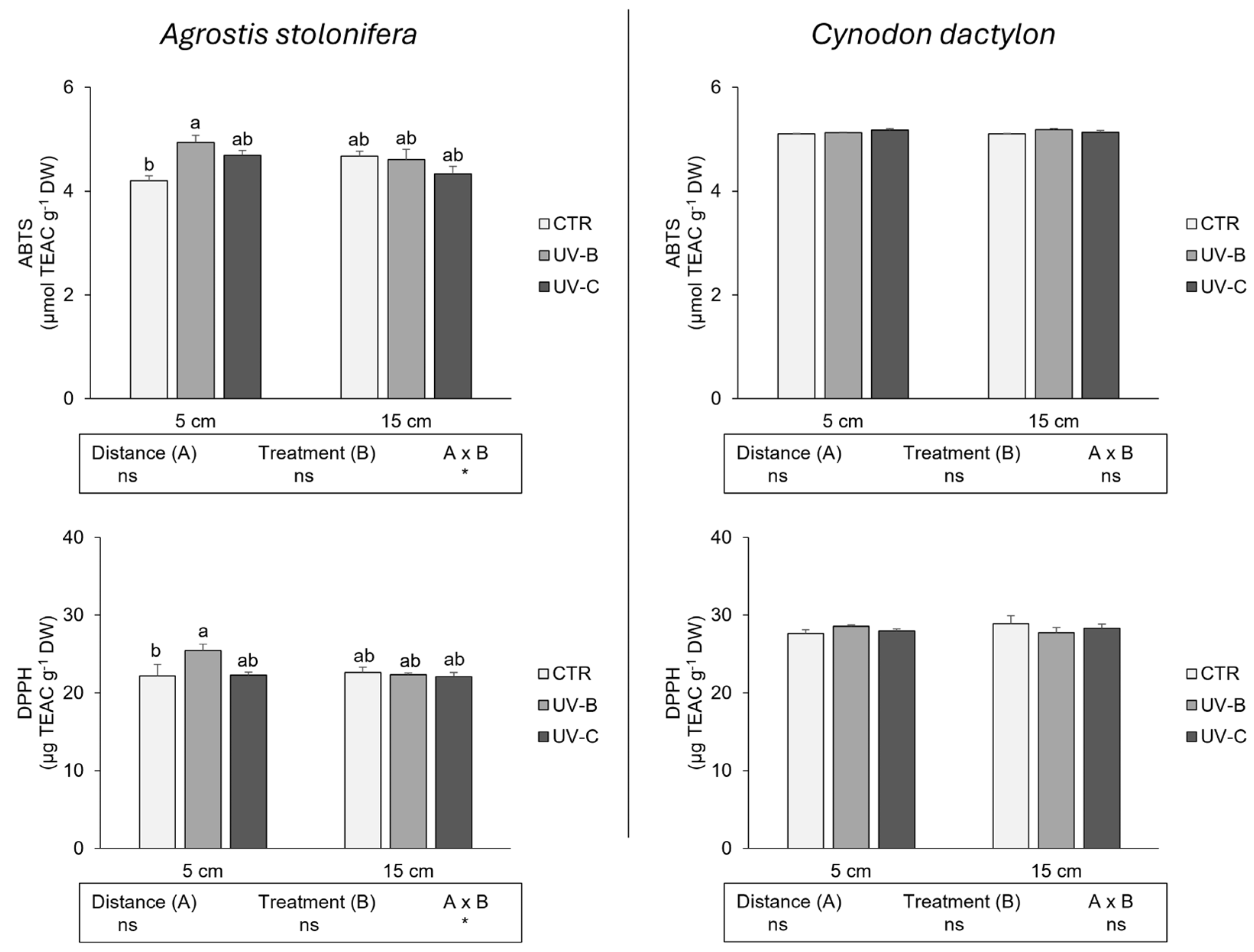
3.3. Evaluation of the Antioxidant Capacity
3.4. Concentration of Photosynthetic Pigments
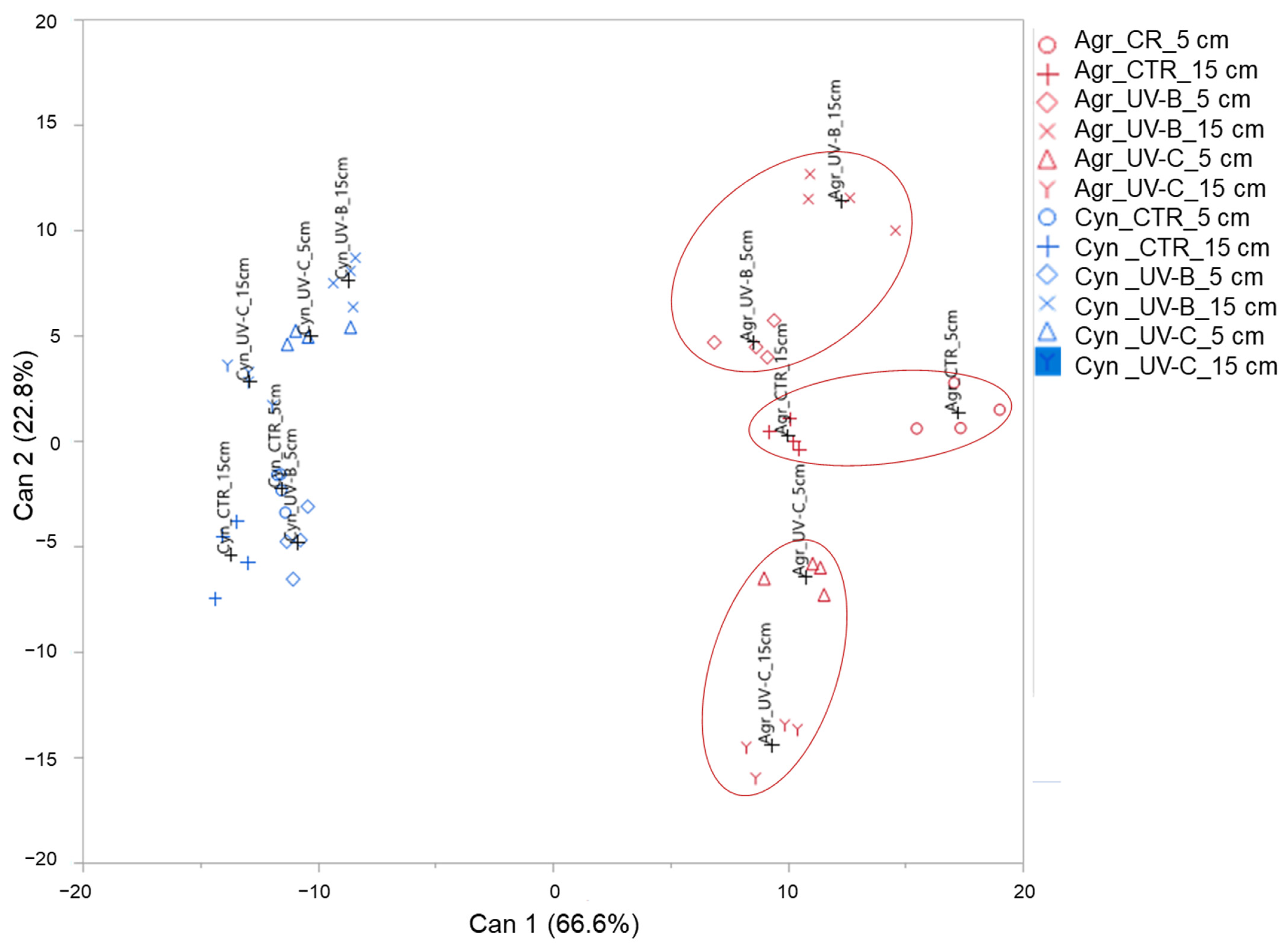
Canonical Discriminant Analysis and Pearson’s Correlation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Haydu, J.J.; Hodges, A.W.; Hall, C.R. Economic Impacts of the Turfgrass and Lawncare Industry in the United States; FE 632; Florida Cooperative Extension Service: Gainesville, FL, USA, 2006. [Google Scholar]
- Lindsey, A.J.; Schiavon, M.; Unruh, J.B.; Kenworthy, K. Urban landscapes: Turgrass benefits. Grasses 2025, 4, 3. [Google Scholar]
- Breuninger, J.M.; Welterlen, M.S.; Augustin, B.J.; Cline, V.; Morris, K. The turfgrass industry. In Turfgrass: Biology, Use, and Management; Stier, J.C., Horgan, B.P., Bonos, S.A., Eds.; ASA-CSSA-SSSA: Madison, WI, USA, 2013. [Google Scholar]
- Morris, K. National Turfgrass Research Initiative; National Turfgrass Federation and National Turfgrass Evaluation Program: Beltsville, MD, USA, 2003. [Google Scholar]
- Jung, H.; Chung, C. Consumers’ WTP for sustainability turfgrass attributes with consideration of aesthetic attributes and water conservation policies. Agriculture 2024, 14, 159. [Google Scholar] [CrossRef]
- Sang, H.; Popko, J.T., Jr.; Jung, G. Evaluation of a Sclerotinia homeocarpa population with multiple fungicide resistance phenotypes under differing selection pressures. Plant Disease 2019, 103, 4. [Google Scholar]
- Bennett, F.T. Dollar spot disease of turf and its causal organism, Sclerotinia homoeocarpa n. sp. Ann. Appl. Biol. 1937, 24, 236–257. [Google Scholar]
- Hu, J.; Zhou, Y.; Geng, J.; Dai, Y.; Ren, H.; Lamour, K. A new Dollar spot disease of turfgrass caused by Clarireedia paspali. Mycol. Prog. 2019, 18, 1423–1435. [Google Scholar]
- Salgado-Salazar, C.; Beirn, L.A.; Ismaiel, A.; Boehm, M.J.; Carbone, I.; Putman, A.I.; Tredway, L.P.; Clarke, B.B.; Crouch, J.A. Clarireedia: A new fungal genus comprising four pathogenic species responsible for Dollar spot disease of turfgrass. Fungal Biol. 2018, 122, 761–773. [Google Scholar]
- Kitchin, E.C.A.; Sneed, H.J.; McCall, D.S. Leveraging deep learning for Dollar spot detection and quantification in turfgrass. Crop Sci. 2024, 65, e21329. [Google Scholar]
- Hu, J.; Zhang, H.; Dong, Y.; Jiang, S.; Lamour, K.; Liu, J.; Chen, Y.; Yang, Z. Global distributions of Clarireedia species and their in vitro sensitivity profiles to fungicides. Agronomy 2021, 11, 2036. [Google Scholar] [CrossRef]
- Zhang, H.; Dong, Y.; Zhou, Y.; Hu, J.; Lamour, K.; Yang, Z. Clarireedia hainanense: A new species is associated with Dollar spot of turfgrass in Hainan, China. Plant Dis. 2022, 106, 996–1002. [Google Scholar]
- Hu, J.; Zhang, H.; Kong, Y.; Lamour, K.; Jung, G.; Yang, Z. Varied sensitivity to boscalid among different Clarireedia species causing Dollar spot in turfgrass. Pestic. Biochem. Physiol. 2024, 204, 106029. [Google Scholar]
- Zhang, H.; Liu, J.; Dong, Y.; Hu, J.; Lamour, K.; Yang, Z. A one-step multiplex PCR assay for the detection and differentiation of four species of Clarireedia causing Dollar spot on turfgrass. Pest Manag. Sci. 2023, 79, 1069–1077. [Google Scholar] [CrossRef] [PubMed]
- Sapkota, S.; Catching, K.E.; Raymer, P.L.; Martinez-Espinoza, A.D.; Bahri, B.A. New approaches to an old problem: Dollar spot of turfgrass. Phytopathol. 2022, 112, 469–480. [Google Scholar]
- Tredway, L.P.; Clarke, B.B.; Kerns, J.P.; Tomaso-Peterson, M. Compendium of Turfgrass Diseases, 4th ed.; The American Phytopathological Society: St Paul, MN, USA, 2022. [Google Scholar]
- Walsh, B.; Ikeda, S.S.; Boland, G.J. Biology and management of Dollar spot (Sclerotinia homoeocarpa); an important disease of turfgrass. HortScience 1999, 34, 13. [Google Scholar]
- Liu, J.; Wu, J.; Jin, P.; Hu, J.; Lamour, K.; Yang, Z. Activity of the succinate dehydrogenase inhibitor fungicide benzovindiflupyr against Clarireedia spp. Plant Disease 2023, 107, 12. [Google Scholar]
- Vanhaelewyn, L.; Prinsen, E.; Van Der Straeten, D.; Vandenbussche, F. Hormone-controlled UV-B responses in plants. J. Exp. Bot. 2016, 67, 4469–4482. [Google Scholar]
- Jenkins, G.I. Photomorphogenic responses to ultraviolet-B light. Plant Cell Environ. 2017, 40, 2544–2557. [Google Scholar]
- Vandenbussche, F.; Yu, N.; Li, W.; Vanhaelewyn, L.; Hamshou, M.; Van Der Straeten, D.; Smagghe, G. An ultraviolet B condition that affects growth and defense in Arabidopsis. Plant Sci. 2018, 268, 54–63. [Google Scholar]
- Reyes, T.H.; Pompeiano, A.; Ranieri, A.; Volterrani, M.; Guglielminetti, L.; Scartazza, A. Photosynthetic performance of five cool-season turfgrasses under UV-B exposure. Plant Physiol. Biochem. 2020, 151, 181–187. [Google Scholar]
- Santin, M.; Giordani, T.; Cavallini, A.; Bernardi, R.; Castagna, A.; Hause, M.-T.; Ranieri, A. UV-B exposure reduces the activity of several cell wall-dismantling enzymes and affects the expression of their biosynthetic genes in peach fruit (Prunus persica L., cv. Fairtime, melting phenotype). Photochem. Photobiol. Sci. 2019, 18, 1280–1289. [Google Scholar]
- Santin, M.; Zeni, V.; Grassi, A.; Ricciardi, R.; Pieracci, Y.; Di Giovanni, F.; Panzani, S.; Frasconi, C.; Agnolucci, M.; Avio, L.; et al. Do changes in Lactuca sativa metabolic performance, induced by mycorrhizal symbionts and leaf UV-B irradiation, play a role towards tolerance to a polyphagous insect pest? Environ. Sci.Pollut. Res. 2023, 30, 56207–56223. [Google Scholar]
- Thomas, T.T.D.; Puthur, J.T. UV-B priming enhances specific secondary metabolites in Oryza sativa (L.) empowering to encounter diverse abiotic stresses. Plant Growth Regul. 2020, 92, 169–180. [Google Scholar] [CrossRef]
- Yavaş, İ.; Ünay, A.; Ali, S.; Abbas, Z. UV-B radiations and secondary metabolites. Turk. J.Agric.-Food Sci. Technol. 2020, 8, 147–157. [Google Scholar] [CrossRef]
- Zeni, V.; Grassi, A.; Santin, M.; Ricciardi, R.; Pieracci, Y.; Flamini, G.; Di Giovanni, F.; Marmugi, M.; Agnolucci, M.; Avio, L.; et al. Leaf UV-B irradiation and mycorrhizal symbionts affect lettuce VOC emissions and defence mechanisms, but not aphid feeding preferences. Insects 2022, 14, 20. [Google Scholar] [CrossRef]
- Escalona, V.H.; Aguayo, E.; Martínez-Hernández, G.B.; Artés, F. UV-C doses to reduce pathogen and spoilage bacterial growth in vitro and in baby spinach. Postharvest Biol. Technol. 2010, 56, 223–231. [Google Scholar] [CrossRef]
- Han, S.; Cai, H.; Yu, H.; Yu, Z.; Wu, Y. Targeted metabolomic and transcriptomic reveal the regulation of UV-C on phenolics biosynthesis of peach fruit during storage. LWT 2023, 190, 115573. [Google Scholar] [CrossRef]
- Liu, C.; Zheng, H.; Sheng, K.; Liu, W.; Zheng, L. Effects of postharvest UV-C irradiation on phenolic acids, flavonoids, and key phenylpropanoid pathway genes in tomato fruit. Sci. Hortic. 2018, 241, 107–114. [Google Scholar] [CrossRef]
- Urban, L.; Charles, F.; de Miranda, M.R.A.; Aarrouf, J. Understanding the physiological effects of UV-C light and exploiting its agronomic potential before and after harvest. Plant Physiol.Biochem. 2016, 105, 1–11. [Google Scholar] [CrossRef]
- Zhang, W.; Jiang, H.; Cao, J.; Jiang, W. UV-C treatment controls brown rot in postharvest nectarine by regulating ROS metabolism and anthocyanin synthesis. Postharvest Biol.Technol. 2021, 180, 111613. [Google Scholar] [CrossRef]
- Hu, C. The Effect of Photocatalytic Titanium Dioxide and Ultraviolet B Radiation on Sclerotinia homoeocarpa Growth and Pathogenicity. Master’s Thesis, The University of Tennessee, Knoxville, TN, USA, 2013. [Google Scholar]
- Berkelmann-loehnertz, B.; Klaerner, S.; Flemming, B.; Keicher, R.; Schwarz, H.-P.; Pflieninger, F.; Loehnertz, O. Enhanced phytosanitary performance in the vineyard and physiological host responses induced by UV-C radiation. J. Plant Pathol. 2013, 1 (Suppl. S1), 75. [Google Scholar]
- Meyer, P.; Van de Poel, B.; De Coninck, B. UV-B light and its application potential to reduce disease and pest incidence in crops. Hortic. Res. 2021, 8, 194. [Google Scholar]
- Karcher, D.E.; Richardson, M.D. Quantifying turfgrass color using digital image analysis. Crop Sci. 2003, 43, 943–951. [Google Scholar]
- Santin, M.; Sciampagna, M.C.; Mannucci, A.; Puccinelli, M.; Angelini, L.G.; Tavarini, S.; Castagna, A. Supplemental UV-B exposure influences the biomass and the content of bioactive compounds in Linum usitatissimum L. sprouts and microgreens. Hortic. 2022, 8, 213. [Google Scholar]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free. Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [PubMed]
- Blois, M.S. Antioxidant determinations by the use of a stable free radical. Nature 1958, 181, 1199–1200. [Google Scholar]
- Castagna, A.; Di Baccio, D.; Tognetti, R.; Ranieri, A.; Sebastiani, L. Differential ozone sensitivity interferes with cadmium stress in poplar clones. Biol. Plant. 2013, 57, 313–324. [Google Scholar]
- Marvin, J.W.; Kerr, R.A.; McCarty, L.B.; Bridges, W.; Martin, S.B.; Wells, C.E. Curative evaluation of biological control agents and synthetic fungicides for Clarireedia jacksonii. HortScience 2020, 55, 1622–1625. [Google Scholar]
- Demkura, P.V.; Ballaré, C.L. UVR8 mediates UV-B-induced Arabidopsis defense responses against Botrytis cinerea by controlling sinapate accumulation. Mol. Plant 2012, 5, 642–652. [Google Scholar]
- Luglio, S.M.; Sportelli, M.; Frasconi, C.; Raffaelli, M.; Gagliardi, L.; Peruzzi, A.; Fortini, V.; Volterrani, M.; Magni, S.; Caturegli, L.; et al. Monitoring Autonomous Mowers Operative Parameters on Low-Maintenance Warm-Season Turfgrass. Appl. Sci. 2023, 13, 7852. [Google Scholar] [CrossRef]
- Onofre, R.B.; Gadoury, D.M.; Stensvand, A.; Bierman, A.; Rea, M.; Peres, N.A. Use of ultraviolet light to suppress powdery mildew in strawberry fruit production fields. Plant Dis. 2021, 105, 2402–2409. [Google Scholar]
- Gadoury, D.M.; Sapkota, S.; Cadle-Davidson, L.; Underhill, A.; McCann, T.; Gold, K.M.; Gambhir, N.; Combs, D.B. Effects of nighttime applications of germicidal ultraviolet light upon powdery mildew (Erysiphe necator), downy mildew (Plasmopara viticola), and sour rot of grapevine. Plant Dis. 2023, 107, 1452–1462. [Google Scholar]
- McDaniel, A.L.; Gadoury, D.M.; Moyer, M.M. Effects of germicidal ultraviolet-C light on grape mealybug (Pseudococcus maritimus). Crop Prot. 2024, 178, 106584. [Google Scholar]
- Hesselsøe, K.J.; Borchert, A.F.; Bekken, M.; Aamlid, T.S.; Hahn, D.; Pramassing, W. FAIRWAYS4FUTURE—A New STERF-Project on Sustainable Management of Golf Course Fairways. 2023. Available online: http://www.sterf.org (accessed on 4 February 2025).
- Sinha, R.P.; Hader, D.P. UV-induced DNA damage and repair: A review. Photochem. Photobiol. Sci. 2002, 4, 225–236. [Google Scholar]
- Li, X.; Cai, M.; Wang, L.; Niu, F.; Yang, D.; Zhang, G. Evaluation survey of microbial disinfection methods in UV-LED water treatment systems. Sci. Tot. Environ. 2019, 659, 1415–1427. [Google Scholar]
- Dat, J.; Vandenabeele, S.; Vranov, E.; Van Montagu, M.; Inzé, D.; Van Breusegem, F. Dual action of the active oxygen species during plant stress responses. Cell Mol. Life Sci. 2000, 57, 779–795. [Google Scholar] [CrossRef]
- Grant, J.; Loake, G.J. Role of reactive oxygen intermediates and cognate redox signaling in disease resistance. Plant Physiol. 2000, 124, 21–29. [Google Scholar] [PubMed]
- Sarkar, D.; Bhowmik, P.C.; Shetty, K. The role of proline-associated pentose phosphate pathway in cool-season turfgrasses after UV-B exposure. Environ. Exp. Bot. 2011, 70, 251–258. [Google Scholar]
- Mackerness, S.A.H. Plant responses to ultraviolet-B (UV-B: 280–320 nm) stress: What are the key regulators? Plant Growth Regul. 2000, 32, 27–39. [Google Scholar]
- Marchica, A.; Ascrizzi, R.; Flamini, G.; Cotrozzi, L.; Tonelli, M.; Lorenzini, G.; Nali, C.; Pellegrini, E. Ozone as eustress for enhancing secondary metabolites and bioactive properties in Salvia officinalis. Ind. Crops Prod. 2021, 170, 113730. [Google Scholar]

| Distance from the Lamps (cm) | PAR (µmol m−2 s−1) | UV-B | UV-C | ||
|---|---|---|---|---|---|
| Irradiance (W m−2) | Total Dose (kJ m−2) | Irradiance (W m−2) | Total Dose (kJ m−2) | ||
| 5 | 370.7 ± 3.5 | 11.9 ± 1.7 | 0.42 ± 0.06 | 25.5 ± 2.0 | 0.89 ± 0.07 |
| 15 | 340.2 ± 4.4 | 6.6 ± 0.6 | 0.23 ± 0.02 | 14.5 ± 1.0 | 0.51 ± 0.04 |
| Distance (cm) | Treatment | Lutein (µg g−1 DW) | Chl b (mg g−1 DW) | Chl a (mg g−1 DW) | Chl a + b (mg g−1 DW) | Chl a/b | β-Carotene (µg g−1 DW) | Chl/Car |
|---|---|---|---|---|---|---|---|---|
| 5 | CTR | 113.5 ± 4.8 | 1.80 ± 0.05 | 3.56 ± 0.05 | 5.36 ± 0.03 | 1.98 ± 0.08 | 50.6 ± 0.7 | 32.7 ± 0.7 |
| UV-B | 118.6 ± 4.2 | 1.70 ± 0.05 | 3.28 ± 0.10 | 4.97 ± 0.07 | 1.94 ± 0.11 | 50.6 ± 0.4 | 29.5 ± 1.0 | |
| UV-C | 110.2 ± 6.5 | 1.68 ± 0.03 | 3.57 ± 0.12 | 5.24 ± 0.14 | 2.13 ± 0.04 | 49.4 ± 1.9 | 33.0 ± 1.8 | |
| 15 | CTR | 114.1 ± 7.9 | 1.72 ± 0.09 | 3.51 ± 0.07 | 5.23 ± 0.09 | 2.06 ± 0.12 | 47.4 ± 0.6 | 32.6 ± 2.2 |
| UV-B | 121.7 ± 2.6 | 1.82 ± 0.05 | 3.41 ± 0.09 | 5.23 ± 0.12 | 1.88 ± 0.05 | 47.7 ± 0.4 | 30.9 ± 0.2 | |
| UV-C | 115.5 ± 5.0 | 1.72 ± 0.08 | 3.45 ± 0.10 | 5.18 ± 0.01 | 2.02 ± 0.14 | 50.6 ± 2.1 | 31.2 ± 1.0 | |
| Mean effect | ||||||||
| 5 | 114.1 ± 2.9 | 1.72 ± 0.03 | 3.47 ± 0.07 | 5.19 ± 0.10 | 2.02 ± 0.05 | 50.2 ± 0.6 | 31.7 ± 0.9 | |
| 15 | 117.1 ± 3.0 | 1.75 ± 0.04 | 3.46 ± 0.05 | 5.21 ± 0.01 | 1.98 ± 0.06 | 48.6 ± 0.8 | 31.6 ± 0.8 | |
| CTR | 113.8 ± 4.1 | 1.76 ± 0.05 | 3.54 ± 0.04 | 5.30 ± 0.05 | 2.02 ± 0.07 | 49.0 ± 0.8 | 32.7 ± 1.0 | |
| UV-B | 120.2 ± 2.3 | 1.76 ± 0.04 | 3.35 ± 0.07 | 5.10 ± 0.09 | 1.91 ± 0.05 | 49.1 ± 0.7 | 30.2 ± 0.6 | |
| UV-C | 112.8 ± 3.9 | 1.70 ± 0.04 | 3.51 ± 0.07 | 5.21 ± 0.07 | 2.07 ± 0.07 | 50.0 ± 1.3 | 32.2 ± 1.0 | |
| Distance (cm) | Treatment | Lutein (µg g−1 DW) | Chl b (mg g−1 DW) | Chl a (mg g−1 DW) | Chl a + b | Chl a/b | β-Carotene (µg g−1 DW) | Chl/Car |
|---|---|---|---|---|---|---|---|---|
| 5 | CTR | 91.7 ± 3.0 | 1.55 ± 0.11 | 2.15 ± 0.13 | 3.70 ± 0.15 | 1.40 ± 0.14 | 36.3 ± 1.2 | 29.0 ± 1.7 |
| UV-B | 93.4 ± 1.2 | 1.57 ± 0.03 | 2.28 ± 0.11 | 3.85 ± 0.12 | 1.45 ± 0.07 | 36.4 ± 1.3 | 29.6 ± 0.9 | |
| UV-C | 95.1 ± 2.2 | 1.46 ± 0.05 | 2.06 ± 0.05 | 3.51 ± 0.09 | 1.41 ± 0.04 | 35.4 ± 1.4 | 27.0 ± 0.1 | |
| 15 | CTR | 87.9 ± 2.6 | 1.53 ± 0.06 | 2.06 ± 0.18 | 3.59 ± 0.20 | 1.35 ± 0.13 | 32.5 ± 1.1 | 29.8 ± 1.6 |
| UV-B | 87.8 ± 3.8 | 1.51 ± 0.07 | 2.22 ± 0.09 | 3.73 ± 0.06 | 1.47 ± 0.12 | 36.9 ± 1.4 | 29.9 ± 0.9 | |
| UV-C | 91.4 ± 1.8 | 1.45 ± 0.04 | 2.15 ± 0.25 | 3.60 ± 0.23 | 1.49 ± 0.19 | 35.2 ± 0.1 | 28.4 ± 1.7 | |
| Mean effect | ||||||||
| 5 cm | 93.4 ± 1.2 | 1.53 ± 0.04 | 2.16 ± 0.06 | 3.69 ± 0.08 | 1.42 ± 0.05 | 36.1 ± 0.7 | 28.5 ± 0.7 | |
| 15 cm | 89.1 ± 1.6 | 1.50 ± 0.03 | 2.14 ± 0.10 | 3.64 ± 0.09 | 1.44 ± 0.08 | 34.9 ±0.8 | 29.4 ± 0.8 | |
| CTR | 89.8 ± 2.0 | 1.54 ± 0.06 | 2.10 ± 0.10 | 3.64 ± 0.12 | 1.38 ± 0.09 | 34.4 ± 1.1 | 29.4 ± 1.1 | |
| UV-B | 90.7 ± 2.2 | 1.55 ± 0.04 | 2.25 ± 0.06 | 3.79 ± 0.06 | 1.46 ± 0.06 | 36.7 ± 0.9 | 29.8 ± 0.6 | |
| UV-C | 93.2 ± 1.5 | 1.45 ± 0.03 | 2.10 ± 0.12 | 3.55 ± 0.11 | 1.45 ± 0.09 | 35.3 ± 0.6 | 27.7 ± 0.8 | |
| Pearson Coefficient (|r|) | ||
|---|---|---|
| Can 1 | Can 2 | |
| Lutein | 0.87 * | 0.04 |
| Chl b | 0.80 * | 0.02 |
| Chl a | 0.96 ** | 0.11 |
| β-carotene | 0.96 ** | 0.09 |
| Chl a/Chl b | 0.87 * | 0.14 |
| Chl a + b | 0.97 ** | 0.10 |
| Chl/car | 0.58 | 0.21 |
| ABTS | 0.79 * | 0.46 |
| DPPH | 0.48 | 0.52 |
| Disease severity | 0.74 * | 0.40 |
| DGCI ratio | 0.45 | 0.82 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santin, M.; Caturegli, L.; Gagliardi, L.; Luglio, S.M.; Magni, S.; Pellegrini, E.; Pisuttu, C.; Raffaelli, M.; Volterrani, M.; Incrocci, L. Innovative Techniques for Managing Dollar Spot in Warm- and Cool-Season Turfgrasses: The Case of UV-B and UV-C Irradiations. Agriculture 2025, 15, 784. https://doi.org/10.3390/agriculture15070784
Santin M, Caturegli L, Gagliardi L, Luglio SM, Magni S, Pellegrini E, Pisuttu C, Raffaelli M, Volterrani M, Incrocci L. Innovative Techniques for Managing Dollar Spot in Warm- and Cool-Season Turfgrasses: The Case of UV-B and UV-C Irradiations. Agriculture. 2025; 15(7):784. https://doi.org/10.3390/agriculture15070784
Chicago/Turabian StyleSantin, Marco, Lisa Caturegli, Lorenzo Gagliardi, Sofia Matilde Luglio, Simone Magni, Elisa Pellegrini, Claudia Pisuttu, Michele Raffaelli, Marco Volterrani, and Luca Incrocci. 2025. "Innovative Techniques for Managing Dollar Spot in Warm- and Cool-Season Turfgrasses: The Case of UV-B and UV-C Irradiations" Agriculture 15, no. 7: 784. https://doi.org/10.3390/agriculture15070784
APA StyleSantin, M., Caturegli, L., Gagliardi, L., Luglio, S. M., Magni, S., Pellegrini, E., Pisuttu, C., Raffaelli, M., Volterrani, M., & Incrocci, L. (2025). Innovative Techniques for Managing Dollar Spot in Warm- and Cool-Season Turfgrasses: The Case of UV-B and UV-C Irradiations. Agriculture, 15(7), 784. https://doi.org/10.3390/agriculture15070784








